Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Concentrate Samples
2.2. Leaching Solutions
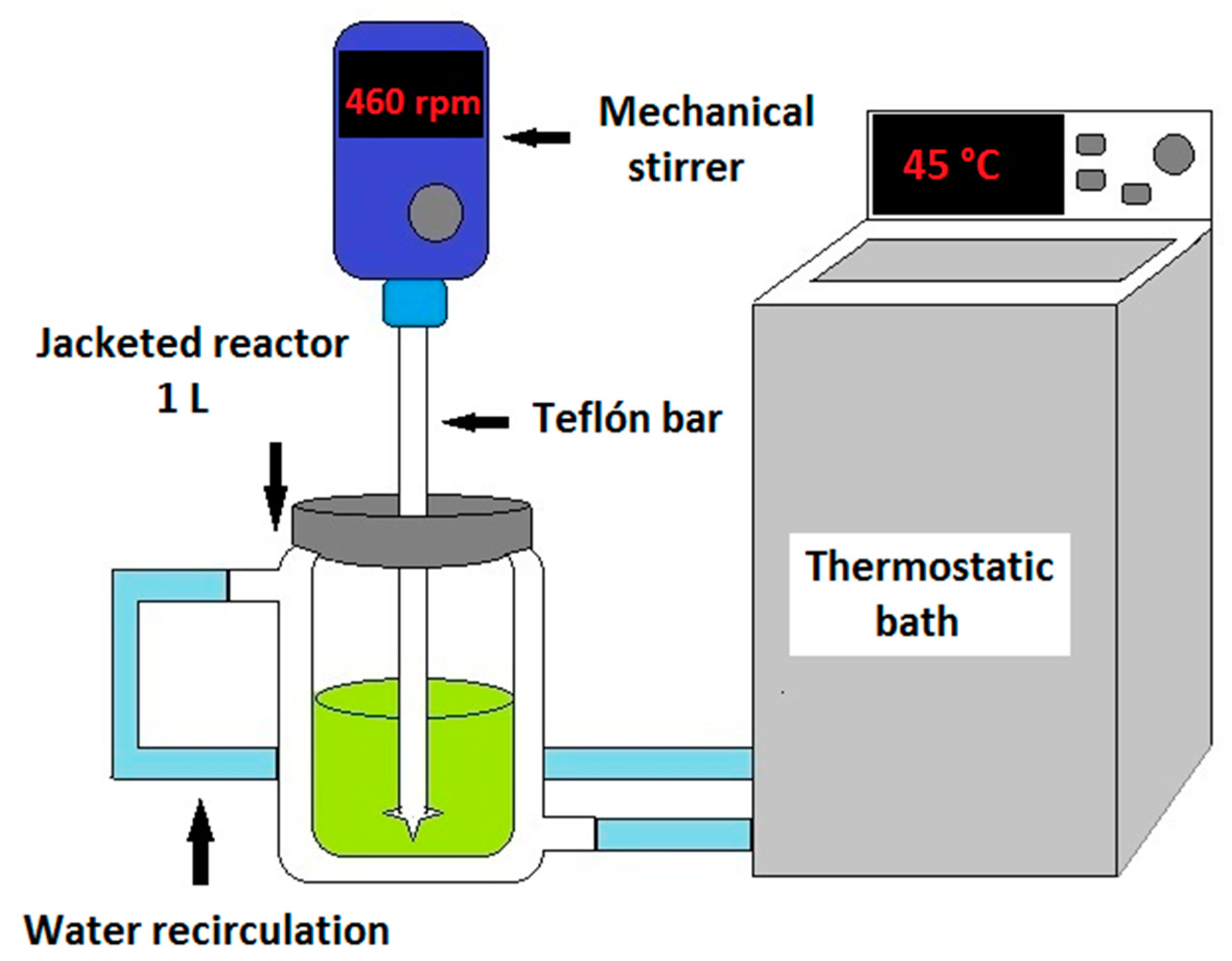
2.3. Experimental Procedure
2.3.1. Leaching Tests
2.3.2. Pre-Treatment Tests
3. Results and Discussion
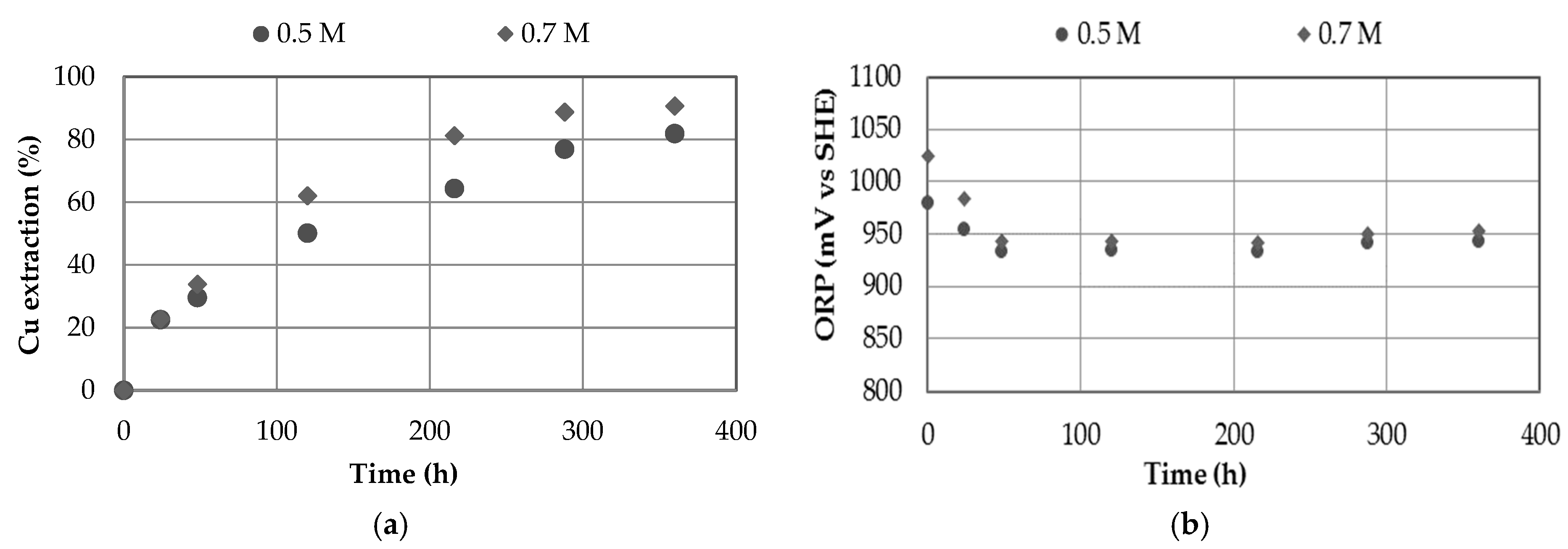
3.1. Effect of Nitrate Concentration
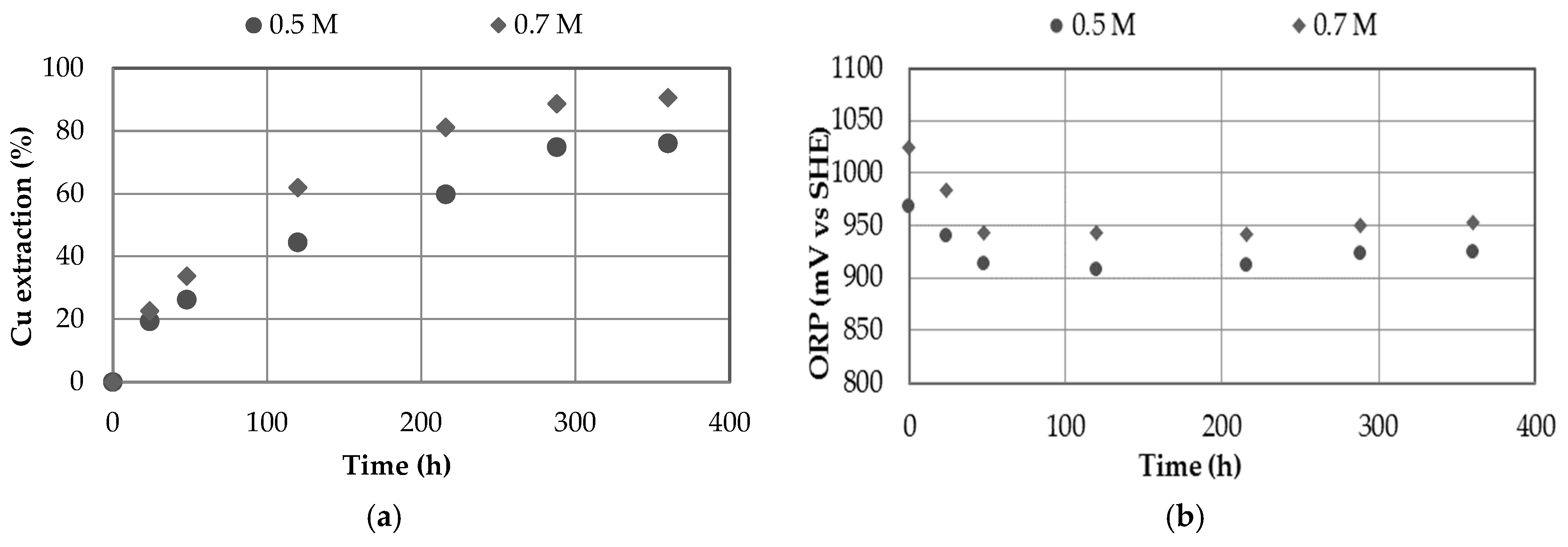
3.2. Effect of Sulfuric Acid Concentration
