Abstract
Coal fly ash contains a considerable number of toxic elements that can be leached into the environment, such as chromium (Cr), thereby quickly leading to severe contaminations. In this research, the leaching behaviors of Cr were analyzed from 14 kinds of coal fly ash samples collected from the electrostatic precipitators of coal-fired thermal power plants in Japan. The level of Cr concentration found in the samples varied from 0.00 to 82.93 μg/L. However, Cr toxicity depends on its valence state; Cr6+ is more toxic than Cr3+. Additive materials containing high calcium content were used to control the leaching concentration of Cr, such as Ca(OH)2, paper sludge ash, and blast furnace cement. This research used several instruments. An X-ray fluorescence was adopted to measure the major chemical composition of the fly ash samples and the additive materials. A thermogravimetric analyzer was used to examine the calcium compounds in the additive materials. Inductively coupled plasma was used to determine the Cr leaching concentrations from the fly ash samples. Findings showed that the three-additive mixture had a promising effect on controlling the Cr leaching concentrations. These results were also supported by FactSage 7.2 simulation.
1. Introduction
The International Energy Agency predicts that coal will still be the largest source of power supply worldwide (35%) in 2024 [1]. In thermal power plants, coal is used as a fossil fuel in the combustion process. The combustion produces coal fly ash, which contains toxic elements. These elements, once in landfills, then leach into water environments after coming into contact with rain. This condition affects the concentration of toxic elements in water environments in that toxic elements could leach from lignite fly ash into aquatic environments when it comes into contact with water [2]. Concentrations of these toxic elements will increase continuously. One of the toxic elements in coal fly ash is chromium (Cr). The Environmental Protection Agency tested coal ash leachate by obtaining waste from numerous operating power plants and found that many ashes and sludge produce Cr-rich leachate [3]. Permissible limit (for the protection of human health) of chromium set by the Ministry of the Environment of the government of Japan is 50 μg/L.
Some researchers in previous studies have used some material to stabilize chromium. Xue, T et al [4] used calcium polysulfide (CaS5) and ferrous sulfate (FeSO4) for reducing Cr leachability from the soil. As a result, they found that CaS5 had a better effect than FeSO4 on the stabilization of chromium. Morales, C et al [5] observed the effects of slag basicity (mass ratio CaO to SiO2) and the addition of FeSO4 or FeS2 into the slag on the stability of the mineralogical species in the slag containing chromium compounds. They found that the lowest chromium concentration levels in the leaching liquors corresponded to slags with CaO/SiO2 = 1 and high FeS2 contents, owing to the stable binding of chromium in the compounds FeCr2O4, Cr3S4, and Ca3Cr2Si3O. Mixing additives in the leaching process could be useful in decreasing As, Se, B, and F leaching concentrations simultaneously from two kinds of fly ash [6]. So, in current research, the utilization of additive material that contained high calcium has been done. The objectives were to use laboratory experiments and FactSage simulations: (1) to determine the Cr leaching levels from different kinds of fly ash, (2) to investigate the effect of additive materials on controlling the Cr leaching from the different types of fly ash, (3) to examine the influence of pH on Cr, and (4) to identify a suitable additive for controlling the Cr leaching concentration.
2. Materials and Methods
2.1. Materials
Fly ash samples (FA A–FA N) were obtained from 14 coal-fired power stations in Japan. The primary chemical composition of the samples, which was determined based on X-ray fluorescence (XRF) analysis results (WDXRF S8 Tiger, Bruker AXS), is provided in Table 1. The table shows that the samples had different CaO contents. The highest CaO content was found in FA B (10.80%), and the lowest was in FA D (0.45%). Previous research has shown that calcium content has an essential role in the release of trace elements from coal fly ash [7].

Table 1.
Chemical composition of coal fly ash samples.
Additive materials containing high calcium content were used to control the leaching concentration of Cr such as calcium hydroxide (Ca(OH)2, paper sludge (PS) ash 8, and blast furnace (BF) cement. The chemical composition of additive materials is provided in Table 2. The calcium compound Ca(OH)2 had 95% purity and was obtained from Kanto Chemical Co. Inc. (Gifu, Japan). PS ash is a waste material formed during the paper manufacturing process using wooden pulp in the paper industry. Based on quantitative analysis, PS ash 8 contains 11.10% CaO, 1.44% Ca(OH)2, and 19.32% CaCO3 [8]. BF cement is a material formed through the mixing of granulated BF slag with Portland cement clinker and gypsum. These contents in BF cement trigger the formation of a compound that helps preserve trace elements in coal fly ash [6]. Both of PS ash 8 and BF cement was obtained from a manufacturing company in Sendai, Japan.

