Analysis of Kaolin Flocculation in Seawater by Optical Backscattering Measurements: Effect of Flocculant Management and Liquor Conditions
Abstract
1. Introduction
2. Methodology
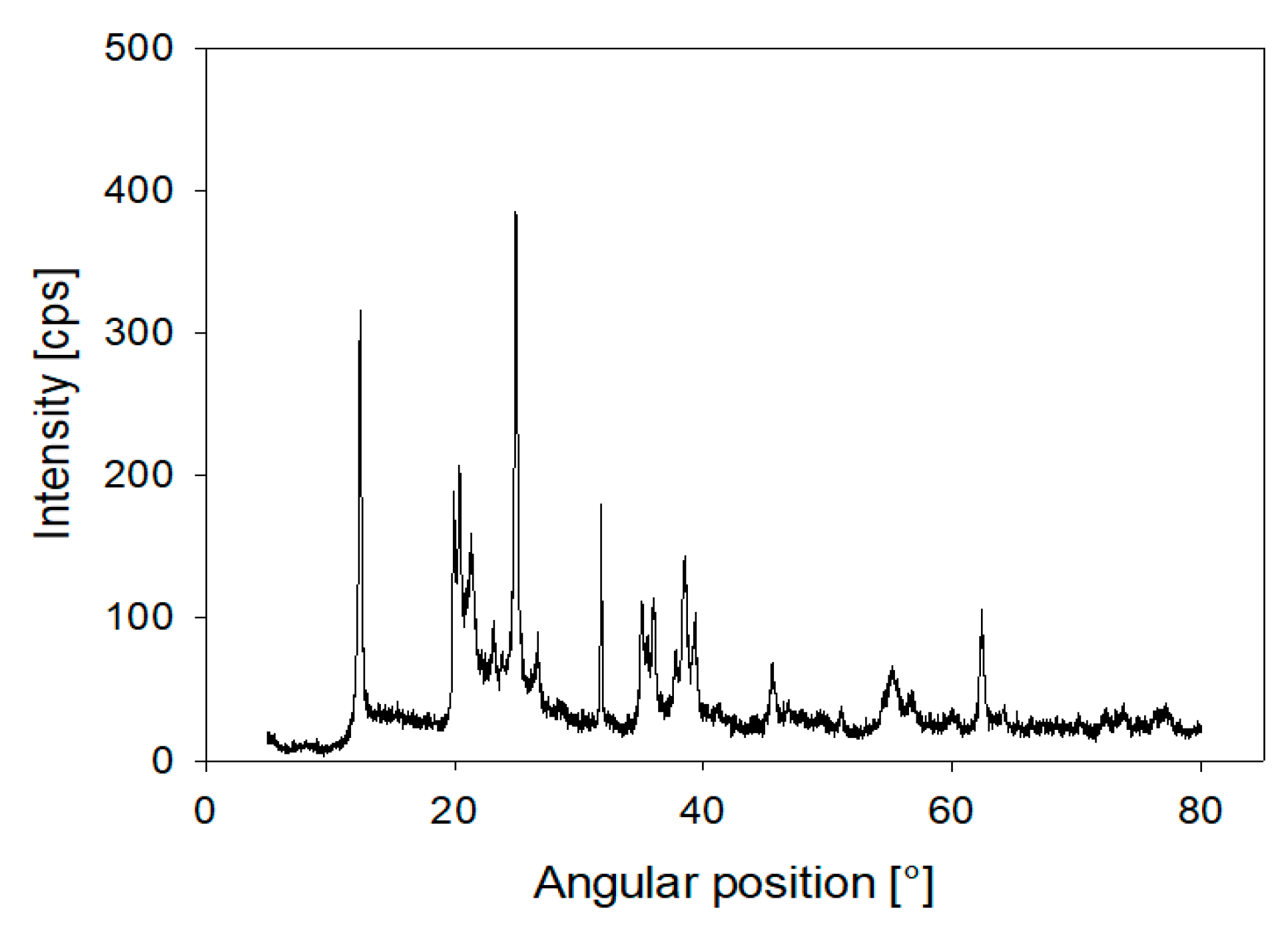
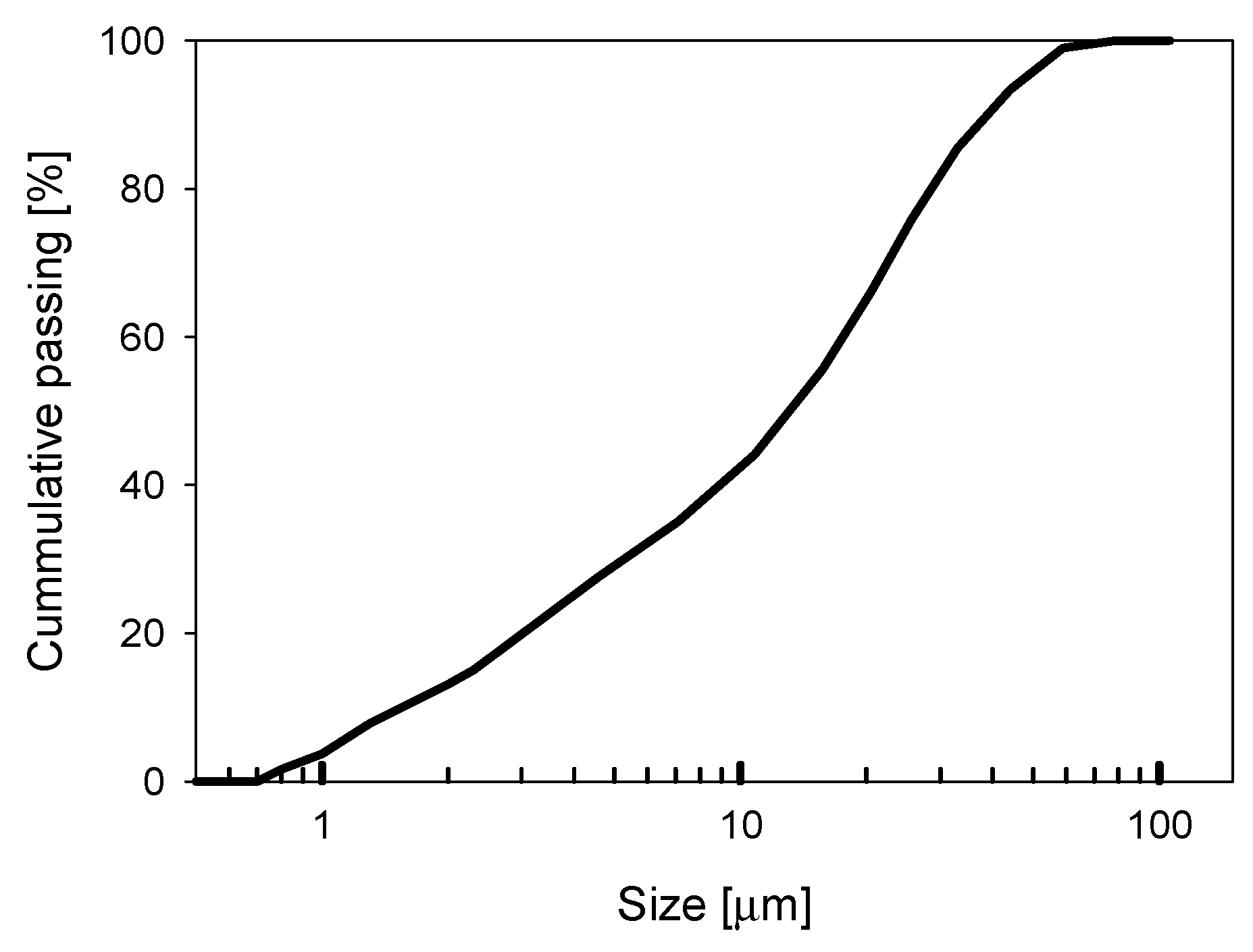
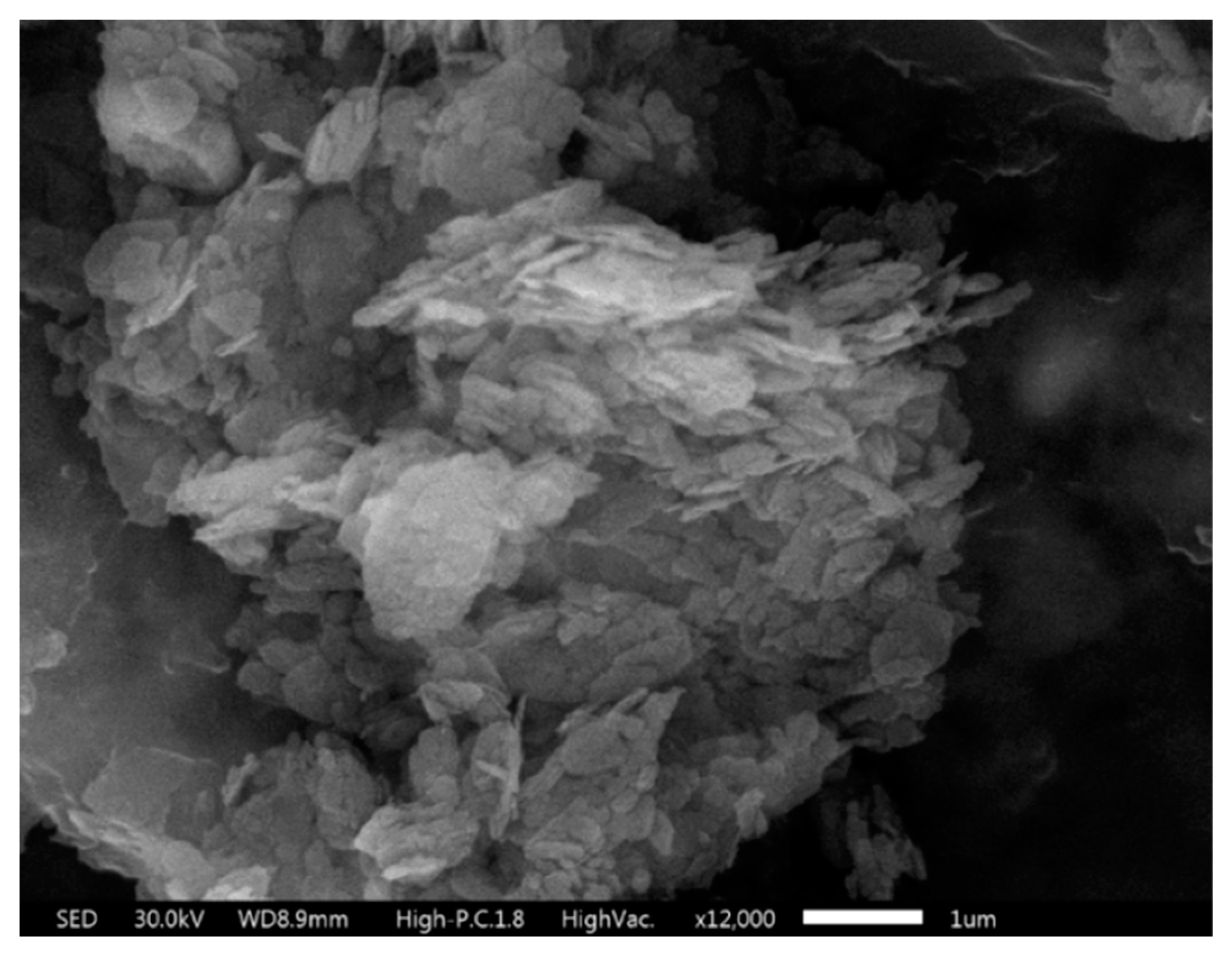
2.1. Materials
2.2. Aggregate Characterisation
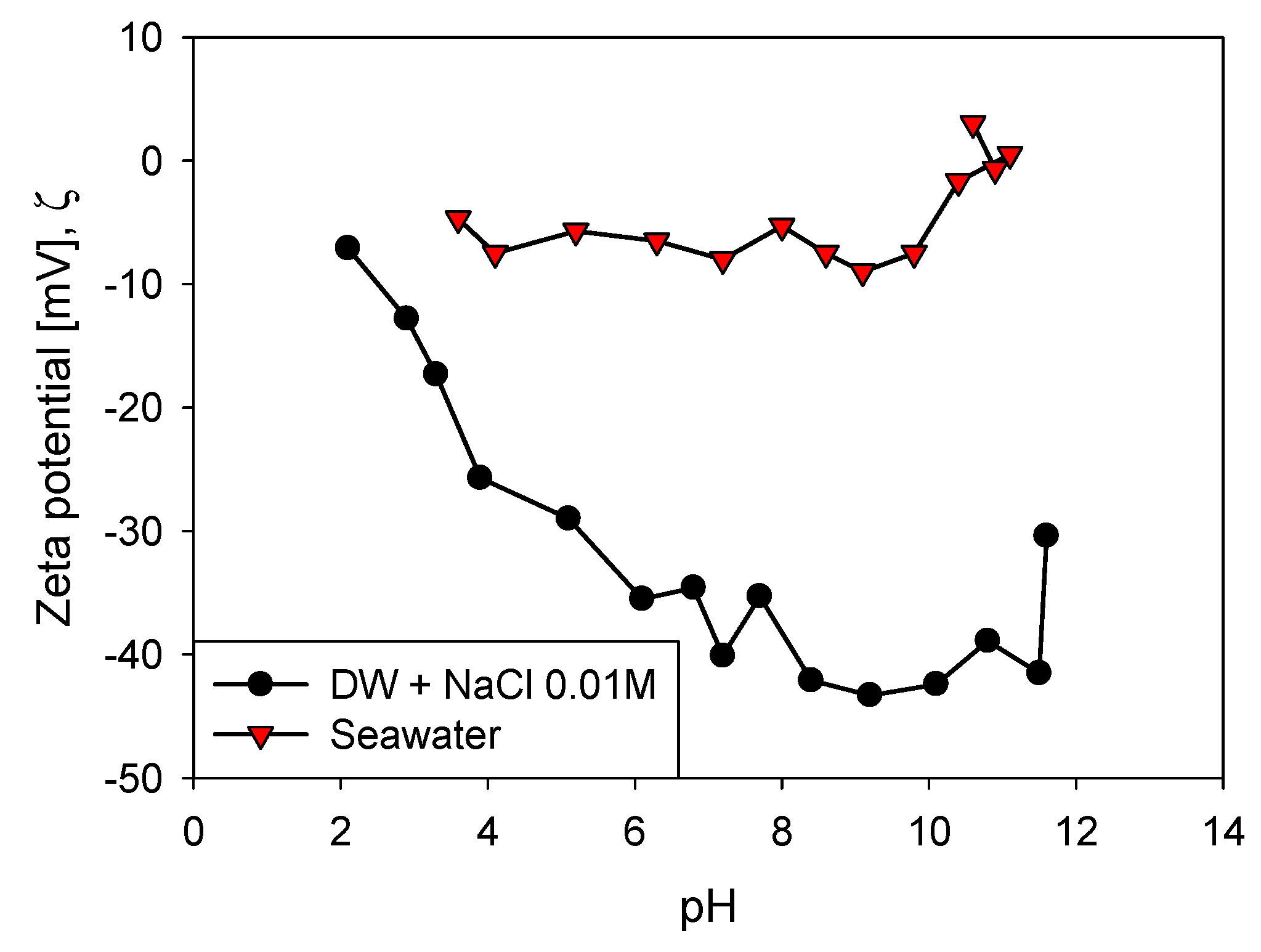
2.3. Zeta Potential
2.4. Flocculation Assays
2.5. Backscattering Scan
3. Results and Discussions
3.1. Aggregation of Primary Particles
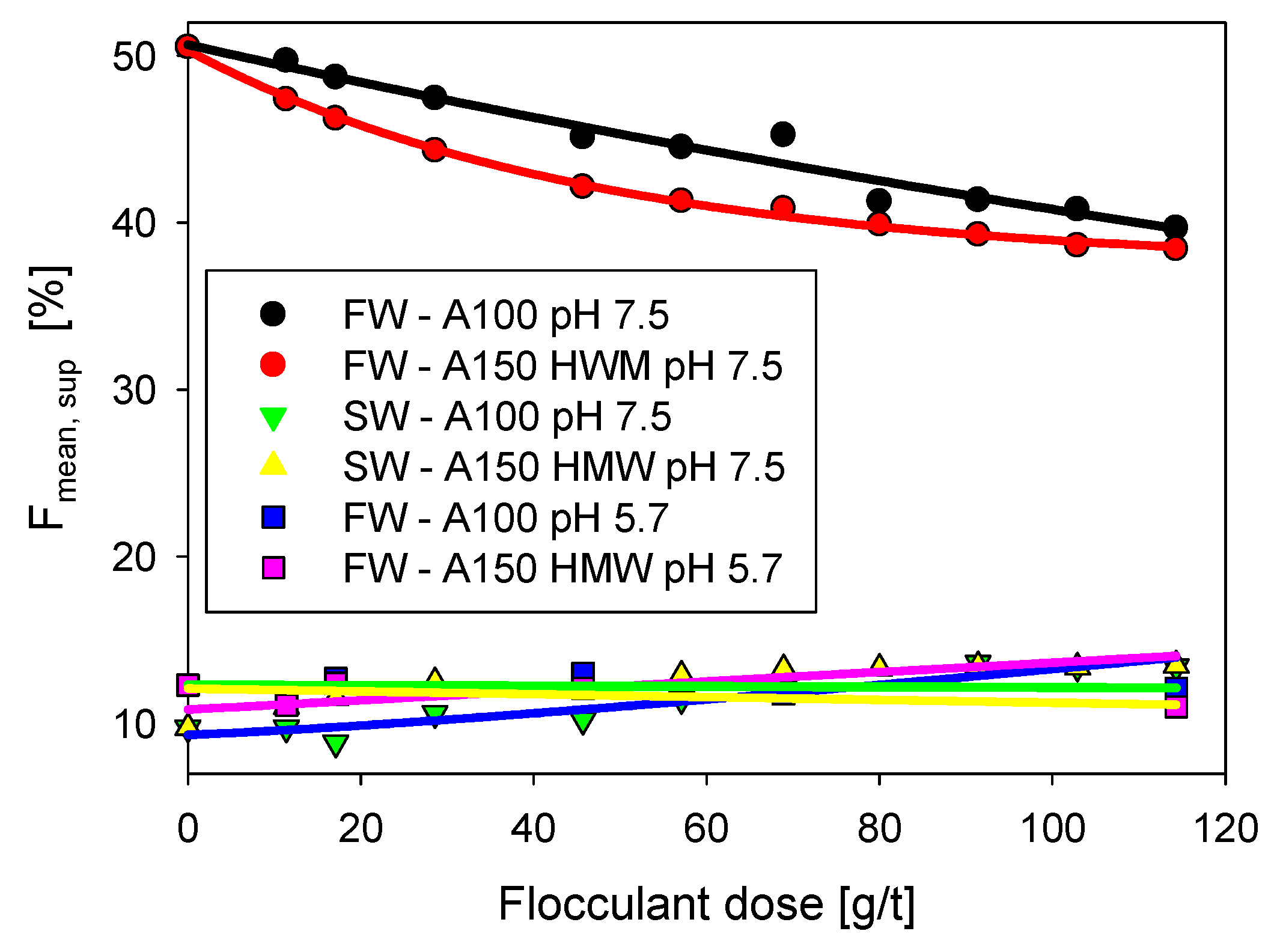
3.2. Supernatant Analysis
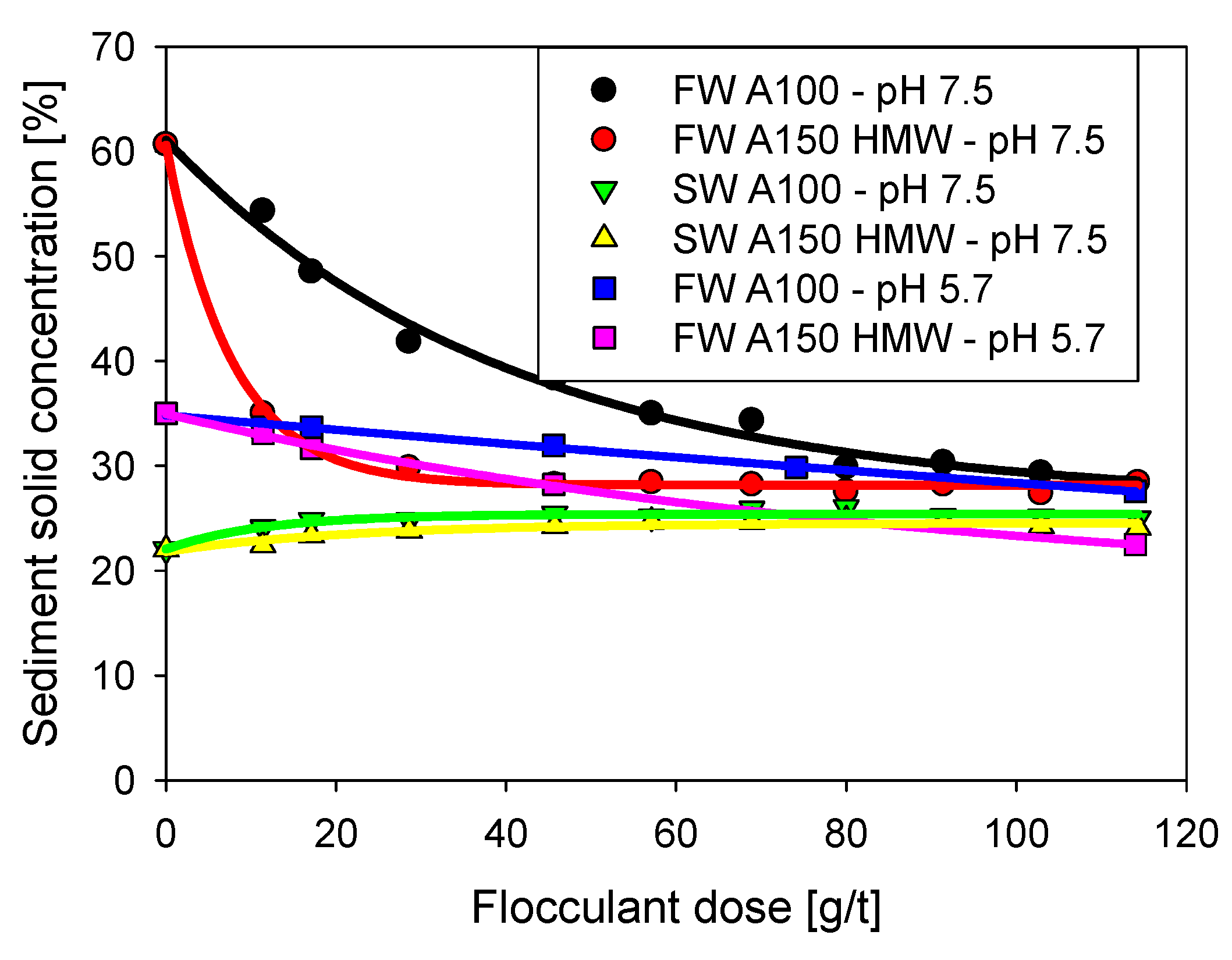
3.3. Sediment Solid Concentration
3.4. Flocculation Performance: Freshwater vs Seawater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scott, K.J. Experimental study of continuous thickening of a flocculated silica slurry. Ind. Eng. Chem. Fundam. 1968, 7, 582–595. [Google Scholar] [CrossRef]
- Scott, K.J. Continuous thickening of flocculated suspensions. Comparison with batch settling tests and effects of floc compression using pyrophyllite pulp. Ind. Eng. Chem. Fundam. 1970, 9, 422–427. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Forbes, L.; Cisternas, L.A. Effect of seawater on sulfide ore flotation: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 369–384. [Google Scholar] [CrossRef]
