Abstract
Previous studies have proved that the lead complexes of benzohydroxamic acid (Pb–BHA) are effective collectors of scheelite flotations; however, the separation of scheelite from calcite needs depressants with high selectivity. In this study, we reported a novel depressant for calcite minerals, and Pb–BHA served as the collector of scheelite. The flotation behavior of polyaspartic acid (PASP) in a scheelite and calcite flotation that uses Pb–BHA was determined via flotation experiments. Furthermore, the selective adsorption of PASP on the mineral surfaces and the effect of PASP on the adsorption of Pb–BHA on the mineral surfaces were investigated through zeta potential measurements, X-ray photoelectron spectroscopy (XPS), crystal chemistry calculations, and Fourier transform infrared spectroscopy (FTIR) measurements. Thus, PASP demonstrated high selectivity in both scheelite and calcite and contributed to the successful separation of scheelite from calcite. PASP exhibited a higher adsorption capacity and stronger chemisorption with the active sites of calcium atoms on the calcite surface. The crystal chemistry calculations indicated that the distance of the PASP functional groups matched with the calcium distance of a calcite mineral surface, which can be attributed to the selectivity of PASP. Furthermore, the adsorption of PASP impeded the adsorption of Pb–BHA on the calcite surfaces, whereas the opposite was the case for scheelite. The mutually reinforcing selectivity of PASP and Pb–BHA considerably contributes to the efficient flotation separation of scheelite from calcite.
1. Introduction
Tungsten and its alloys have been widely used in metallurgical machinery, petrochemical, construction, aerospace, and defense engineering [1,2,3]. However, as the chief commercial source of tungsten, scheelite (CaWO4) tends to coexist with other calcium-containing minerals, such as fluorite (CaF2) and calcite (CaCO3), in ore deposits [4,5,6]. The flotation separation of scheelite from gangue minerals is problematic because of their similar surface physicochemical characteristics; furthermore, the traditional anion collectors adsorb onto the surface of these minerals by identifying active Ca2+ sites [5,7,8]. Fortunately, previous studies have proved that the lead complexes of benzohydroxamic acid (Pb–BHA) were effective collectors of scheelite flotation [9,10,11]. Here, fluorite was scarcely collected in the flotation of the Pb–BHA complexes, implying that scheelite cannot be separated from fluorite without depressants [11,12]. However, calcite exhibits high recovery in the case of the Pb–BHA flotation, and it would be impossible to separate scheelite from calcite in the absence of depressants [13,14]. Therefore, to obtain a high-quality concentrate, a reagent that could selectively depress the floatability of calcite in Pb–BHA flotation should be developed.
Many depressants have been reported to generate scheelite from calcite. These depressants can be generally categorized as inorganic and organic depressants. Sodium silicate (Na2SiO3) is the most widely used inorganic depressant because it is cheap and easily accessible [15,16]. However, it was less selective for scheelite and calcite in fatty acids and the Pb–BHA flotation [14,15,17]. Sodium silicate was modified using sulfuric acid and diverse metal ions to improve its selectivity and was observed to inhibit scheelite to some extent [18,19]. Meanwhile, the presence of a large amount of sodium silicate leads to difficulty with respect to water reuse, resulting in prominent environmental issues [9]. Alternative inorganic depressants, such as polyphosphates, have failed to achieve satisfactory results in practice [20]. Recently, organic depressants have become the focus of research because of their variety and large number of sources. Some organic depressants, such as tannin, calcium lignosulfonate, sodium alginate, carboxymethyl cellulose, and pectin, have been used as a calcite depressant in scheelite or fluorite flotation when sodium oleate was used as the collector [21,22,23,24,25,26,27,28]. However, the majority of the research on organic depressants remains confined to the laboratory and has not been applied in practice, which can be attributed to the fact that organic depressants have certain drawbacks, including high cost, poor solubility, and their non-biodegradable or polluting nature [29]. Fortunately, polyaspartic acid (PASP) does not exhibit these disadvantages and can be considered to be an ecofriendly and efficient depressant. Furthermore, it is non-toxic, water soluble, and highly biodegradable, and the final degradation products are ammonia, carbon dioxide, and water, which are harmless to the environment [30,31]. PASP belongs to a class of polycarboxylic acids, the molecular structure of which contains several carboxyl groups that tend to interact with the calcium ions in a solution [32], making it possible for PASP to be used in the flotation of calcium-containing minerals. The selectivity of reagents is highly dependent on the matching degree of the surface atom distance and the functional group [33]. Interestingly, in the study of crystal chemistry, the distance of the PASP functional groups seemed to match the distance between the calcium atoms on the calcite surfaces, enabling the application of PASP to selectively depress calcite. Pb–BHA complexes, as a new collector, recognize the oxygen atom group during an interaction with calcium-containing minerals, which are fundamentally different from the traditional anion collectors [34,35,36]. Therefore, a scheme that combines PASP and Pb–BHA for separating scheelite from calcite has considerable application potential.
In this study, PASP and Pb–BHA were used for the selective separation of scheelite from calcite. The flotation behaviors of PASP in the scheelite and calcite flotation using Pb–BHA complexes as the collector were investigated via pure mineral flotation experiments. The selective adsorption mechanisms of PASP on the scheelite and calcite surfaces were investigated via the zeta potential measurements, XPS measurements, and crystal chemistry calculations. Furthermore, the competitive adsorption of PASP and Pb–BHA on the mineral surfaces was clarified through zeta potential and FTIR measurements.
