Using Complementary Methods of Synchrotron Radiation Powder Diffraction and Pair Distribution Function to Refine Crystal Structures with High Quality Parameters—A Review
Abstract
1. Introduction
2. Basic Principles
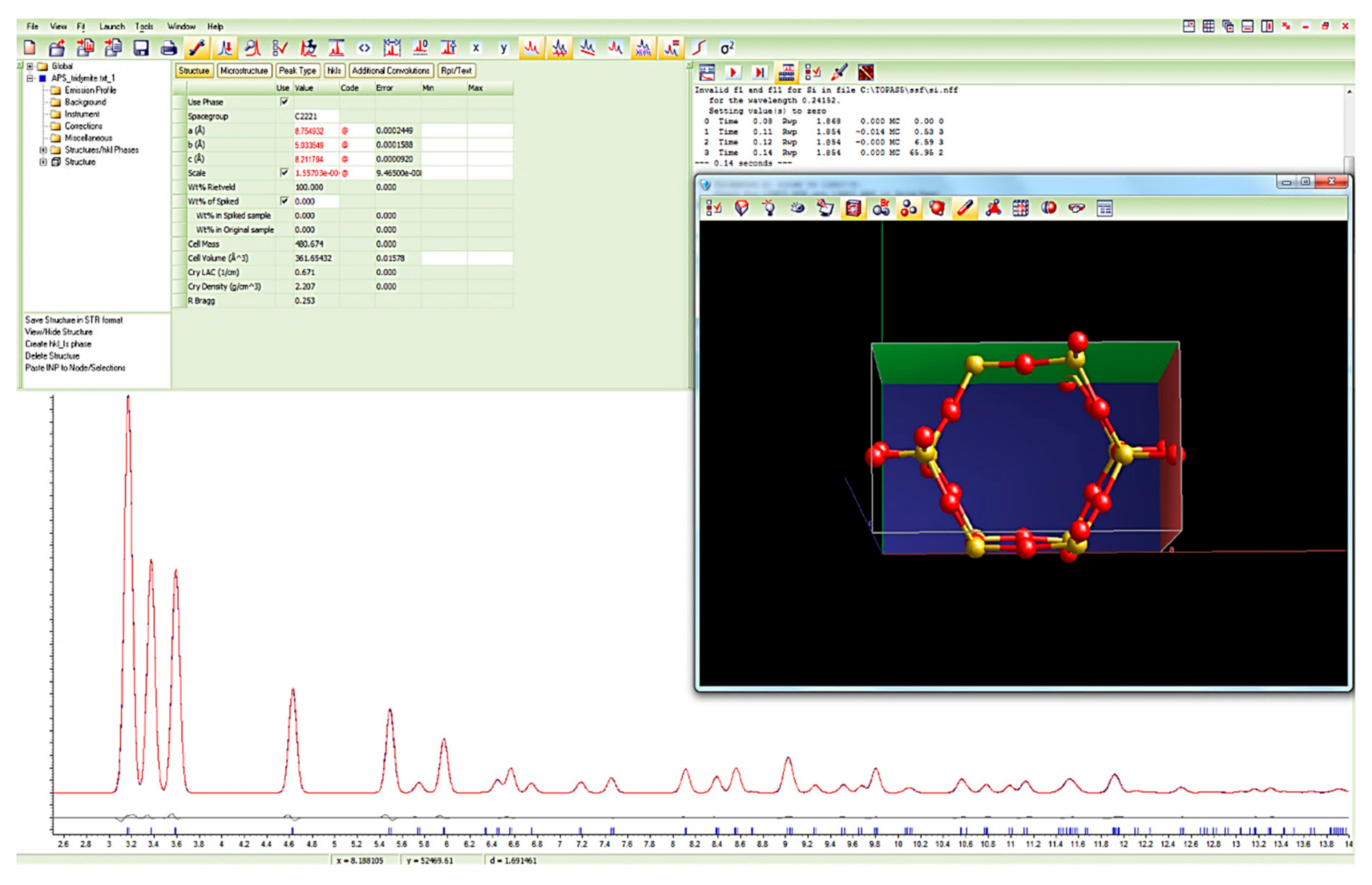
2.1. Rietveld Refinement of Powder XRD Data
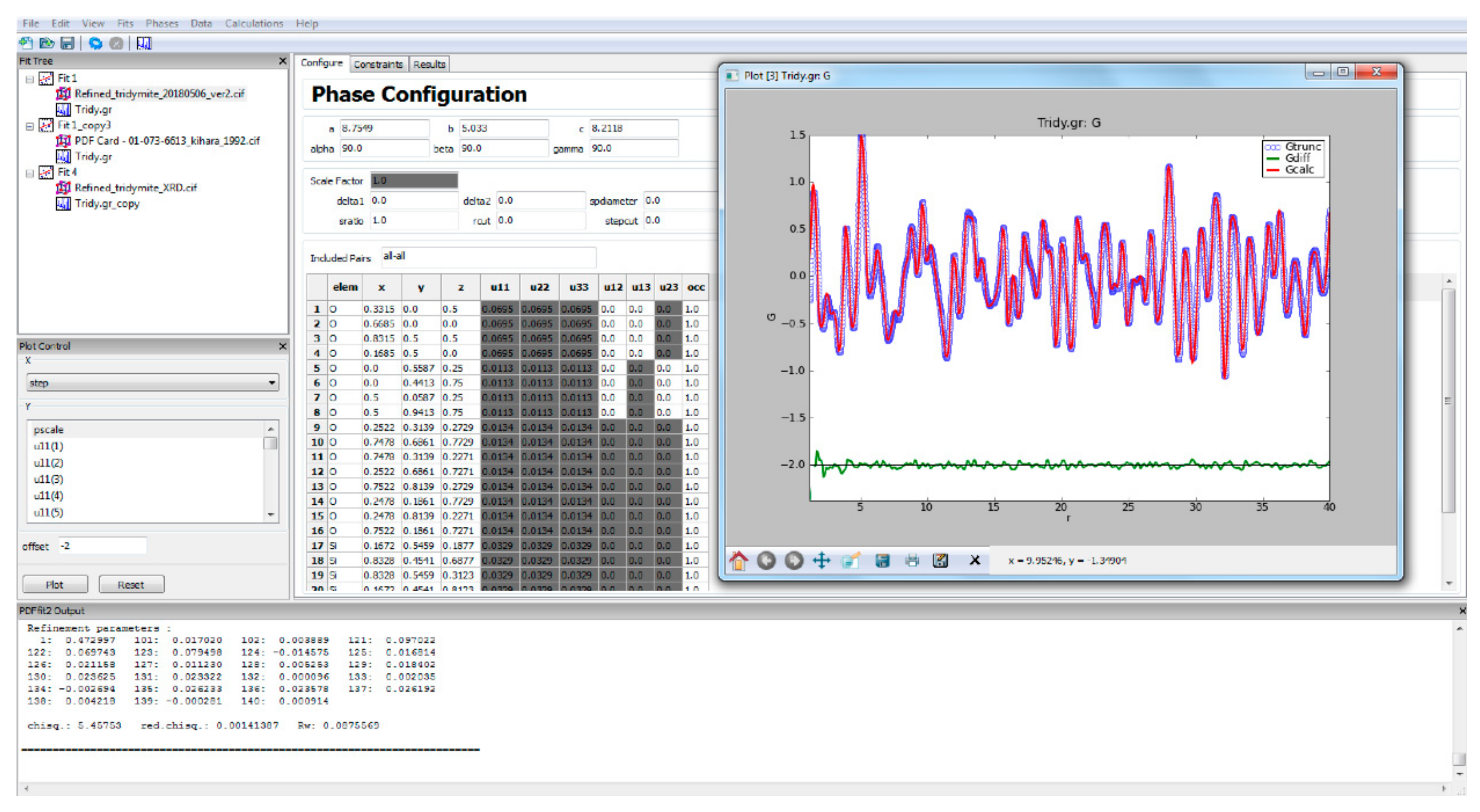
2.2. Pair Distribution Function
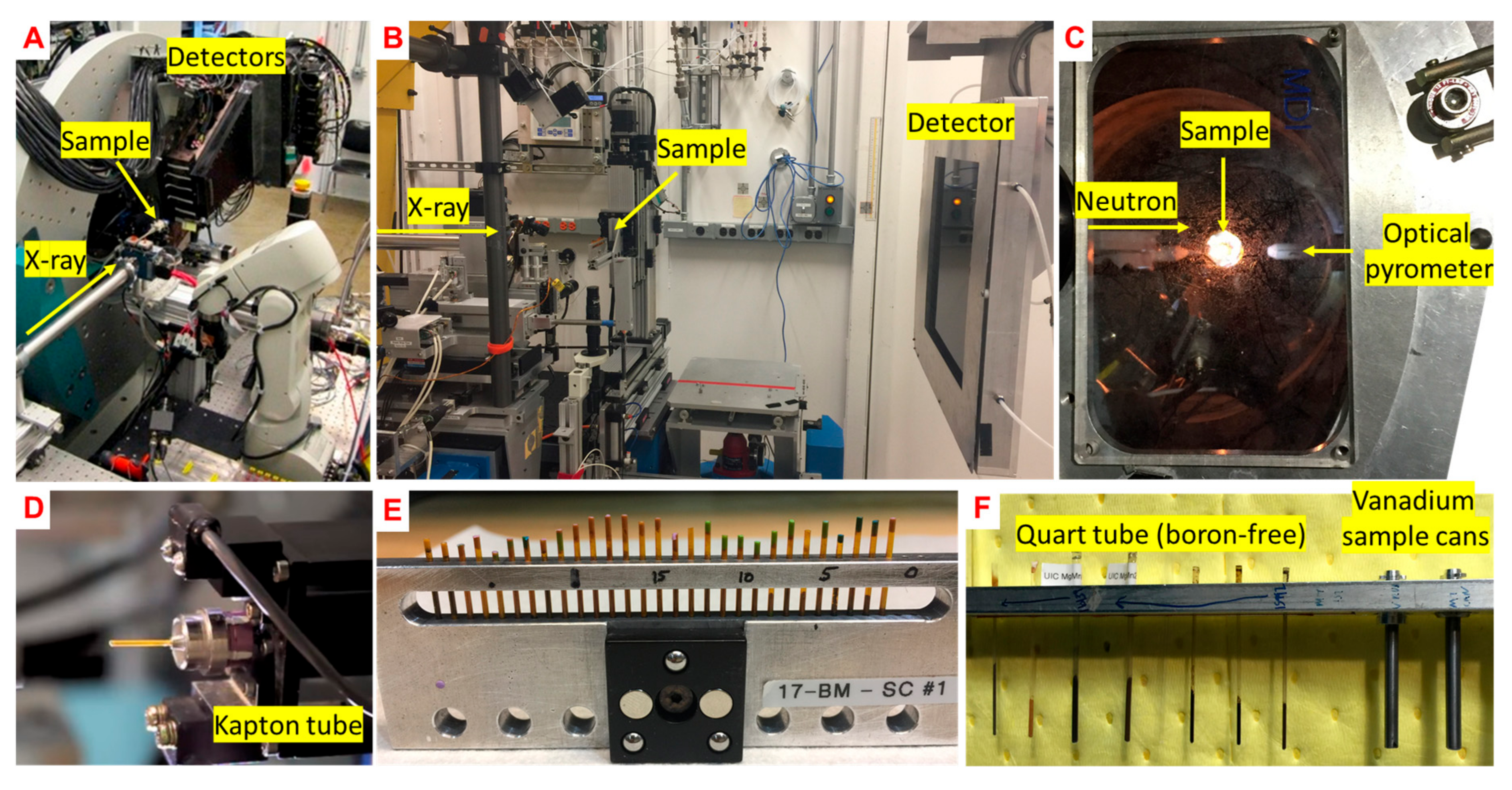
3. Experimental Method
4. Case Studies
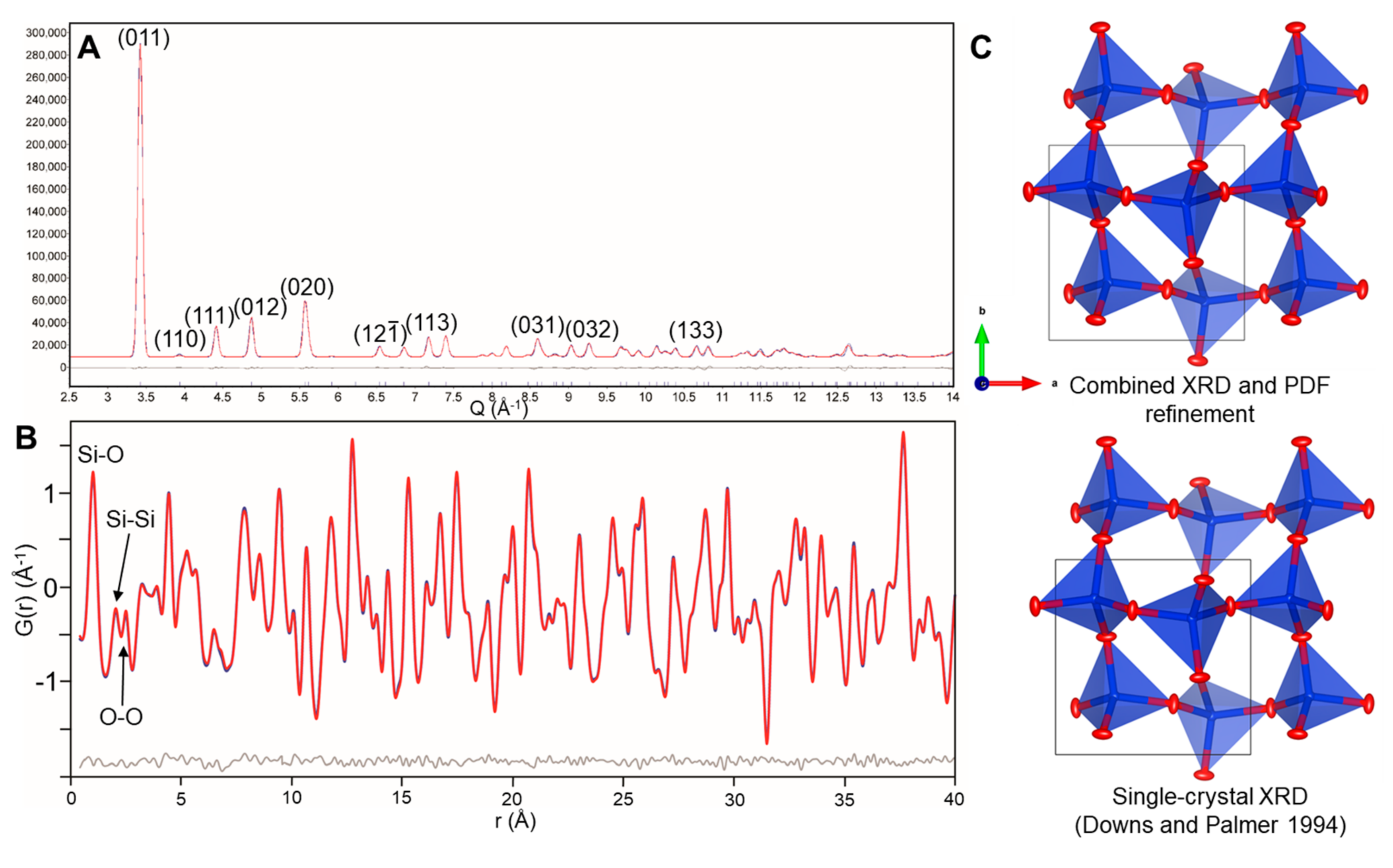
4.1. Low-Crystobalite
4.2. Kaolinite
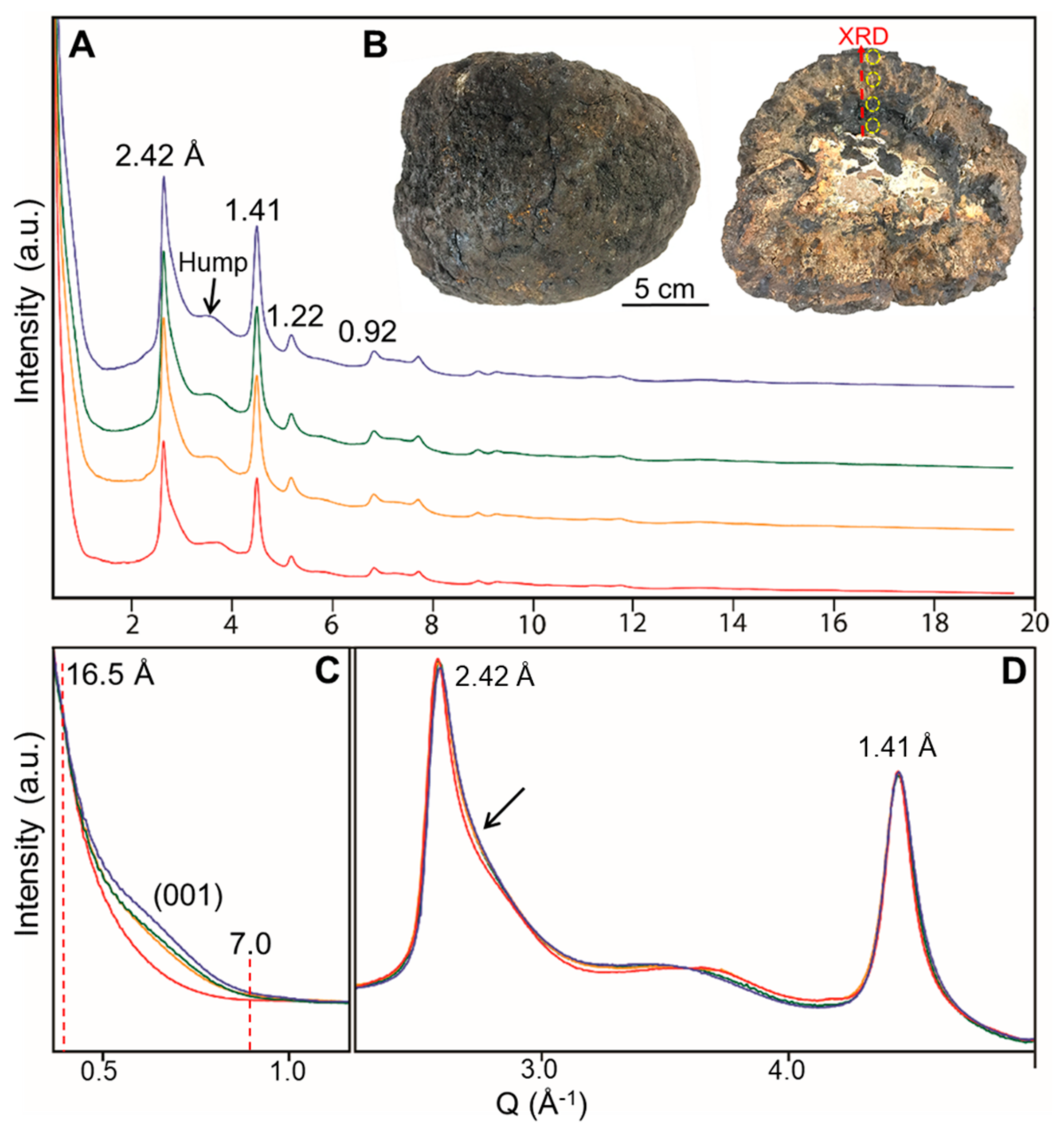
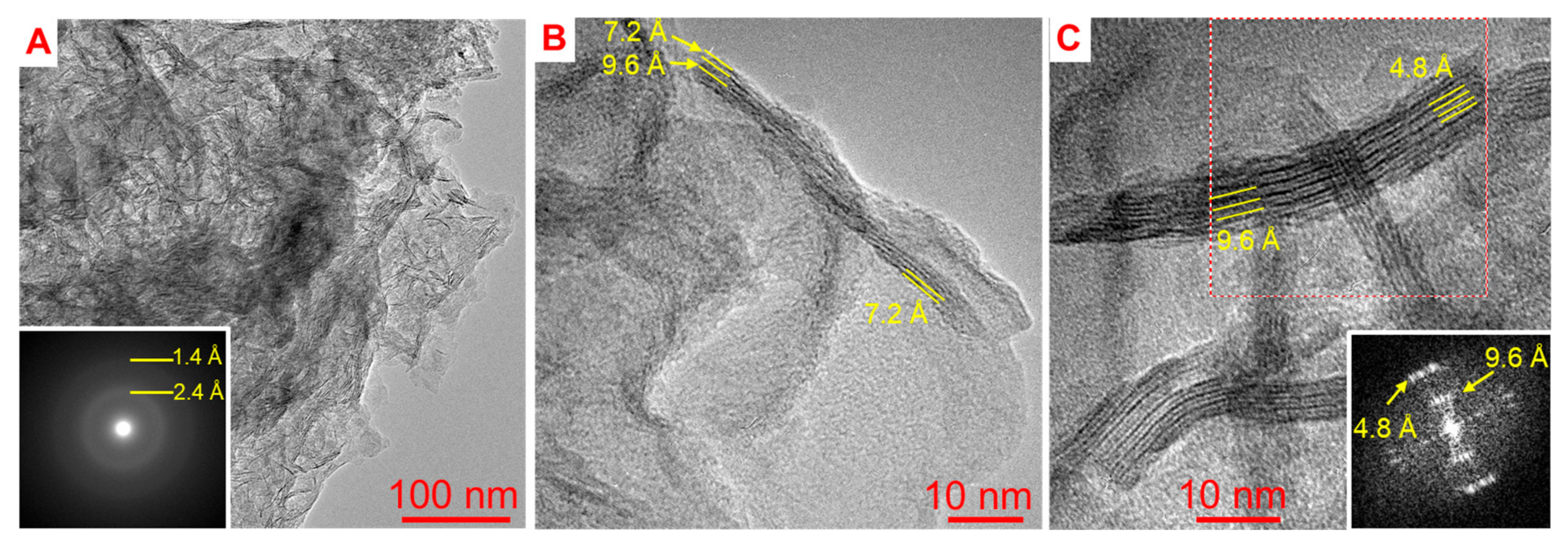
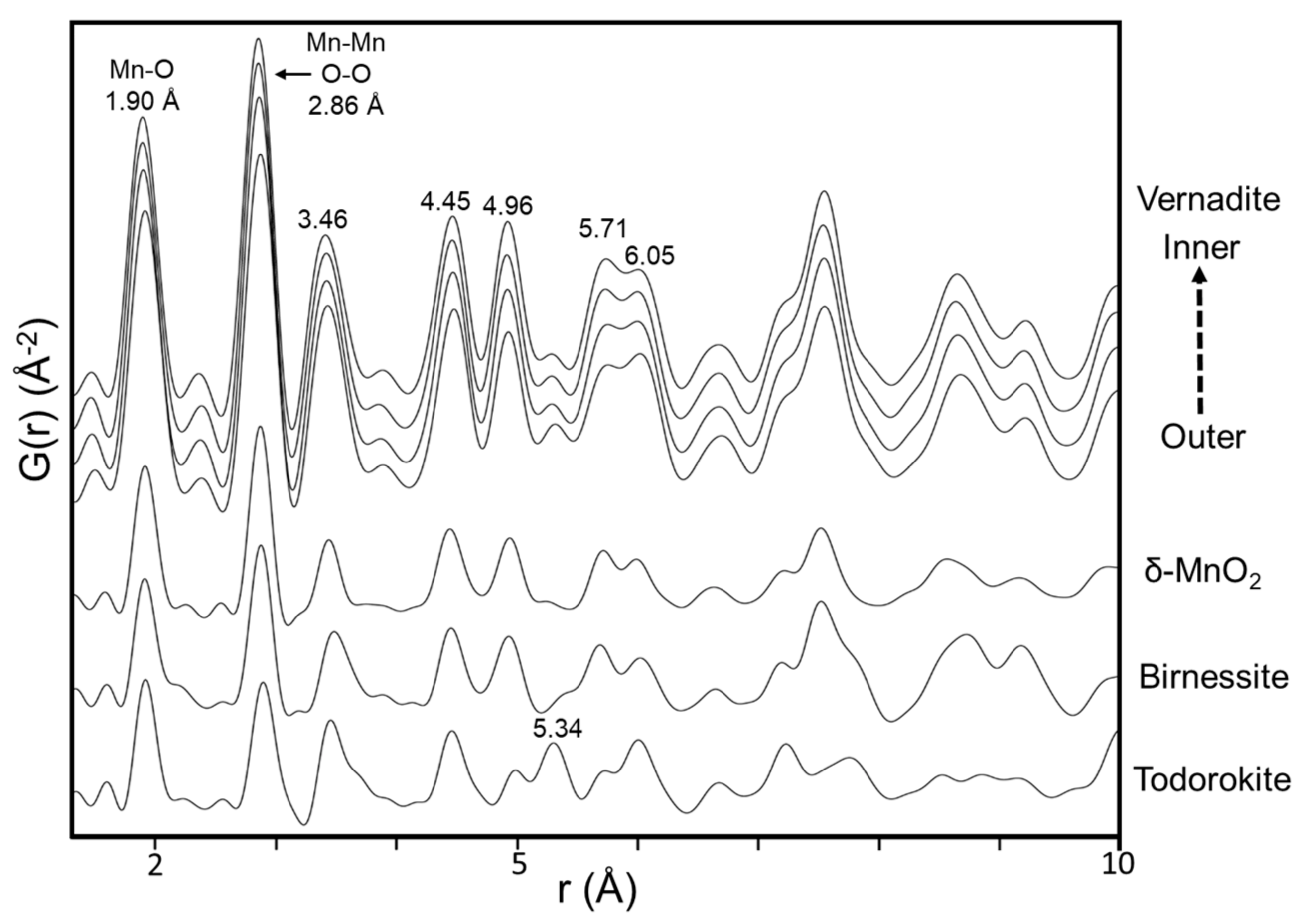
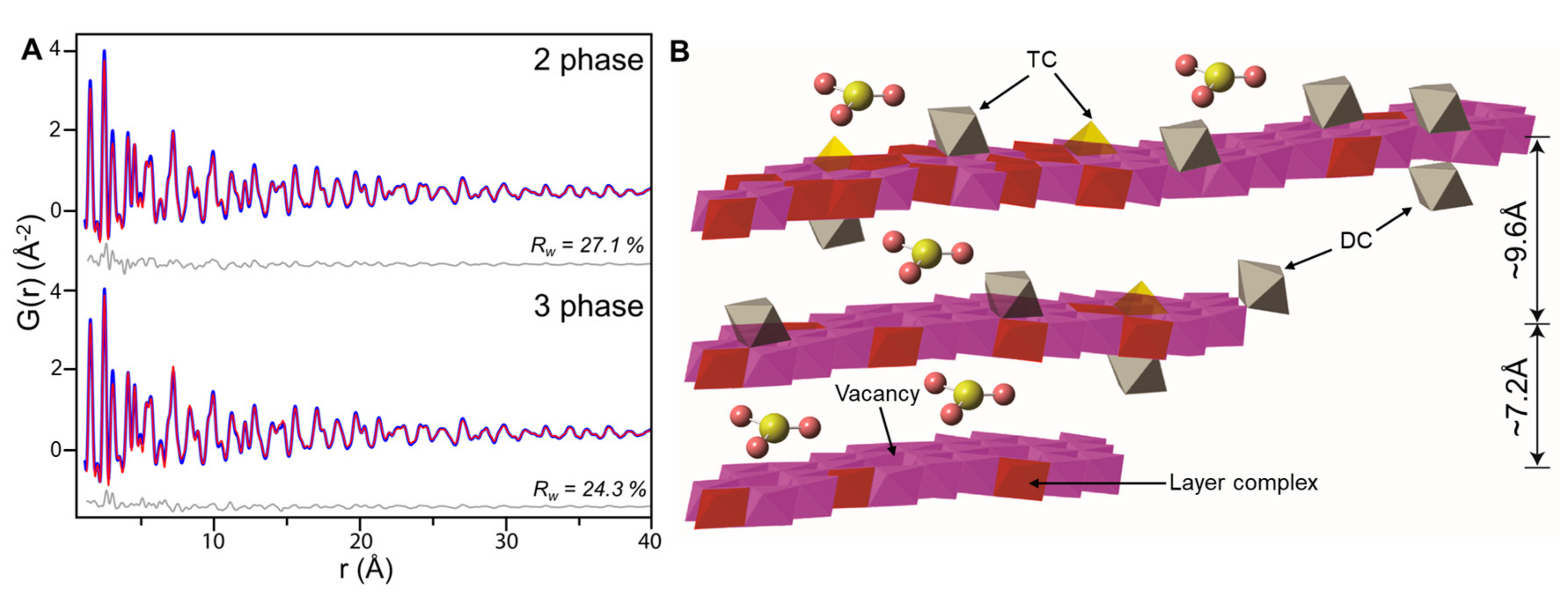
4.3. Vernadite
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, D.; Sali, A. Protein structure prediction and structural genomics. Science 2001, 294, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.-J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K. High-resolution crystal structure of an engineered human β2-adrenergic G protein–coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Merkulov, V.I.; Guillorn, M.A.; Lowndes, D.H.; Simpson, M.L.; Voelkl, E. Shaping carbon nanostructures by controlling the synthesis process. Appl. Phys. Lett. 2001, 79, 1178–1180. [Google Scholar] [CrossRef]
- Egami, T.; Billinge, S.J. Underneath the Bragg Peaks: Structural Analysis of Complex Materials; Pergamon: Oxford, UK, 2012; Volume 16, pp. 10–32. [Google Scholar]
- Jin, S.; Xu, H.; Lee, S.; Fu, P. Jinshajiangite: Structure, twinning and pseudosymmetry. Acta Cryst. B 2018, 74, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jin, S.; Noll, B.C. Incommensurate density modulation in a Na-rich plagioclase feldspar: Z-contrast imaging and single-crystal X-ray diffraction study. Acta Cryst. B 2016, 72, 904–915. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. Using powder XRD and pair distribution function to determine anisotropic atomic displacement parameters of orthorhombic tridymite and tetragonal cristobalite. Acta Cryst. B 2019, 75, 160–167. [Google Scholar] [CrossRef]
