The Behaviour of Mixtures of Sodium Iso-Butyl Xanthate and Sodium Di-Ethyl Dithiophosphate during the Flotation of a Cu-Ni-Pt Ore in Degrading Water Quality
Abstract
1. Introduction
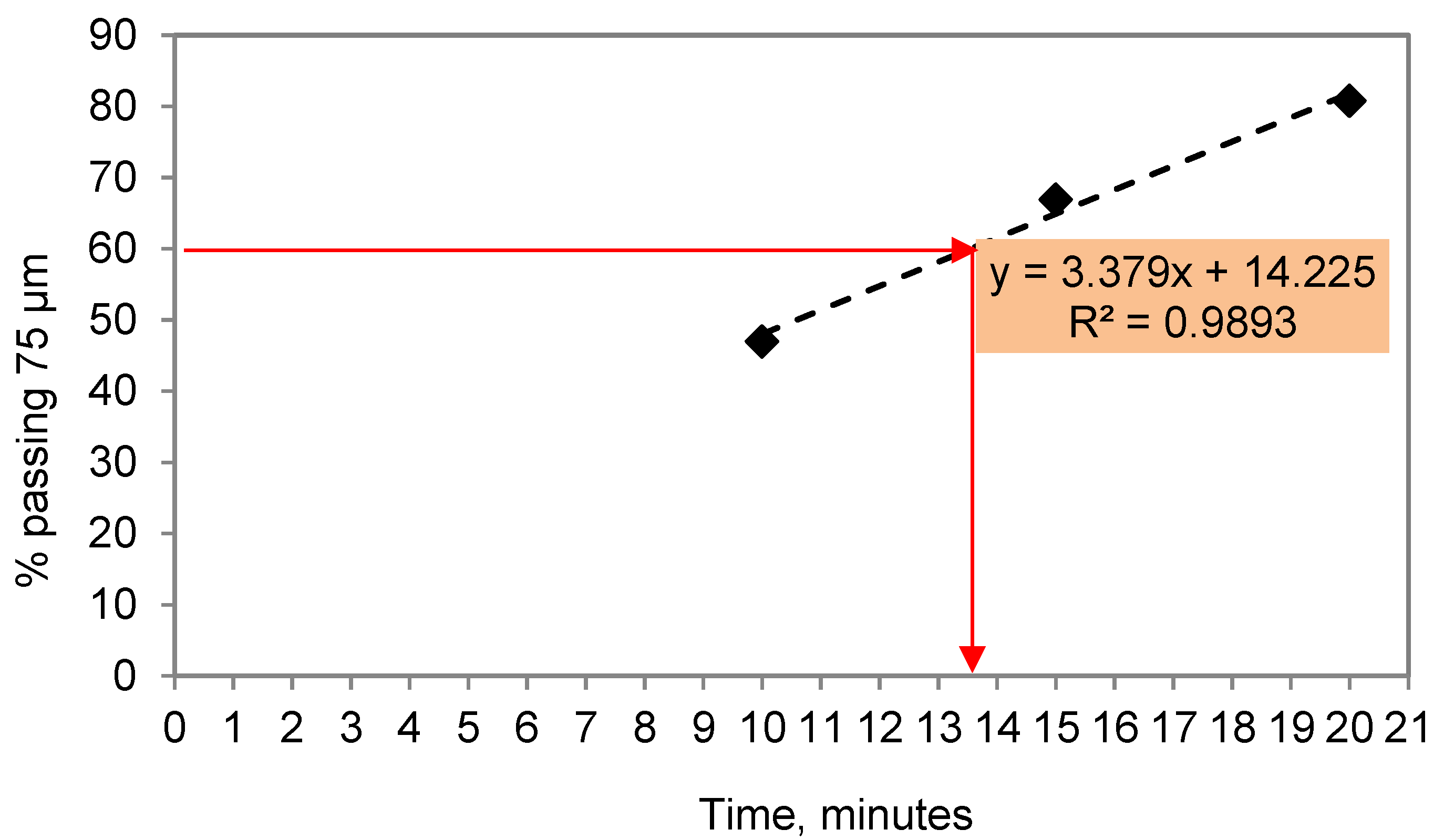
2. Experimental Procedure
3. Results and Discussion
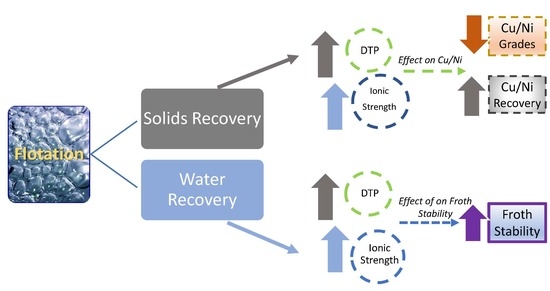
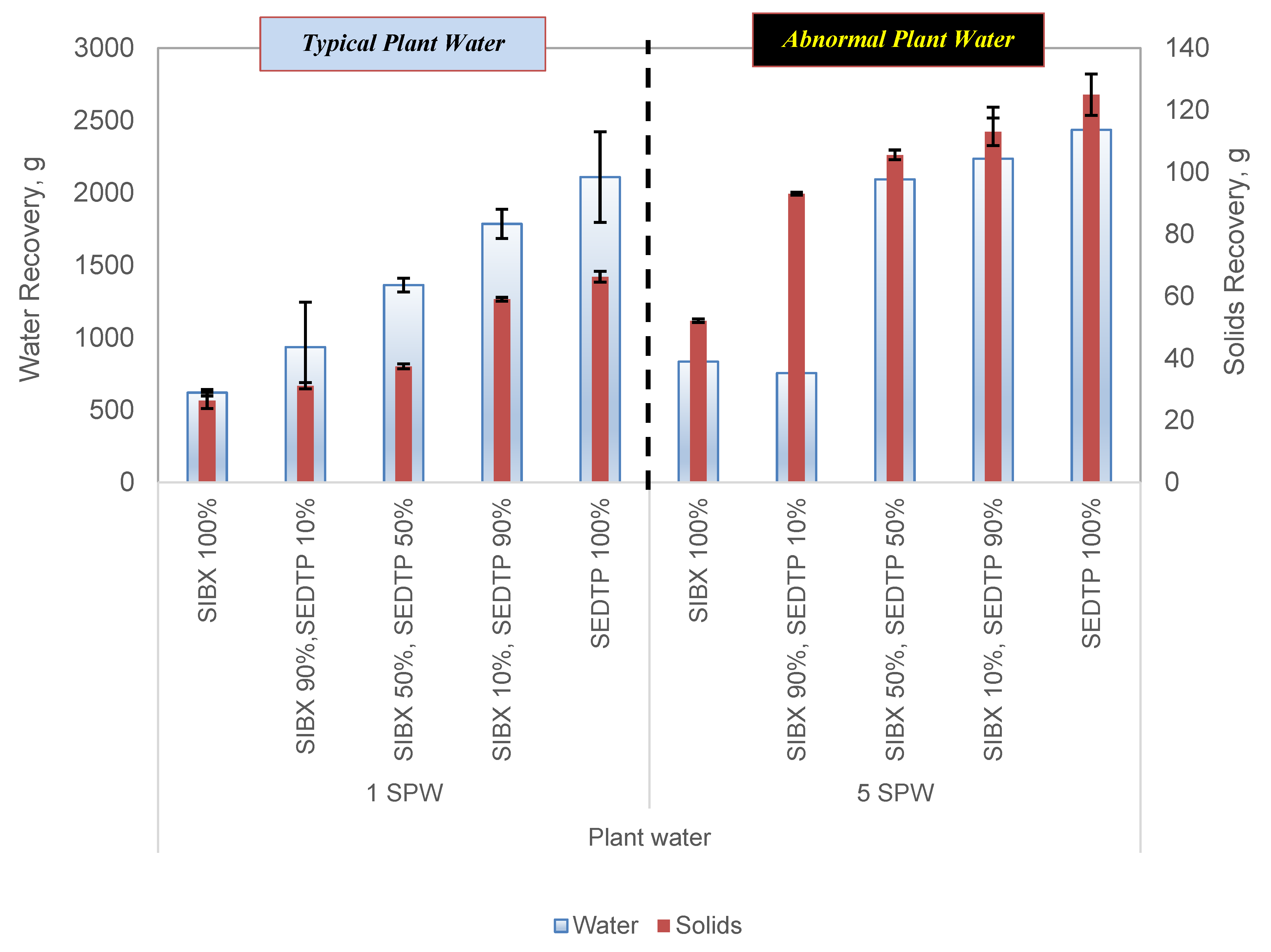
3.1. The Effect of Varying Thiol Collector Mixture and Ionic Strength of Plant Water on Solids and Water Recoveries
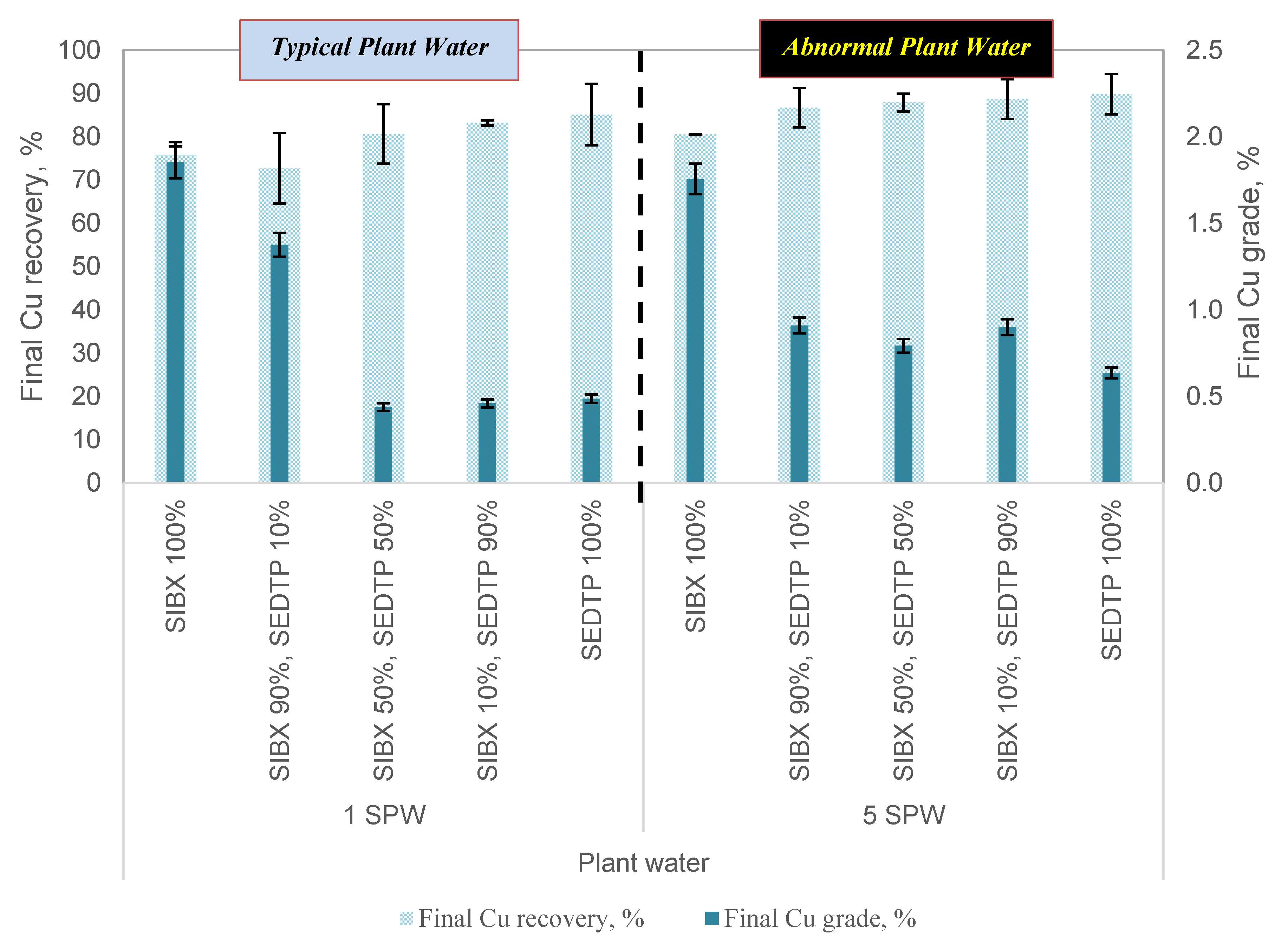
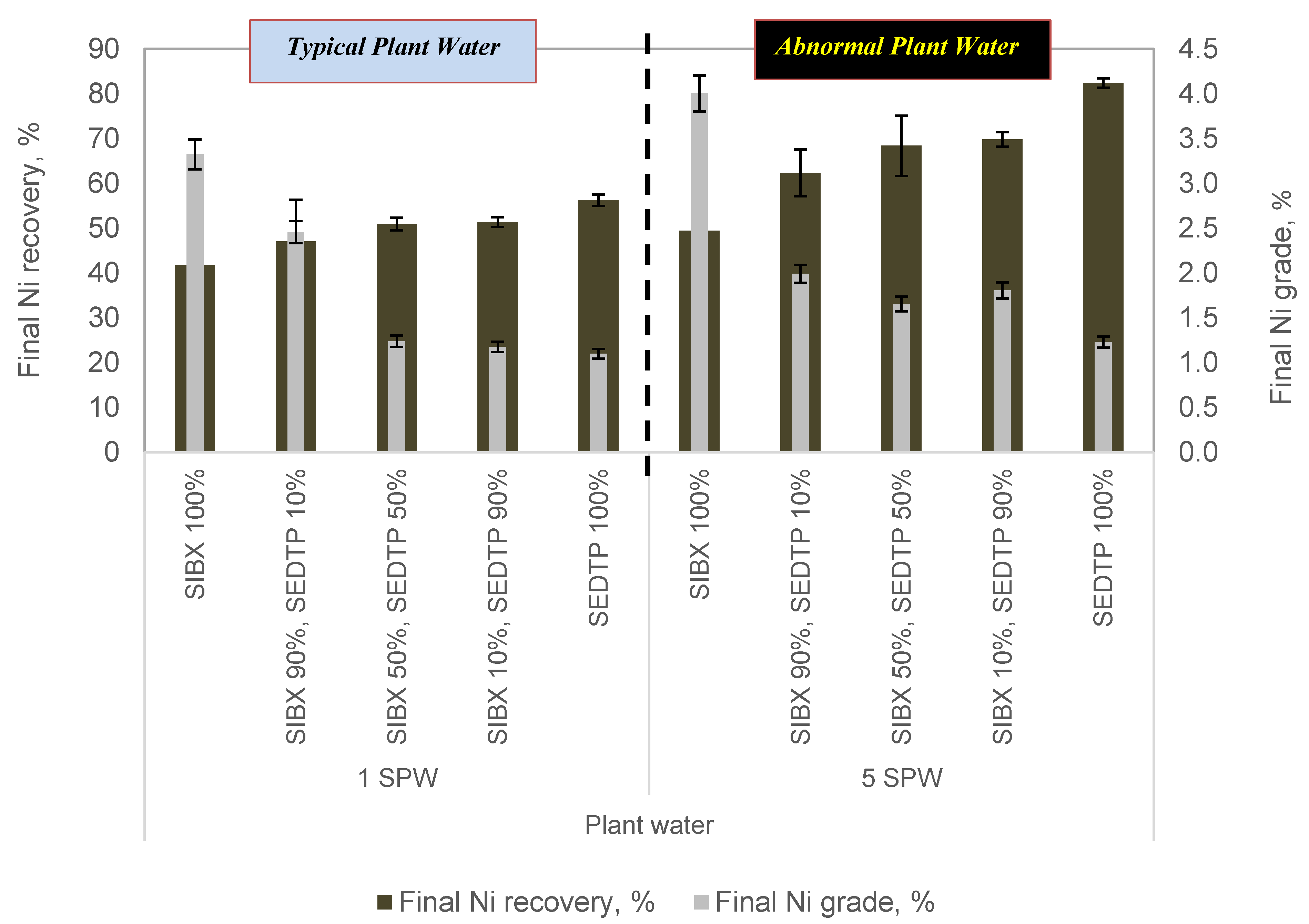
3.2. The Effect of Varying Thiol Collector Mixture and Ionic Strength of Plant Water Cu and Ni Grades and Recoveries
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Connor, C.; Wiese, J.; Corin, K.; McFadzean, B. On the management of gangue minerals in the flotation of platinum group minerals. Min. Metall. Explor. 2019, 36, 55–62. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. Perspectives from literature on the influence of inorganic electrolytes present in plant water on flotation performance. Physicochem. Probl. Miner. Process. 2018, 54, 1191–1214. [Google Scholar]
- Moimane, T.M.; Corin, K.C.; Wiese, J.G. The effect of varying pulp reagent chemistry on the flotation performance of a South African PGM ore. Miner. Eng. 2016, 95, 155–160. [Google Scholar] [CrossRef]
- Sheni, N.; Corin, K.; Wiese, J. Considering the effect of pulp chemistry during flotation on froth stability. Miner. Eng. 2018, 115, 15–23. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. Water quality effects on a sulfidic PGM ore: Implications for froth stability and gangue management. Physicochem. Probl. Miner. Process. 2018, 54, 1253–1265. [Google Scholar]