- Castro, S. Physico-chemical factors in flotation of Cu-Mo-Fe ores with seawater: A critical review. Physicochem. Probl. Miner. Process. 2018, 54, 1223–1236. [Google Scholar] [CrossRef]
- Franks, G.V. Zeta potentials and yield stresses of silica suspensions in concentrated monovalent electrolytes: Isoelectric point shift and additional attraction. J. Colloid Interface Sci. 2002, 249, 44–51. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Piceros, E.C.; Leiva, W.H.; Toledo, P.G.; Quezada, G.R.; Robles, P.A.; Valenzuela, J. Analysis of silica pulp viscoelasticity in saline media: The effect of cation size. Minerals 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Liu, D.; Edraki, M.; Berry, L. Investigating the settling behaviour of saline tailing suspensions using kaolinite, bentonite, and illite clay minerals. Powder Technol. 2018, 326, 228–236. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, Q.; Liu, Q.; Zeng, H. Effect of solution salinity on settling of mineral tailings by polymer flocculants. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 29–38. [Google Scholar] [CrossRef]
- Shaikh, S.M.R.; Nasser, M.S.; Magzoub, M.; Benamor, A.; Hussein, I.A.; El-Naas, M.H.; Qiblawey, H. Effect of electrolytes on electrokinetics and flocculation behavior of bentonite-polyacrylamide dispersions. Appl. Clay Sci. 2018, 158, 46–54. [Google Scholar] [CrossRef]