2. Materials and Methods
2.1. Minerals and Reagents
The pure scheelite, calcite, and fluorite samples used in the experiments were obtained from the Chaishan mine in the Hunan Province, China. The X-ray powder diffraction spectra of scheelite and calcite are presented in Figure S1. The purities of the mineral samples were more than 98.5%. The measured exposed planes of scheelite are {1 0 1} and {1 1 2}, whereas those of calcite is {1 0 4}, which is also the most common cleavage plane for scheelite and calcite during crushing or grinding [37]. Blocky pure mineral samples were crushed by a crusher and powdered using a laboratory ceramic ball mill. The −74 + 38 μm mineral samples were screened for use via flotation experiments and FTIR and XPS measurements, and samples with size fractions of −2 µm were prepared for the zeta potential measurements.
Benzohydroxamic acid (BHA), polyaspartic acid (PASP), lead nitrate, and terpineol were of analytical grade and obtained from the Kefei Chemical Industry, Tianjin, China. The molecular weight of PASP is 1000–5000. The pH regulators in all the experiments were HCl and NaOH, and distilled water with a conductivity of more than 17.0 MΩ·cm was used throughout the experiment.
2.2. Micro-Flotation Experiments
Pure mineral flotation experiments were conducted, and the flowsheet is presented in Figure 1 [9,34,38]. An XFG flotation machine containing a 40 mL cell at 1800 rpm was employed for all the flotation experiments. A total of 2.0 g mineral samples were used for each experiment, and the mineral pulp was treated using desired reagents. The pulp pH was adjusted using HCl or NaOH solutions. After flotation, the concentrate and tailings were collected, dried, and weighed, and the recovered amount was calculated. Each experiment was repeated thrice, and the average value was calculated as the final result.
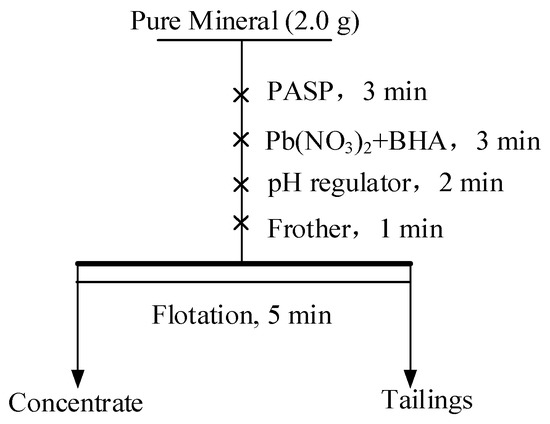
Figure 1.
Flowsheet of the micro-flotation test.
2.3. Zeta Potential Measurements
A Coulter Delsa440sx Zeta analyzer (Beckman Coulter Inc., USA) was used for performing the zeta potential measurements. 20 mg of minerals (−2 μm) and 40 mL of a KCl background electrolyte (10−3 mol/L) were used for preparing the mineral suspension. This was left to settle for 10 min under room temperature (25 °C) after the reagents were added at the desired concentration; subsequently, supernatant liquor was used for conducting the zeta potential measurements.
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Measurements
A Bruker Alpha FTIR spectrometer (Nicolet 6700, Thermo Scientific, USA) was employed for performing the IR measurements. The mineral samples were conditioned in desired reagent solutions at pH 10.0 and 25 °C, and the suspensions were centrifuged. The precipitation was washed thrice using deionized water and then vacuum dried at 40 °C. The IR measurements were performed in the range of 400–4000 cm−1. The spectral resolution was 2.0 cm−1.
2.5. X-Ray Photoelectron Spectroscopy (XPS) Measurements
The mineral samples for XPS measurements were prepared in accordance with the experimental procedure used for preparing samples for the FTIR measurements. The XPS spectra of the samples were recorded using a Thermo Fisher Scientific K-Alpha 1063 X system (Thermo fisher scientific, UK). The standard C1s binding energy was calibrated at 284.8 eV, and the test results were fitted using XPS Peak 4.1.
3. Results and Discussion
3.1. Pure Mineral Flotation Results
The collecting abilities of the Pb–BHA complexes with respect to scheelite, calcite, and fluorite are shown in Figure 2. The result demonstrated that fluorite was barely collected, the flotation recoveries of scheelite and calcite exhibit a similar tendency with changing pH, and high recovery of both the minerals can be achieved in pH 8–10.The highest recoveries of scheelite and calcite are 95.5% and 86.7% at pH = 9.0, respectively, and the difference between their recoveries is very small. This illustrated that the separation of scheelite from calcite in a Pb–BHA flotation is almost impossible without any depressant, which is in agreement with previous studies [9,10].