Table 2.
Chemical composition of additive materials.
2.2. Sample Preparation and Leaching Test
The coal fly ash samples mixed with the additives in a specific ratio before the leaching process. The ratio of the additives in the total sample is provided in Table 3. The percentage of additive used was based on the total amount of sample (50 g). Then, the mixture was poured into a bowl and distilled water was added (25% of the total sample) until the mixture was perfectly mixed (3 min). After that, it was air-dried for 7 days and then sieved with a 2 mm sieve. The prepared sample was tested by leaching test based on JLT-13 (Environment Agency Notification No.13, 1973). First, 5 g of a sample that had been mixed with additives was added to 50 mL distilled water, and then was shaken for 6 h at room temperature with shaking speed of 200 rpm by Shaker SA400 YAMATO. The solid-liquid was centrifuged by AS-ONE HSIANGTAI centrifuge for 20 min at a speed of 3000 rpm. A nitrocellulose membrane filter of 0.45 μm was used to separate leachates.

Table 3.
Ratio of additive in fly ash for leaching test.
2.3. Analysis and Instrumentation
The calcium compound in the additive was measured by thermogravimetric (TG) analysis (TG/DTA6300 SII EXSTAR 6000, Hitachi, Hong Kong, China). A sample of 8–12 mg was heated with a measurement temperature from 30 to 1000 °C at a heating rate of 10 °C/min under a nitrogen atmosphere at a flow rate of 200 mL/min. To determine the Cr leaching concentration, the leachate obtained from the leaching process was analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES, ULTIMA 2, HORIBA, Tokyo, Japan). Cr standard stock solution (1000 mg/L) was purchased from Kanto Chemical Co., Inc., Tokyo, Japan. Working standards of concentrations 0, 50, 100, 150, and 200 μg/L were added to HNO3 60% and diluting with deionized water. Their absorbances were measured, and five-point linear calibration curves were established. Then, samples solutions were aspirated into the atomic spectrometer and direct readings of total chromium concentrations were recorded. At least two replicate determinations were carried out for each sample resulting in a relative standard deviation lower than 4%. The blanks were also run before chromium determination. The leachate alkalinity was analyzed by a pH ion meter (D-53, HORIBA) to check the effect of pH on the Cr leaching concentration. XRF analysis (WDXRF S8 Tiger, Bruker AXS, Yokohama, Japan) was used to measure the chemical composition of the fly ash samples and the additive materials.
2.4. Analysis by FactSage
FactSage 7.2 was employed to predict the Cr speciation based on the minimization of the free Gibbs energy to simulate the chemical reaction equilibrium and processes. FactSage provides information about formed compounds and their phases, mass or mole fraction, and thermodynamic properties for a range of pressures and temperatures. Understanding the effect of temperature on Cr speciation during the combustion process is essential for controlling Cr emissions. The FactSage contains a compound database of all coal fly ash components. These calculations were used to predict the possible Cr-bearing compounds in coal fly ash. The data search used in this analysis included FactPS and FToxid. In the combustion process, the elemental composition of coal fly ash sample was used as input data. The calculation was performed at different temperature intervals between 100 to 1600 °C, at atmospheric pressure. For the leaching process, the predicted Cr compound from the combustion process was used as input data. Output from these analyses was aqueous, gases, and solid species.
3. Results
3.1. Analysis of Calcium Compound in Additive Material
Ca(OH)2 and CaCO3 were used as standards in analyzing the calcium compounds in the PS ash 8 and BF cement. The Ca(OH)2, and CaCO3 in the PS ash 8 and BF cement were thermally decomposed as shown in Figure 1. Ca(OH)2 started losing weight in the temperature range of 350–450 °C and then decomposed into CaO and H2O. CaCO3 decomposed into CaO and CO2 at temperature around 600–790 °C. The weight of PS ash decreased twice: first from 380 °C to 430 °C (calculated as Ca(OH)2 decomposition) and then from 600 to 700 °C (as CaCO3). Similar to PS ash 8, the weight of BF cement decreased twice: from 380 to 400 °C and then from 580 to 680 °C. This condition describes the decomposition of the calcium compound contained in the PS ash 8 and BF cement. Then, the calculation of Ca(OH) 2 and CaCO3 percentage carried out by Equations (1) and (2).