- Billinge, S.J.; Kanatzidis, M. Beyond crystallography: The study of disorder, nanocrystallinity and crystallographically challenged materials with pair distribution functions. Chem. Commun. 2004, 7, 749–760. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H.; Xu, W.; Sun, X. The structure and crystal chemistry of vernadite in ferromanganese crusts. Acta Cryst. B 2019, 75, 591–598. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H.; Xu, H.; Jacobs, R.; Morgan, D. Valleyite: A new magnetic mineral with the sodalite-type structure. Am. Mineral. 2019, 104, 1238–1245. [Google Scholar] [CrossRef]
- Post, J.; Bish, D. Rietveld refinement of crystal structures using powder X-ray diffraction data. In Modern Powder Diffraction; Mineralogical Society of America: Chantilly, VA, USA, 1989; Volume 20, pp. 277–308. [Google Scholar]
- Lee, S.; Xu, H. XRD and TEM studies on nanophase manganese oxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan. Clays Clay Miner. 2016, 64, 523–536. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.; Hastings, J. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. Powder XRD and TEM study on crystal structure and interstratification of Zn-chlorite (baileychlore). Powder Diffr. 2017, 32, 118–123. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. The crystal structure and Gibbs free energy of formation of chukanovite as an oxidation product of carbon steel in human liver. Chem. Geol. 2018, 488, 180–188. [Google Scholar] [CrossRef]
- O’Connor, B.H.; Raven, M.D. Application of the Rietveld refinement procedure in assaying powdered mixtures. Powder Diffr. 1988, 3, 2–6. [Google Scholar] [CrossRef]
- Enderle, R.; Götz-Neunhoeffer, F.; Göbbels, M.; Müller, F.; Greil, P. Influence of magnesium doping on the phase transformation temperature of β-TCP ceramics examined by Rietveld refinement. Biomaterials 2005, 26, 3379–3384. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Venturelli, P. In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. Am. Mineral. 1999, 84, 895–904. [Google Scholar] [CrossRef]
- Nelmes, R.; McMahon, M. High-pressure powder diffraction on synchrotron sources. J. Synchrotron Radiat. 1994, 1, 69–73. [Google Scholar] [CrossRef]
- Wenk, H.-R.; Lutterotti, L.; Kaercher, P.; Kanitpanyacharoen, W.; Miyagi, L.; Vasin, R. Rietveld texture analysis from synchrotron diffraction images. II. Complex multiphase materials and diamond anvil cell experiments. Powder Diffr. 2014, 29, 220–232. [Google Scholar] [CrossRef]
