This section presents data for the study of distribution features and forms of occurrence of PGE (Pt, Pd, Ru, Rh, Ir and Os) in arsenopyrite and pyrite from the Natalkinskoe gold deposit. The data were obtained through local (SEM-EDX and LA-ICP-MS) and, so to speak, “semi-local” analyses (PCA and ADSSC). That is, the methods are not exactly local but were by no means bulk analysis, with a resolution at the level of different size fractions (PCA) or the size of individual crystals (ADSSC). The combination of local analysis and statistics for the compositions of individual monocrystals of different sizes is a unique feature of our approach.
4.2. Results of the Study of Pt, Pd, Ru and Rh in Arsenopyrites and Pyrites by AAS-ADSSC
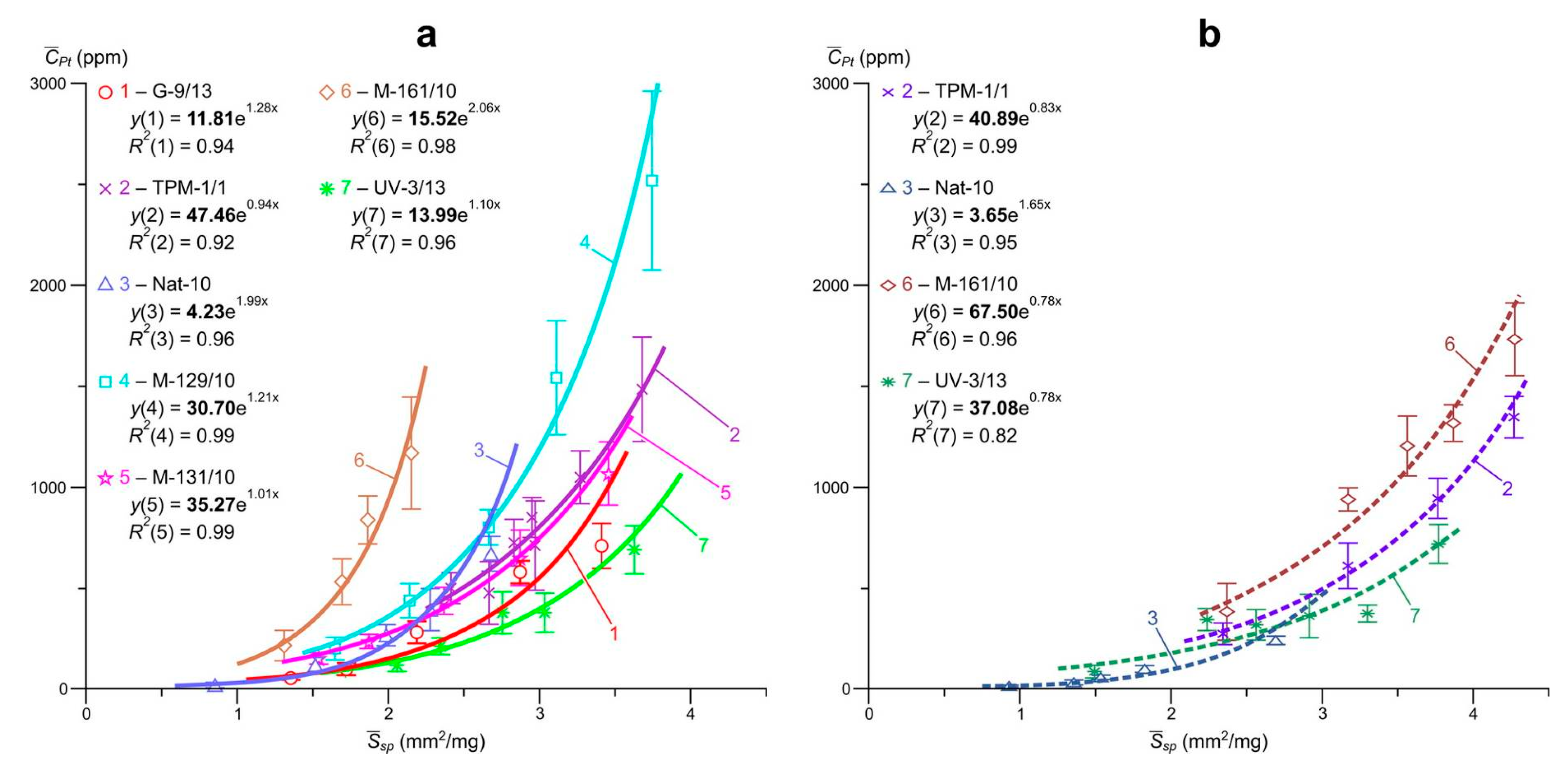
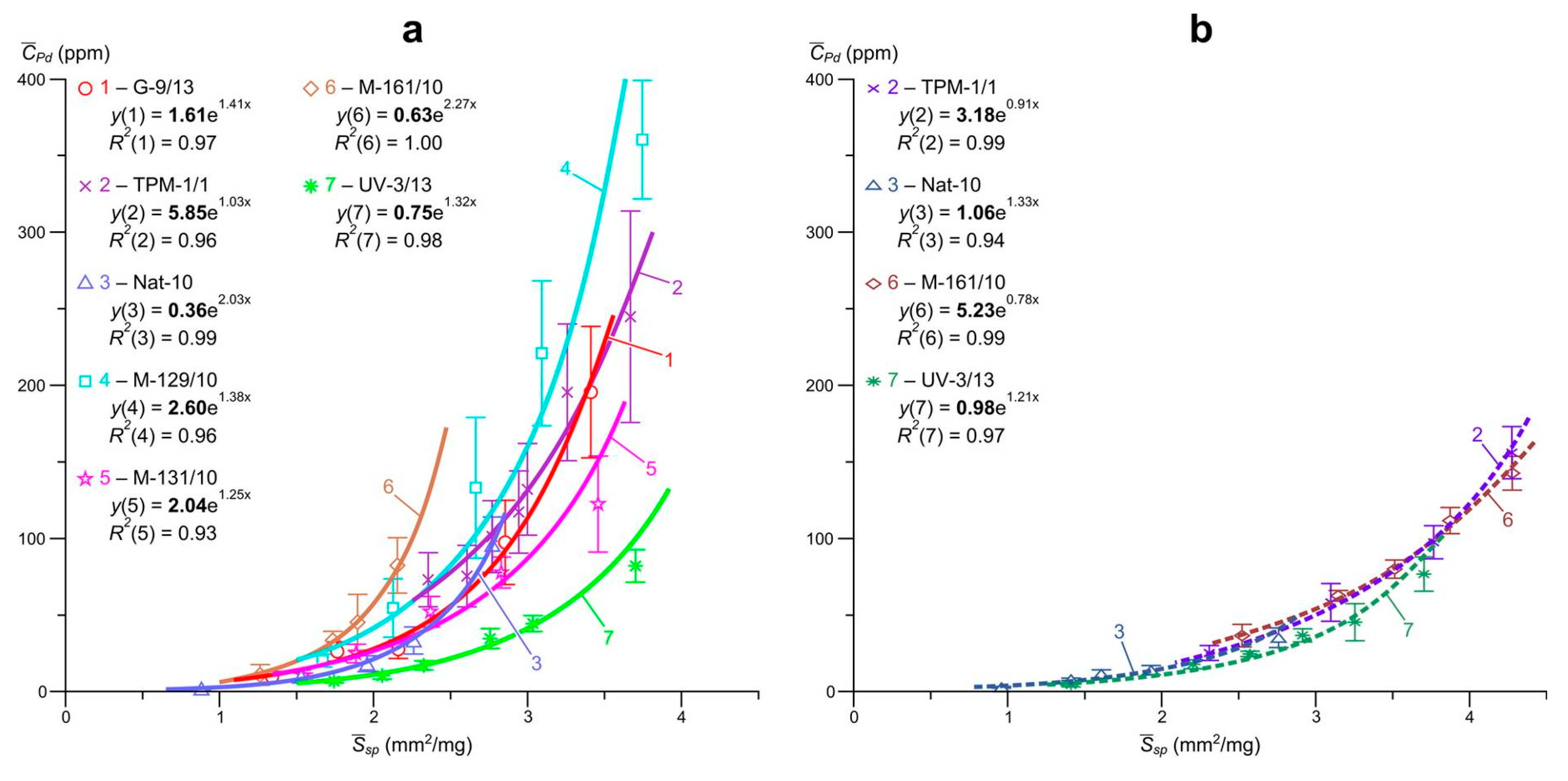
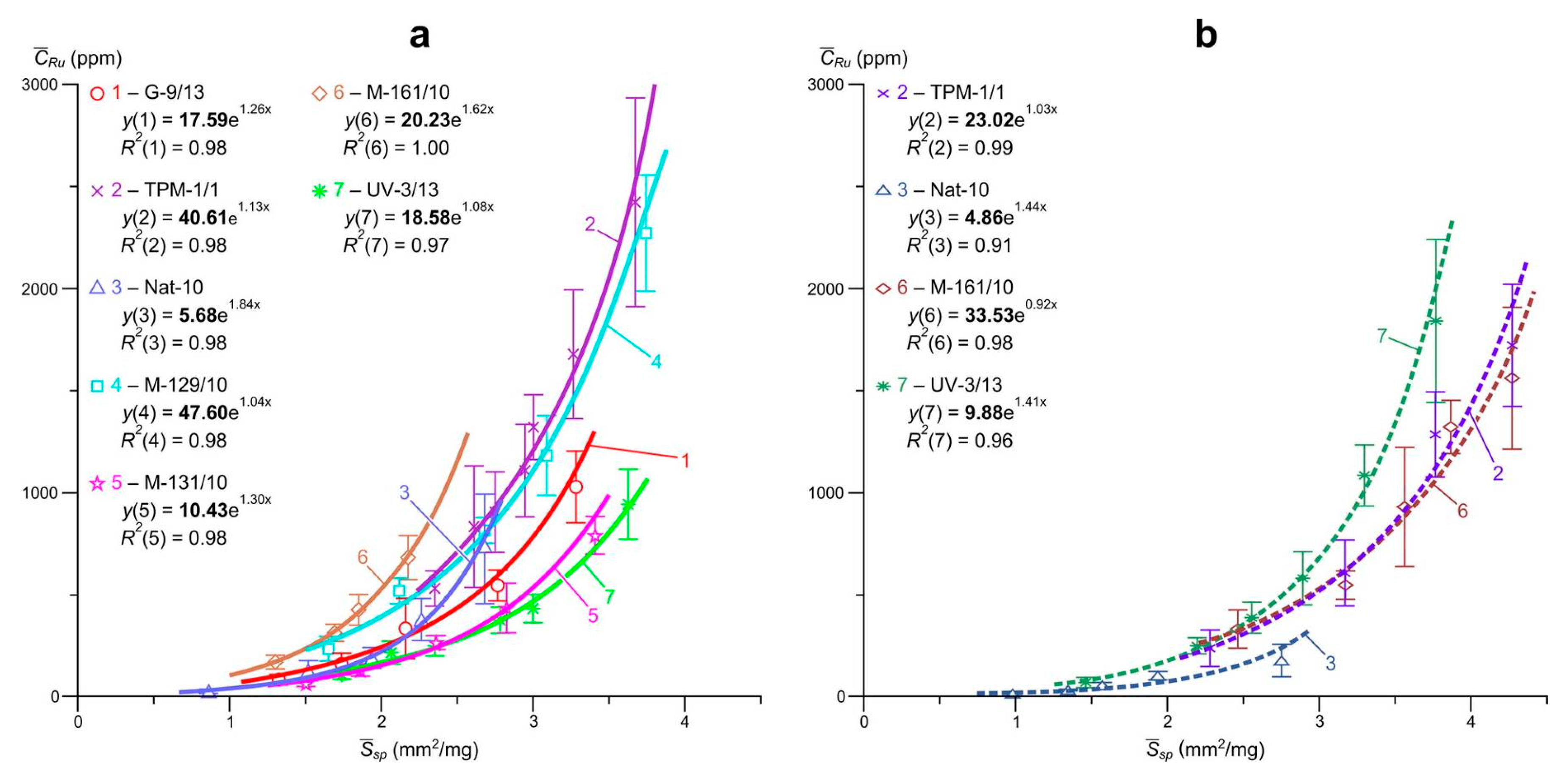
Data on the so-called “invisible” uniformly distributed impurity of Pt, Pd and Ru and the ratio of their structural and surface-bound forms in arsenopyrites and pyrites of the Natalkinskoe deposit are presented in
Figure 5,
Figure 6 and