- Costine, A.; Cox, J.; Travaglini, S.; Lubansky, A.; Fawell, P.; Misslitz, H. Variations in the molecular weight response of anionic polyacrylamides under different flocculation conditions. Chem. Eng. Sci. 2018, 176, 127–138. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Piceros, E.C.; Leiva, W.H.; Toledo, P.G.; Herrera, N. Viscoelasticity and yielding properties of flocculated kaolinite sediments in saline water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 1009–1015. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, R.I.; Fawell, P.D.; Toledo, P.G. Use of molecular dynamics to study the conformation of an anionic polyelectrolyte in saline medium and its adsorption on a quartz surface. Miner. Eng. 2018, 129, 102–105. [Google Scholar] [CrossRef]
- Jeldres, M.; Piceros, E.C.; Toro, N.; Robles, P.; Nieto, S.; Quezada, G.R.; Jeldres, R.I. Enhancing the sedimentation of clay-based tailings in seawater by magnesium removal treatment. Sep. Purif. Technol. 2020, 242, 116762. [Google Scholar] [CrossRef]
- Concha, F.; Rulyov, N.N.; Laskowski, J.S. Settling velocities of particulate systems 18: Solid flux density determination by ultra-flocculation. Int. J. Miner. Process. 2012, 104–105, 53–57. [Google Scholar] [CrossRef]
- Concha, F. Solid-Liquid Separation in the Mining Industry; Springer International Publishing: Basel, Switzerland, 2013; ISBN 3319024841. [Google Scholar]