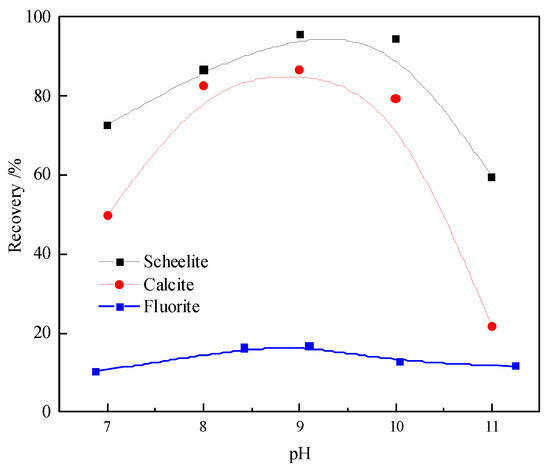
Figure 2.
Floatability of scheelite, calcite, and fluorite using Pb–BHA as the collector (CBHA = 2.0 × 10−4 mol/L, CPb = 2.0 × 10−4 mol/L, and Cterpineol = 12.5 μL/L).
PASP was added as a depressant into the Pb–BHA flotation to increase the difference with respect to the recovery of scheelite and calcite. The effect of PASP dosage on the floatability of scheelite and calcite was investigated and is shown in Figure 3. The results show that the flotation recovery of scheelite and calcite decreased with increasing PASP dosage; however, the recovery of scheelite decreased slowly, whereas that of calcite decreased rapidly. When the concentration of PASP is less than 4 mg/L, the recovery of scheelite barely changes with increasing PASP concentration, whereas that of calcite drastically decreases with a small increase in PASP, indicating that PASP does not significantly affect the floatability of scheelite and considerably influences the flotation of calcite. With the further increase in PASP dosage, although the recoveries of the two minerals correspondingly decreased, the recovery of calcite decreased more intensely than that of scheelite. When the PASP dosage was 4 mg/L, recovery rates of 86.7% and 30.87% were obtained for scheelite and calcite, respectively, based on which 4 mg/L can be considered to be the optimum concentration for separating scheelite from calcite.

Figure 3.
Effect of PASP dosage on the flotation recovery of scheelite and calcite (pH = 9.0 ± 0.1, CBHA = 2.0 × 10−4 mol/L, CPb = 2.0 × 10−4 mol/L, and Cterpineol = 12.5 μL/L).
The effect of pH on scheelite and calcite flotation was investigated after PASP treatment at its optimum concentration. As denoted in Figure 4, the floatability of calcite was poor throughout the pH range, whereas scheelite demonstrated good floatability when pH = 8.5–10.5. At pH = 10, scheelite achieved its highest recovery of 93.24%, whereas the lowest recovery of 12.14% was obtained in the case of calcite; thus, the maximum difference in recovery could become approximately 80%. The scheelite and calcite in the Pb–BHA flotation can be effectively separated after adding PASP as a depressant at approximately pH = 10.0.
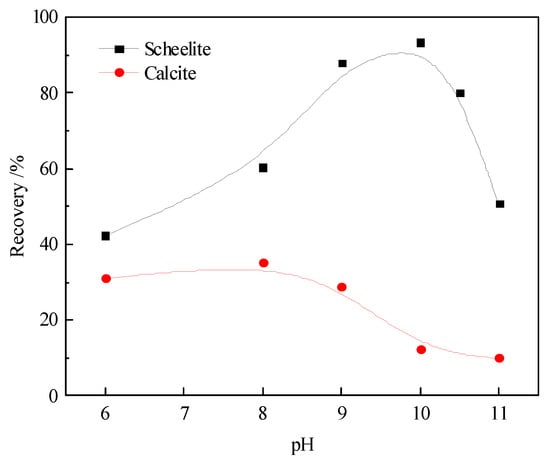
Figure 4.
Floatability of scheelite and calcite in Pb–BHA after PASP treatment (CBHA = 2.0 × 10−4 mol/L, CPb = 2.0 × 10−4 mol/L, CPASP = 4 mg/L, and Cterpineol = 12.5 μL/L).
The flotation experiments that have been conducted with respect to pure minerals indicate that PASP has excellent selectivity for scheelite and calcite and that they can be separated using 4 mg/L of PASP when pH = 10.0. This proved that the scheme in which Pb–BHA complexes are employed as the collector and PASP is considered to be the depressant demonstrates high efficiency with respect to the selective separation of scheelite from calcite. However, further studies will be required to identify the adsorption mechanism of PASP and the Pb–BHA complexes on the mineral surfaces, including the selective adsorption of PASP on mineral surfaces and the competitive adsorption of PASP and Pb–BHA on mineral surfaces.
3.2. Selective Adsorption of PASP on the Scheelite and Calcite Surfaces
3.2.1. Solution Chemistry Analysis of PASP
Silverman et al. [39] and Wu and Grant [32] modeled PASP as a molecule containing four aspartyl residues, denoted as H4L. The protonation reactions of the PASP molecule and the equilibrium constants can be given as follows:
H+ + L4− = HL3− logK1 = 5.30
H+ + HL3− = H2L2− logK2 = 4.22
H+ + H2L2− = H3L− logK3 = 3.72
H+ + H3L− = H4L logK4 = 2.40
The species distribution of PASP was obtained using Equations (1)–(4), as presented in Figure 5. The PASP molecules gradually deprotonated with increasing pH of the solution. When pH > 7, L4− is the dominant component of the PASP solution. Therefore, within the optimal flotation pH range (8.5–10.5), PASP interacts with mineral surfaces in the form of L4− in a solution.

Figure 5.