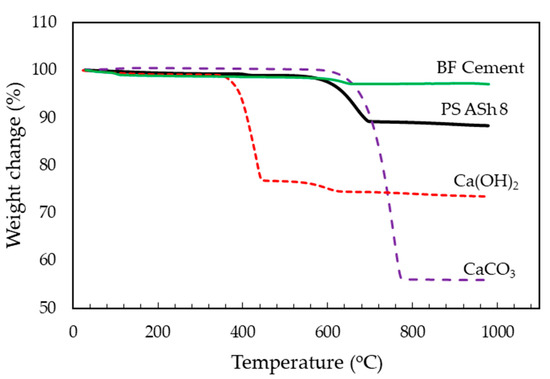
Figure 1.
Thermogravimetric (TG) curves show the thermal decomposition of Ca(OH)2, CaCO3, PS ash 8, and BF cement in a N2 atmosphere.
In this equation, W1 is the weight between temperature decomposition end, and temperature decomposition start. Mr is the relative molecular mass or molecular weight of molecules. From this calculation it was found that PS ash 8 contains 1.44% Ca(OH)2 and 19.32% CaCO3. BF cement contains 0.31% Ca(OH) 2 and 6.45% CaCO3. BF cement contains less calcium compound than PS ash 8. It can be shown in Figure 1 that BF cement loses less weight compared PS ash 8.
3.2. Effect of Additive on Chromium Leaching Concentration from Fly Ash
The total Cr leaching concentration from the 14 coal fly ash samples was measured by ICP-AES, as shown in Figure 2.
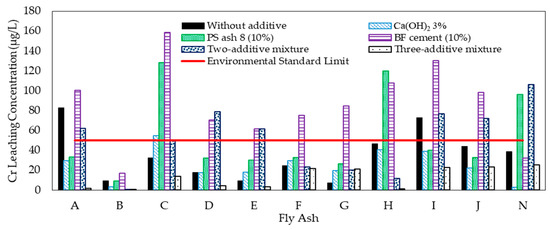
Figure 2.
Effect of additive on Cr leaching concentration from coal fly ash.
The concentration varied from 0.00 μg/L to 82.93 μg/L. The amount of Cr in two fly ash samples (A and I) exceeded the environmental quality standard (50 μg/L). In contrast, Cr was not detected in fly ash samples K, L, and M. Then, the additive materials were added into fly ash samples A, B, C, D, E, F, G, H, I, J, and N to investigate the effect of the additive materials on controlling the Cr leaching concentration. Findings showed that the addition of Ca(OH)2 can decrease the Cr leaching concentration from fly ash samples A, B, H, I, J, and N. The addition of PS ash 8 can decrease the concentration of only A, I, and J, whereas the BF cement yielded the opposite effect. The Cr leaching concentration increased after the addition of the BF cement. This condition was caused by the Cr leaching concentration from the PS ash 8 and BF cement, which was 0.32 μg/L and 228.73 μg/L, respectively. Therefore, the two-additive mixture cannot decrease all of kinds of Cr leaching concentrations. By contrast, the three-additive mixture had a good effect on decreasing the Cr leaching concentration. The Cr leaching concentration from all the fly ash samples except FA G was decreased by the three-additive mixture. Nonetheless, even though the Cr leaching concentration increased in FA G, it remained under the environmental quality standard. As shown in Figure 2, the three-additive mixture gave a significantly decreased concentration compared to the other additives. These results differ slightly from those of our previous research; although not as good as the three-additive mixture, two-additive mixtures can also reduce the level of As, Se, B, and F leaching concentration until under the environmental quality standard [6]. Therefore, the use of a three-additive mixture is beneficial for simultaneously controlling the leaching concentration of As, Se, B, F, and Cr.