- Michaux, B.; Rudolph, M.; Reuter, M.A. Challenges in predicting the role of water chemistry in flotation through simulation with emphasis an emphasis on the influence of electrolytes. Miner. Eng. 2018, 125, 252–264. [Google Scholar] [CrossRef]
- Hancer, M.; Celik, M.S.; Miller, J.D. The significance of interfacial water structure in soluble salt flotation systems. J. Colloid Interface Sci. 2001, 235, 150–169. [Google Scholar] [CrossRef]
- October, L.; Corin, K.; Schreithofer, N.; Manono, M.; Wiese, J. Water quality effects on bubble-particle attachment of pyrrhotite. Miner. Eng. 2019, 131, 230–236. [Google Scholar] [CrossRef]
- October, L.L.; Corin, K.; Schreithofer, N.; Manono, M.; Wiese, J. Considering the Ionic Strength and pH of Process Water on Bubble-particle Attachment of Sulfide Minerals: Implications for Froth Flotation in Saline Water. In Mine Water-Technological and Ecological Challenges; Khayrulina, E., Wolkersdorfer, C.H., Polyakova, S., Bogush, A., Eds.; Perm State University: Perm, Russia, 2019; pp. 437–445. [Google Scholar]
- Wang, B.; Peng, Y. Effect of Saline Water on Mineral Flotation. Ph.D. Thesis, Unversity of Queensland, Brisbane, Australia, 2014. [Google Scholar]
- Ikotun, B.D.; Adams, F.V.; Ikotun, A.G. Application of three xanthates collectors on the recovery of nickel and pentlandite in a low-grade nickel sulfide ore using optimum flotation parameters. Part. Sci. Technol. 2017, 35, 462–471. [Google Scholar] [CrossRef]