Figure 7 and
Table 3,
Table 4 and
Table 5. In all cases, highly deterministic (
R2 = 0.82–1.0) dependences of the average element content in the size sample on the specific surface area of the average crystal in it were obtained. The highest
R2 values were set for arsenopyrite: Pt, 0.92–0.99; Pd, 0.93–1.0 and Ru, 0.97–1.0 and were slightly lower for pyrite: Pt, 0.82–0.99; Pd, 0.94–0.99 and Ru, 0.91–0.99.
Extrapolation of these dependences to zero specific surface area (
, mm
2/mg,
Table 3,
Table 4 and
Table 5), i.e., on a conditionally infinite crystal in the first case of arsenopyrite, and in the second pyrite, yields the following estimates of the contents of the structural form of the elements (
, ppm): for arsenopyrite Pt, 4.2–48; Pd, 0.4–5.9 and Ru, 5.7–48; for pyrite Pt, 3.7–68; Pd, 1.0–5.2 and Ru 4.9–34 (
Figure 5,
Figure 6 and
Figure 7). The average contents of surface-bound form of elements (
, ppm) are given in
Table 3,
Table 4 and
Table 5 and for arsenopyrite amount to the following values: Pt, 89–714; Pd, 8.1–114 and Ru, 79–1083; for pyrite Pt, 35–890; Pd, 4.9–62 and Ru, 30–690.