- Lutterotti, L.; Vasin, R.; Wenk, H.-R. Rietveld texture analysis from synchrotron diffraction images. I. Calibration and basic analysis. Powder Diffr. 2014, 29, 76–84. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Antonio, S.G.; Rosa, P.C.P.; de Oliveira Paiva-Santos, C. Crystal structure determination of mebendazole form A using high-resolution synchrotron x-ray powder diffraction data. J. Pharm. Sci. 2010, 99, 1734–1744. [Google Scholar] [CrossRef]
- Bhakar, A.; Pandey, A.H.; Singh, M.; Upadhyay, A.; Sinha, A.; Gupta, S.; Ganguli, T. Structural analysis of lead magnesium niobate using synchrotron powder X-ray diffraction and the Rietveld method. Acta Cryst. B 2016, 72, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lee, S.; Xu, H. Luogufengite: A new nano-mineral of Fe2O3 polymorph with giant coercive field. Am. Mineral. 2017, 102, 711–719. [Google Scholar] [CrossRef]
- Chupas, P.J.; Qiu, X.; Hanson, J.C.; Lee, P.L.; Grey, C.P.; Billinge, S.J. Rapid-acquisition pair distribution function (RA-PDF) analysis. J. Appl. Crystallogr. 2003, 36, 1342–1347. [Google Scholar] [CrossRef]
- Toby, B.; Egami, T. Accuracy of pair distribution function analysis applied to crystalline and non-crystalline materials. Acta Cryst. A 1992, 48, 336–346. [Google Scholar] [CrossRef]
- Billinge, S.J. The rise of the X-ray atomic pair distribution function method: A series of fortunate events. Philos. Trans. R. Soc. A 2019, 377, 20180413. [Google Scholar] [CrossRef]
- Soper, A.K.; Barney, E.R. Extracting the pair distribution function from white-beam X-ray total scattering data. J. Appl. Crystallogr. 2011, 44, 714–726. [Google Scholar] [CrossRef]
- Davis, T.; Johnson, M.; Billinge, S.J. Toward Phase Quantification at the Nanoscale Using the Total Scattering Pair Distribution Function (TSPDF) Method: Recrystallization of Cryomilled Sulfamerazine. Cryst. Growth Des. 2013, 13, 4239–4244. [Google Scholar] [CrossRef]
- Nilsson, A.; Pettersson, L.G. The structural origin of anomalous properties of liquid water. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Billinge, S.J.; Levin, I. The problem with determining atomic structure at the nanoscale. Science 2007, 316, 561–565. [Google Scholar] [CrossRef]
