Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments
Abstract
1. Introduction
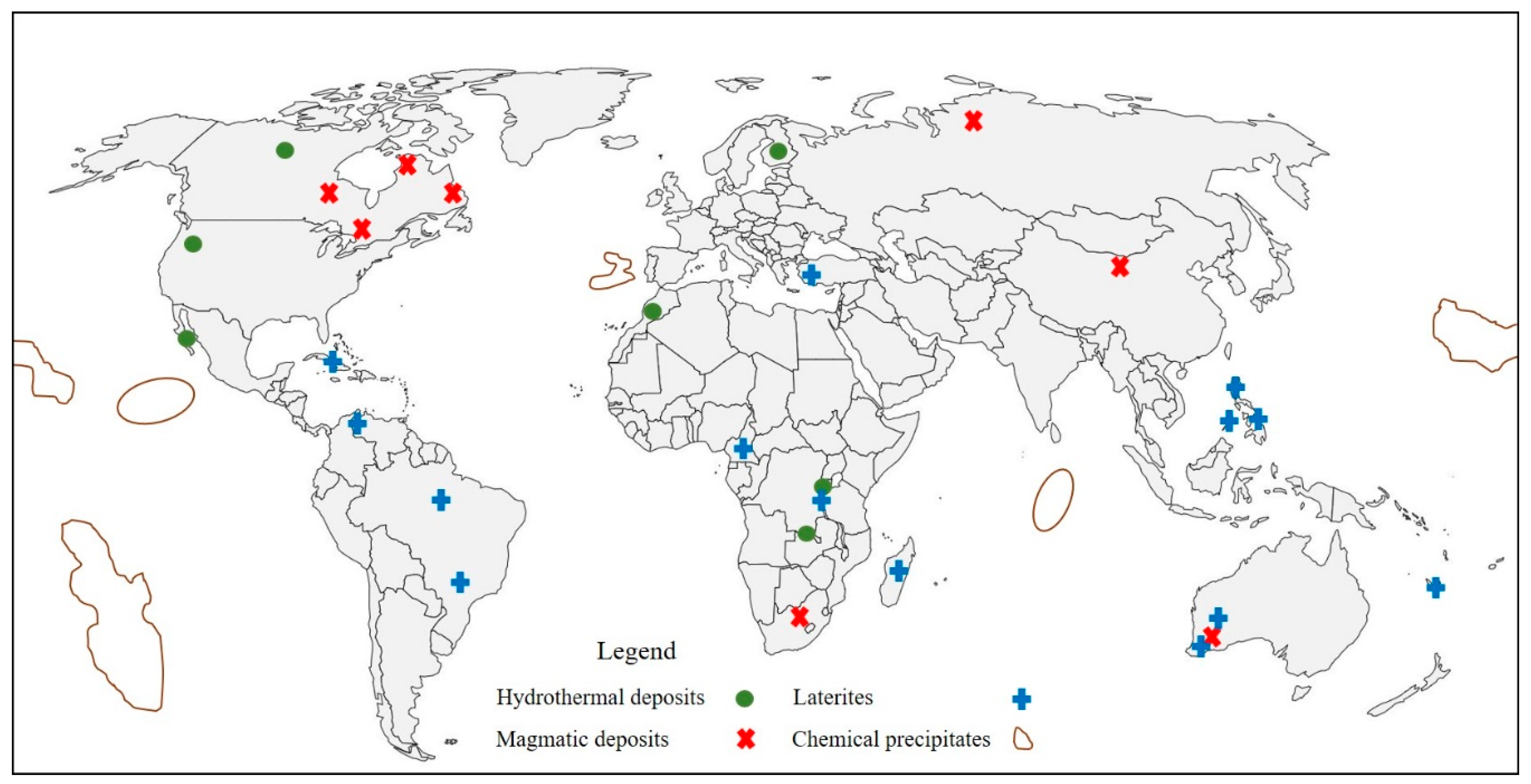
2. Geology and Characteristics of Co-Bearing Ore Deposits
2.1. Hydrothermal Deposits
2.2. Magmatic Deposits
2.3. Laterites
2.4. Chemical Precipitates
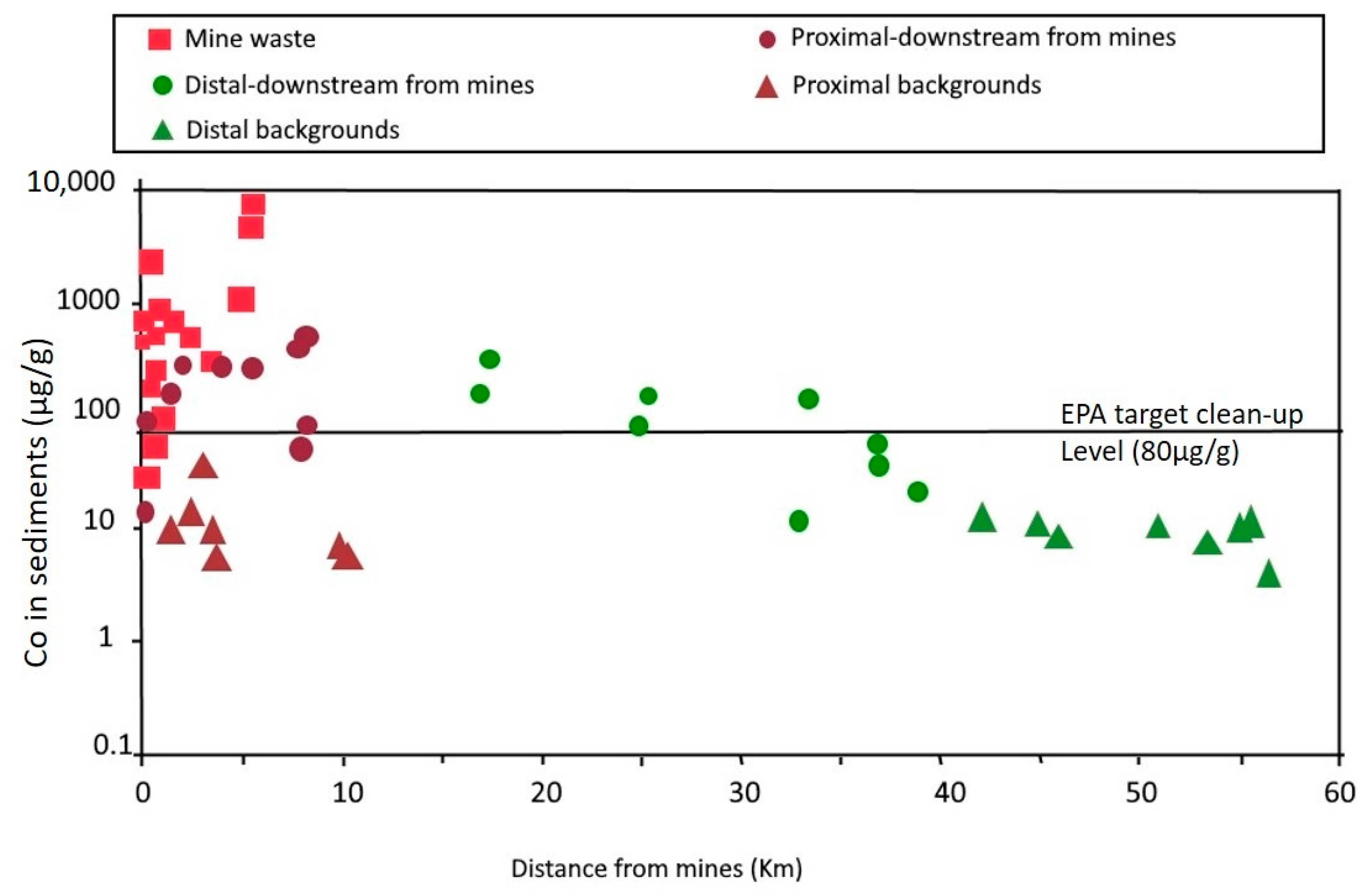
3. Geochemistry of Cobalt in Mine Wastes
3.1. Cobalt in Mine Waters
| Mine/Region | Ore/Deposit Type | Period of Mining | Type | Mean/Range Co Concentration (µg/L) | Reference |
|---|---|---|---|---|---|
| Kabwe mine, Zambia | Pb–Zn | 1903–1994 | Sludge resulting from chemical leaching | 34,400,000 | Sracek et al., 2010 [35] |
| Cobalt, Ontario, Canada | Ag–As–Bi–Co | Not recorded | Ground water samples | 140–1800 | Percival, et al., 1996 [36] |
| Cobalt, Ontario, Canada | Ag–As–Bi–Co | Not recorded | Surface water samples | 0.5–2028 | Percival, et al., 1996 [36] |
| Idaho Cobalt Belt (ICB), USA | Co–Cu–Au | Early 1900s–1967 | Mine water (adits and open pits) | 11,000 | Gray and Eppinger, 2012 [38] |
| San Telmo mine, Spain | pyrite | 1970–1989 | Pyrite leachate, pH 0.61–0.82 | 18,689 | España et al., 2008 [40] |
| Peña de Hierro mine, Spain | Pyrite–Cu | Mid-19th century–1966 | Stream water from the mine | 599–6100 | Romero et al., 2011 [41] |
| Savage River mine, Tasmania, Australia | magnetite | 1967–1982 | Pore waters from old tailings | 5000 | Jackson and Parbhakar-Fox, 2016 [42] |
| Katanga province, DRC | Co–Cu | Before 1960–present | Mining effluent and water | 3164 | Atibu et al., 2013 [39] |
| Rio Piscinas area, Italy | Pb–Zn | beginning of 19th century–1992 | Groundwater samples | 1500–2900 | Concas et al., 2006 [43] |
| Pyrite–uranium mine at Rudki, Poland | pyrite–U | Early 1900–1968 | Acid pool waters from the mine tailings | 303–1439 | Migaszewski et al., 2015 [44] |
| Darrehzar porphyry copper mine, Iran | Cu | Not recorded | Mine water flowing from the mine | 831 | Soltani et al., 2014 [45] |
| Haveri mine, Finland | Au–Cu | 1942–1961 | Ground and surface water | 10–866 | Parviainen, 2009 [46] |
| Banjas area, northern Portugal | As–Au | 1864–1890 | Spring and groundwater proximal to the mine | 11 | Carvalho et al., 2014 [47] |
3.2. Cobalt in Tailings and Mine-Affected Soils and Sediments
| Mine/Region | Ore/Deposit Type | Period of Mining | Tonnage/Type | Mean/Range Co Concentration (mg/kg) | Reference |
|---|---|---|---|---|---|
| Kabwe mine, Zambia | Pb–Zn | 1903–1984 | Oxidised tailings pond | 14,165 | Sracek et al., 2010 [35] |
| Katanga province, DRC | Co–Cu | Before 1960–present | Freshly processed tailings | 6100 | Lutandula and Maloba, 2013 [58] |
| Haveri mine, Finland | Au–Cu | 1942–1961 | 1.5 Mt Oxidised, weathered | 24–329 | Parviainen 2009 [46] |
| Algares area, Portugal | Pb–As sulphides | 1963–1971 | Oxidised zone | 97–157 | Bobos et al., 2006 [63] |