3.3. Different Equivalent Sodium Nitrate and Sulfuric Acid Concentration
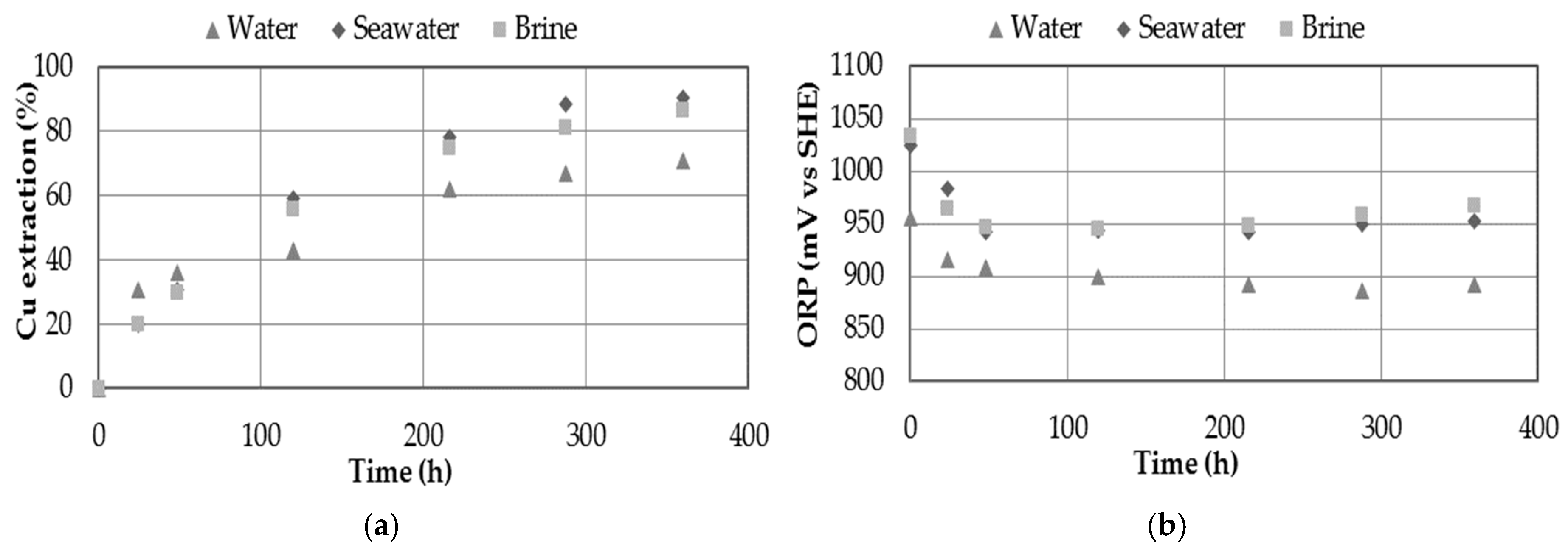
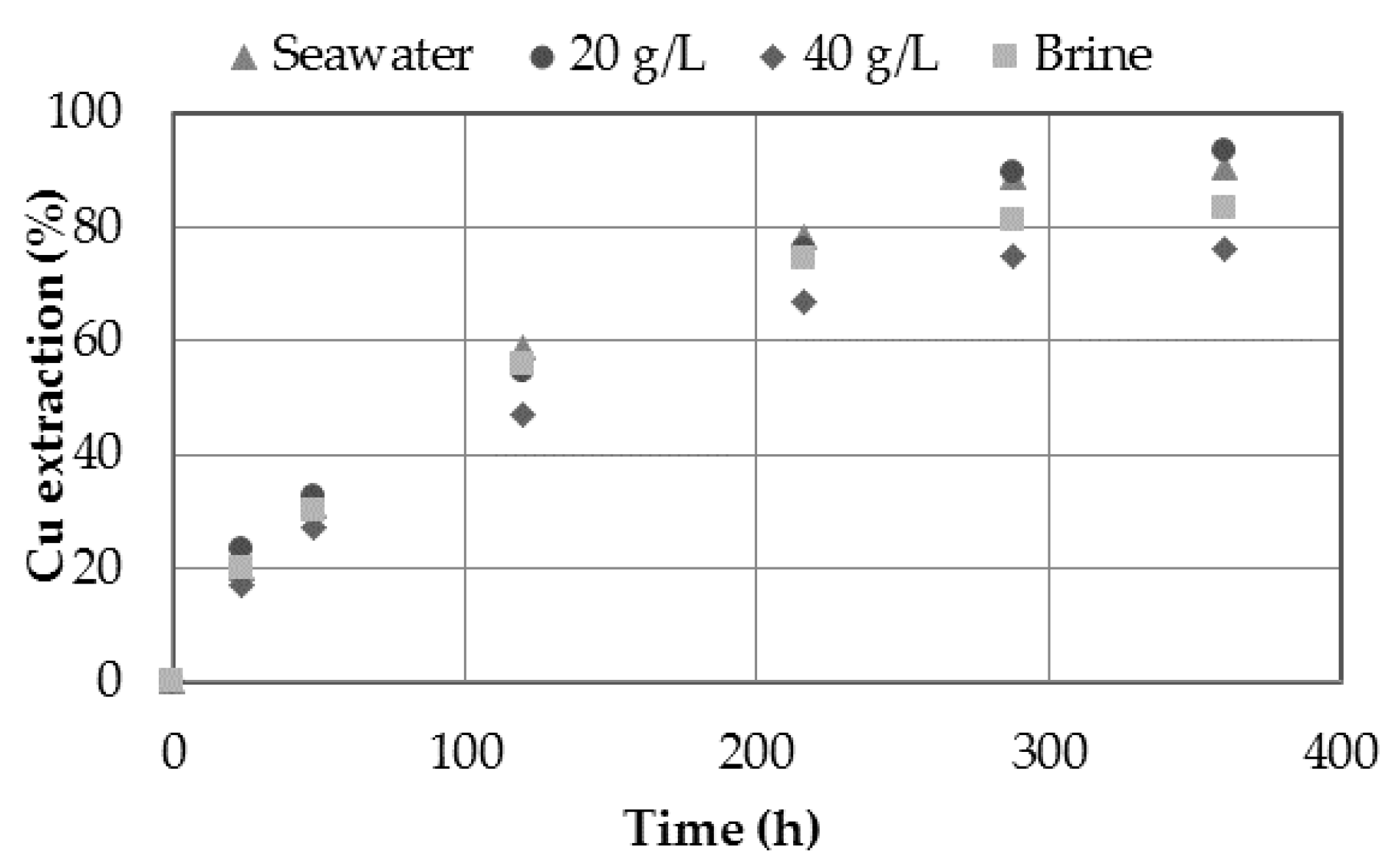
3.4. Effect of Dissolvent
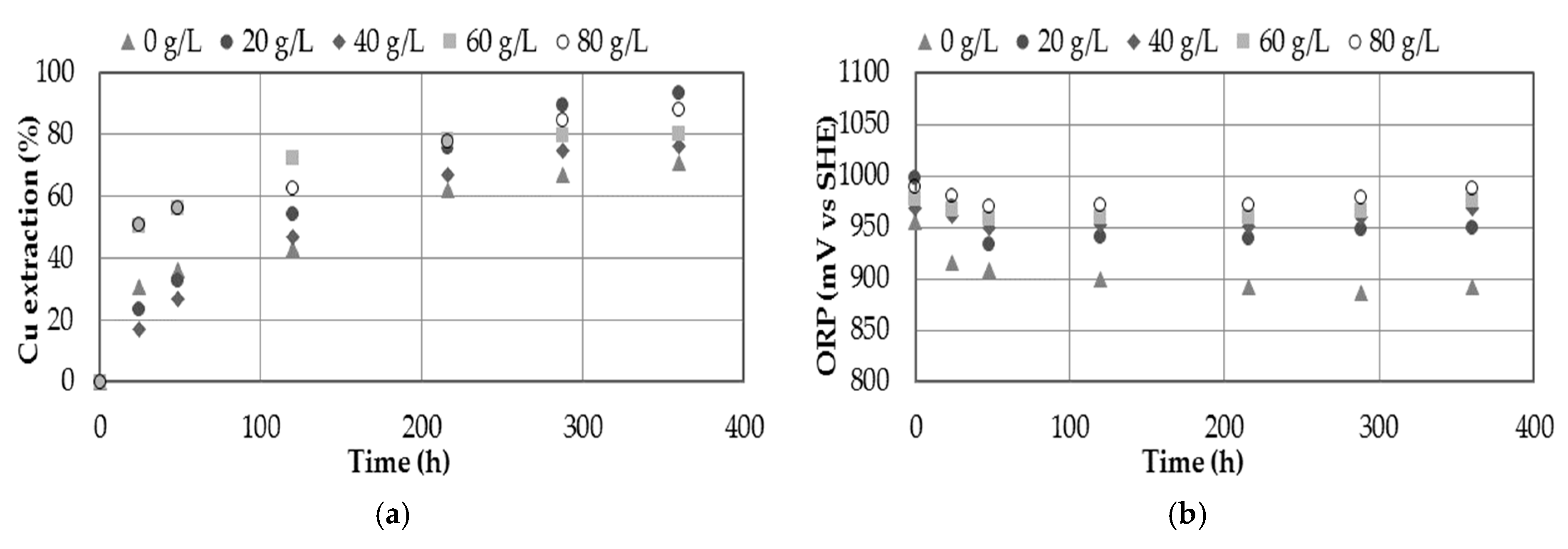
3.5. Effect of Chloride Concentration
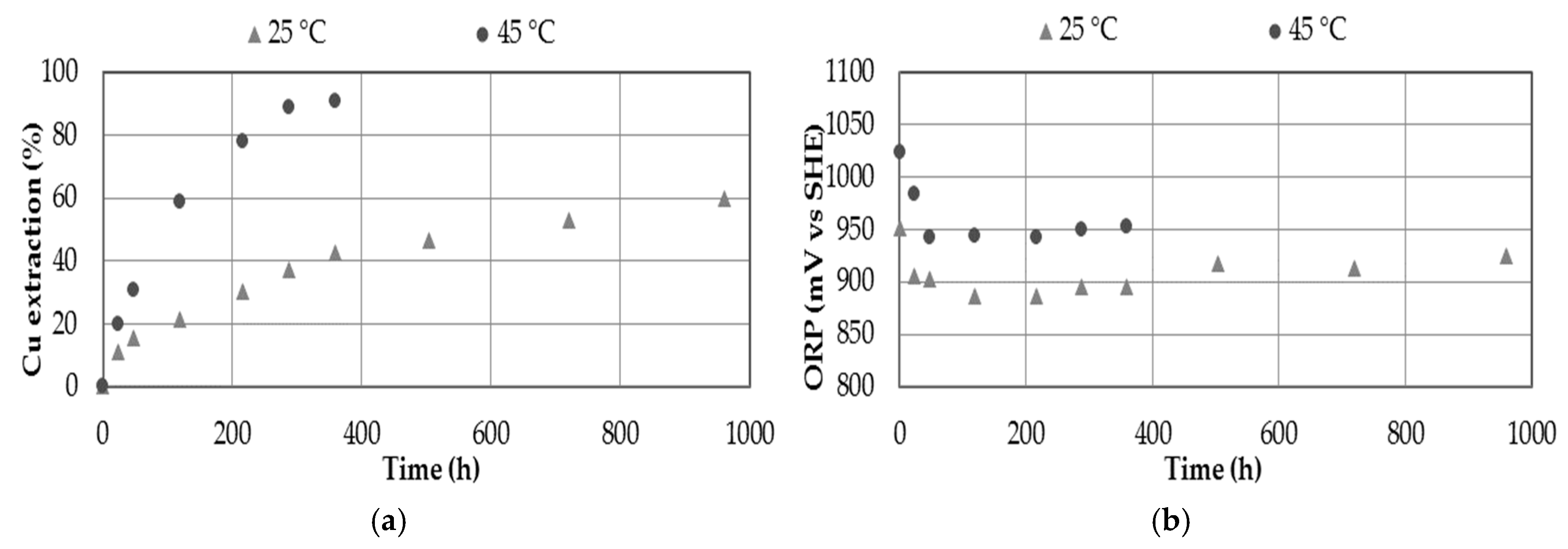
3.6. Effect of Temperature
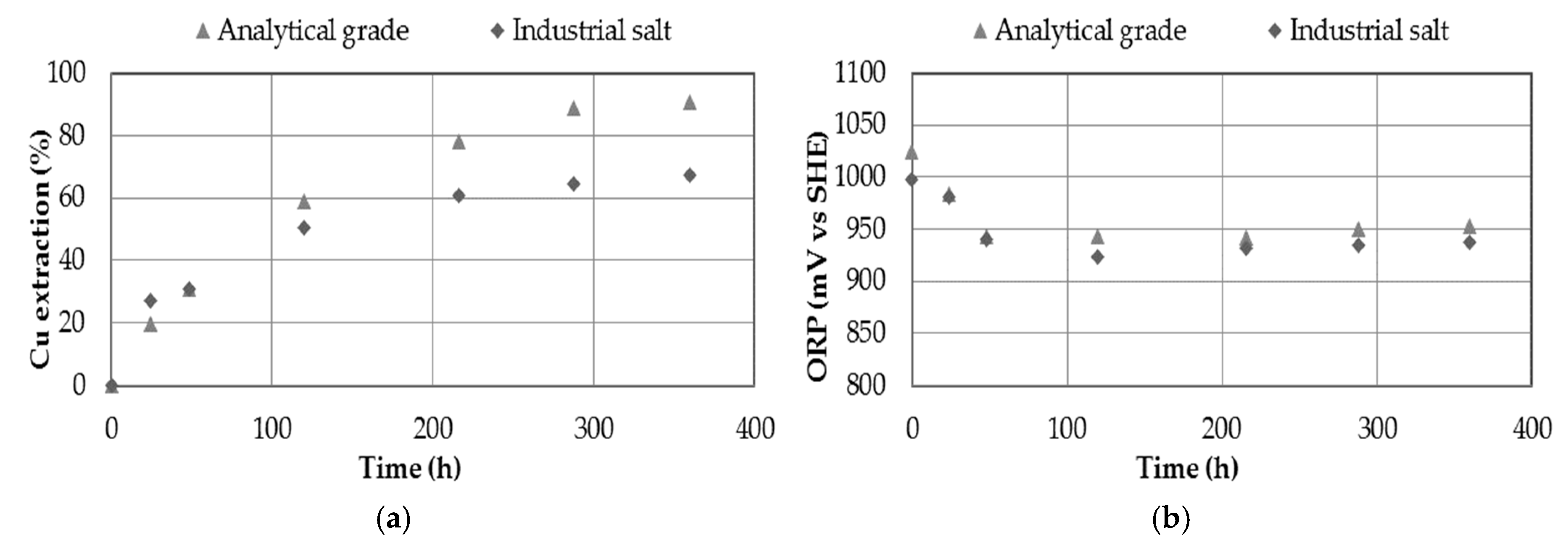
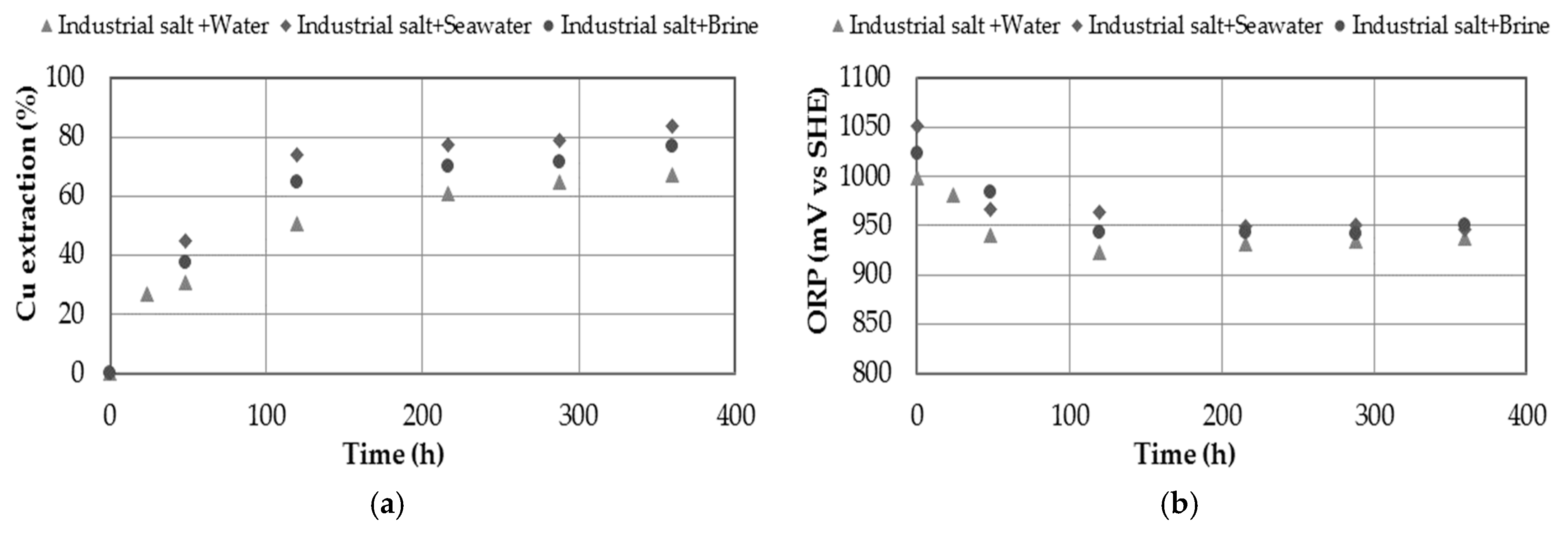
3.7. Effect of Using Industrial Salt as a Source of Nitrate