- Hunter, T.N.; Peakall, J.; Egarr, D.; Cowell, D.M.J.; Freear, S.; Tonge, A.S.; Horton, L.; Rice, H.P.; Smith, I.; Malone, K.; et al. Concentration profiling of a horizontal sedimentation tank utilising a bespoke acoustic backscatter array and CFD simulations. Chem. Eng. Sci. 2020, 218, 115560. [Google Scholar] [CrossRef]
- Owen, A.T.; Fawell, P.D.; Swift, J.D.; Labbett, D.M.; Benn, F.A.; Farrow, J.B. Using turbulent pipe flow to study the factors affecting polymer-bridging flocculation of mineral systems. Int. J. Miner. Process. 2008, 87, 90–99. [Google Scholar] [CrossRef]
- MacIver, M.R.; Pawlik, M. Measurement of optical backscattering height scans from flocculated mineral sediments. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 38–46. [Google Scholar] [CrossRef]
- Downing, J. Twenty-five years with OBS sensors: The good, the bad, and the ugly. Cont. Shelf Res. 2006, 26, 2299–2318. [Google Scholar] [CrossRef]
- Bunt, J.A.C.; Larcombe, P.; Jago, C.F. Quantifying the response of optical backscatter devices and transmissometers to variations in suspended particulate matter. Cont. Shelf Res. 1999, 19, 1199–1220. [Google Scholar] [CrossRef]
- Campbell, D.E.; Spinrad, R.W. The relationship between light attenuation and particle characteristics in a turbid estuary. Estuar. Coast. Shelf Sci. 1987, 25, 53–65. [Google Scholar] [CrossRef]