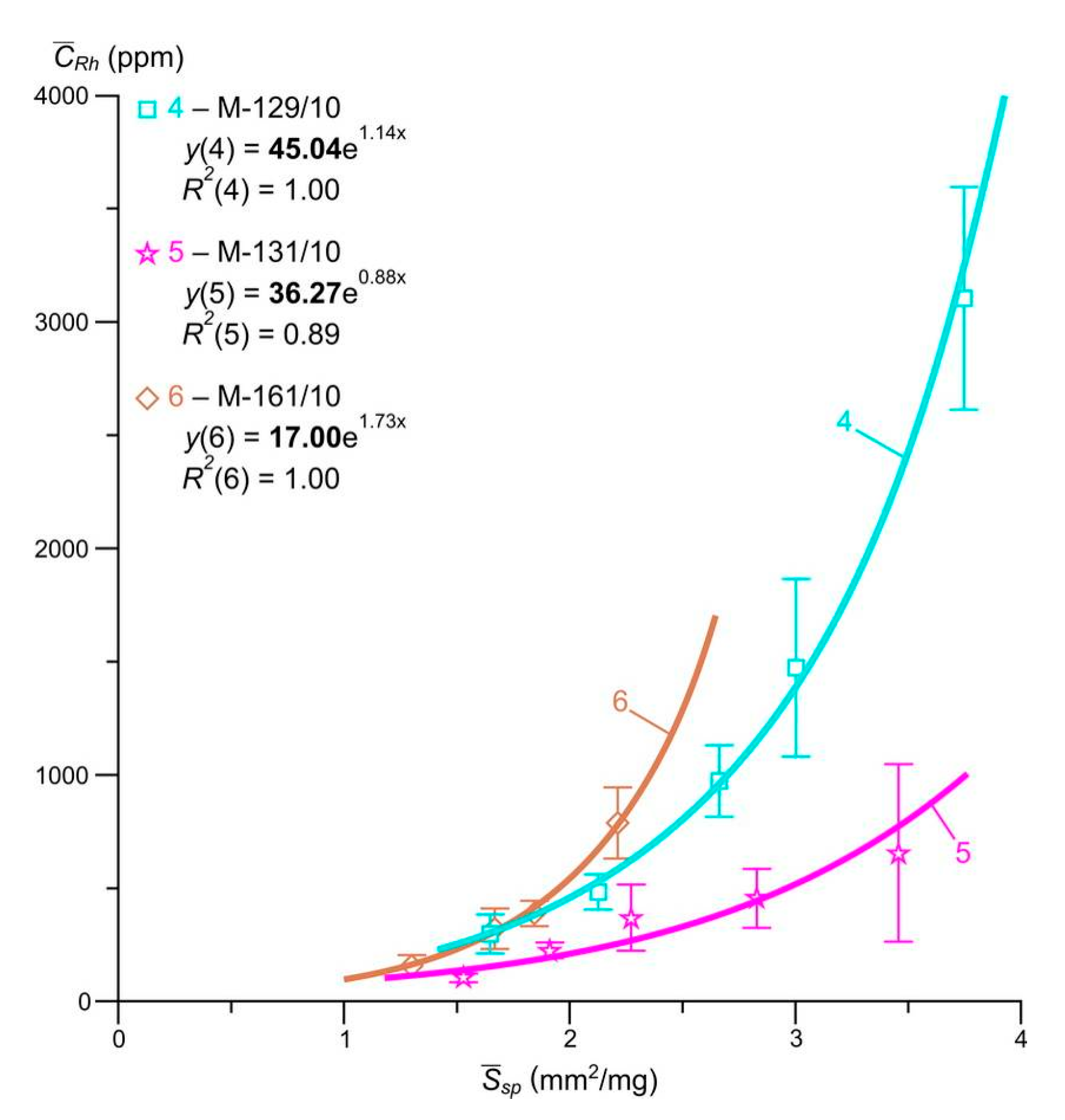
By now, the range of PGE studied by the AAS-ADSSC method has expanded. The first results for the forms of occurrence of Rh, one more element of the platinum group in crystals of arsenopyrite, were obtained. These data are still limited and preliminary. The results of the study of the content of evenly distributed Rh and the ratio of its structural and surface-bound forms in arsenopyrites from vein and streaky-vein ores of the Natalkinskoe deposit are presented in
Figure 8 and in
Table 6. In all cases, as well as for Pt, Pd and Ru, highly deterministic (
R2 = 0.89–1.0) dependences of the average Rh content in the size fraction on the specific surface area of the average arsenopyrite crystal in it were obtained. Extrapolation of these dependences to a zero-specific surface, i.e., to a conditionally infinite crystal, yielded estimates of the contents of the structural form of this element of 17–45 ppm. The average grades of the surface-associated forms were 154–678 ppm for arsenopyrite (see
Table 6).
The data presented (PCA and AAS-ADSSC) suggest that arsenopyrite also acts as a concentrator mineral for Rh. Structural and surface-bound forms in arsenopyrite (as well as in pyrite), as the main uniformly distributed forms, are characteristic not only of Pt, Pd and Ru, but also Rh. The data obtained confirms that arsenopyrites and pyrites are major mineral concentrators of PGE at the Natalkinskoe gold ore deposit. It can also be assumed that the size dependence of the content of evenly distributed Pt, Pd, Ru and Rh, and the confinement of a significant part to the surface of the crystals, is not a unique phenomenon for sulphide minerals of ore deposits. Structurally and superficially bound forms, as the main uniformly distributed forms, may be typical of both the remaining PGE (Os and Ir) and of other noble metals—Au and Ag [
11,
37,
38,
39,
41,
42,
43,
44,
45].
New data for Pt and Pd forms in arsenopyrites, both structural and surface-bound, confirmed earlier conclusions [
11]. The uniformly distributed Pt, Pd and Ru and, as we believe, Rh in arsenopyrites and pyrites are forms of elements which are chemically bound in the structure of these minerals and in nanoscale nonautonomous phases (NAPs) on the surface of arsenopyrite and pyrite crystals. The latter usually dominates over the former according to the content of these elements. Recent experimental and natural data [
37,
38,
46] suggest that Pt, Pd, Ru and Rh, as well as Au, can be incorporated into NAP formed on the surface of arsenopyrite and pyrite crystals in a very thin surface layer (~100–500 nm). These elements are absorbed by NAP from the hydrothermal solution even more efficiently than gold, which is incompatible in pyrite.