- Farrow, C.; Juhas, P.; Liu, J.; Bryndin, D.; Božin, E.; Bloch, J.; Proffen, T.; Billinge, S. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Condens. Matter Phys. 2007, 19, 335219. [Google Scholar] [CrossRef]
- McCusker, L.; Von Dreele, R.; Cox, D.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Merritt, E.A. Expanding the model: Anisotropic displacement parameters in protein structure refinement. Acta Cryst. D 1999, 55, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, R.E.; Goossens, D.J.; Welberry, T.R. Total scattering and pair distribution function analysis in modelling disorder in PZN (PbZn1/3Nb2/3O3). IUCrJ 2016, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Ida, T.; Ando, M.; Toraya, H. Extended pseudo-Voigt function for approximating the Voigt profile. J. Appl. Crystallogr. 2000, 33, 1311–1316. [Google Scholar] [CrossRef]
- Farrow, C.L.; Billinge, S.J. Relationship between the atomic pair distribution function and small-angle scattering: Implications for modeling of nanoparticles. Acta Cryst. A 2009, 65, 232–239. [Google Scholar] [CrossRef]
- Juhás, P.; Farrow, C.L.; Yang, X.; Knox, K.R.; Billinge, S.J. Complex modeling: A strategy and software program for combining multiple information sources to solve ill posed structure and nanostructure inverse problems. Acta Cryst. A 2015, 71, 562–568. [Google Scholar] [CrossRef]
- Wang, J.; Toby, B.H.; Lee, P.L.; Ribaud, L.; Antao, S.M.; Kurtz, C.; Ramanathan, M.; Von Dreele, R.B.; Beno, M.A. A dedicated powder diffraction beamline at the advanced photon source: Commissioning and early operational results. Rev. Sci. Instrum. 2008, 79, 085105. [Google Scholar] [CrossRef]
- Neuefeind, J.; Feygenson, M.; Carruth, J.; Hoffmann, R.; Chipley, K.K. The nanoscale ordered materials diffractometer NOMAD at the spallation neutron source SNS. Nucl. Instrum. Methods Phys. Res. 2012, 287, 68–75. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Juhás, P.; Davis, T.; Farrow, C.L.; Billinge, S.J. PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013, 46, 560–566. [Google Scholar] [CrossRef]