| pyrrhotite mine, Morocco | pyrrhotite | 1964–1981 | >0.4 Mt Oxidised tailings | 60–80 | Hakkou et al., 2008 [64] |
| Azegour mine, Morocco | Cu–Mo–W | 1932–1971 | 850,000 t oxidised tailing impoundments, | 40–440 | Goumih et al., 2013 [65] |
| Skellefte district sulphide ore field, Sweden | Zn–Cu | Not recorded | Freshly processed tailings | 57.8 | Gleisner and Herbert, 2002 [59] |
| The Aljustrel mine (SW Portugal | pyrite | Not recorded | Tailings from roasting pyrite | 59 | Candeias et al., 2011 [66] |
| Virgina Au–pyrite belt, USA | Au–pyrite | 1909–1945 | 120,000 t primary unoxidised and oxidised | 44 | Seal II et al., 2008 [67] |
| Rio Piscinas area, Italy | Pb–Zn | beginning of 19th century–1992 | Not described | 15–43 | Concas et al., 2006 [43] |
| Kidston gold mine, Australia | Au | 1985–2001 | Un-oxidised tailings | 2.32–29.20 | Edraki et al., 2019 [68] |
| Mine/Region | Ore/Deposit Type | Period of Mining | Material Type | Mean/Range Co Concentration (mg/kg) | Reference |
|---|---|---|---|---|---|
| Kolwezi district, Province of Lualaba, DRC | Co–Cu | Before 1960–present | Stream sediments | 19.4–18,434 | Atibu et al., 2018 [69] |
| Kolwezi district, Province of Lualaba, DRC | Co–Cu | Before 1960–present | Soil samples | 6.4–21,134 | Atibu et al., 2018 [69] |
| Katanga province, DRC | Co–Cu | Before 1960–present | Stream sediments | 59.7–13,199 | Atibu et al., 2013 [39] |
| Idaho Cobalt Belt (ICB), USA | Co–Cu–Au | Early 1900s–1967 | Stream sediments | 14–520 | Gray and Eppinger, 2012 [38] |
| Idaho Cobalt Belt (ICB), USA | Co–Cu–Au | Early 1900s–1967 | Soil samples | 29–940 | Gray and Eppinger, 2012 [38] |
| Rio Piscinas area, Italy | Pb–Zn | beginning of 19th century–1992 | Stream sediments | 9–38 | Concas et al., 2006 [43] |
| The Kettara Mine, Morocco | Ochre–pyrrhotite | 1933–1982 | Soil samples | 25.14 | El Amari et al., 2014 [70] |
| The Kettara Mine, Morocco | Ochre-pyrrhotite | 1933–1982 | Stream sediments | 27.62 | El Amari et al., 2014 [70] |
| Maldon, Victoria, Australia | Au | 1850s–not reported | Soil samples | 25 | Abraham et al., 2018 [71] |
| Hagan Mine, Egypt Bay, Maine, USA | Cu–Ag | 1877–1885 | Soil samples | 1.9–21.3 | Osher et al., 2006 [72] |
| Panasqueira mine area, Portugal | Sn–W | 1898–2001 | Soil samples | 7–8 | Candeias et al., 2015 [73] |
| Alto da Várzea radium mine, Portugal | Ra–U | 1911–1922 | Stream sediments | 3.8–4.8 | Antunes et al., 2018 [74] |
3.3. Cobalt in Mine-Affected Plants
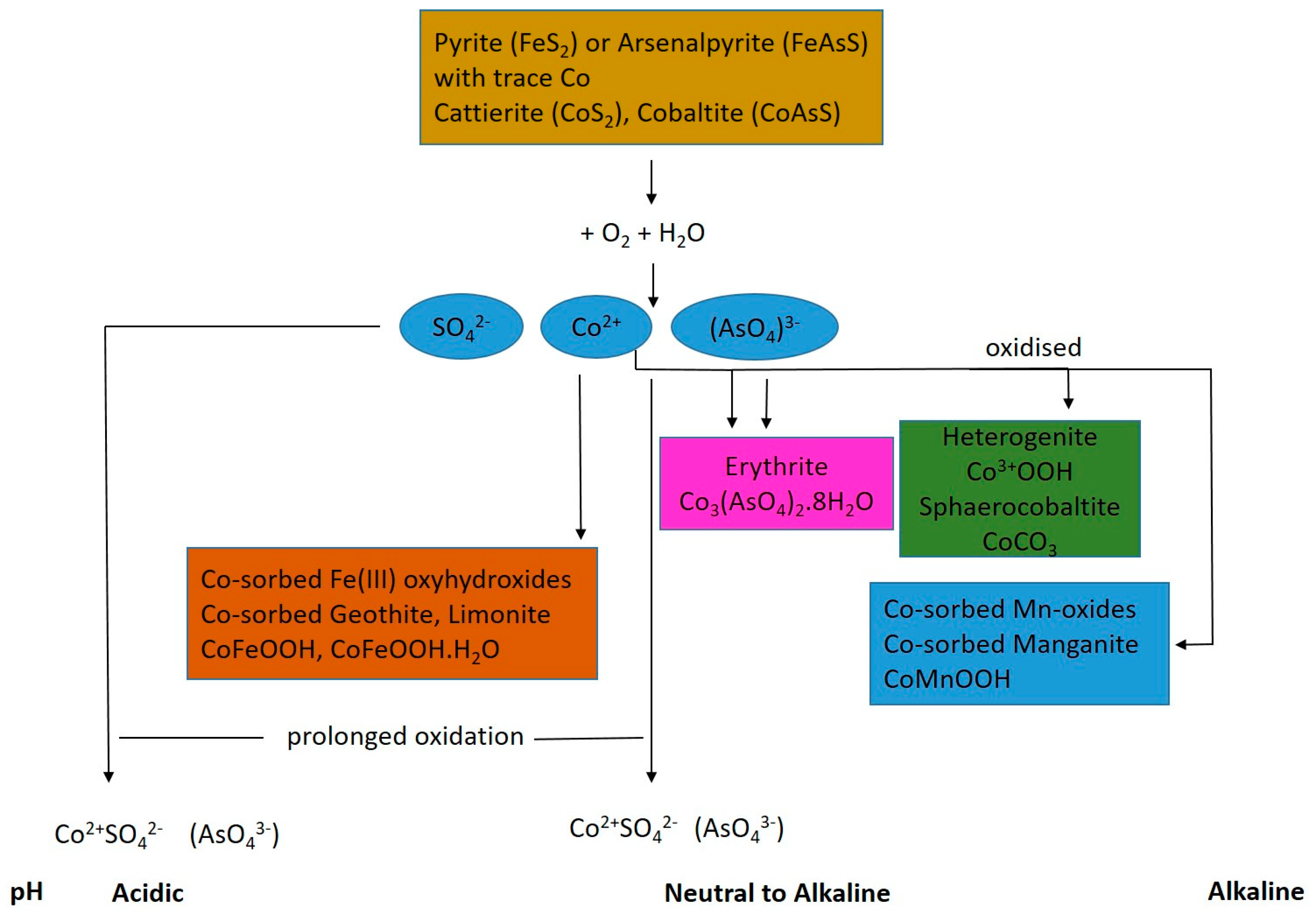
4. Mineralogy of Cobalt in Mine Wastes
| Mine/Region | Ore/Deposit Type | Period of Mining | Type | Mean/Range Co Concentration (mg/kg) | Reference |
|---|---|---|---|---|---|
| Kolwezi district, Province of Lualaba, DRC | Co–Cu | Before 1960–present | Phalaris arundinacea L. | 9–5050.80 | Atibu et al., 2018 [69] |
| Copperbelt Province, Zambia | Co–Cu | Before 1960–present | Cassava leaves (Manihot esculenta crantz) | 24 | Kříbek et al., 2014 [82] |