There is currently high demand for studies of the surface layer of sulphide minerals as possible hosts for “invisible” forms of trace elements, the nature of their distribution and concentration levels. Nanoscale NAPs enriched with Pt, Pd, Ru and Rh form a part of the so-called impurity component on the surface of arsenopyrite and pyrite crystals. The importance of its investigation is obvious. It is assumed that there is a common mechanism of absorption of all noble metals for the studied sulphides due to the active role of the surface during the growth of the crystal from a supersaturated hydrothermal solution [
38,
39]. This mechanism is closely related to the conditions of ore formation. This is an important point that must be taken into account in practical terms, for example, in the concentration of ores that include sulphides, in our case arsenopyrite and pyrite.
The structurally bound form of PGE, primarily Pt, despite a lower content compared to the surface form, is also extremely important. As shown earlier, data on the structural form of Au, Ag and Pt make it possible to make comparative estimates of the contents of these elements in the ore-forming fluids that form gold deposits. It is the only form of the element occurrence that can be used as an indicator of element activity in hydrothermal solution [
37,
46]. For example, the experimental data on the distribution and segregation of trace elements in crystals of ore minerals grown in hydrothermal systems were used to assess the content of Au and Pt in the ore-forming fluid (
) of the Natalkinskoe and Degdekan gold deposits. The content of the element in the structural form in natural pyrite, and the distribution coefficient (
) for the same form between pyrite and hydrothermal solution according to experimental data, were used for estimating element content in the ore-forming fluid [
38]. In
Table 7 these results are supplemented by the data from the present work on samples M-161/10, TPM-1/1 and Nat-10.
The average value of
is 0.1 for Au and 21 for Pt [
41,
46]. The evaluation of Au and Pt content in the ore-forming fluid showed that Au content in the fluids of the Degdekan and Natalkinskoe gold deposits was mainly at the level of 1.3–3.2 ppm, and it reached a maximum value of 18 ppm for the Nat-10 sample. Pt grade ranged from 0.1 to 3.2 ppm and, on average, was clearly elevated at the Natalkinskoe deposit compared to Degdekan. The fluid of the Natalkinskoe deposit was rich in both elements compared to the fluid of the Degdekan deposit. Au and Pt contents in solution were also comparable for most samples from the Natalkinskoe deposit (with the exception of the anomalous Au in sample Nat-10), whereas Au dominated over Pt in the Degdekan deposit. This is another argument in favor of studying platinum-bearing gold deposits, of which the Natalkinskoe deposit is no exception. Information about the Pt content in the fluid is of particular value in view of the complex behavior of Pt and the heterogeneity of its distribution under the capture of gas–liquid inclusions, the composition of which is determined by LA-ICP-MS as a proxy of the ore fluid composition [
47].
4.3. Study of the Chemical Composition of the Surface of Crystals of Arsenopyrite and Pyrite Using the SEM-EDX Method
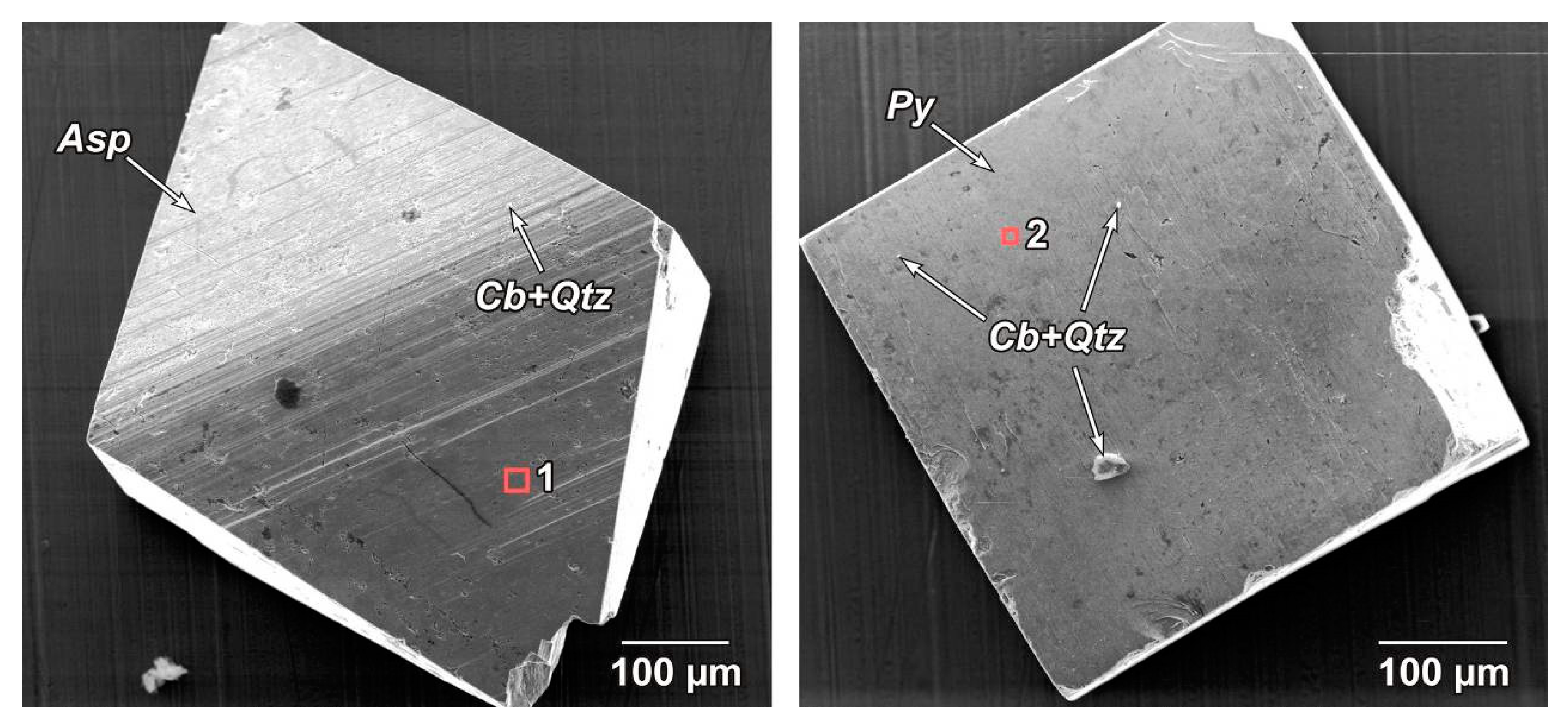
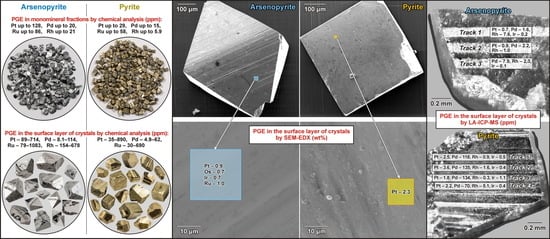
Nearly isometric pseudo-orthorhombic crystals of arsenopyrite and cubic crystals of pyrite without visible inclusions of ore minerals and with a minimum number of non-metallic microinclusions (mainly quartz and carbonate) were selected for the study (
Figure 9). The so-called “clean” areas virtually lacking visible inclusions and significant defects were then selected to study the composition of the “invisible” impurity component on the surface of these crystals (
Figure 10). The size (in μm) of such areas was 10 × 10, 20 × 20, and it was at least 30 × 30. Sections with low roughness were selected using the scanning probe microscopy technique (see above).