3.3. Effect of pH on Chromium Leaching Concentration
The pH value is an important factor that can influence the leaching of many potential contaminants from coal fly ash [9,10,11]. Leaching characteristics of coal combustion by-products under different pH conditions shows Cr elements reach a maximum value under acidic condition, then as the pH increases, the concentration of Cr drops slowly [12]. In the current research, additive materials were applied to increase the pH value. The effect of additive materials on the pH value and the Cr leaching concentration is shown in Figure 3. Figure 3a shows the effect of pH on the Cr leaching concentration with single additives. The pH value range of the leachate without additives was 7.94–13.19, with the Cr concentration ranging between 0.00 and 82.93 μg/L. Every single additive material increased the pH value of the leachate, but only Ca(OH)2 could decrease the Cr leaching concentration and increase the pH value simultaneously. The pH value range with Ca(OH)2 addition was 12.11–12.72, with the Cr leaching concentration being 3.17–54.95 μg/L. The increase in the Cr leaching concentration with the addition of the PS ash 8 and BF cement was caused by the Cr content in said additives. Figure 3b reveals that both additive mixtures could increase the pH value, but only the three-additive mixture could decrease the Cr leaching concentration. The pH value range with the three-additive mixture was 12.44–12.59, with the Cr leaching concentration being 0.00–25.37 μg/L. Increasing the pH enhanced the stability of Cr in the fly ash. Therefore, the role of calcium, which was contained in the additives, was needed to increase the pH value. Although the alkalinity of leachate is strong, it is decreased over time to a pH between 8 and 9 because of carbonate buffering, and a consequent precipitation of calcite in the atmosphere under field conditions has been reported [13], while chromium is transformed into highly stable Ca3Cr2 (SiO4)3.
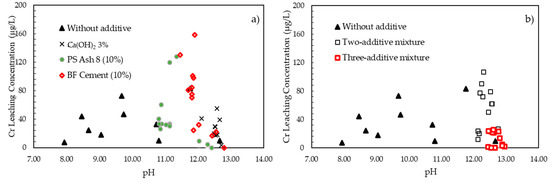
Figure 3.
Effect of pH on chromium leaching concentration: (a) single additives; (b) mixed additives.
3.4. Analysis by FactSage 7.2.
Chromium partitioning behavior characteristics in interaction with mineral in fly ash (FA A) after combustion process, as analyzed using FactSage, was summarized in Table 4. Chromium reacted with CaO, Na2O, K2O, and SO3 to form CaCr2O4(s), Na2CrO4(s), Cr2(SO4)3(s), and K2CrO4(s), respectively, which inhibited chromium volatilization. No reaction or interaction between chromium and SiO2, Al2O3, TiO2, Fe2O3, MgO, and P2O5 were observed. The effect of combining minerals on chromium volatilization are also studied as illustrated in interaction no. 12–16 in Table 4. In the Cr-O2-K2O-SO3, Cr-O2-K2O-SO3-SiO2, and Cr-O2-K2O-SO3-SiO2-CaO, chromium existed as K2CrO4(s), by adding Fe2O3 to system analysis (Cr-O2-K2O-SO3-SiO2-CaO-Fe2O3), chromium existed as CrO2(s). In interactions of chromium and all mineral components in fly ash, chromium existed as CrO2(s) with small contributions of CrO2 and CrO3 in gaseous phases. To better understand the combustion process of chromium from fly ash, the possible species of chromium were predicted by a FactSage model, as depicted in Figure 4, at different temperatures. All the species studied were present in the gas phase during the combustion stage. CrO3(g) formed at a temperature above 1100 °C and became the main gaseous species with a small contribution of CrO2(g). Below 1200 °C, Cr was in the form of Cr2O3 and CrO2 in the solid phase. Cr becomes volatile only at high combustion temperatures, and gaseous species leave the combustion zone and cooling condition [14]. Cr2O3(s) became the main species at cooling temperatures from 1200 to 200 °C. At temperatures below 200 °C, Cr existed in the solid phase in the form of CrO2, and the formation of CrO2 increased in the final combustion process (cooling process), indicating that CrO2 in the solid phase was the chromate species contained in the coal fly ash. This process can be explained by the following reaction of generating CrO2.

Table 4.
Interaction of chromium with mineral compounds in fly ash (FA A) after combustion process (T = 100 °C and P = 1 atm.).
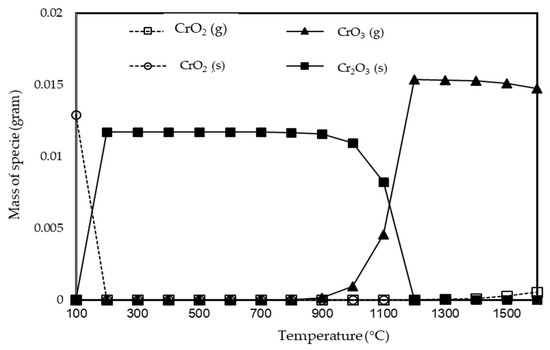
Figure 4.