- Peterson, P.; Gutmann, M.; Proffen, T.; Billinge, S. PDFgetN: A user-friendly program to extract the total scattering structure factor and the pair distribution function from neutron powder diffraction data. J. Appl. Crystallogr. 2000, 33, 1192. [Google Scholar] [CrossRef]
- Downs, R.T.; Palmer, D.C. The pressure behavior of α cristobalite. Am. Mineral. 1994, 79, 9–14. [Google Scholar]
- Pluth, J.; Smith, J.; Faber, J., Jr. Crystal structure of low cristobalite at 10, 293, and 473 K: Variation of framework geometry with temperature. J. Appl. Phys. 1985, 57, 1045–1049. [Google Scholar] [CrossRef]
- Saikia, N.; Bharali, D.; Sengupta, P.; Bordoloi, D.; Goswamee, R.; Saikia, P.; Borthakur, P. Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Appl. Clay Sci. 2003, 24, 93–103. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B. The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J. Hazard. Mater. 2006, 134, 130–139. [Google Scholar] [CrossRef]
- King, R. Kaolinite. Geol. Today 2009, 25, 75–78. [Google Scholar] [CrossRef]
- Bish, D.L. Rietveld refinement of the kaolinite structure at 1.5 K. Clays Clay Miner. 1993, 41, 738–744. [Google Scholar] [CrossRef]
- Bish, D.L.; Von Dreele, R.B. Rietveld refinement of non-hydrogen atomic positions in kaolinite. Clays Clay Miner. 1989, 37, 289–296. [Google Scholar] [CrossRef]
- Hochella Jr, M.F.; Kasama, T.; Putnis, A.; Putnis, C.V.; Moore, J.N. Environmentally important, poorly crystalline Fe/Mn hydrous oxides: Ferrihydrite and a possibly new vernadite-like mineral from the Clark Fork River Superfund Complex. Am. Mineral. 2005, 90, 718–724. [Google Scholar] [CrossRef]
- Lanson, B.; Marcus, M.A.; Fakra, S.; Panfili, F.; Geoffroy, N.; Manceau, A. Formation of Zn–Ca phyllomanganate nanoparticles in grass roots. Geochim. Cosmochim. Acta 2008, 72, 2478–2490. [Google Scholar] [CrossRef][Green Version]
- Bargar, J.R.; Fuller, C.C.; Marcus, M.A.; Brearley, A.J.; De la Rosa, M.P.; Webb, S.M.; Caldwell, W.A. Structural characterization of terrestrial microbial Mn oxides from Pinal Creek, AZ. Geochim. Cosmochim. Acta 2009, 73, 889–910. [Google Scholar] [CrossRef]
- Bodeï, S.; Manceau, A.; Geoffroy, N.; Baronnet, A.; Buatier, M. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments. Geochim. Cosmochim. Acta 2007, 71, 5698–5716. [Google Scholar] [CrossRef]