| Co-Ni-mine, Southern Morocco | Co–Ni | Not reported | Parsley (Petroselinum vulgare) | 20.2–69.4 | El Hamiani et al., 2015 [83] |
| Rosemary (Rosmarinus officinalis) | 39.1–54.4 | ||||
| Fava bean (Vicia faba) | 74.6 | ||||
| Ishiagu, South East Nigeria | Pb–Zn | Not reported | Roots (Clotalariaretusa and Andropogontectorum) | 13.40–89.75 | Ogbonna et al., 2015 [97] |
| Stems (Imperatacylindrica and Alchorneacordifolia) | 2.20–78.20 | ||||
| Palão and Pinheiro mines, Portugal | Pb–Zn | Not reported | Elatine macropoda | 127.8 | Prasad et al., 2006 [98] |
| Shangla District, Pakistan | Cr | Not reported | Roots (N. cataria) | 23 | Nawab et al., 2015 [99] |
| Sukinda chromite mine, India | Cr | Not reported | Solanum surattense | 9.9 | Samantaray et al., 2001 [100] |
| Mineral Name | Elemental Composition | References |
|---|---|---|
| Cobaltite | (Co,Fe)AsS | Harris et al., 2003 [101]; Kelly et al., 2007 [87]; Percival et al., 2007 [88]; Loredo et al., 2008 [85] |
| Carrollite | CoCu2S4 | Chen et al., 2016 [102] |
| Sphaerocobaltite | CoCO3 | Vítková et al., 2010 [103] |
| Cobaltpentlandite | (Co-Fe)9S8 | Vítková et al., 2010 [103] |
| Safflorite | (Co,Fe,Ni)As2 | Clarke, 2017 [93] |
| Skutterudite | (Co,Ni,Fe)As3–x | Clarke, 2017 [93] |
| Erythrite | Co3(AsO4)2·8H2O | Percival et al., 2007 [88]; Loredo et al., 2008 [85]; Clarke, 2017 [93] |
| Bieberite | CoSO4·7H2O | Sracek et al., 2010 [35]; Mees et al., 2013 [104] |
| Moorhouseite | CoSO4·H2O | Sracek et al., 2010 [35] |
| Mineral Name | Elemental Composition | References |
|---|---|---|
| Fe oxyhydroxides | FeOOH | Holmström and Öhlander, 2001 [105]; Sracek et al., 2010 [96]; Queiroz et al., 2018 [106] |
| Pyrite | FeS2 | Moncur et al., 2005 [107]; Jackson and Parbhakar-Fox, 2016 [42]; Zhang et al., 2020 [108] |
| Arsenopyrite | FeAsS | Assawincharoenkij et al., 2018 [109] |
| Pyrrhotite | Fe(1–x)S | Moncur et al., 2005 [107]; Heikkinen and Räisänen, 2008 [110] |
| Co-poor bloedite | Na2(Co,Mg)(SO4)2·4H2O | Sracek et al., 2010 [35] |
| Bornite | Cu5FeS4 | Loredo et al., 2008 [85] |
| Chalcocite | Cu2S | Loredo et al., 2008 [85] |
| Covellite | CuS | Loredo et al., 2008 [85] |
| Chalcopyrite | CuFeS2 | Assawincharoenkij et al., 2018 [109] |
5. Microbiology of Cobalt in Mine Wastes
6. Geochemical-Mineralogical-Microbiological Controls on Cobalt Mobility in Mining-Affected Environments
6.1. Impact of Eh-pH on Co Geochemistry
6.2. Impact of Microbial Activity
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.G. Always the bridesmaid, never the bride: Cobalt geology and resources. Appl. Earth Sci. 2001, 110, 75–80. [Google Scholar] [CrossRef]
- Faroon, O.M.; Abadin, H.; Keith, S.; Osier, M.; Chappell, L.L.; Diamond, G.; Sage, G. Toxicological Profile for Cobalt; US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp33.pdf (accessed on 5 November 2020).