3.8. Effect of Concentrate Grade
| FeS2 + NO3− + 4H+ = Fe3+ + NO + 2H2O + 2S | ∆G45 °C = −31.8 kcal | (1) |
| 3FeS2 + 2NO3− + 8H+ + 6Cl− = 3FeCl2 + 2NO + 4H2O + 6S | ∆G45 °C = −24.1 kcal | (2) |
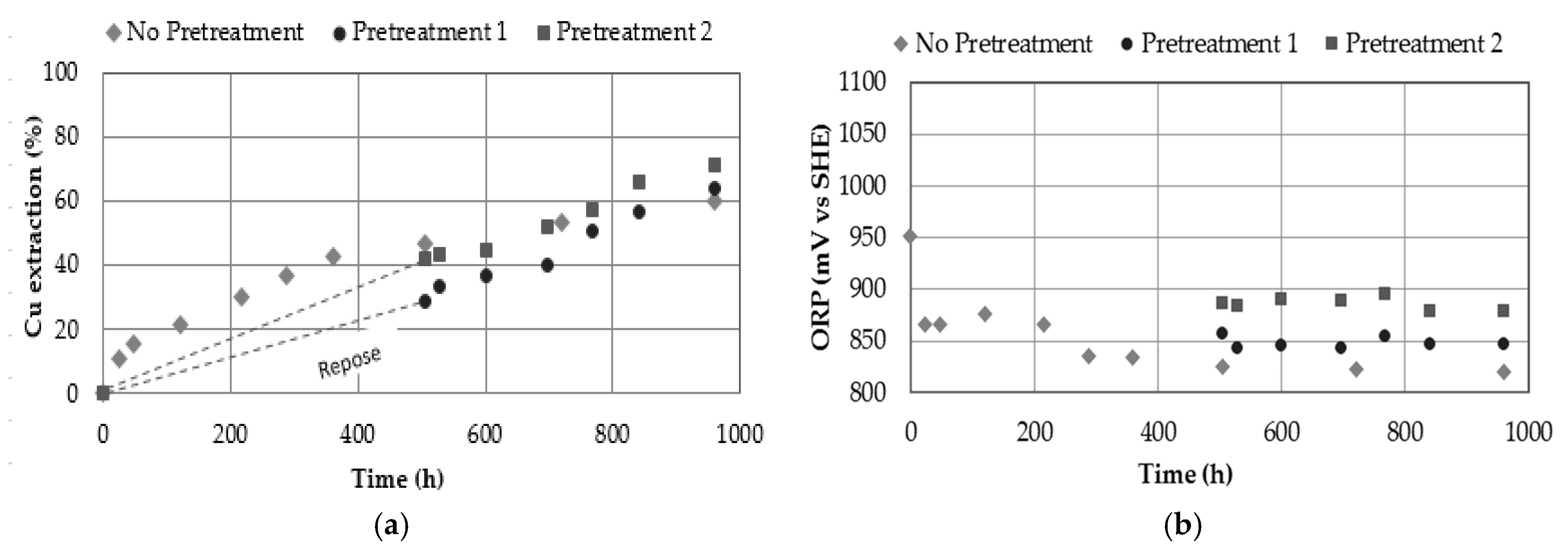
3.9. Effect of Pre-Treatment
4. Conclusions
- Increasing the nitrate concentration from 0 to 0.7 M showed positive effects on copper extraction, although the most significant effect was observed by increasing the concentration of sulfuric acid from 0.5 to 0.7 M.
- The presence of chloride in the medium showed increasing on copper extraction. The presence of chloride ions can be provided through the use of seawater, discard brines from desalination plants or by adding sodium chloride, obtaining a maximum extraction of 93.5% Cu when 20 g/L Cl− was used.
- The effect of temperature significantly influences dissolution kinetics of copper, reaching 90.6% Cu at 45 °C after 15 days of leaching in comparison to 42.4% Cu at 25 °C in the same conditions.
- The use of industrial salt providing 0.7 M of nitrate in a leaching medium with 0.7 M of sulfuric acid did not obtain better extraction percentages than using analytical salt, due to the presence of other impurities that affect the dissolution system. In any case, it is a possible alternative to evaluate, obtaining percentages of copper extraction of 67%.
- The leaching of two samples of concentrates showed different copper extraction values, mainly due to the mineralogical and chemical composition of each concentrate. Sample A has a greater presence of pyrite, which could contribute ferric ion to the medium, which would help leach chalcopyrite, as this is an oxidizing ion.