- Ostwald, J. Ferruginous vernadite in an Indian Ocean ferromanganese nodule. Geol. Mag. 1984, 121, 483–488. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Geoffroy, N. Natural speciation of Ni, Zn, Ba, and As in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction. Geochim. Cosmochim. Acta 2007, 71, 95–128. [Google Scholar] [CrossRef]
- Marcus, M.A.; Manceau, A.; Kersten, M. Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule1. Geochim. Cosmochim. Acta 2004, 68, 3125–3136. [Google Scholar] [CrossRef]
- Palumbo, B.; Bellanca, A.; Neri, R.; Roe, M. Trace metal partitioning in Fe–Mn nodules from Sicilian soils, Italy. Chem. Geol. 2001, 173, 257–269. [Google Scholar] [CrossRef]
- Xue, T.; Xiaoming, S.; Gaowen, H.; Shengwei, W.; Hongfeng, L.; Mei, Z. Geochemistry of PGE and Au in ferromanganese crusts from seamounts in the west Pacific Ocean. In Mineral Deposit Research: Meeting the Global Challenge; Springer: Berlin, Germany, 2005; pp. 207–209. [Google Scholar]
- Simonin, L.; Colin, J.-F.; Ranieri, V.; Canévet, E.; Martin, J.-F.; Bourbon, C.; Baehtz, C.; Strobel, P.; Daniel, L.; Patoux, S. In situ investigations of a Li-rich Mn–Ni layered oxide for Li-ion batteries. J. Mater. Chem. 2012, 22, 11316–11322. [Google Scholar] [CrossRef]
- Lafferty, B.J.; Ginder-Vogel, M.; Zhu, M.; Livi, K.J.; Sparks, D.L. Arsenite oxidation by a poorly crystalline manganese-oxide. 2. Results from X-ray absorption spectroscopy and X-ray diffraction. Environ. Sci. Technol. 2010, 44, 8467–8472. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Progress in Solid State Chemistry 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Zaharieva, I.; Chernev, P.; Risch, M.; Klingan, K.; Kohlhoff, M.; Fischer, A.; Dau, H. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Environ. Sci. Technol. 2012, 5, 7081–7089. [Google Scholar] [CrossRef]
- Zhu, M.; Farrow, C.L.; Post, J.E.; Livi, K.J.; Billinge, S.J.; Ginder-Vogel, M.; Sparks, D.L. Structural study of biotic and abiotic poorly-crystalline manganese oxides using atomic pair distribution function analysis. Geochim. Cosmochim. Acta 2012, 81, 39–55. [Google Scholar] [CrossRef]