- Gál, J.; Hursthouse, A.; Tatner, P.; Stewart, F.; Welton, R. Cobalt and secondary poisoning in the terrestrial food chain: Data review and research gaps to support risk assessment. Environ. Int. 2008, 34, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability. COM 474 Final. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474 (accessed on 7 November 2020).
- Barton, I.F.; Yang, H.; Barton, M.D. The mineralogy, geochemistry, and metallurgy of cobalt in the rhombohedral carbonates. Can. Mineral. 2014, 52, 653–670. [Google Scholar] [CrossRef]
- Slack, J.F.; Kimball, B.E.; Shedd, K.B. Cobalt (No. 1802-F); US Geological Survey: Reston, VA, USA, 2017.
- Hannis, S.; Bide, T. Cobalt; British Geological Survey: Nottingham, UK, 2009.
- Barceloux, D.G.; Barceloux, D. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef]
- Banza, C.L.N.; Nawrot, T.S.; Haufroid, V.; Decrée, S.; De Putter, T.; Smolders, E.; Nemery, B. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009, 109, 745–752. [Google Scholar] [CrossRef]
- Cheyns, K.; Nkulu, C.B.L.; Ngombe, L.K.; Asosa, J.N.; Haufroid, V.; De Putter, T.; Nemery, B. Pathways of human exposure to cobalt in Katanga, a mining area of the DR Congo. Sci. Total Environ. 2014, 490, 313–321. [Google Scholar] [CrossRef]
- Pourret, O.; Lange, B.; Houben, D.; Colinet, G.; Shutcha, M.; Faucon, M.P. Modeling of cobalt and copper speciation in metalliferous soils from Katanga (Democratic Republic of Congo). J. Geochem. Explor. 2015, 149, 87–96. [Google Scholar] [CrossRef]
- Roberts, S.; Gunn, G. Cobalt. In Critical Metals Handbook; John Wiley & Sons, Inc.: New York, NY, USA, 2014; pp. 122–149. [Google Scholar]
- Ahmed, A.H.; Arai, S.; Ikenne, M. Mineralogy and paragenesis of the Co-Ni arsenide ores of Bou Azzer, Anti-Atlas, Morocco. Econ. Geol. 2009, 104, 249–266. [Google Scholar] [CrossRef]
- Mudd, G.M.; Weng, Z.; Jowitt, S.M.; Turnbull, I.D.; Graedel, T.E. Quantifying the recoverable resources of by-product metals: The case of cobalt. Ore Geol. Rev. 2013, 55, 87–98. [Google Scholar] [CrossRef]
- Van Langendonck, S.; Muchez, P.; Dewaele, S.; Kalubi, A.K.; Cailteux, J. Petrographic and mineralogical study of the sediment-hosted Cu-Co ore deposit at Kambove West in the central part of the Katanga Copperbelt (DRC). Geol. Belg. 2013, 16, 91–104. [Google Scholar]
- Annels, A.E.; Simmonds, J.R. Cobalt in the Zambian copperbelt. Precambrian Res. 1984, 25, 75–98. [Google Scholar] [CrossRef]
- Nold, J.L. The Idaho cobalt belt, northwestern United States—A metamorphosed Proterozoic exhalative ore district. Miner. Depos. 1990, 25, 163–168. [Google Scholar] [CrossRef]
- Slack, J.F.; Johnson, C.A.; Causey, J.D.; Lund, K.; Schulz, K.J.; Gray, J.E.; Eppinger, R.G. Descriptive and Geoenvironmental Model for Co-Cu-Au Deposits in METASEDIMENTARY Rocks: Chapter G in Mineral Deposit Models for Resource Assessment (No. 2010-5070-G); US Geological Survey: Reston, VA, USA, 2013.
- Carr, M.H.; Turekian, K.K. The geochemistry of cobalt. Geochim. Cosmochim. Acta 1961, 23, 9–60. [Google Scholar] [CrossRef]
- Schmandt, D.; Broughton, D.; Hitzman, M.W.; Plink-Bjorklund, P.; Edwards, D.; Humphrey, J. The Kamoa copper deposit, Democratic Republic of Congo: Stratigraphy, diagenetic and hydrothermal alteration, and mineralization. Econ. Geol. 2013, 108, 1301–1324. [Google Scholar] [CrossRef]
- Slack, J.F. Strata-bound Fe-Co-Cu-Au-Bi-Y-REE deposits of the Idaho cobalt belt: Multistage hydrothermal mineralization in a magmatic-related iron oxide copper-gold system. Econ. Geol. 2012, 107, 1089–1113. [Google Scholar] [CrossRef]
- Cailteux, J.L.H.; Kampunzu, A.B.; Lerouge, C.; Kaputo, A.K.; Milesi, J.P. Genesis of sediment-hosted stratiform copper–cobalt deposits, central African Copperbelt. J. Afr. Earth Sci. 2005, 42, 134–158. [Google Scholar] [CrossRef]
- Hitzman, M.W.; Bookstrom, A.A.; Slack, J.F.; Zientek, M. Cobalt—Styles of Deposits and the Search for Primary Deposits; U.S. Geological Survey Open-File Report: Reston, VA, USA, 2017; p. 47.
- Eckstrand, O.R.; Hulbert, L.J. Magmatic nickel-copper-platinum group element deposits. Mineral deposits of canada: A synthesis of major deposit types, district metallogeny, the evolution of geological provinces, and exploration methods. Geol. Assoc. Can. Miner. Depos. Div. Spec. Publ. 2007, 5, 205–222. [Google Scholar]
- Butt, C.R.; Cluzel, D. Nickel laterite ore deposits: Weathered serpentinites. Elements 2013, 9, 123–128. [Google Scholar] [CrossRef]
- Elias, M. Overview of the Australian Nickel-Cobalt Industry. AUSIMM Monogr. 2013, 2, 1723–1727. [Google Scholar]
- Dublet, G.; Juillot, F.; Brest, J.; Noël, V.; Fritsch, E.; Proux, O.; Morin, G. Vertical changes of the Co and Mn speciation along a lateritic regolith developed on peridotites (New Caledonia). Geochim. Cosmochim. Acta 2017, 217, 1–15. [Google Scholar] [CrossRef]
- Berger, V.I.; Singer, D.A.; Bliss, J.D.; Moring, B.C. Ni-Co Laterite Deposits of the World; Database and Grade and Tonnage Models; U.S. Geological Survey Open-File Report: Reston, VA, USA, 2017; p. 26.