- The use of pre-treatment for 20 days produces extractions close to 28.4% and 42.3% Cu, using pre-treatment 1 and pre-treatment 2 at 25 °C, respectively. Copper extraction can increase to values close to 63.8 and 71.4% by leaching these pretreated concentrates for an additional 20 days. A leach at 25 °C for 40 days obtained a 60% copper extraction. It is necessary to evaluate the cost of pretreating the concentrate and leaving it at rest, versus leaching it.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Consejo-Minero. Cifras Actualizadas de la Minería; Consejo-Minero: Santiago de Chile, Chile, 2020. [Google Scholar]
- COCHILCO. Consumo de Agua en la Minería del Cobre al 2018; Ministerio de Minería, G.d.C.: Santiago de Chile, Chile, 2019.
- Wang, S. Copper leaching from chalcopyrite concentrates. JOM J. Miner. Met. Mater. Soc. 2005, 57, 48–51. [Google Scholar] [CrossRef]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.; Gerson, A. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Torres, D.; Toro, N. Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, M.E.; Jamett, N.E.; Ghorbani, Y.; Hernández, P.C. Effect of Pretreatment on Leaching Primary Copper Sulfide in Acid-Chloride Media. Minerals 2017, 8, 1. [Google Scholar] [CrossRef]
- Torres, C.M.; Ghorbani, Y.; Hernández, P.C.; Justel, F.J.; Aravena, M.I.; Herreros, O.O. Cupric and Chloride Ions: Leaching of Chalcopyrite Concentrate with Low Chloride Concentration Media. Minerals 2019, 9, 639. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Nila, C.; Gonzalez, I. Thermodynamics of Cu−H2SO4−Cl−−H2O and Cu−NH4Cl−H2O based on predominance-existence diagrams and Pourbaix-type diagrams. Hydrometallurgy 1996, 42, 63–82. [Google Scholar] [CrossRef]
- Herreros, O.; Viñals, J. Leaching of sulfide copper ore in a NaCl–H2SO4–O2 media with acid pre-treatment. Hydrometallurgy 2007, 89, 260–268. [Google Scholar] [CrossRef]
- Torres, C.; Taboada, M.; Graber, T.; Herreros, O.; Ghorbani, Y.; Watling, H. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Hernández, P.; Taboada, M.; Herreros, O.; Torres, C.; Ghorbani, Y. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy 2015, 157, 325–332. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leão, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Watling, H.; Shiers, D.; Li, J.; Chapman, N.; Douglas, G. Effect of water quality on the leaching of a low-grade copper sulfide ore. Miner. Eng. 2014, 58, 39–51. [Google Scholar] [CrossRef]
- Taboada, M.E.; Hernández, P.C.; Galleguillos, H.R.; Flores, E.K.; Graber, T.A. Behavior of sodium nitrate and caliche mineral in seawater: Solubility and physicochemical properties at different temperatures and concentrations. Hydrometallurgy 2012, 113–114, 160–166. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Moreno, L.; Gálvez, E.D.; Cisternas, L.A. Seawater leaching of caliche mineral in column experiments. Hydrometallurgy 2013, 139, 79–87. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Graber, T.A.; Ghorbani, Y. Leaching of Chalcopyrite in Acidified Nitrate Using Seawater-Based Media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Castellón, C.I.; Hernández, P.C.; Velásquez-Yévenes, L.; Taboada, M.E. An Alternative Process for Leaching Chalcopyrite Concentrate in Nitrate-Acid-Seawater Media with Oxidant Recovery. Metals 2020, 10, 518. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Matković, V.; Živković, D.; Štrbac, N.; Stojanović, J. Kinetics and mechanism of sphalerite leaching by sodium nitrate in sulphuric acid solution. J. Min. Metall. Sect. B Metall. 2012, 48, 185–195. [Google Scholar] [CrossRef]
- Torres, M.A.; Meruane, G.E.; Graber, T.A.; Gutiérrez, P.C.; Taboada, M.E. Recovery of nitrates from leaching solutions using seawater. Hydrometallurgy 2013, 133, 100–105. [Google Scholar] [CrossRef]
- Bogorodskii, E.; Rybkin, S.; Barankevich, V. Kinetics of the interaction of iron, copper, and nickel sulfides with a sodium nitrate-sodium carbonate mixture. Russ. J. Inorg. Chem. 2011, 56, 831–834. [Google Scholar] [CrossRef]
- Sokić, M.D.; Marković, B.; Živković, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Narangarav, T.; Nyamdelger, S.; Ariunaa, G.; Azzaya, T.; Burmaa, G. Dissolution behavior of copper concentrate in acidic media using nitrate ions. Mong. J. Chem. 2014, 15, 79–84. [Google Scholar] [CrossRef]
- Shiers, D.; Collinson, D.; Kelly, N.; Watling, H. Copper extraction from chalcopyrite: Comparison of three non-sulfate oxidants, hypochlorous acid, sodium chlorate and potassium nitrate, with ferric sulfate. Miner. Eng. 2016, 85, 55–65. [Google Scholar] [CrossRef]