- Liu, L.; Rao, S.R.; Finch, J.A. Technical note: Laboratory study of effect of recycle water on flotation of a Cu/Zn sulphide ore. Miner. Eng. 1993, 6, 1183–1190. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Herrara-Urbina, R.; McGlashan, D.W. Studies on the applicability of chelating agents as universal collectors for copper minerals. Int. J. Miner. Process. 1999, 58, 15–33. [Google Scholar] [CrossRef]
- Özün, S.; Ergen, G. Determination of optimum parameters for flotation of galena: effect of chain length and chain structure of xanthates on flotation recovery. ACS Omega. 2019, 4, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Zanin, M.; Lambert, H.; du Plessis, C.A. Lime use and functionality in sulphide mineral flotation: A review. Miner. Eng. 2019, 143, 105922. [Google Scholar] [CrossRef]
- Corin, K.C.; Wiese, J.G. Investigating froth stability: A comparative study of ionic strength and frother dosage. Miner. Eng. 2014, 66–68, 130–134. [Google Scholar] [CrossRef]
- Dishon, M.; Zohar, O.; Sivan, U. From repulsion to attraction and back to repulsion: The effect of NaCl, KCl and CsCl on the force between silica surfaces in aqueous solution. Langmuir 2009, 25, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Ejtemaei, M.; Plackowaski, C.; Nguyea, A.V. The effect of calcium, magnesium and sulphate ions on the surface properties of copper activated sphalerite. Miner. Eng. 2016, 89, 42–51. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. An investigation into the effect of various ions and their ionic strength on the flotation performance of a platinum bearing ore from Merensky reef. Miner. Eng. 2012, 36, 231–236. [Google Scholar] [CrossRef]
- Shengo, L.M.; Gaydardzhiev, S.; Kaleng, N.M. Assessment of water quality effects on flotation of copper-cobalt oxide ore. Miner. Eng. 2014, 65, 145–148. [Google Scholar] [CrossRef]