Species distribution of PASP versus pH (C = 4 mg/L).
3.2.2. Zeta Potential Measurements
The zeta potential of scheelite after being treated with PASP is shown in Figure 6a. The zeta potential of scheelite and PASP (in colloid) were negative at pH = 7.0–12.0, implying that the PASP and scheelite were negatively charged in the tested pH range, which is in agreement with previous studies [40,41]. After being treated with PASP, the surface charge of scheelite slightly changed, indicating that a smaller adsorption level of PASP was obtained on the scheelite surface. The zeta potential of calcite after being treated with PASP is shown in Figure 6b. The zeta potential of calcite was almost positive at around pH = 6–11, and the isoelectric point (IEP) of calcite was approximately 10.9. Here, there is some inconsistency with respect to the previous findings regarding the IEP of calcite, which may be attributed to the different sources of calcite samples [42]. The result presented in Figure 6b shows that the positively charged calcite drastically shifted to a negative charge after the addition of negatively charged PASP, indicating that a higher adsorption level of PASP could be observed in the case of the calcite surface. There was an apparent difference with respect to the degree of the zeta potential negative shift caused by PASP between scheelite and calcite. Therefore, PASP exhibited weaker adsorption on the scheelite surface and stronger adsorption on the calcite surface, consistent with the flotation experiment results.

Figure 6.
Zeta potential of (a) scheelite and (b) calcite before and after treatment with PASP (CPASP = 4 mg/L).
3.2.3. XPS Analysis Results
Scheelite and calcite were subjected to XPS measurements before and after being treated with PASP to investigate the role of chemical adsorption in the selective depression of PASP on the mineral surfaces. Furthermore, the atomic concentrations of specific elements on the scheelite and calcite surfaces before and after being treated with PASP were measured, as shown in Table 1. The adsorption of PASP on the mineral surfaces could result in the variation of atomic concentrations, and N can be used as the characteristic element of PASP under this research system. After being treated with PASP, the atomic concentrations of C1s, O1s, W4f, Ca2p, and N1s on the scheelite surface slightly changed, with the atomic concentration of N1s increasing by 1.04%, indicating the modest adsorption of PASP on the scheelite surfaces. Meanwhile, the atomic concentration of N1s on the calcite surface increased by 1.60% after being treated with PASP, denoting that PASP is extensively adsorbed on the calcite surfaces. After comparing the atomic concentrations of N1s on the mineral surfaces, the higher adsorption amount of PASP was obtained from the surface of calcite, which is in accordance with the zeta potential measurement results.

Table 1.
Atomic concentration of elements on the mineral surfaces before and after treatment with PASP.
PASP mainly exists in the form of L4− in alkaline pulp; because the deprotonated carboxyl groups in PASP molecules tend to react with calcium ions to form chelates [32,39], the oxygen atoms of carboxyl in PASP are believed to combine with the calcium ions on mineral surfaces to form complexes. Therefore, the XPS analysis of Ca2p and O1s was used to characterize the adsorption strength of PASP on the mineral surfaces. The O1s spectra of scheelite before and after PASP treatment are shown in Figure 7, with the peaks at 530.54 and 531.84 eV corresponding to O1s in the W–O and Ca–O groups, respectively [11,43]. After treatment using PASP, the binding energies of the O1s peaks became 530.39 and 531.76 eV, with the displacements of the two peaks observed to be 0.15 and 0.08 eV, respectively. The Ca2p spectra of scheelite before and after PASP treatment are shown in Figure 8. The peak values of 347.04 and 350.54 eV were attributed to the Ca2p of Ca2p3/2 and Ca2p1/2, respectively. After PASP treatment, the two peaks slightly shifted to 347.09 and 350.59 eV, respectively. The binding energy changes of both the peaks were 0.05eV, which is within the measurement error associated with the instrument. Based on these insignificant shifts, PASP can be concluded to weakly adsorb on the scheelite surface.

Figure 7.
O1s spectra of the scheelite surface with/without PASP.

Figure 8.
Ca2p spectra of the scheelite surface with/without PASP.
As shown in Figure 9, the peaks at 531.52 and 532.94 eV correspond to the O1s in the C–O and Ca–O groups [26,44]. When PASP was added, the peaks at 531.52 and 532.94 eV shifted to 531.39 and 532.70 eV, respectively, with the displacement of the two peaks observed to be 0.13 and 0.24 eV, respectively. Figure 10 shows the Ca2p spectra of the untreated and treated calcite. When PASP was added, the peaks at 346.91 and 350.46 eV shifted to 347.15 and 350.70 eV, and the shift in the two peaks was 0.24 and 0.25 eV, respectively. Here, the significant shift in O1s and Ca2p on the calcite surface indicates the chemisorption of PASP on the calcite surface. This also illustrated that PASP exhibited higher chemical adsorption on calcite surfaces when compared with that on scheelite surfaces. Furthermore, the significant shift in the Ca–O group and Ca2p on the calcite surface indicated that calcium cations could be the active sites of PASP adsorption on the calcite surface. During the adsorption process, the oxygen atoms of PASP bond with the calcium cations of the calcite surface, enabling the absorption of PASP on the mineral surface.

Figure 9.
O1s spectra of the calcite surface with/without PASP.

Figure 10.
Ca2p spectra of the calcite surface with/without PASP.