- Dzemua, G.L.; Gleeson, S.A. Petrography, mineralogy, and geochemistry of the Nkamouna serpentinite: Implications for the formation of the Cobalt-Manganese Laterite Deposit, Southeast Cameroon. Econ. Geol. 2012, 107, 25–41. [Google Scholar] [CrossRef]
- Dzemua, G.L.; Gleeson, S.A.; Schofield, P.F. Mineralogical characterization of the Nkamouna Co–Mn laterite ore, Southeast Cameroon. Miner. Depos. 2013, 48, 155–171. [Google Scholar] [CrossRef]
- Glasby, G.P.; Ren, X.; Shi, X.; Pulyaeva, I.A. Co–Rich Mn crusts from the Magellan Seamount cluster: The long journey through time. Geo Mar. Lett. 2007, 27, 315–323. [Google Scholar] [CrossRef]
- Canterford, J.H. Sulphuric acid leaching of cobalt-bearing manganese wad. Hydrometallurgy 1985, 14, 35–46. [Google Scholar] [CrossRef]
- Krupka, K.M.; Serne, R.J. Geochemical Factors Affecting the Behaviour of Antimony, Cobalt, Europium, Technetium, and Uranium in Vadose Zone Sediments (No. PNNL-14126); Pacific Northwest National Lab (PNNL): Richland, WA, USA, 2002.
- Sracek, O.; Veselovský, F.; Kříbek, B.; Malec, J.; Jehlička, J. Geochemistry, mineralogy and environmental impact of precipitated efflorescent salts at the Kabwe Cu–Co chemical leaching plant in Zambia. Appl. Geochem. 2010, 25, 1815–1824. [Google Scholar] [CrossRef]
- Percival, J.B.; Dumaresq, C.G.; Kwong, Y.T.J.; Hendry, K.B.; Michel, F.A. Arsenic in surface waters, Cobalt, Ontario. Curr. Res. 1996, 100, 137–146. [Google Scholar]
- Zhu, Y.N.; Zhang, X.H.; Chen, Y.D.; Zeng, H.H.; Liu, J.; Liu, H.L.; Wang, X.M. Characterisation, dissolution and solubility of synthetic erythrite [Co3 (AsO4) 2.8 H2O] and annabergite [Ni3 (AsO4) 2.8 H2O] at 25 °C. Can. Metall. Q. 2013, 52, 7–17. [Google Scholar] [CrossRef]
- Gray, J.E.; Eppinger, R.G. Distribution of Cu, Co, As, and Fe in mine waste, sediment, soil, and water in and around mineral deposits and mines of the Idaho Cobalt Belt, USA. Appl. Geochem. 2012, 27, 1053–1062. [Google Scholar] [CrossRef]
- Atibu, E.K.; Devarajan, N.; Thevenon, F.; Mwanamoki, P.M.; Tshibanda, J.B.; Mpiana, P.T.; Poté, J. Concentration of metals in surface water and sediment of Luilu and Musonoie Rivers, Kolwezi-Katanga, Democratic Republic of Congo. Appl. Geochem. 2013, 39, 26–32. [Google Scholar] [CrossRef]
- España, J.S.; Toril, E.G.; Pamo, E.L.; Amils, R.; Ercilla, M.D.; Pastor, E.S.; San Martín-Úriz, P. Biogeochemistry of a hyperacidic and ultraconcentrated pyrite leachate in San Telmo mine (Iberian Pyrite Belt, Spain). Water Air Soil Pollut. 2008, 194, 243–257. [Google Scholar] [CrossRef]
- Romero, A.; González, I.; Galán, E. Stream water geochemistry from mine wastes in Peña de Hierro, Riotinto area, SW Spain: A case of extreme acid mine drainage. Environ. Earth Sci. 2011, 62, 645–656. [Google Scholar] [CrossRef]
- Jackson, L.M.; Parbhakar-Fox, A. Mineralogical and geochemical characterization of the Old Tailings Dam, Australia: Evaluating the effectiveness of a water cover for long-term AMD control. Appl. Geochem. 2016, 68, 64–78. [Google Scholar] [CrossRef]
- Concas, A.; Ardau, C.; Cristini, A.; Zuddas, P.; Cao, G. Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 2006, 63, 244–253. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Dołęgowska, S.; Hałas, S.; Krzciuk, K.; Gebus, B. Assessing the impact of Serwis mine tailings site on farmers’ wells using element and isotope signatures (Holy Cross Mountains, south-central Poland). Environ. Earth Sci. 2015, 74, 629–647. [Google Scholar] [CrossRef]
- Soltani, N.; Moore, F.; Keshavarzi, B.; Sharifi, R. Geochemistry of trace metals and rare earth elements in stream water, stream sediments and acid mine drainage from Darrehzar Copper Mine, Kerman, Iran. Water Qual. Expo. Health 2014, 6, 97–114. [Google Scholar] [CrossRef]
- Parviainen, A. Tailings mineralogy and geochemistry at the abandoned Haveri Au–Cu mine, SW Finland. Mine Water Environ. 2009, 28, 291. [Google Scholar] [CrossRef]
- Carvalho, P.C.; Neiva, A.M.; Silva, M.M.; da Silva, E.A.F. Geochemical comparison of waters and stream sediments close to abandoned Sb-Au and As-Au mining areas, northern Portugal. Geochemistry 2014, 74, 267–283. [Google Scholar] [CrossRef]
- Hubau, A.; Guezennec, A.G.; Joulian, C.; Falagán, C.; Dew, D.; Hudson-Edwards, K.A. Bioleaching to reprocess sulfidic polymetallic primary mining residues: Determination of metal leaching mechanisms. Hydrometallurgy 2020, 197, 105484. [Google Scholar] [CrossRef]
- Cornell, R.M. Simultaneous incorporation of Mn, Ni and Co in the goethite (α-FeOOH) structure. Clay Miner. 1991, 26, 427–430. [Google Scholar] [CrossRef]
- Ohnuki, T.; Kozai, N. Sorption behavior of cobalt on manganese dioxide, smectite and their mixture. Radiochim. Acta 1995, 68, 203–207. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Gomes, M.E.P. Hydrochemistry of superficial waters in the Adoria mine area (Northern Portugal): Environmental implications. Environ. Earth Sci. 2012, 65, 363–372. [Google Scholar] [CrossRef]
- Nagpal, N.K. Technical Report, Water Quality Guidelines for Cobalt; Water Protection Section, Water, Air and Climate Change Branch, Ministry of Water, Land and Air Protection: Victoria, BC, Canada, 2004; p. 59.
- CCME (Canadian Council of Ministers of the Environment). Canadian Environmental Quality Guidelines Summary Table. 2010. Available online: http://st-ts.ccme.ca (accessed on 26 August 2020).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: First Addendum to The Fourth Edition; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- NYSDEC. Water Quality Regulations: Surface and Groundwater Classifications and Standards. New York State Codes, Rules and Regulations; Title 6, Chapter X, Parts 700–705; NY State Department of Conservation: Albany, NY, USA, 1986.
- USEPA (United States Environmental Protection Agency). Cleanup Regulations and Standards. 2011. Available online: http://www.epa.gov/cleanup/regs.htm (accessed on 26 August 2020).