- Habashi, F. Nitric acid in the hydrometallurgy of sulfides. In Proceedings of the EPD Congress 1999, San Diego, CA, USA, 28 February–4 March 1999; pp. 357–364. [Google Scholar]
- Hernández, P.C.; Dupont, J.; Herreros, O.O.; Jimenez, Y.P.; Torres, C.M. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals 2019, 9, 250. [Google Scholar] [CrossRef]
- Hernández, P.; Gahona, G.; Martínez, M.; Toro, N.; Castillo, J. Caliche and Seawater, Sources of Nitrate and Chloride Ions to Chalcopyrite Leaching in Acid Media. Metals 2020, 10, 551. [Google Scholar] [CrossRef]
- Sokić, M.D.; Matković, V.L.; Marković, B.R.; Štrbac, N.D.; Živković, D.T. Passivation of chalcopyrite during the leaching with sulphuric acid solution in presence of sodium nitrate. Hemijska Industrija 2010, 64, 343–350. [Google Scholar]
- Lu, Z.Y.; Jeffrey, M.I.; Lawson, F. The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Carneiro, M.F.C.; Leão, V.A. Lixiviação da calcopirita com cloreto férrico e cloreto de sódio. REM: Revista Escola Minas 2005, 58, 231–235. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Copper chloride leaching from chalcopyrite and bornite concentrates containing high levels of impurities and minor elements. Hydrometallurgy 2013, 138, 40–47. [Google Scholar] [CrossRef]
- Ruiz, M.; Montes, K.; Padilla, R. Chalcopyrite leaching in sulfate–chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Rautenbach, D.; Velasques, L.; Van Buuren, C. The development of heap leaching in chloride solutions for primary and secondary copper minerals. In Percolation Leaching: The Status of Globally and in Southern Africa 2011; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2011; pp. 183–200. [Google Scholar]
- Kimball, B.E.; Rimstidt, J.D.; Brantley, S.L. Chalcopyrite dissolution rate laws. Appl. Geochem. 2010, 25, 972–983. [Google Scholar] [CrossRef]



| Chemical Formula | Mineral | Amount (%) | |
|---|---|---|---|
| Sample A | Sample B | ||
| CuFeS2 | Chalcopyrite | 37.2 | 74.0 |
| CuS | Covellite | 12.5 | - |
| Cu9S5 | Digenite | 2.9 | - |
| CuFeO2 | Delafossite | 0.8 | - |
| FeS2 | Pyrite | 34.0 | 2.0 |
| SiO2 | Quartz | 12.6 | 2.5 |
| Gangue | - | 21.5 | |
| Total | 100.0 | 100.0 | |
| Chemical Method | Atomic Absorption Spectrometry | Volumetric Analysis | Gravimetric Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ionic Species | Na+ | K+ | Mg2+ | Ca2+ | Cu2+ | NO3− | Cl− | HCO3− | SO42− |
| Seawater | 11,250 | 401 | 1256 | 427 | 0.07 | 2.40 | 20.289 | 149 | 2758 |
| Brine | 19,768 | 746 | 2297 | 355 | 0.14 | 6.40 | 36,074 | 236 | 5063 |
| N° | H2SO4 (M) | NaNO3 (M) | T (°C) | Disolvente | Sample | Special Characteristic | Cu (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.7 | 0.7 | 45 | Water | A | - | 70.9 |
| 2 | 0.7 | 0.7 | 45 | Seawater | A | - | 90.6 |
| 3 | 0.7 | 0.7 | 45 | Brine | A | - | 86.6 |
| 4 | 0.7 | 0.7 | 45 | Water | A | [Cl−] = 20 (g/L) | 93.5 |
| 5 | 0.7 | 0.7 | 45 | Water | A | [Cl−] = 40 (g/L) | 76.1 |
| 6 | 0.7 | 0.7 | 45 | Water | A | [Cl−] = 60 (g/L) | 80.4 |
| 7 | 0.7 | 0.7 | 45 | Water | A | [Cl−] = 80 (g/L) | 88.2 |
| 8 | 0.7 | 0.7 | 45 | Water | A | Industrial salt | 67.2 |
| 9 | 0.7 | 0.7 | 45 | Seawater | A | Industrial salt | 83.5 |
| 10 | 0.7 | 0.7 | 45 | Brine | A | Industrial salt | 77.0 |
| 11 | 0.7 | 0 | 45 | Seawater | A | - | 79.2 |
| 12 | 0.7 | 0.7 | 25 | Seawater | A | - | 60.0 |
| 13 | 0.5 | 0.7 | 45 | Seawater | A | - | 81.9 |
| 14 | 0.7 | 0.5 | 45 | Seawater | A | - | 88.0 |
| 15 | 0.5 | 0.5 | 45 | Seawater | A | - | 76.1 |
| 16 | 0.7 | 0.7 | 45 | Seawater | B | - | 80.8 |
| 17 | 0.7 | 0.7 | 25 | Seawater | A | Pre-treatment (Method 1) | 63.8 |
| 18 | 0.7 | 0.7 | 25 | Seawater | A | Pre-treatment (Method 2) | 71.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, P.; Dorador, A.; Martínez, M.; Toro, N.; Castillo, J.; Ghorbani, Y. Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching. Minerals 2020, 10, 477. https://doi.org/10.3390/min10050477
Hernández P, Dorador A, Martínez M, Toro N, Castillo J, Ghorbani Y. Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching. Minerals. 2020; 10(5):477. https://doi.org/10.3390/min10050477
Chicago/Turabian StyleHernández, Pía, Alexis Dorador, Monserrat Martínez, Norman Toro, Jonathan Castillo, and Yousef Ghorbani. 2020. "Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching" Minerals 10, no. 5: 477. https://doi.org/10.3390/min10050477
APA StyleHernández, P., Dorador, A., Martínez, M., Toro, N., Castillo, J., & Ghorbani, Y. (2020). Use of Seawater/Brine and Caliche’s Salts as Clean and Environmentally Friendly Sources of Chloride and Nitrate Ions for Chalcopyrite Concentrate Leaching. Minerals, 10(5), 477. https://doi.org/10.3390/min10050477