The zeta potential measurements showed that the negatively charged PASP tended to adsorb on the positively charged calcite surface. Furthermore, the zeta potential and XPS measurements indicate that PASP exhibits a higher adsorption capacity and stronger chemisorption with the active sites of calcium atom on the surface of calcite when compared with those observed on the surface of scheelite. This demonstrates the excellent selective adsorption capacity of PASP on the scheelite and calcite surfaces.
3.2.4. Crystal Chemistry Calculations
The distribution of the active Ca atoms on the exposed surfaces of scheelite and calcite is presented in Table 2. The number of Ca atoms in each unit cell area of scheelite {1 0 1} and {1 1 2} and calcite {1 0 4} were 1, 2 and 2, respectively, and the Ca density was 5.06, 6.58 and 8.22 mol/m2, respectively, indicating that more Ca atoms were exposed on the calcite surface. This was supported by the XPS results of the atomic concentration of Ca on the mineral surfaces, as shown in Table 1. The high Ca density on the calcite surface provides more active sites for PASP adsorption.

Table 2.
Distribution of Ca atoms on the exposed surfaces of scheelite and calcite.
The selective adsorption of PASP on the calcium minerals is highly related to the surface structure of the mineral and the molecular structure of PASP. According to the molecular simulation results, as shown in Figure 11, the distance between the two oxygen atoms of carboxyl in the PASP ion was 2.615 Å. As shown in Figure 12, the distance between two adjacent calcium atoms on the {1 0 1} and {1 1 2} scheelite planes was 3.867 Å, whereas those observed in the case of calcite {1 0 4} was 4.047 and 4.988 Å. This indicated that the distance between two adjacent Ca atoms on the scheelite and calcite surfaces was much longer than that between two oxygen atoms of carboxyl in the PASP ion, implying that the two oxygen atoms in the PASP ion are likely to bond with the Ca atoms on the scheelite and calcite surfaces with a bidentate coordination rather than a bridged coordination [45,46]. Because of its polymer structure, several carboxyls are present in PASP. The existence of carboxyl allows interaction and binding with the calcium points on the mineral surfaces. However, the distance between the calcium absorption sites on the scheelite and calcite mineral surfaces are different. Therefore, the selectivity of PASP may be related to the matching degree between the active sites and the functional group of the reagent PASP [33,47]. Figure 11 shows that the closest distance between the oxygen atoms in two adjacent carboxyl groups in the polymer structure of PASP ion ranges from 4.278 to 4.399 Å and 5.098 to 5.188 Å. Based on the comparison of the distance between two adjacent calcium atoms on the scheelite and calcite surfaces (Figure 12), the distance between the oxygen atoms in two adjacent carboxyl groups of PASP was closer to that between the calcium atoms on the calcite surface, implying that the carboxyl groups of PASP could match with Ca–Ca on the calcite surface better than that on the scheelite surface. Although the atomic distance is not very precisely matched, it is very beneficial for the selective adsorption of PASP polymers on the surfaces of the two minerals. Therefore, a high matching degree between the PASP functional groups and the active calcium atoms on the calcite surface strengthens the PASP adsorption on the calcite surface, resulting in a higher shift with respect to the zeta potential and the element binding energy of calcite.
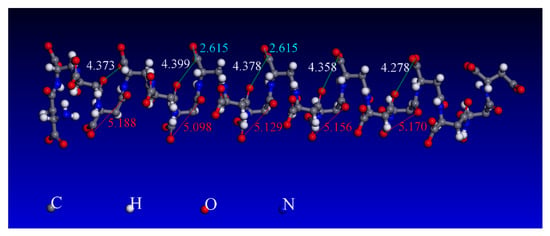
Figure 11.
Molecular diagram of the steady-state deprotonated PASP.

Figure 12.
Molecular geometries of the scheelite {1 0 1} (a) and {1 1 2} (b) as well as calcite {1 0 4} (c) cleavage plane.
3.3. Effect of PASP on the Adsorption of Pb–BHA on the Mineral Surfaces
3.3.1. Zeta Potential Analysis Results
The effect of PASP on the adsorption of Pb–BHA on mineral surfaces was investigated via zeta potential measurements. Figure 13 shows the zeta potential for scheelite and calcite after treatment with Pb–BHA and PASP. Figure 13a shows that the zeta potential of scheelite became positively charged after being treated with positively charged Pb–BHA, whereas that of the scheelite treated with PASP and Pb–BHA was very close to that treated only with Pb–BHA, implying that the pre-adsorption of PASP has little influence on the adsorption of Pb–BHA on scheelite. The results presented in Figure 13b show that there were negative shifts with respect to the zeta potential of calcite after reacting with Pb–BHA, which is different when compared with the results observed in the case of scheelite. Even though this unique result may be attributed to the special effect of Pb–BHA adsorption on the zeta potential of the calcite surfaces, a more detailed adsorption mechanism must be identified through further research. Here, we continue to focus on the influence of PASP on the adsorption of Pb–BHA on the calcite surfaces. Figure 13b shows that the zeta potentials of calcite treated with PASP and Pb–BHA experience remarkable drops (approximately 30 mV), suggesting that PASP can strongly interact with calcite and impact the adsorption of collector on the calcite surfaces. The zeta potential measurements revealed that the collecting abilities of the Pb–BHA complexes are much more powerful in the case of scheelite when compared with those of calcite in the presence of PASP.