- Fisher, K.G. Cobalt processing developments. In Proceedings of the Sixth Southern African Base Metals Conference, Phalaborwa, South Africa, 18–20 July 2011; Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2011; pp. 237–258. [Google Scholar]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Gleisner, M.; Herbert Jr, R.B. Sulfide mineral oxidation in freshly processed tailings: Batch experiments. J. Geochem. Explor. 2002, 76, 139–153. [Google Scholar] [CrossRef]
- Gavelin, S. Sulphide mineralization in the Skellefte district, Northern Sweden, and its relation to regional granitization. Econ. Geol. 1955, 50, 814–831. [Google Scholar] [CrossRef]
- Pourret, O.; Lange, B.; Bonhoure, J.; Colinet, G.; Decrée, S.; Mahy, G.; Faucon, M.P. Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo). Appl. Geochem. 2016, 64, 43–55. [Google Scholar] [CrossRef]
- Narendrula, R.; Nkongolo, K.K.; Beckett, P. Comparative soil metal analyses in sudbury (Ontario, Canada) and lubumbashi (Katanga, DR-Congo). Bull. Environ. Contam. Toxicol. 2012, 88, 187–192. [Google Scholar] [CrossRef]
- Bobos, I.; Durães, N.; Noronha, F. Mineralogy and geochemistry of mill tailings impoundments from Algares (Aljustrel), Portugal: Implications for acid sulfate mine waters formation. J. Geochem. Explor. 2006, 88, 1–5. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussiere, B. Acid mine drainage at the abandoned Kettara mine (Morocco): 2. Mine waste geochemical behavior. Mine Water Environ. 2008, 27, 160–170. [Google Scholar] [CrossRef]
- Goumih, A.; El Adnani, M.; Hakkou, R.; Benzaazoua, M. Geochemical behaviour of mine tailings and waste rock at the abandoned Cu–Mo–W Azegour mine (Occidental High Atlas, Morocco). Mine Water Environ. 2013, 32, 121–132. [Google Scholar] [CrossRef]
- Candeias, C.; Da Silva, E.F.; Salgueiro, A.R.; Pereira, H.G.; Reis, A.P.; Patinha, C.; Avila, P.H. Assessment of soil contamination by potentially toxic elements in the Aljustrel mining area in order to implement soil reclamation strategies. Land Degrad. Dev. 2011, 22, 565–585. [Google Scholar] [CrossRef]
- Seal, R.R., II; Hammarstrom, J.M.; Johnson, A.N.; Piatak, N.M.; Wandless, G.A. Environmental geochemistry of a Kuroko-type massive sulfide deposit at the abandoned Valzinco mine, Virginia, USA. Appl. Geochem. 2008, 23, 320–342. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Mulligan, D.; Fegan, W.; Munawar, A. Geochemical characteristics of rehabilitated tailings and associated seepages at Kidston gold mine, Queensland, Australia. Int. J. Min. Reclam. Environ. 2019, 33, 133–147. [Google Scholar] [CrossRef]
- Atibu, E.K.; Lacroix, P.; Sivalingam, P.; Ray, N.; Giuliani, G.; Mulaji, C.K.; Poté, J. High contamination in the areas surrounding abandoned mines and mining activities: An impact assessment of the Dilala, Luilu and Mpingiri Rivers, Democratic Republic of the Congo. Chemosphere 2018, 191, 1008–1020. [Google Scholar] [CrossRef]
- El Amari, K.; Valera, P.; Hibti, M.; Pretti, S.; Marcello, A.; Essarraj, S. Impact of mine tailings on surrounding soils and ground water: Case of Kettara old mine, Morocco. J. Afr. Earth Sci. 2014, 100, 437–449. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. Assessment of potentially toxic metal contamination in the soils of a legacy mine site in Central Victoria, Australia. Chemosphere 2018, 192, 122–132. [Google Scholar] [CrossRef]
- Osher, L.J.; Leclerc, L.; Wiersma, G.B.; Hess, C.T.; Guiseppe, V.E. Heavy metal contamination from historic mining in upland soil and estuarine sediments of Egypt Bay, Maine, USA. Estuar. Coast. Shelf Sci. 2006, 70, 169–179. [Google Scholar] [CrossRef]
- Candeias, C.; Ávila, P.F.; Da Silva, E.F.; Teixeira, J.P. Integrated approach to assess the environmental impact of mining activities: Estimation of the spatial distribution of soil contamination (Panasqueira mining area, Central Portugal). Environ. Monit. Assess. 2015, 187, 135. [Google Scholar] [CrossRef]
- Antunes, I.M.H.R.; Neiva, A.M.R.; Albuquerque, M.T.D.; Carvalho, P.C.S.; Santos, A.C.T.; Cunha, P.P. Potential toxic elements in stream sediments, soils and waters in an abandoned radium mine (central Portugal). Environ. Geochem. Health 2018, 40, 521–542. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Gandy, C.J.; Smith, J.W.N.; Jarvis, A.P. Attenuation of mining-derived pollutants in the hyporheic zone: A review. Sci. Total Environ. 2007, 373, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.C.; Harvey, J.W. Reactive uptake of trace metals in the hyporheic zone of a mining-contaminated stream, Pinal Creek, Arizona. Environ. Sci. Technol. 2000, 34, 1150–1155. [Google Scholar] [CrossRef]
- Apfelbaum, S.I.; Sams, C.E. Ecology and control of reed canary grass (Phalaris arundinacea L.). Nat. Areas J. 1987, 7, 69–74. [Google Scholar]
- Comes, R.D. The Biology and Control of Reed Canary Grass (Phalaris arundinacea L.) on Irrigation Ditchbanks. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1971. [Google Scholar]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Bakkaus, E.; Gouget, B.; Gallien, J.P.; Khodja, H.; Carrot, F.; Morel, J.L.; Collins, R. Concentration and distribution of cobalt in higher plants: The use of micro-PIXE spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. Beam Interact. Mater. At. 2005, 231, 350–356. [Google Scholar] [CrossRef]
- Kříbek, B.; Majer, V.; Knésl, I.; Nyambe, I.; Mihaljevič, M.; Ettler, V.; Sracek, O. Concentrations of arsenic, copper, cobalt, lead and zinc in cassava (Manihot esculenta Crantz) growing on uncontaminated and contaminated soils of the Zambian Copperbelt. J. Afr. Earth Sci. 2014, 99, 713–723. [Google Scholar] [CrossRef]
- El Hamiani, O.; El Khalil, H.; Sirguey, C.; Ouhammou, A.; Bitton, G.; Schwartz, C.; Boularbah, A. Metal concentrations in plants from mining areas in South Morocco: Health risks assessment of consumption of edible and aromatic plants. CLEAN Soil Air Water 2015, 43, 399–407. [Google Scholar] [CrossRef]
- Luo, D.; Zheng, H.; Chen, Y.; Wang, G.; Fenghua, D. Transfer characteristics of cobalt from soil to crops in the suburban areas of Fujian Province, southeast China. J. Environ. Manag. 2010, 91, 2248–2253. [Google Scholar] [CrossRef]
- Loredo, J.; Álvarez, R.; Ordóñez, A.; Bros, T. Mineralogy and geochemistry of the Texeo Cu–Co mine site (NW Spain): Screening tools for environmental assessment. Environ. Geol. 2008, 55, 1299–1310. [Google Scholar] [CrossRef]