- Chakraborti, R.K.; Gardner, K.H.; Atkinson, J.F.; Van Benschoten, J.E. Changes in fractal dimension during aggregation. Water Res. 2003, 37, 873–883. [Google Scholar] [CrossRef]
- Peng, F.F.; Di, P. Effect of multivalent salts-calcium and aluminum on the flocculation of kaolin suspension with anionic polyacrylamide. J. Colloid Interface Sci. 1994, 164, 229–237. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Piceros, E.C.; Wong, L.; Leiva, W.H.; Herrera, N.; Toledo, P.G. Dynamic moduli of flocculated kaolinite sediments: effect of salinity, flocculant dose, and settling time. Colloid Polym. Sci. 2018, 296, 1935–1943. [Google Scholar] [CrossRef]
- Castillo, C.; Ihle, C.F.; Jeldres, R.I. Chemometric optimisation of a copper sulphide tailings flocculation process in the presence of clays. Minerals 2019, 9, 582. [Google Scholar] [CrossRef]
- Quezada, G.R.; Ramos, J.; Jeldres, R.I.; Robles, P.; Toledo, P.G. Analysis of the flocculation process of fine tailings particles in saltwater through a population balance model. Sep. Purif. Technol. 2019, 116319. [Google Scholar] [CrossRef]
- Jeldres, M.; Piceros, E.C.; Toro, N.; Torres, D.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Copper tailing flocculation in seawater: Relating the yield stress with fractal aggregates at varied mixing conditions. Metals 2019, 9, 1295. [Google Scholar] [CrossRef]