- Manceau, A.; Marcus, M.A.; Grangeon, S.; Lanson, M.; Lanson, B.; Gaillot, A.-C.; Skanthakumar, S.; Soderholm, L. Short-range and long-range order of phyllomanganate nanoparticles determined using high-energy X-ray scattering. J. Appl. Crystallogr. 2013, 46, 193–209. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. Size-dependent phase map and phase transformation kinetics for nanometric iron (III) oxides (γ → ε → α pathway). J. Phys. Chem. C 2016, 120, 13316–13322. [Google Scholar] [CrossRef]
- Hill, T.R.; Konishi, H.; Hobbs, F.; Lee, S.; Xu, H. Precipitates of α-cristobalite and silicate glass in UHP clinopyroxene from a Bohemian Massif eclogite. Am. Mineral. 2019, 104, 1402–1415. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H. The role of ε-Fe2O3 nano-mineral and domains in enhancing magnetic coercivity: Implications for the natural remanent magnetization. Minerals 2018, 8, 97. [Google Scholar] [CrossRef]
- Drits, V.A.; Lanson, B.; Gaillot, A.-C. Birnessite polytype systematics and identification by powder X-ray diffraction. Am. Mineral. 2007, 92, 771–788. [Google Scholar] [CrossRef]
- Grangeon, S.; Fernandez-Martinez, A.; Claret, F.; Marty, N.; Tournassat, C.; Warmont, F.; Gloter, A. In-situ determination of the kinetics and mechanisms of nickel adsorption by nanocrystalline vernadite. Chem. Geol. 2017, 459, 24–31. [Google Scholar] [CrossRef]
- Grangeon, S.; Fernandez-Martinez, A.; Warmont, F.; Gloter, A.; Marty, N.; Poulain, A.; Lanson, B. Cryptomelane formation from nanocrystalline vernadite precursor: A high energy X-ray scattering and transmission electron microscopy perspective on reaction mechanisms. Geochem. Trans. 2015, 16, 1–16. [Google Scholar] [CrossRef]
- Lee, S.; Shen, Z.; Xu, H. Study on nanophase iron oxyhydroxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan, with implications for the adsorption of As and heavy metals. Am. Mineral. 2016, 101, 1986–1995. [Google Scholar] [CrossRef]
- Yang, P.; Lee, S.; Post, J.E.; Xu, H.; Wang, Q.; Xu, W.; Zhu, M. Trivalent manganese on vacancies triggers rapid transformation of layered to tunneled manganese oxides (TMOs): Implications for occurrence of TMOs in low-temperature environment. Geochim. Cosmochim. Acta 2018, 240, 173–190. [Google Scholar] [CrossRef]