Figure 13.
Zeta potential of (a) scheelite and (b) calcite after treatment with PASP and Pb–BHA (CBHA = 2 × 10−4 mol/L, CPb = 2 × 10−4 mol/L, and CPASP = 4 mg/L).
3.3.2. FTIR Analysis Results
FTIR measurements were employed to study the influence of PASP on the adsorption of collectors on the mineral surfaces. Figure 14 shows the infrared spectrum of minerals and its interaction with the reagents. Infrared spectra of PASP is shown in Figure S2. In the case of scheelite, as shown in Figure 14a, the characteristic absorption peaks of tungstate were 807 and 446 cm−1. Furthermore, after interaction with Pb–BHA, the absorption peaks of C=O and C–N were 1599 and 1151 cm−1 and the vibration of the benzene ring skeleton was 1562 and 1480 cm−1, respectively [20,34,48]. This proves the adsorption of Pb–BHA on the scheelite surface. In the infrared spectra of scheelite treated with PASP and Pb–BHA, the characteristic absorption peaks of Pb–BHA barely changed, indicating that PASP has little influence on the adsorption of Pb–BHA on the scheelite surfaces. In the case of calcite, as shown in Figure 14b, the characteristic absorption peaks of CO32− were 712, 876, and 1428 cm–1, which were caused by the in-plane bending vibration, out-of-plane bending vibration, and asymmetric stretching vibration, respectively [49]. After being treated with Pb–BHA, the peaks of C=O and C–N were 1609 and 1150 cm−1, respectively. This shows that Pb–BHA adsorbed on the calcite surface. All the characterized peaks induced by Pb–BHA disappeared from the spectra of the calcite treated with PASP and Pb–BHA, implying the considerable influence of the presence of PASP with respect to the adsorption of Pb–BHA on the calcite surfaces. This is in good agreement with the zeta potential measurement results.

Figure 14.
Infrared spectra of (a) scheelite and (b) calcite after treatment with Pb–BHA and PASP.
The zeta potential and FTIR measurements proved that PASP impedes the adsorption of the Pb–BHA complexes on the calcite surfaces; however, only a minor influence could be observed in the case of scheelite. This implies that Pb–BHA has a more powerful adsorption ability when compared with that of scheelite surfaces treated with PASP, whereas the opposite is true in the case of calcite.
3.4. Adsorption Model of PASP and Pb–BHA on the Mineral Surfaces
The adsorption models of PASP and Pb–BHA were built on the scheelite and calcite surfaces to clearly explain their adsorption mechanisms, as shown in Figure 15 (PASP was simplified to the shown model). Scheelite exposes more O atoms on the typical cleavage planes, and the preferential dissociation of the calcium ions in the solution results in excess WO42− on the surface, indicating that scheelite has a negatively charged oxygen-rich surface [50]. Meanwhile, more Ca atoms expose the usual cleavage planes of calcite, and the preferential dissociation of the CO32− in the solution results in excess Ca2+ on the surface, indicating that calcite has a positively charged calcium-rich surface [51,52]. In terms of PASP, a negatively charged PASP colloid tended to adsorb on a positively charged calcite surface; however, this was problematic in the case of a negatively charged scheelite surface. Furthermore, the experiments demonstrated that PASP has a higher adsorption capacity and exhibits stronger chemisorption with the active sites of calcium atom on the calcite surfaces; however, a lower adsorption capacity and weaker chemisorption could be observed in the case of scheelite surfaces. With regard to the collector of the Pb–BHA complexes, the positively charged Pb–BHA complexes could easily adsorb on the negatively charged scheelite surfaces, with the lead group of collectors bonding with the oxygen atoms on the scheelite surface via chemical adsorption. Therefore, PASP and Pb–BHA complexes exhibit opposite charges with respect to the pulp and action sites in the adsorption process of reagents and mineral surfaces. However, this difference in the property of the collector and depressant is highly favorable for the separation of scheelite from calcite. When Pb–BHA is employed as the collector and PASP is employed as the depressant, their selectivity is mutually reinforced and fully utilized. Thus, fewer PASP and more Pb–BHA were obtained in the case of the scheelite surface, rendering it strongly hydrophobic, such that it stuck to air bubbles and floated. More PASP and fewer Pb–BHA were observed on the calcite surface, making it strongly hydrophilic, such that it sunk to the bottom of the slurry. Thus, the collaborative selectivities of the Pb–BHA complexes and PASP denote that this novel scheme presents a promising protocol for realizing the highly efficient separation of scheelite from calcite.
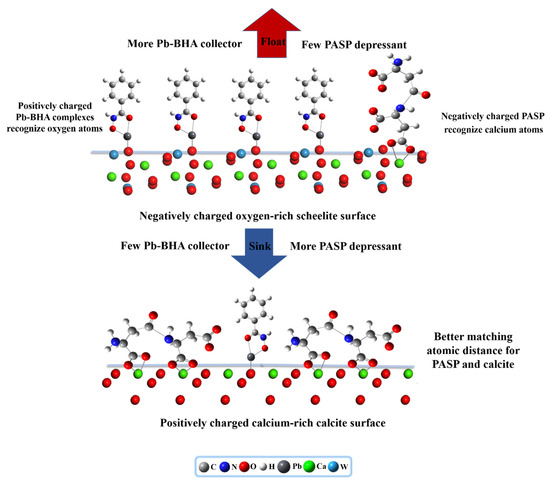
Figure 15.