- Shen, W.Z.; Fornasiero, D.; Ralston, J. The flotation of sphalerite and pyrite in the presence of sodium sulfite. Int. J. Miner. Process. 2001, 63, 17–28. [Google Scholar] [CrossRef]
- Kirjavanein, V.; Schreithofer, N.; Heiskanen, K. Effect of calcium and thiosulfate ions on flotation selectivity of Nickel-Copper ores. Miner. Eng. 2002, 15, 1–5. [Google Scholar] [CrossRef]
- Atkin, R.; Craig, V.S.; Wanless, E.J.; Biggs, S. The influence of chain length and electrolyte on the adsorption kinetics of cationic surfactants at the silica-aqueous solution interface. J. Colloid Interface Sci. 2003, 266, 236–244. [Google Scholar] [CrossRef]
- Subramanian, V.; Ducker, W.A. Counter Ion effects on adsorbed miceller shape: Experimental study of the role of polarizability and charge. Langmuir 2000, 16, 4447–4454. [Google Scholar] [CrossRef]
- Xu, S.; Boyd, S.A. Cationic surfactant sorption to a vermiculitic subsoil via hydrophobic bonding. Environ. Sci. Technol. 1995, 29, 321. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.T.L.; Hao, F.P.; Smith, L.K.; Chau, T.T.; Bruckard, W.J. The effect of particle shape and hydrophobicity in flotation. Int. J. Miner. Process. 2009, 93, 128–134. [Google Scholar] [CrossRef]
- Schwarz, S.; Grano, S. Effect of particle hydrophobicity on particle and water transport across a flotation froth. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 157–164. [Google Scholar] [CrossRef]
- Taggart, A. Handbook of Mineral Processing; Wiley: New York, NY, USA, 1950. [Google Scholar]