- Jeldres, M.; Piceros, E.; Robles, P.A.; Toro, N.; Jeldres, R.I. Viscoelasticity of quartz and kaolin slurries in seawater: Importance of magnesium precipitates. Metals 2019, 9, 1120. [Google Scholar] [CrossRef]
- Zhou, Z.; Gunter, W.D. The nature of the surface charge of kaolinite. Clays Clay Miner. 1992, 40, 365–368. [Google Scholar] [CrossRef]
- Grabsch, A.F.; Yahyaei, M.; Fawell, P.D. Number-sensitive particle size measurements for monitoring flocculation responses to different grinding conditions. Miner. Eng. 2020, 145. [Google Scholar] [CrossRef]
- Arinaitwe, E.; Pawlik, M. A method for measuring the degree of anionicity of polyacrylamide-based flocculants. Int. J. Miner. Process. 2009, 91, 50–54. [Google Scholar] [CrossRef]
- Kyoda, Y.; Costine, A.D.; Fawell, P.D.; Bellwood, J.; Das, G.K. Using focused beam reflectance measurement (FBRM) to monitor aggregate structures formed in flocculated clay suspensions. Miner. Eng. 2019, 138, 148–160. [Google Scholar] [CrossRef]
- Herrington, T.M.; Clarke, A.Q.; Watts, J.C. The surface charge of kaolin. Colloids Surf. 1992, 68, 161–169. [Google Scholar] [CrossRef]