Selective adsorption model for PASP and Pb–BHA on the mineral surfaces.
4. Conclusions
It is impossible to separate scheelite from calcite without using any depressants in a Pb–BHA flotation. However, our flotation experiments involving pure minerals indicated that PASP exhibits excellent selectivity for scheelite and calcite at pH = 10.0. Scheelite can be separated from calcite using 4 mg/L of PASP. Thus, the experiments proved that a scheme that employs Pb–BHA as the collector and PASP as the depressant is highly efficient in terms of the selective separation of scheelite from calcite. The XPS and zeta potential measurements reveal that PASP has a higher adsorption capacity and stronger chemisorption with the active sites of calcium atom on the calcite surfaces, whereas the opposite is true in the case of scheelite. The crystal chemistry calculations indicate that PASP is likely to bond with the Ca atoms on the mineral surfaces in a bidentate coordination. The increased matching degree between the PASP functional groups and the active calcium atoms on the calcite surface strengthens the PASP adsorbed on the calcite surface, which probably can be attributed to the selectivity of PASP. The zeta potential and FTIR measurements proved that PASP impedes the adsorption of the Pb–BHA complexes on the calcite surfaces but has little influence in the case of scheelite. Fewer PASP and more Pb–BHA complexes were obtained on the scheelite surface when compared with those on the calcite surface. Therefore, the collaborative selectivity of the Pb–BHA complexes and PASP contributed to the highly efficient separation of scheelite from calcite. In summary, the employment of PASP as the depressant and Pb–BHA as the collector resulted in a novel scheme with highly selective reagents. Furthermore, the cooperative selectivity of PASP and Pb–BHA presents a promising protocol for realizing the highly efficient separation of scheelite from other calcium-containing minerals.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/10/6/561/s1, Figure S1: XRD of pure scheelite and calcite, Figure S2: Infrared spectra of PASP.
Author Contributions
Z.W.: Writing—original draft, review and editing, investigation. J.F.: writing—original draft, software. H.H.: conceptualization, methodology. W.S.: writing—review and editing. T.Y.: conceptualization, methodology. L.W.: software. L.S.: methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 51804340), the State Key Laboratory of Mineral Processing (No. BGRIMM-KJSKL-2019), the Innovation Driven Plan of Central South University (No. 2018CX036), National Key Scientific Research Project (2018YFC1901601 and 2018YFC1901602), and the Collaborative Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rao, N.K. Beneficiation of tungsten ores in India: A review. Bull. Mater. Sci. 1996, 19, 201–265. [Google Scholar] [CrossRef]
- Ilhan, S.; Kalpakli, A.O.; Kahruman, C.; Yusufoglu, I. The investigation of dissolution behavior of gangue materials during the dissolution of scheelite concentrate in oxalic acid solution. Hydrometallurgy 2013, 136, 15–26. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Kelsall, G.H. The surface chemical properties of scheelite (CaWO4) I. The scheelite/water interface and CaWO4 solubility. Colloids Surf. 1987, 25, 369–385. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mechanism of fatty acid adsorption in salt-type mineral flotation. Miner. Eng. 1991, 4, 879–890. [Google Scholar]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; et al. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Han, H.; Xiao, Y.; Hu, Y.; Sun, W.; Nguyen, A.V.; Tang, H.; Gui, X.; Xing, Y.; Wei, Z.; Wang, J. Replacing Petrov’s process with atmospheric flotation using Pb-BHA complexes for separating scheelite from fluorite. Miner. Eng. 2020, 145, 1–10. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Z.; Han, H.; Sun, W.; Hu, Y.; Wang, J.; Wang, L.; Liu, H.; Yang, Y.; Zhang, C.; et al. Fluorite particles as a novel calcite recovery depressant in scheelite flotation using Pb-BHA complexes as collectors. Miner. Eng. 2019, 132, 84–91. [Google Scholar] [CrossRef]
- Han, H.; Liu, W.; Hu, Y.; Sun, W.; Li, X. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb-BHA complexes in Shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.L.; Sun, W.J.; Wang, J.J.; Gao, Z.Y.; Wang, L.; Zhang, C.Y.; et al. Selective Separation of Scheelite from Calcite by Self-Assembly of H2SiO3 Polymer Using Al3+ in Pb-BHA Flotation. Minerals 2019, 9, 43. [Google Scholar] [CrossRef]
- Bo, F.; Xianping, L.; Jinqing, W.; Pengcheng, W. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar] [CrossRef]
- Yang, Y.H.; Xu, L.H.; Tian, J.; Liu, Y.C.; Han, Y.X. Selective flotation of ilmenite from olivine using the acidified water glass as depressant. Int. J. Miner. Process. 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jiao, F.; Qin, W.Q.; Zhu, H.L.; Jia, W.H. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Deng, R.D.; Yang, X.F.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Ma, Y.Q. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Mercade, V. Effect of polyvalent metal-silicate hydrosols on the flotation of calcite. Trans. Soc. Min. Eng. AIME 1981, 268, 1842–1846. [Google Scholar]