Equilibrium composition of Cr considering Cr FA A component interactions.
The coal fly ash, which contained CrO2 from the combustion process, was tested for the leaching process with additives. The leaching of Cr from coal fly ash during the coal fly ash-water-additive interaction depends on many factors, such as the phases associated with the Cr in the coal fly ash, pH of the leachate, ash properties, leaching environment, and temperature of the leaching process. The possible formation of Cr-bearing species during the leaching process with additives was illustrated in Table 5. Once, chromium as CrO2 reacted with potassium as K2O to form K2CrO4(s) at −5 °C and Cr2O3(s) was observed at 25 °C with small contribution of Cr3+ and CrO42− in aqueous species. On the other hand, the interaction of CrO2 with SiO2, Al2O3, TiO2, Fe2O3, CaO, MgO, Na2O, P2O5, and SO3: Cr2O3(s) was observed as the same as the interaction of CrO2 and H2O, indicating that this minerals are not effective on chromium during the leaching process. In the above multiple interaction analysis, K2O reacted more easily than other minerals in fly ash and addictives. The prediction of chromium species in solution phases is also observed. Five types of chromium species, Cr3+, CrO42−, CrO72−, HCrO4−, and Cr(OH)2+, in turn appeared along with the increase of temperature studied.

Table 5.
Interaction of predicted chromium (CrO2) with mineral components during leaching process.
To better understand the leaching process of chromium from fly ash with addictive material, the possible species of chromium were predicted, as demonstrated in Figure 5a, at different temperatures. It obviously indicates that the leachability of chromium is dependent on the temperature. At a temperature of −5 °C, Cr reacted with K2O to form K2CrO4 and minimize the Cr3+ and CrO42− released into the water environment. However, an increase in the temperature of the leaching process to 0 °C caused Cr to easily leach into the water environment in the form of Cr3+ and CrO42− and eliminate the effect of potassium to control the leaching chromium. Here, CrO42− tends to stabilize by increasing temperature in the range 0–25 °C. Immobilization of chromium tends to proceed preferentially under colder conditions; aging at higher temperatures enhances the leachability of chromium in some coal fly ash samples [15]. This analysis indicating that potassium (K) was the most influential factor in Cr transformation. K2CrO4(s) was formed from the predicted CrO2 during the leaching process with K2O in the additive material at −5 °C, as explained by Equation (2).
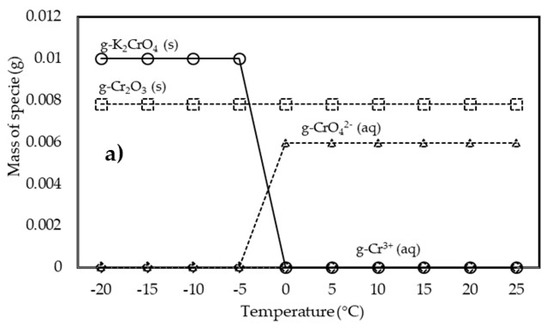
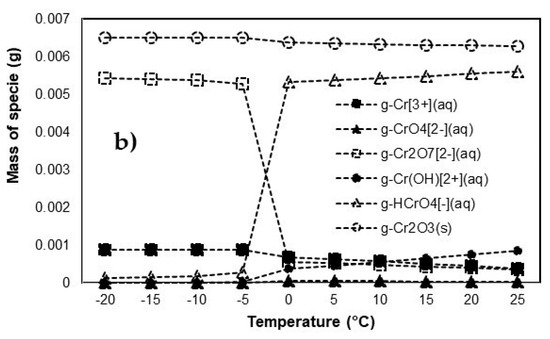
Figure 5.
Leaching mechanism of predicted chromate (CrO2) as function of temperature: (a) with addictive and (b) without addictive.
On the other hand, the leaching process of chromium without addictive material is shown in Figure 5b. Regarding the concentration of chromium species in solution, increasing the temperature of the leaching process caused increases of HCrO4−, Cr(OH)2+, and CrO42−. At the same time, increasing the temperature caused decreased Cr2O72− and Cr3+, indicating the leaching process of chromium without addictive is not effective to control the leaching of chromium species in to water environment.