Faces with striation of the same crystallographic type were selected during scanning in order to eliminate the effect of capturing various impurities by faces of different crystallographic types during crystal growth: for arsenopyrite—prism {141}, for pyrite—cube {100}.
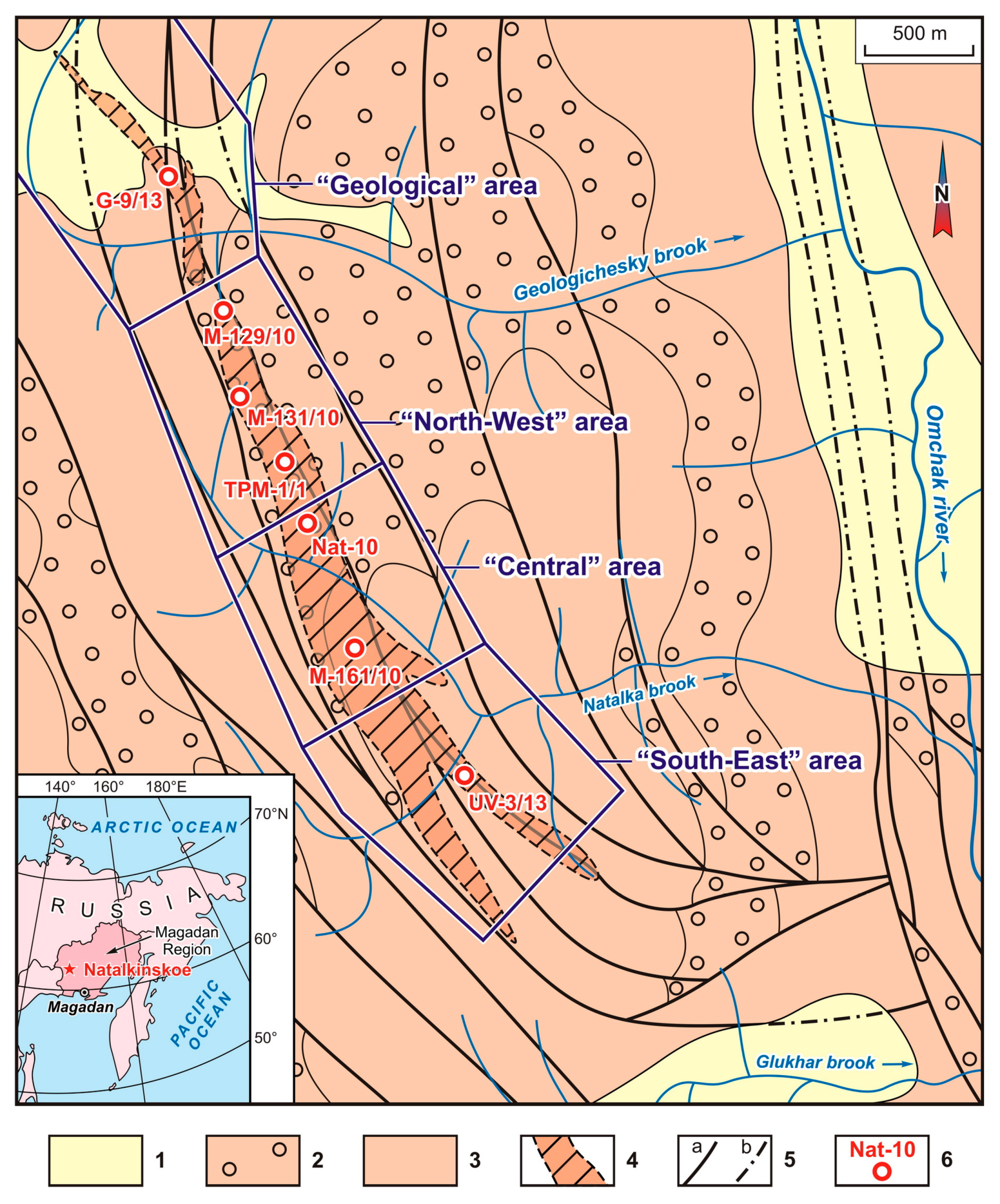
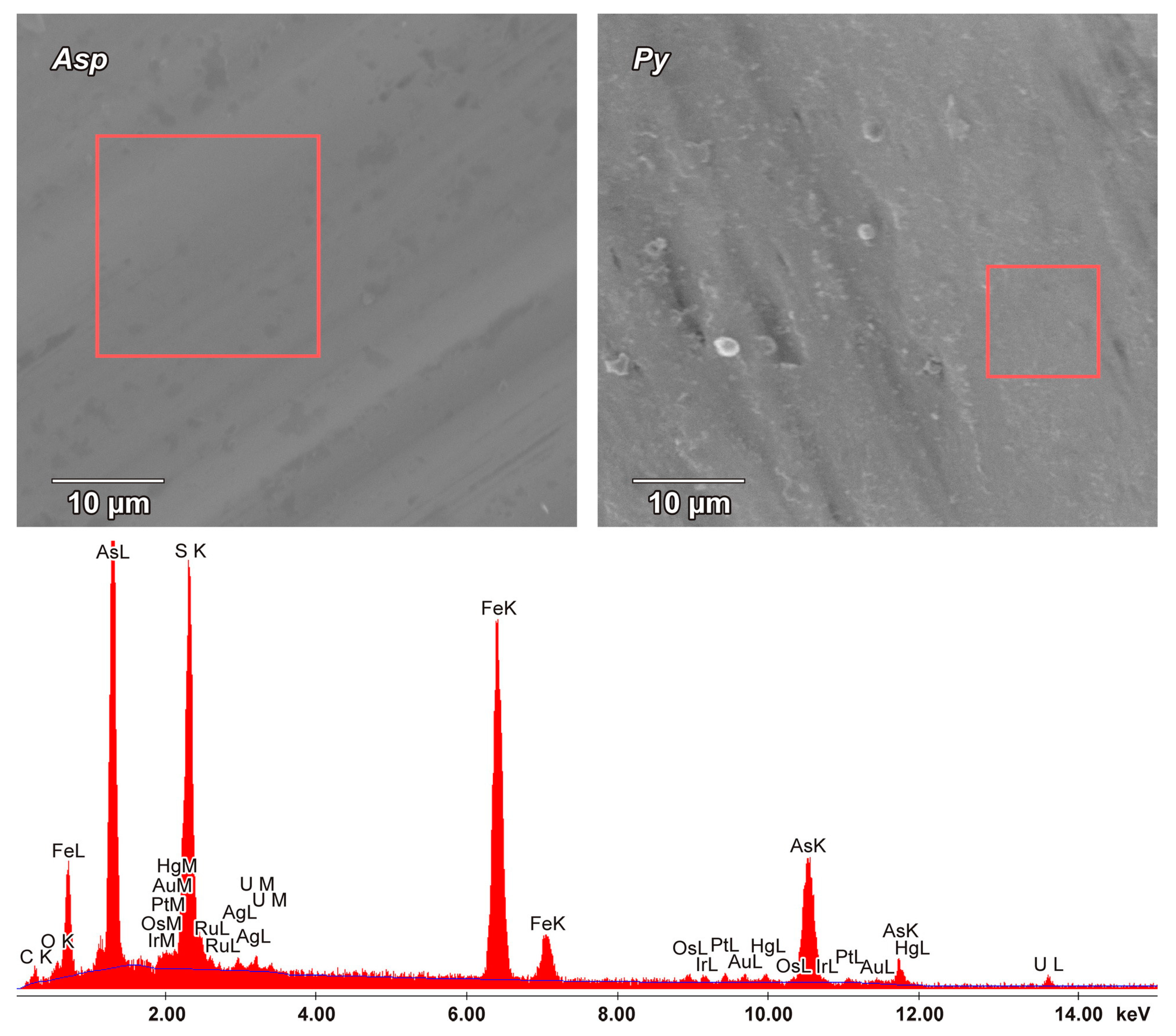
The surfaces of arsenopyrites and pyrites selected from the Natalkinskoe ore deposit (samples G-9/13, TPM-1/1, M-129/10, M-131/10, M-161/10 and Nat-10) clearly demonstrated the peculiarities of its elemental composition. On the “clean” surface of arsenopyrite, PGEs were thus represented by the following elements (in wt%): Pt (up to 2.1), Ru (1.4), Ir (up to 0.7) and Os (up to 0.7). Other ore elements were also determined (in wt%): U (up to 2.0), Hg (up to 1.1), Au (up to 1.0), rarely Ag (up to 0.7) and Cu (up to 0.6) (
Table 8). The only PGE present on the “clean” surface of pyrite was Pt (up to 2.9 wt%), and other ore elements were represented by As (up to 2.2 wt%) and Cu (up to 0.7 wt%) (
Table 9).
The SEM-EDX method confirmed the presence of Au and Pt on the surface of arsenopyrite crystals from the Natalkinskoe deposit, and high contents of them were detected earlier [
11]. It is the first time such PGEs as Ru, Os, Ir and a number of ore elements—Ag, Hg, Cu and U—were detected on the surface of arsenopyrite. Pt and As are shown for the first time to be constantly present on the surface of pyrite crystals. Comparing the data from the AAS-ADSSC and SEM-EDX methods regarding the surface contents of Au, Pt and Ru, it is necessary to recall that the data on