- Robles, P.; Piceros, E.; Leiva, W.H.; Valenzuela, J.; Toro, N.; Jeldres, R.I. Analysis of sodium polyacrylate as a rheological modifier for kaolin suspensions in seawater. Appl. Clay Sci. 2019, 183, 105328. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Santa Barbara, CA, USA, 2011; ISBN 9780123919274. [Google Scholar]
- Yu, W.; Gregory, J.; Campos, L.; Li, G. The role of mixing conditions on floc growth, breakage and re-growth. Chem. Eng. J. 2011, 171, 425–430. [Google Scholar] [CrossRef]
- Gladman, B.J.; Usher, S.P.; Scales, P.J. Understanding the thickening process. In Paste; Jewell, R.J., Lawson, S., Newman, P., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2016; pp. 5–12. [Google Scholar]
- Deng, X.; Davé, R.N. Breakage of fractal agglomerates. Chem. Eng. Sci. 2017, 161, 117–126. [Google Scholar] [CrossRef]
- Benn, F.A.; Fawell, P.D.; Halewood, J.; Austin, P.J.; Costine, A.D.; Jones, W.G.; Francis, N.S.; Druett, D.C.; Lester, D. Sedimentation and consolidation of different density aggregates formed by polymer-bridging flocculation. Chem. Eng. Sci. 2018, 184, 111–125. [Google Scholar] [CrossRef]
- Kohl, R.A.; Taylor, S.A. Hydrogen bonding between the carbonyl group and wyoming bentonite. Soil Sci. 1961, 91, 223–227. [Google Scholar] [CrossRef]
- Nasser, M.S.; James, A.E. Effect of polyacrylamide polymers on floc size and rheological behaviour of kaolinite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 311–322. [Google Scholar] [CrossRef]