- Yongxin, L.; Changgen, L. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. I. Selective flotation of scheelite. Int. J. Miner. Process. 1983, 10, 205–218. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.M.; Zhang, G.F.; Liu, D.Z.; Li, L.F. Selective flotation of scheelite from calcite using calcium lignosulphonate as depressant. Miner. Eng. 2018, 119, 73–75. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective flotation of scheelite from calcite using xanthan gum as depressant. Miner. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Yin, W.; Liang, X. Flotation separation of scheelite from calcite using carboxyl methyl cellulose as depressant. Miner. Eng. 2018, 127, 329–333. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. New insights into the carboxymethyl cellulose adsorption on scheelite and calcite: Adsorption mechanism, AFM imaging and adsorption model. Appl. Surf. Sci. 2019, 463, 105–114. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Han, H.; Sun, W.; Hu, Y. Improved flotation separation of cassiterite from calcite using a mixture of lead (II) ion/benzohydroxamic acid as collector and carboxymethyl cellulose as depressant. Miner. Eng. 2017, 113, 68–70. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Wu, Q.; Zheng, Y.; Cui, Y.; Yan, W.; Deng, J.; Peng, T. Comparative study on adsorption and depressant effects of carboxymethyl cellulose and sodium silicate in flotation. J. Mol. Liq. 2018, 268, 140–148. [Google Scholar] [CrossRef]
- Li, J.; Ping, Y.; Yunbai, L. Synthesis and scale inhibition of polyaspartic acid derivative. Ind. Water Treat. 2006, 26, 21–23. [Google Scholar]
- Liu, Z.; Sun, Y.; Zhou, X.; Wu, T.; Tian, Y.; Wang, Y. Synthesis and scale inhibitor performance of polyaspartic acid. J. Environ. Sci. 2011, 23, S153–S155. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Grant, C. Effect of Chelation Chemistry of Sodium Polyaspartate on the Dissolution of Calcite. Langmuir 2002, 18, 6813–6820. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Liu, Y. Flotation separation of smithsonite from calcite using 2-phosphonobutane-1,2,4-tricarboxylic acid as a depressant. Powder Technol. 2019, 352, 11–15. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.D.; Chen, K.F.; Zhu, Y.G.; Nguyen, A.V.; Tian, M.J.; Wang, L.; Yue, T.; et al. Novel catalysis catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the-surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W. Configurations of lead(II)–benzohydroxamic acid complexes in colloid and interface: A new perspective. J. Colloid Interface Sci. 2020, 562, 342–351. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Zhu, Y.; Li, B.; Song, Z. Structures of Pb-BHA Complexes Adsorbed on Scheelite Surface. Front. Chem. 2019, 7, 645. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef]
- Yue, T.; Han, H.; Hu, Y.; Wei, Z.; Wang, J.; Wang, L.; Sun, W.; Yang, Y.; Sun, L.; Liu, R.; et al. Beneficiation and Purification of Tungsten and Cassiterite Minerals Using Pb–BHA Complexes Flotation and Centrifugal Separation. Minerals 2018, 8, 566. [Google Scholar] [CrossRef]
- Silverman, D.; Kalota, D.; Stover, F. Effect of pH on corrosion inhibition of steel by polyaspartic acid. Corrosion 1995, 51, 818–825. [Google Scholar] [CrossRef]
- Kang, J.; Khoso, S.A.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Utilisation of 1-Hydroxyethylidene-1, 1-diphosphonicacid as a selective depressant for the separation of scheelite from calcite and fluorite. Colloid Surf. A 2019, 582. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Jiao, F.; Jia, W. Flotation separation of fluorite from calcite using polyaspartate as depressant. Miner. Eng. 2018, 120, 80–86. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Y.; Zhu, H.; Sun, W.; Qin, W.; Liu, R.; Gao, Z. Inhibition performance and adsorption of polycarboxylic acids in calcite flotation. Miner. Eng. 2019, 133, 60–68. [Google Scholar] [CrossRef]
- Capece, A.M.; Polk, J.E.; Shepherd, J.E. X-ray photoelectron spectroscopy study of BaWO4 and Ba2CaWO6. J. Electron. Spectrosc. Relat. Phenom. 2014, 197, 102–105. [Google Scholar] [CrossRef]
- Liu, X.; Huang, G.-Y.; Li, C.-X.; Cheng, R.-J. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Li, C.W.; Sun, W.; Hu, Y.H. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Ai, G.; Liu, C.; Zhang, W. Utilization of sodium humate as selective depressants for calcite on the flotation of scheelite. Sep. Sci. Technol. 2018, 53, 2136–2143. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y.; Liu, X. Surface energies and appearances of commonly exposed surfaces of scheelite crystal. Trans. Nonferrous Met. Soc. China 2013, 23, 2147–2152. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, L.; Cui, X.; Hu, Y.; Sun, W.; Zeng, H. Probing Anisotropic Surface Properties and Surface Forces of Fluorite Crystals. Langmuir 2018, 34, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Hiçyilmaz, C.; Özbayoglu, G. The effects of amine and electrolytes on the zeta-potential of scheelite from Uludag, Turkey. Miner. Eng. 1992, 5, 945–951. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).