of AAS-ADSSC method are related to the entire average crystal, whereas the SEM-EDX method virtually defines local content of the element at the local area (“point”) on the surface. It would be more correct to compare these results with the content of the element within NAP, which can be calculated under certain assumptions [
46]. According to such calculations, Au in NAP on pyrite amounts to 0.3–0.5 wt% [
38], which is comparable by an order of magnitude to the data in
Table 8 and
Table 9 on Au and Pt. Increased platinum on pyrite (see
Table 9) is qualitatively consistent with the fact that, according to AAS-ADSSC,
Pt >
Au (see
Table 7).
4.4. Study of the Chemical Composition of the Surface of Arsenopyrite and Pyrite Crystals by LA-ICP-MS
When studying the surface layers of arsenopyrite and pyrite crystals using the LA-ICP-MS method, attention was largely focused on Pt and Pd. Ten arsenopyrite crystals and six pyrite crystals were analyzed (
Table 10 and
Table 11). For all crystals of arsenopyrite (samples M-129/10, M-131/10, M-161/10 and TPM-1/1) and pyrite (samples M-161/10 and TPM-1/1) a track depth of 0.5–0.6 μm per laser pass was obtained. Unfortunately, we do not know the NAP thickness of the studied crystals. If we focus on the previously obtained data [
44,
48], the thickness of NAP on pyrite is ≤0.5 μm, and for arsenopyrite it is, according to preliminary data, much less [
11]. The first pass of the laser thus best represents the composition of NAP, although significant “contamination” by the material underlying NAP is likely. The parameters of the laser were adjusted for one of the arsenopyrite crystals selected for detailed study (see
Table 10, sample M-129/10, crystal 2), in such a way that a layer of ~100 nm thick would be burned in one pass (see above). In this case, the maximum depth of the laser-dig groove was 600 nm.