- Bradshaw, D.; Harris, P.; O’Connor, C. Synergistic interactions between reagents in sulphide flotation. J. South Afr. Inst. Min. Metall. 1998, 98, 189–193. [Google Scholar]
- Heilbig, C.; Bradshaw, D.; Harris, P.; O’Connor, C.T.; Baldauf, H. The synergistic interactions of mixtures of thiol collectors in the flotation of sulphide minerals. In Proceedings of the XXI International Mineral. Processing Congress, Rome, Italy, 23–27 July 2000. [Google Scholar]
- Wiese, J.G.; Harris, P.; Bradshaw, D. The influence of the reagent suite in the flotation of ores from the Merensky reef. Miner. Eng. 2005, 18, 189–198. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Ravishankar, S.A. Flotation reagents—A critical overview from an industry perspective. In Froth Flotation: A Century of Innovation; Fuerstenau, M.C., Jameson, G.J., Yoon, R., Eds.; Society of Mining, Metallurgy, and Exploration, Inc. (SME): Littleton, CO, USA, 2007; pp. 375–413. [Google Scholar]
- O’Connor, C.T.; Bradshaw, D.J.; Upton, A.E. The uses of dithiophosphates and dithiocarbonates for the flotation of arsenopyrite. Miner. Eng. 1990, 3, 447–459. [Google Scholar] [CrossRef]
- Pienaar, D.; Jordaan, T.; McFadzean, B.; O’Connor, C.T. The synergistic interaction between dithiophosphate collectors and frothers at the air-water and sulphide mineral interface. Miner. Eng. 2019, 138, 125–132. [Google Scholar] [CrossRef]
- Corin, K.C.; Bezuidenhout, J.C.; O’Connor, C.T. The role of dithiophosphate as co-collector in the flotation of platinum group mineral ore. Miner. Eng. 2012, 36–38, 100–104. [Google Scholar] [CrossRef]
- Hangone, G.; Bradshaw, D.J.; Ekmekci, Z. Flotation of copper sulphide ore from Okiep uisng thiol collectors and thier mixtures. S. Afr. Inst. Min. Metall. 2008, 105, 199–206. [Google Scholar]