- Álvarez, R.; Ordóñez, A.; Pérez, A.; De Miguel, E.; Charlesworth, S. Mineralogical and environmental features of the asturian copper mining district (Spain): A review. Eng. Geol. 2018, 243, 206–217. [Google Scholar] [CrossRef]
- Kelly, J.; Champagne, P.; Michel, F. Assessment of metal attenuation in a natural wetland system impacted by alkaline mine tailings, Cobalt, Ontario, Canada. Mine Water Environ. 2007, 26, 181–190. [Google Scholar] [CrossRef]
- Percival, J.B.; Kwong, Y.J.; Dumaresq, C.G.; Michel, F.A. Distribution of As, Ni and Co in tailings and surface waters in the cobalt area, Ontario. In Proceedings of the Mining and the Environment IV Conference, Sudbury, ON, Canada, 19–26 October 2007; pp. 19–27. [Google Scholar]
- Luís, A.T.; Teixeira, P.; Almeida, S.F.P.; Matos, J.X.; da Silva, E.F. Environmental impact of mining activities in the Lousal area (Portugal): Chemical and diatom characterization of metal-contaminated stream sediments and surface water of Corona stream. Sci. Total Environ. 2011, 409, 4312–4325. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, A.; Janardhan, M.R. Arsenic contamination in groundwater of the areas surrounding Ingaldhal, Chitradurga district, Karnataka state. Int. J. Geol. Earth Environ. Sci. 2016, 6, 1–7. [Google Scholar]
- Parafiniuk, J.; Siuda, R. Schwertmannite precipitated from acid mine drainage in the Western Sudetes (SW Poland) and its arsenate sorption capacity. Geol. Q. 2006, 50, 475–486. [Google Scholar]
- Rollinson, G.; Le Boutillier, N.; Selley, R. Cobalt mineralisation in Cornwall—A new discovery at Porthtowan. Geosci. South West Engl. 2018, 14, 176–187. [Google Scholar]
- Clarke, J. The Characterization of Arsenic Mineral Phases from Legacy Mine Waste and Soil near Cobalt, Ontario. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2017. [Google Scholar]
- Boyle, R.W.; Dass, A.S. The geochemistry of the supergene processes in the native silver veins of the Cobalt-South Lorrain area, Ontario. Can. Mineral. 1971, 11, 358–390. [Google Scholar]
- Markl, G.; Marks, M.A.; Derrey, I.; Gühring, J.E. Weathering of cobalt arsenides: Natural assemblages and calculated stability relations among secondary Ca-Mg-Co arsenates and carbonates. Am. Mineral. 2014, 99, 44–56. [Google Scholar] [CrossRef]
- Sracek, O.; Mihaljevič, M.; Kříbek, B.; Majer, V.; Veselovský, F. Geochemistry and mineralogy of Cu and Co in mine tailings at the Copperbelt, Zambia. J. Afr. Earth Sci. 2010, 57, 14–30. [Google Scholar] [CrossRef]
- Ogbonna, C.E.; Otuu, F.C.; Ugbogu, O.C.; Nwaugo, V.O.; Ugbogu, A.E. Public health implication of heavy metal contamination of plants growing in the Lead Zinc Mining Area of Ishiagu, Nigeria. Int. J. Biodivers. Environ. Sci. 2015, 7, 76–86. [Google Scholar]
- Prasad, M.N.V.; Pratas, J.; Freitas, H. Trace Elements in Plants and Soils of Abandoned Mines in Portugal: Significance for Phytomanagement and Biogeochemical Prospecting; CRC Press-Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006; pp. 507–521. [Google Scholar]
- Nawab, J.; Khan, S.; Shah, M.T.; Khan, K.; Huang, Q.; Ali, R. Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species. Int. J. Phytoremediation 2015, 17, 801–813. [Google Scholar] [CrossRef]
- Samantaray, S.; Rout, G.R.; Das, P. Heavy metal and nutrient concentration in soil and plants growing on a metalliferous chromite minespoil. Environ. Technol. 2001, 22, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.L.; Lottermoser, B.G.; Duchesne, J. Ephemeral acid mine drainage at the Montalbion silver mine, north Queensland. Aust. J. Earth Sci. 2003, 50, 797–809. [Google Scholar] [CrossRef]
- Chen, G.; Yang, H.; Li, H.; Tong, L. Recovery of cobalt as cobalt oxalate from cobalt tailings using moderately thermophilic bioleaching technology and selective sequential extraction. Minerals 2016, 6, 67. [Google Scholar] [CrossRef]
- Vítková, M.; Ettler, V.; Johan, Z.; Kribek, B.; Sebek, O.; Mihaljevic, M. Primary and secondary phases in copper-cobalt smelting slags from the Copperbelt Province, Zambia. Mineral. Mag. 2010, 74, 581–600. [Google Scholar] [CrossRef]
- Mees, F.; Masalehdani, M.N.N.; De Putter, T.; D’Hollander, C.; Van Biezen, E.; Mujinya, B.B.; Van Ranst, E. Concentrations and forms of heavy metals around two ore processing sites in Katanga, Democratic Republic of Congo. J. Afr. Earth Sci. 2013, 77, 22–30. [Google Scholar] [CrossRef]
- Holmström, H.; Öhlander, B. Layers rich in Fe-and Mn-oxyhydroxides formed at the tailings-pond water interface, a possible trap for trace metals in flooded mine tailings. J. Geochem. Explor. 2001, 74, 189–203. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Nóbrega, G.N.; Ferreira, T.O.; Almeida, L.S.; Romero, T.B.; Santaella, S.T.; Otero, X.L. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci. Total Environ. 2018, 637, 498–506. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L. Release, transport and attenuation of metals from an old tailings impoundment. Appl. Geochem. 2005, 20, 639–659. [Google Scholar] [CrossRef]
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of cobalt from Cu/Co-rich sulfidic mine tailings from the polymetallic Rammelsberg mine, Germany. Hydrometallurgy 2020, 197, 105443. [Google Scholar] [CrossRef]
- Assawincharoenkij, T.; Hauzenberger, C.; Ettinger, K.; Sutthirat, C. Mineralogical and geochemical characterization of waste rocks from a gold mine in northeastern Thailand: Application for environmental impact protection. Environ. Sci. Pollut. Res. 2018, 25, 3488–3500. [Google Scholar] [CrossRef]
- Heikkinen, P.M.; Räisänen, M.L. Mineralogical and geochemical alteration of Hitura sulphide mine tailings with emphasis on nickel mobility and retention. J. Geochem. Explor. 2008, 97, 1–20. [Google Scholar] [CrossRef]
- Piatak, N.M.; Seal II, R.R. Mineralogy and environmental geochemistry of historical iron slag, Hopewell Furnace National Historic Site, Pennsylvania, USA. Appl. Geochem. 2012, 27, 623–643. [Google Scholar] [CrossRef]
- Campbell, G.A. The cobalt market revisited. Miner. Econ. 2020, 33, 21–28. [Google Scholar] [CrossRef]
- Olson, G.J.; Sakai, C.K.; Parks, E.J.; Brinckman, F.E. Bioleaching of cobalt from smelter wastes by Thiobacillus ferrooxidans. J. Ind. Microbiol. 1990, 6, 49–52. [Google Scholar] [CrossRef]
- Coto, O.; Galizia, F.; Hernandez, I.; Marrero, J.; Donati, E. Cobalt and nickel recoveries from laterite tailings by organic and inorganic bio-acids. Hydrometallurgy 2007, 94, 18–22. [Google Scholar] [CrossRef]
- Brisson, V.L.; Zhuang, W.Q.; Alvarez-Cohen, L. Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng. 2016, 113, 339–348. [Google Scholar] [CrossRef]