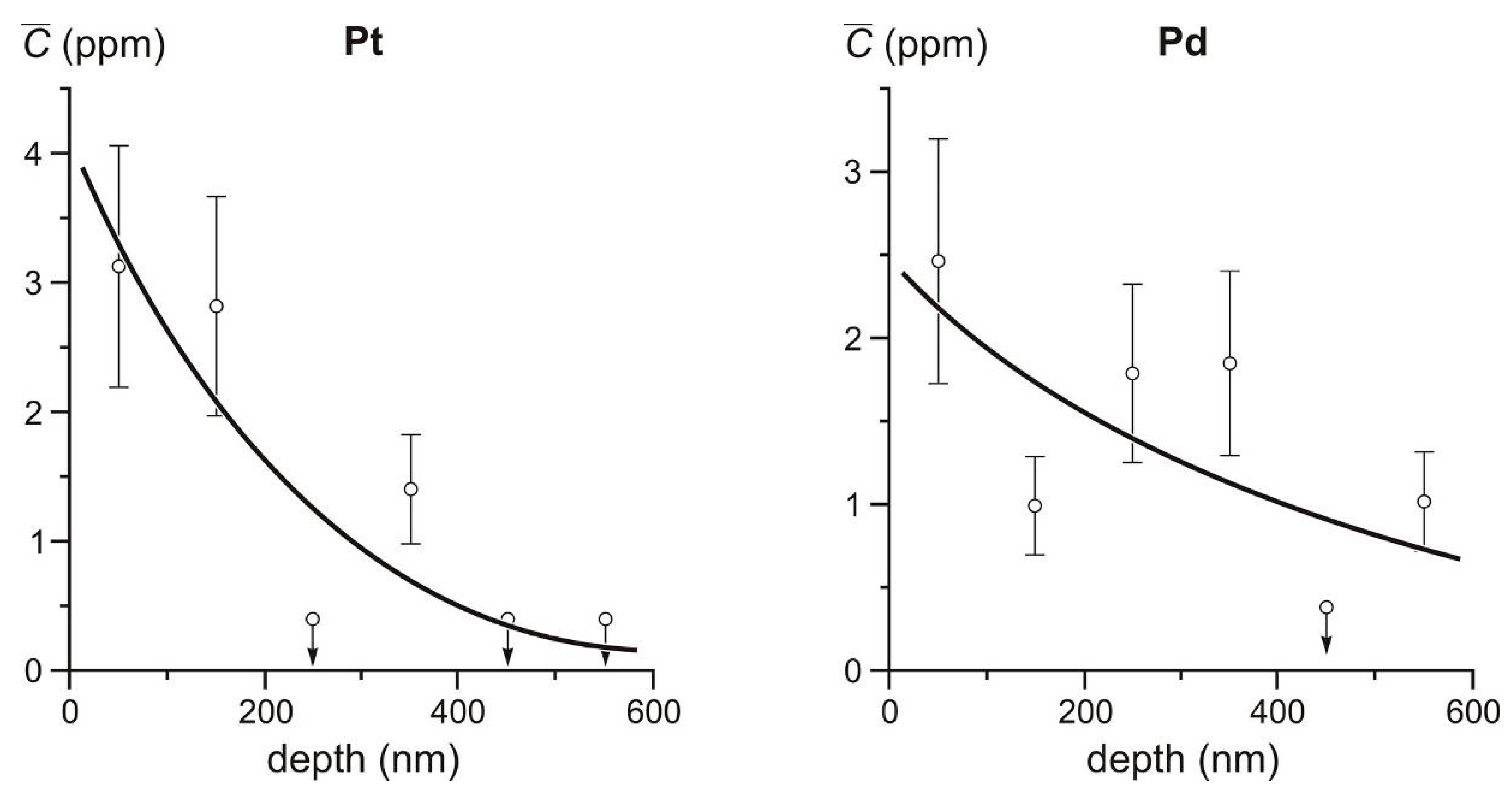
Figure 11 shows graphs of the detailed distribution of Pt and Pd contents in the surface layer of Crystal 2 from the M-129/10 sample.
A generalization of the results of previous studies [
11] and the new data suggest that the increased content of Pt and Pd is confined to the surface layer of not only arsenopyrite crystals, but also pyrite. It can also be assumed that the tendency for a Pt and Pd concentration in the surficial layer at <500 nm thick is common for both minerals (see
Figure 11 and
Table 11). Several exceptions can be noted in
Table 10, however, which may be due to inhomogeneity in the impurity distribution because of the evolution of NAP during crystal growth [
39].
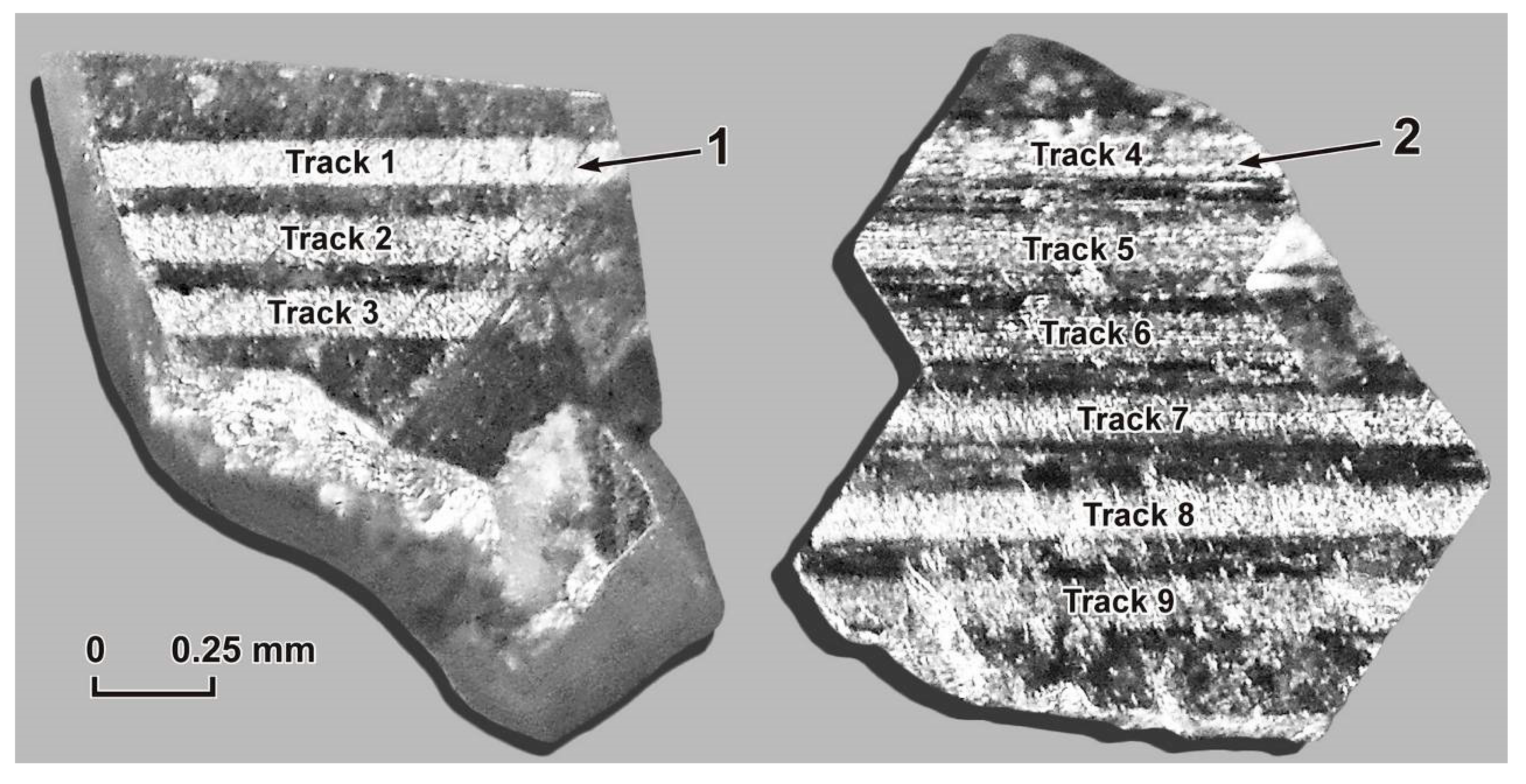
Determination of the contents of a wider range of elements belonging to the group of PGEs—Pt, Pd, Rh and Ir—was carried out in the example of studying the surfaces of arsenopyrite and pyrite crystals taken from the UV-3/13 sample (
Figure 12 and
Figure 13). The surface layer of these crystals was studied to a depth of 6 µm (depth per pass is 1 µm). Ru and Os contents were not determined due to their absence in the standards we used. According to LA-ICP-MS analysis, the surface layers of arsenopyrite and pyrite, along with Pt and Pd, concentrated PGEs such as Rh and Ir. The contents of elements (ppm) in the surface layers of arsenopyrite were Pt up to 5.2, Pd up to 8.4, Rh up to 8.9 and Ir up to 0.2, and in the surface layers of pyrite were Pt up to 13, Pd up to 896, Rh up to 6.7 and Ir up to 4.1. The maximum contents of Pt, Pd and Ir were established in pyrite, and Rh was established in arsenopyrite. Like Pt and Pd, all the highest contents of Rh and Ir were confined to the first removed layer (1 µm thick). There was a distinct tendency for the content of all these elements to decrease with depth (
Table 12 and
Table 13).