- Maree, W.; Kloppers, L.; Hangone, G.; Oyekola, S. The effects of mixtures of potassium amyl xanthate (PAX) and isopropyl ethyl thionocarbamate (IPETC) collectors on grade and recovery in the froth flotation of a nickel sulfide ore. S. Afr. J. Chem. Eng. 2017, 24, 116–121. [Google Scholar] [CrossRef]
- McFadzean, B.; Mhlanga, S.S.; O’Connor, C.T. The effect of thiol collector mixtures on the flotation of pyrite and galena. Miner. Eng. 2013, 50–51, 121–129. [Google Scholar] [CrossRef]
- Corin, K.C.; Reddy, A.; Miyen, L.; Wiese, J.G.; Harris, P.J. The effect of ionic strength of plant water on valuable mineral and gangue recovery in a platinum bearing ore from Merensky reef. Miner. Eng. 2011, 24, 131–137. [Google Scholar] [CrossRef]
- Wiese, J.G. Investigating Depressant Behaviour in the Flotation of Selected Merensky Ores. Master’s Thesis, Faculty of Engineering and the Built Environment, Chemical Engineering, University of Cape Town, Cape Town, South Africa, 2009. [Google Scholar]
- Bournival, G.; Pugh, R.J.; Ata, S. Examination of NaCl and MIBC as bubble coalescence inhibitor in relation to froth flotation. Miner. Eng. 2012, 25, 47–53. [Google Scholar] [CrossRef]
- Craig, V.S.; Ninham, B.W.; Pashley, R.M. The effect of electrolytes on bubble coalescence in water. J. Phys. Chem. 1993, 97, 10192–10197. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. The effect of ionic strength of plant water on foam stability: A 2-phase flotation study. Miner. Eng. 2013, 40, 42–47. [Google Scholar] [CrossRef]
- Marrucci, G.; Nicodemo, L. Coalescence of gas bubbles in aqueous solutions of inorganic electrolytes. Chem. Eng. Sci. 1967, 22, 1257–1265. [Google Scholar] [CrossRef]