According to the above discussion, potassium (K) can control the leaching of Cr from coal fly ash by reacting with the Cr at a temperature of −5 C°. The effect of the other minerals in the additive materials to control the leaching of Cr3+ during the leaching process was also analyzed under different temperatures, as shown in Figure 6. Not only K had the potential to control the leaching of Cr3+ during the leaching process; Ca, Na, and Mg also interacted with Cr3+. Therefore, K, Ca, Na, and Mg could control the leaching of Cr3+ from the fly ash. Previous research has found that the chemical compounds CaO and Mg on fly ash surfaces can control the pH of fly ash and soil leachate [12]. The content of these metals in the additive materials in the current study is provided in Table 3. Hydrocaluminate (Ca4Al2(OH)12(OH)2·6H2O) and ettringite (Ca6Al2(OH)12(SO4)3·26H2O), which form during the leaching process, increase the pH value and also reduce the concentration of heavy metals [16,17,18].
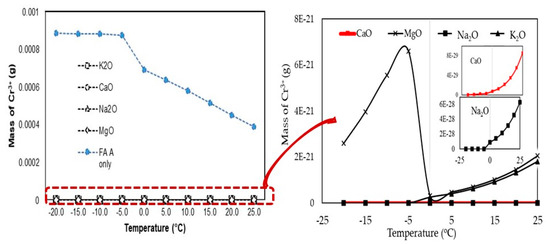
Figure 6.
Mineral effect on controlling Cr3+ during leaching process.
3.5. Comparison Result between Experiment and Simulation
Similar to the laboratory experiment, the FactSage simulation was done by the addition of single and mixed additive materials. The result from FactSage also revealed that the three-additive mixture was useful in controlling the Cr leaching concentration from the fly ash. Additive materials’ capability is the capability of an additive material to control the Cr leaching concentration from the fly ash. As shown in Figure 7, there were no significant differences between the laboratory experiments and FactSage simulations. This figure shows the capability of the three-additive mixture to control the leaching of Cr from fly ash A and B. The Cr controlled from fly ash A by FactSage and the laboratory experiments were 99.98% and 96.91%, respectively; those from FA B were 99.92% and 97.78%, respectively, where error percentages of experimental analysis to FactSage analysis of FA A and FA B were 3.07% and 2.15%, respectively. In a previous research, leaching test results were compared with solution equilibrium calculation results to consider the leaching mechanisms; however, the experimental results were lower than the equilibrium calculation results [19]. The possible reason is the kinetic control during leaching test due to lack of agitation and short residence time [19], therefore, thermodynamic calculation (FactSage 7.2) does not consider the reaction time, which is necessary for the theoretical equilibrium state to be reacted [20].
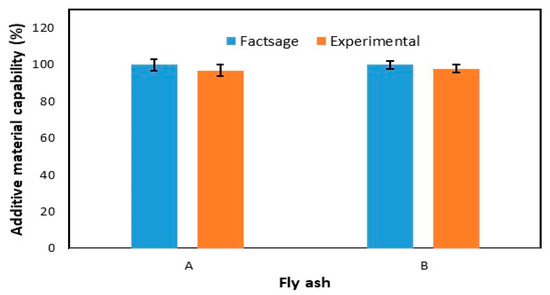
Figure 7.
Leaching rate comparison between laboratory experiment and FactSage simulation.
4. Conclusions
A mixture of the additives Ca(OH)2, PS ash 8, and BF cement yielded promising effects in controlling the Cr leaching concentration from coal fly ash. An analysis of 14 fly ash samples revealed that this three-additive mixture could reduce the Cr leaching concentration to a level within the environmental quality standard. The pH value also played a role in decreasing the Cr leaching concentration. Analysis by FactSage showed that not only Ca had a role in controlling the concentration; K, Mg, and Na, which are difficult to break down even at high temperatures, were also useful. The results from both laboratory experiments and simulation by FactSage found that the mixture of the three additives decreased the Cr leaching concentration by more than 97%.
Author Contributions
S.K. conceived and designed the experiment and contributed to the materials, reagents and instrumentation; E.R.D. and U.M.S. performed the experiments; E.R.D., U.M.S., and Y.H. discussed and analyzed the data; and E.R.D. and U.M.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Energy Agency, Fuel Report. Coal 2019 Analysis and Forecasts to 2024. 2019. Available online: https://www.iea.org/reports/coal-2019/ (accessed on 14 February 2020).