High Pd content and Pd/Pt ratios on the pyrite surface (see
Table 13) contradict the AAS-ADSSC and SEM-EDX data presented above, according to which the Pt content was more than an order of magnitude higher than Pd. The reason for this contradiction requires special research and may be related to the existence of an unidentified surface form of Pd (for example, Pd nanoparticles), which is inhomogeneously distributed over crystals. The SEM-EDX method was also local (locality is even higher than for LA-ICP-MS), and therefore cannot serve to confirm this hypothesis. In the AAS-ADSSC method, crystals with such an excess of Pd over the average content simply will not be included in the final sample.
The maximum PGE contents at the Natalkinskoe deposit are currently known to be associated with sulphide minerals. High levels of PGE in the sulphide gravity concentrate of this deposit ore were established by sample decomposition using fluoro oxidants [
49] and amounted to (in ppm) Pt, 12.9–92.8; Pd, 0.62–2.97; Ru, 0.11–3.27; Rh, 0.95–1.54 and Ir, 0.1–0.3. The speculation about the possible role of sulphide minerals as Pt concentrators at the Natalkinskoe gold deposit was previously made by L.P. Plyusnina and co-authors [
14]; however, microprobe analysis did not reveal high content of noble metals in conventional preparations of arsenopyrite and pyrite [
3]. Arsenopyrite concentrates of not only Au, but also Pt and Pd, were later demonstrated at the Natalkinskoe deposit [
11]. The new data confirmed and supplemented the earlier conclusion. The highest contents of PGE (in ppm) observed in arsenopyrite were Pt up to 128, Pd up to 20, Ru up to 86 and Rh up to 21, and they were less high in pyrite: Pt up to 29, Pd up to 15, Ru up to 58 and Rh up to 5.9. AAS-ADSSC found that the high content of PGE largely was due to the existence of non-autonomous phases, causing their surface concentration. The fact that the nature of high contents of PGE in arsenopyrite and pyrite is mainly superficial was confirmed by the data obtained by SEM-EDX and (to some extent) LA-ICP-MS. If the data from the first two methods are comparable (taking into account the above considerations), the latter gives different results, which can be explained by the following reasons. First, there is some dispersion of the material on the surface after the first and each subsequent laser pass, which increases the error of determination. Second, the analysis of surface areas of several crystals does not have the necessary statistical reliability for determining the average contents, as is the case in the analytical technology of AAS-ADSSC. It is quite possible to meet with an “empty” crystal or, conversely, a crystal with the surface strongly enriched with impurity. This is all the more likely because the heterogeneity of the distribution of PGE is well known. For example, in pyrite from the intercumulus sulphide phases of the main sulphide zone of Great Dyke (Zimbabwe), the ion-microprobe analysis revealed a strong heterogeneity in the Pt distribution—from 0.4 to 244 ppm in different grains of the same sample [
50]. Thirdly, significant errors can occur due to the lack of external calibration on such surface structures, for example, deposited on the crystal films of sulphide material with a certain content of admixture of the studied element. In the current state of the study of the surface composition of sulphides by LA-ICP-MS, although the history of such studies seems to date back 12 years [
51], it is possible to speak only about the general tendency consisting of the enrichment of PGE of a surface of pyrite and arsenopyrite crystals; however, this trend is fully confirmed by other methods.
The reason for this phenomenon involves the features of the crystal growth mechanism and the dualism of the element distribution coefficient in the mineral-hydrothermal solution system, which is an order of magnitude higher for NAP compared to the crystal volume [
38,
39]. Non-autonomous phases are extremely important, but they are still rarely considered components of synthetic materials and minerals, the geochemical role of which lies in their ability to accumulate impurity elements in ultra-high contents [
52]. Such phases occur on the surface of a growing crystal due to the chemical modification and structural reconstruction of its surface layer, which is in local equilibrium with a supersaturated solution. NAP contain defects, chemical bonds and valence states of elements unusual for crystal volume, for example AsV, AsIII, AsII and FeIII on pyrite [
41], which provides additional opportunities for the incorporation of minor and trace elements, especially incompatible, into their structures. Since the stability field of arsenopyrite is adjacent to the pyrite field, according to the principle of continuity of phase formation on mineral surfaces and the correspondence of chemical forms of elements on them [
53], the surface NAP on arsenopyrite can have a defective pyrite structure. Since platarsite Pt(As,S)
2 has the structure of pyrite
Pa3, we should therefore expect an increase in the solubility of Pt in the surficial phase compared to the volume of the crystal.
Gold and PGE nanoparticles are increasingly, especially recently, found in ores of different genesis deposits [
7,
8,
54,
55,
56]. Although no PGE mineral forms were identified at the Natalkinskoe deposit, the need to continue research in this direction is obvious. This may be demonstrated by the Degdekan deposit, another gold project in the northeast of Russia, which is in the territory of Pre Kolyma. Its ores, classed as black shale formation, yielded the first findings of a number of platinum group minerals of submicron dimensions: native osmium, routheniridosmine, osmirid, ruthenosmirid, laurite, iridarsenite, ruthenium arsenide and arsenide of osmium and iridium [
8]. The studies by [
5,
6] established the existence of different mineral forms of platinum metals, including native platinum, phases of Pt–Cu–Fe system, sperrylite, cooperite, and minerals of the Pd–Bi system in gold ores of the Sukhoi Log deposit (Lena ore district, Irkutsk region, Russia). Their size mainly ranges within 1–10 μm. Later, native Pt nanoparticles, with a dominant size of 0.5–20 nm, were found in the concentrates of insoluble carbonaceous matter of shales containing ore mineralization [
7]. It follows that there is a high probability of nanoscale mineral forms of PGE existing at the Natalkinskoe deposit, according to the model of surface NAP evolution in sulphide minerals, suggesting their partial transformation and aggregation with the formation of nano- and microinclusions of separate (autonomous) phases of trace elements [
39]. The possibility of finding nano-sized particles of PGE in sulphides is confirmed by experimental data. For example, nanometer-size PtS
2 inclusions in synthetic Pt-containing pyrite were detected with high-resolution transmission electron microscopy [
57].