- Nagaraj, D.R. The chemistry and application of chelating or complexing agents in minerals separations. In Reagents in Mineral, Technology; Somasundaran, P., Moudgil, B.M., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1988; pp. 387–409. [Google Scholar]
- Bagci, E.; Ekmekci, Z.; Bradshaw, D. Adsorption behavior of xanthate and dithiophosphinate from thiol mixture on chalcopyrite. Miner. Eng. 2007, 20, 1047–1053. [Google Scholar] [CrossRef]
- Kloppers, L.; Maree, W.; Oyekola, O.; Hangone, G. Froth flotation of a Merensky Reef Platinum bearing ore using mixtures of SIBX with a dithiophosphate and a dithiocarbonate. Miner. Eng. 2016, 87, 54–58. [Google Scholar] [CrossRef]
- Becker, M.; Harris, P.J.; Wiese, J.G.; Bradshaw, D.J. Mineralogical characterisation of naturally floatable gangue in Merensky reef ore flotation. Int. J. Miner. Process. 2009, 93, 246–255. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. The effect of the ionic strength of process water on the interaction of talc and CMC: Implications of recirculated water on floatable gangue depression. Minerals 2019, 9, 231. [Google Scholar] [CrossRef]
| Water Type | Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | NO3− (mg/L) | CO32− (mg/L) | TDS (mg/L) | IS [M] | Class |
|---|---|---|---|---|---|---|---|---|---|---|
| 1SPW | 80 | 70 | 153 | 287 | 240 | 176 | 17 | 1023 | 0.0242 | Typical |
| 5SPW | 400 | 350 | 765 | 1435 | 1200 | 880 | 85 | 5115 | 0.1212 | Abnormal |
| Collector | Molar Ratios (%) | ||||
|---|---|---|---|---|---|
| SIBX:DTP | 100:0 | 90:10 | 50:50 | 10:90 | 0:100 |