- Lindsay, M.B.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Marrero, J.; Coto, O.; Goldmann, S.; Graupner, T.; Schippers, A. Recovery of nickel and cobalt from laterite tailings by reductive dissolution under aerobic conditions using Acidithiobacillus species. Environ. Sci. Technol. 2015, 49, 6674–6682. [Google Scholar] [CrossRef]
- Cabrera, G.; Gómez, J.M.; Hernández, I.; Coto, O.; Cantero, D. Different strategies for recovering metals from CARON process residue. J. Hazard. Mater. 2011, 189, 836–842. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khezri, M.; Abdollahzadeh, A.A.; Askari, M. Bioleaching of copper, nickel and cobalt from the low grade sulfidic tailing of Golgohar Iron Mine, Iran. Hydrometallurgy 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Morin, D.H.R.; d’Hugues, P. Bioleaching of a cobalt-containing pyrite in stirred reactors: A case study from laboratory scale to industrial application. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 35–55. [Google Scholar]
- Gadd, G.M. Heavy metal accumulation by bacteria and other microorganisms. Experientia 1990, 46, 834–840. [Google Scholar] [CrossRef]
- Valix, M.; Usai, F.; Malik, R. Fungal bio-leaching of low grade laterite ores. Miner. Eng. 2001, 14, 197–203. [Google Scholar] [CrossRef]
- Yang, Y.; Ferrier, J.; Csetenyi, L.; Gadd, G.M. Direct and indirect bioleaching of cobalt from low grade laterite and pyritic ores by Aspergillus niger. Geomicrobiol. J. 2019, 36, 940–949. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, S.; Paul, A.K. Biosorption of cobalt by fungi from serpentine soil of Andaman. Bioresour. Technol. 2006, 97, 1253–1258. [Google Scholar] [CrossRef]
- Ali Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Ilyas, S.; Chi, R.A.; Lee, J.C. Fungal bioleaching of metals from mine tailing. Mineral. Process. Extr. Metall. Rev. 2013, 34, 185–194. [Google Scholar] [CrossRef]
- Newsome, L.; Arguedas, A.S.; Coker, V.S.; Boothman, C.; Lloyd, J.R. Manganese and cobalt redox cycling in laterites; Biogeochemical and bioprocessing implications. Chem. Geol. 2020, 531, 119330. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Alexandria, VA, USA, 1985.
- Hem, J.D.; Roberson, C.E.; Lind, C.J. Thermodynamic stability of CoOOH and its coprecipitation with manganese. Geochim. Cosmochim. Acta 1985, 49, 801–810. [Google Scholar] [CrossRef]
- De Putter, T.; Decrée, S.; Banza, C.L.N.; Nemery, B. Mining the Katanga (DRC) Copperbelt: Geological aspects and impacts on public health and the environment—Towards a holistic approach. In Proceedings of the Mining and the Environment in Africa, Inaugural Workshop, IGCP/SIDA, Kitwe, Zambia, 17–18 October 2011; pp. 14–17, No. 594. [Google Scholar]
- Decree, S.; Pourret, O.; Baele, J.M. Rare earth element fractionation in heterogenite (CoOOH): Implication for cobalt oxidized ore in the Katanga Copperbelt (Democratic Republic of Congo). J. Geochem. Explor. 2015, 159, 290–301. [Google Scholar] [CrossRef]
- Crowther, D.L.; Dillard, J.G.; Murray, J.W. The mechanisms of Co (II) oxidation on synthetic birnessite. Geochim. Cosmochim. Acta 1983, 47, 1399–1403. [Google Scholar] [CrossRef]
- Tanaka, K.; Yu, Q.; Sasaki, K.; Ohnuki, T. Cobalt (II) oxidation by biogenic Mn oxide produced by Pseudomonas sp. strain NGY-1. Geomicrobiol. J. 2013, 30, 874–885. [Google Scholar] [CrossRef]
- Mwema, M.D.; Mpoyo, M.; Kafumbila, K. Use of sulphur dioxide as reducing agent in cobalt leaching at Shituru hydrometallurgical plant. J. South. Afr. Inst. Min. Metall. 2002, 102, 1–4. [Google Scholar]
- Singh, S.K.; Subramanian, V.; Gibbs, R.J. Hydrous Fe and Mn oxides—Scavengers of heavy metals in the aquatic environment. Crit. Rev. Environ. Control 1984, 14, 33–90. [Google Scholar] [CrossRef]
- Ardizzone, S.; Formaro, L. Cobalt (II) adsorption on haematite at room temperature. Surf. Technol. 1983, 19, 283–288. [Google Scholar] [CrossRef]
- Kobal, I.; Hesleitner, P.; Matijević, E. Adsorption at solid/solution interfaces. 6. Interactions of Co2+ ions with spherical hematite particles. Colloids Surf. 1988, 33, 167–174. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Lovley, D.R. Fe (III) and Mn (IV) reduction. In Environmental Microbe-Metal Interactions; American Society of Microbiology: Washington, DC, USA, 2000; pp. 3–30. [Google Scholar]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation. Rev. iMineral. 1997, 35, 361–390. [Google Scholar]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I.; Jerzykiewicz, M.; Trynda, J. Wheat straw biochar as a specific sorbent of cobalt in soil. Materials 2020, 13, 2462. [Google Scholar] [CrossRef]
- Cárdenas González, J.F.; Rodríguez Pérez, A.S.; Vargas Morales, J.M.; Martínez Juárez, V.M.; Rodríguez, I.A.; Cuello, C.M.; Muñoz Morales, A. Bioremoval of Cobalt (II) from aqueous solution by three different and resistant fungal biomasses. Bioinorg. Chem. Appl. 2019, 2019, 1–8. [Google Scholar] [CrossRef]

| Type of Limit | Limit Value (mg/kg) | Organisation | Reference |
|---|---|---|---|
| Drinking water | No data | CCME | CCME, 2010 [53] |
| Surface water | 5 | NYSDEC | NYSDEC, 1986 [55] |
| Freshwater for aquatic life | 5 | NYSDEC | NYSDEC, 1986 [55] |
| Agriculture | 50 Irrigation 1000 Livestock | CCME | CCME, 2010 [53] |
| Residential soil quality guidelines | 23 | USEPA | USEPA, 2011 [56] |
| Industrial soil quality guidelines | 300 | USEPA | USEPA, 2011 [56] |
| Sediment Quality Guidelines for the Protection of Aquatic Life | 35 | CCME | CCME, 2010 [53] |
| CCME: Canadian Council of Ministers of the Environment NYSDEC: New York State Department of Environmental Conservation USEPA: United States Environmental Protection Agency | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziwa, G.; Crane, R.; Hudson-Edwards, K.A. Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments. Minerals 2021, 11, 22. https://doi.org/10.3390/min11010022
Ziwa G, Crane R, Hudson-Edwards KA. Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments. Minerals. 2021; 11(1):22. https://doi.org/10.3390/min11010022
Chicago/Turabian StyleZiwa, Gabriel, Rich Crane, and Karen A. Hudson-Edwards. 2021. "Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments" Minerals 11, no. 1: 22. https://doi.org/10.3390/min11010022
APA StyleZiwa, G., Crane, R., & Hudson-Edwards, K. A. (2021). Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments. Minerals, 11(1), 22. https://doi.org/10.3390/min11010022