- Lee, L.T.; Rahbari, R.; Lecourtier, J.; Chauveteau, G. Adsorption of polyacrylamides on the different faces of kaolinites. J. Colloid Interface Sci. 1991, 147, 351–357. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeldres, R.I.; Jeldres, M.; MacIver, M.R.; Pawlik, M.; Robles, P.; Toro, N. Analysis of Kaolin Flocculation in Seawater by Optical Backscattering Measurements: Effect of Flocculant Management and Liquor Conditions. Minerals 2020, 10, 317. https://doi.org/10.3390/min10040317
Jeldres RI, Jeldres M, MacIver MR, Pawlik M, Robles P, Toro N. Analysis of Kaolin Flocculation in Seawater by Optical Backscattering Measurements: Effect of Flocculant Management and Liquor Conditions. Minerals. 2020; 10(4):317. https://doi.org/10.3390/min10040317
Chicago/Turabian StyleJeldres, Ricardo I., Matías Jeldres, Michael R. MacIver, Marek Pawlik, Pedro Robles, and Norman Toro. 2020. "Analysis of Kaolin Flocculation in Seawater by Optical Backscattering Measurements: Effect of Flocculant Management and Liquor Conditions" Minerals 10, no. 4: 317. https://doi.org/10.3390/min10040317
APA StyleJeldres, R. I., Jeldres, M., MacIver, M. R., Pawlik, M., Robles, P., & Toro, N. (2020). Analysis of Kaolin Flocculation in Seawater by Optical Backscattering Measurements: Effect of Flocculant Management and Liquor Conditions. Minerals, 10(4), 317. https://doi.org/10.3390/min10040317