| Collector Name | Abbreviation | Mr, g/mol | % Purity |
|---|---|---|---|
| Sodium iso-butyl xanthate | SIBX | 200.00 | 97 |
| Sodium di-ethyl dithiophosphate | SEDTP | 208.21 | 45 |
| Depressant | Mr, g/mol | Density, g/mL | % Purity | Degree of Substitution |
|---|---|---|---|---|
| CMC: depramin 267 | 325,000 | 1.43 | 72 | 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manono, M.S.; Matibidi, K.; Otunniyi, I.O.; Thubakgale, C.K.; Corin, K.C.; Wiese, J.G. The Behaviour of Mixtures of Sodium Iso-Butyl Xanthate and Sodium Di-Ethyl Dithiophosphate during the Flotation of a Cu-Ni-Pt Ore in Degrading Water Quality. Minerals 2020, 10, 123. https://doi.org/10.3390/min10020123
Manono MS, Matibidi K, Otunniyi IO, Thubakgale CK, Corin KC, Wiese JG. The Behaviour of Mixtures of Sodium Iso-Butyl Xanthate and Sodium Di-Ethyl Dithiophosphate during the Flotation of a Cu-Ni-Pt Ore in Degrading Water Quality. Minerals. 2020; 10(2):123. https://doi.org/10.3390/min10020123
Chicago/Turabian StyleManono, Malibongwe S., Katlego Matibidi, Iyiola O. Otunniyi, Catherine K. Thubakgale, Kirsten C. Corin, and Jenny G. Wiese. 2020. "The Behaviour of Mixtures of Sodium Iso-Butyl Xanthate and Sodium Di-Ethyl Dithiophosphate during the Flotation of a Cu-Ni-Pt Ore in Degrading Water Quality" Minerals 10, no. 2: 123. https://doi.org/10.3390/min10020123
APA StyleManono, M. S., Matibidi, K., Otunniyi, I. O., Thubakgale, C. K., Corin, K. C., & Wiese, J. G. (2020). The Behaviour of Mixtures of Sodium Iso-Butyl Xanthate and Sodium Di-Ethyl Dithiophosphate during the Flotation of a Cu-Ni-Pt Ore in Degrading Water Quality. Minerals, 10(2), 123. https://doi.org/10.3390/min10020123