- Darakas, E.; Tsiridis, V.; Petala, M.; Kungolos, A. Hexavalent chromium release from lignite fly ash and related ecotoxic effects. J. Environ.Sci. Health A 2013, 48, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Kosson, D.S.; Sanchez, F.; Kariher, P.; Turner, L.H.; Delapp, R.; Seignette, P. Characterization of Coal Combustion Residues from Electric Utilities–Leaching and Characterization Data; U.S. EPA: Washington, DC, USA, 2009; EPA-600/R-09/151.
- Xue, Q.; Wei, M. Leachability and stability of hexavalent-chromium-contaminated soil stabilized by ferrous sulfate and calcium polysulfide. Appl. Sci. 2018, 8, 1431. [Google Scholar]
- Morales, C.M.; Serano, A.R.; Zeifert, B.; Ramirez, A.H.; Ramirez, A.C.; Labra, M.P. Stabilization of Chromium in Synthetic Slags with FeSO4 and FeS2. Trans. Indian Inst. Met. 2017, 70, 1399–1407. [Google Scholar]
- Hanum, F.F.; Desfitri, E.R.; Hayakawa, Y.; Kambara, S. Preliminary study on additives for controlling As, Se, B, and F leaching from coal fly as. Minerals 2019, 8, 493. [Google Scholar] [CrossRef]
- Desfitri, E.R.; Hanum, F.F.; Hayakawa, Y.; Kambara, S. Calcium performance in paper sludge ash as suppressing material. IOP Conf. Ser. Mater. Sci. 2019, 543, 012092. [Google Scholar] [CrossRef]
- Narukawa, T.; Riley, K.W.; French, D.H.; Chiba, K. Speciation of chromium in Australian fly ash. Talanta 2007, 73, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Vollpracht, A.; van der Sloot, H.A. pH dependent leaching characterization of major and trace elements from fly ash and metakaolin geopolymers. Cem. Concr. Res. 2019, 125, 105889. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Finkelman, R.B.; French, D.; Graham, I.T.; Yang, Y.; Li, J.; Yang, P. Leaching behavior of trace elements from fly ashes of five Chinese coal power plants. Int. J. Coal. Geol. 2020, 219, 103381. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, S.; Finkelman, R.B.; Graham, I.T.; French, D.; Hower, J.C.; Li, X. Leaching characteristics of alkaline coal combustion by-products: A case study from a coal-fired power plant, Hebei Province, China. Fuel 2019, 255, 115710. [Google Scholar] [CrossRef]
- Roy, W.; Berger, P.M. Geochemical controls of coal fly ash leachate pH. CCGP 2011, 3, 63–66. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Yu, L.; Oakey, J. Thermodynamic equilibrium study of trace element transformation during underground coal gasification. Fuel Process. Technol. 2006, 87, 209–215. [Google Scholar] [CrossRef]
- Leelarungroj, K.; Likitlersuang, S.; Chompoorat, T.; Janjaroen, D. Leaching mechanisms of heavy metals from fly ash stabilised soils. Waste Manag. Res. 2018, 36, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Sakakibara, K.; Wang, L.; Sato, K.; Inoue, C. Immobilization of B, F, Cr, and As in alkaline coal fly ash through an aging process with water. Environ. Monit. Assess. 2014, 186, 6757–6770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Reardon, E.J. Removal of B, Cr, Mo, and Se from wastewater by incorporation into hydrocalumite and ettringite. Environ. Sci. Technol. 2003, 37, 2947–2952. [Google Scholar] [CrossRef] [PubMed]
- Mahedi, M.; Cetin, B.; Dayioglu, A.Y. Effect of cement incorporation on the leaching characteristics of elements from fly ash and slag treated soils. J. Environ. Manag. 2020, 253, 109720. [Google Scholar] [CrossRef] [PubMed]
- Hartuti, S.; Kambara, S.; Takeyama, A.; Hanum, F.F. Leaching characteristic of arsenic in coal fly ash. JMSE-B, 2017, 7, 19–26. JMSE-B 2017, 7, 19–26. [Google Scholar]
- Neupane, G.; Donahoe, R.J. Leachability of elements in alkaline and acidic coal fly ash samples during batch and column leaching tests. Fuel 2013, 104, 758–770. [Google Scholar] [CrossRef]
- Roy, B.; Choo, L.W.; Battacharya, S. Prediction of distribution of trace elements under Oxy-fuel combustion condition using Victorian brown coals. Fuel 2013, 114, 135–142. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).