- Tomaszewski, E.J.; Lee, S.; Rudolph, J.; Xu, H.; Ginder-Vogel, M. The reactivity of Fe (II) associated with goethite formed during short redox cycles toward Cr (VI) reduction under oxic conditions. Chem. Geol. 2017, 464, 101–109. [Google Scholar] [CrossRef]

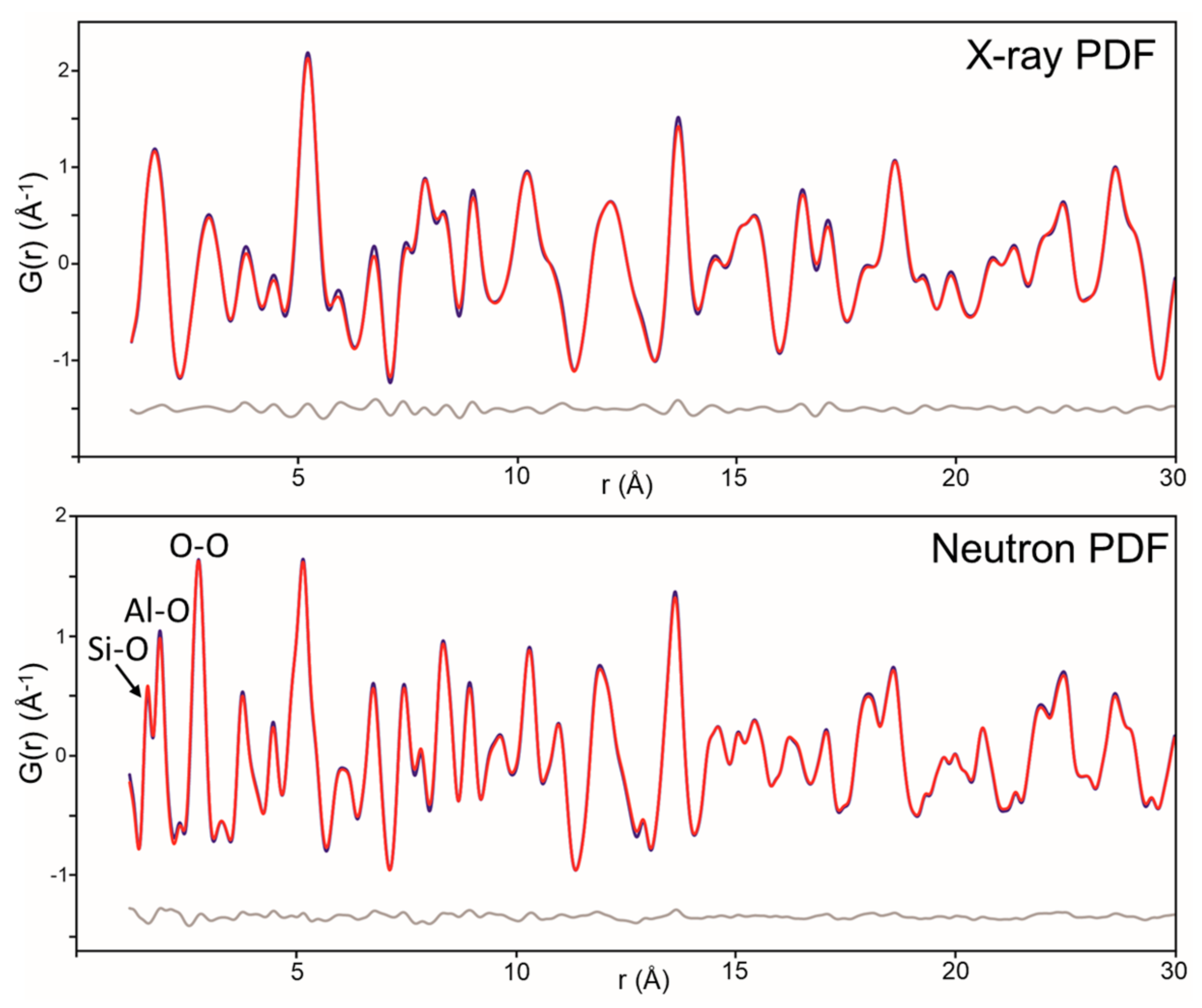
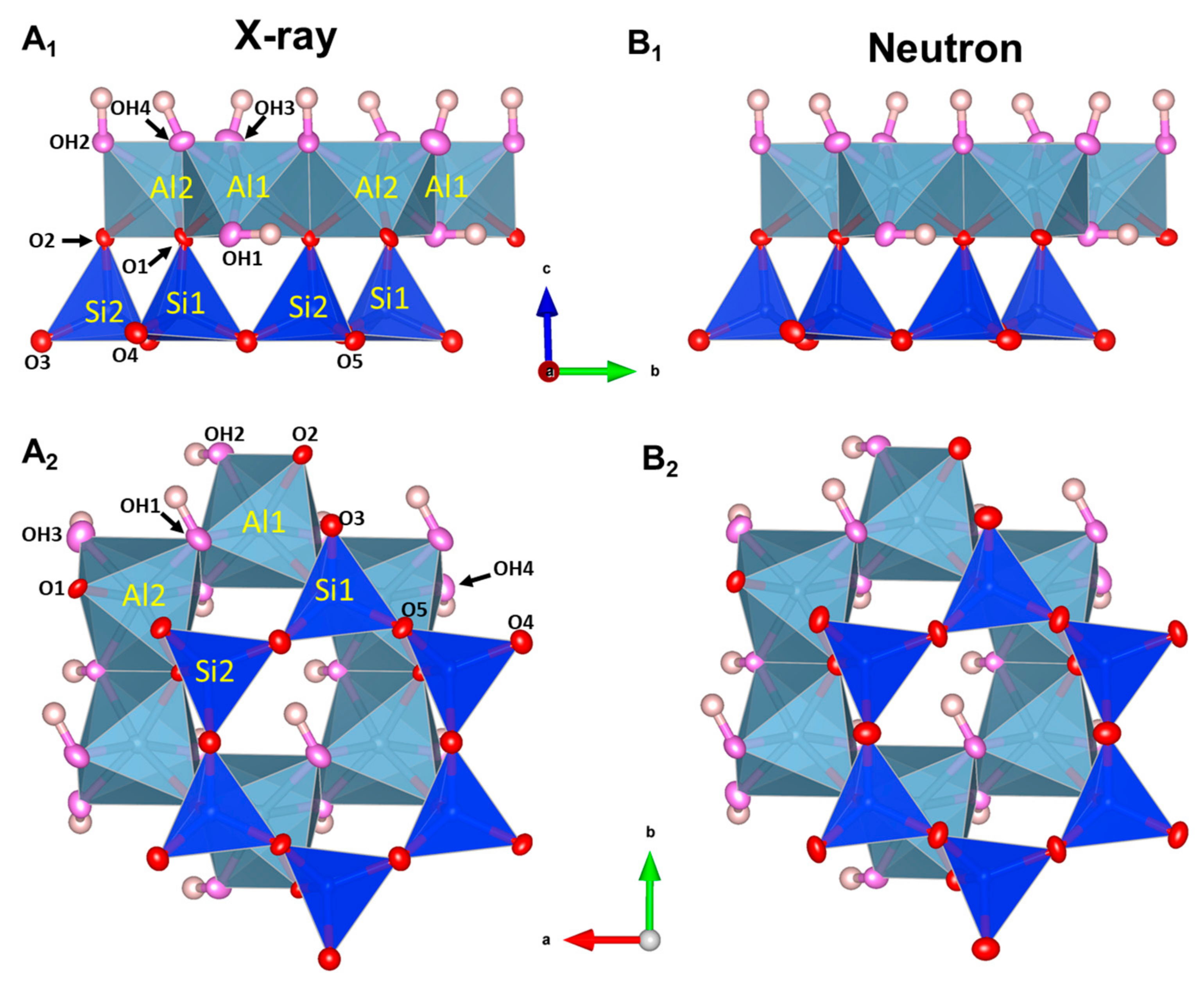
| (This Study) | (This Study) | Bish 1988 [52] | Bish 1993 [51] | |
|---|---|---|---|---|
| Location | Arkansas | Arkansas | Keokuk | Keokuk |
| Source | X-ray | Neutron | X-ray | Neutron |
| Temp | 298 K | 298 K | 298 K | 1.5 K |
| a (Å) | 5.1546(9) | 5.1528(6) | 5.1554(1) | 5.1535(3) |
| b | 8.9425(11) | 8.9415(7) | 8.9448(2) | 8.9419(5) |
| c | 7.4033(15) | 7.3985(9) | 7.4048(2) | 7.3906(4) |
| α (°) | 91.704(8) | 91.715(7) | 91.700(2) | 91.926(2) |
| β | 104.735(13) | 104.756(6) | 104.862(1) | 105.046(2) |
| γ | 89.852(9) | 89.866(7) | 89.822(1) | 89.797(2) |
| Volume | 164.94(12) | 164.74(8) | 164.94(2) | 164.35(5) |
| Si(1)–O(1) | 1.617(5) | 1.618(5) | 1.626(5) | 1.618(4) |
| –O(3) | 1.623(4) | 1.614(4) | 1.609(5) | 1.611(4) |
| –O(4) | 1.620(5) | 1.619(5) | 1.628(5) | 1.620(4) |
| –O(5) | 1.615(4) | 1.617(4) | 1.617(5) | 1.619(4) |
| Avg. | 1.619(5) | 1.617(4) | 1.620(5) | 1.617(4) |
| Si(2)–O(2) | 1.612(4) | 1.613(4) | 1.630(5) | 1.612(4) |
| –O(3) | 1.627(4) | 1.620(4) | 1.614(5) | 1.617(4) |
| –O(4) | 1.616(4) | 1.616(4) | 1.597(6) | 1.616(4) |
| –O(5) | 1.615(5) | 1.611(5) | 1.611 (5) | 1.608(4) |
| Avg. | 1.618(4) | 1.615(4) | 1.613(5) | 1.613(4) |
| Al(1)–O(1) | 1.947(4) | 1.955(5) | 1.930(6) | 1.927(6) |
| –O(2) | 1.987(5) | 1.983(6) | 1.965(5) | 1.930(6 |
| –OH(1) | 1.921(6) | 1.909(6) | 1.932(6) | 1.913(6) |
| –OH(2) | 1.853(7) | 1.852(7) | 1.880(6) | 1.890(6) |
| –OH(3) | 1.855(6) | 1.859(6) | 1.892(6) | 1.865(6) |
| –OH(4) | 1.859(6) | 1.872(6) | 1.868(6) | 1.915(6) |
| Avg. | 1.904(6) | 1.905(6) | 1.911(6) | 1.907(6) |
| Al(2)–O(1) | 1.981(5) | 1.976(5) | 1.969(5) | 1.931(6) |
| –O(2) | 1.945(5) | 1.942(5) | 1.936(5) | 1.919(6) |
| –OH(1) | 1.918(6) | 1.912(6) | 1.920(6) | 1.912(6) |
| –OH(2) | 1.886(7) | 1.875(7) | 1.884(6) | 1.896(6) |
| –OH(3) | 1.867(6) | 1.865(6) | 1.900(6) | 1.886(6) |
| –OH(4) | 1.855(6) | 1.869(6) | 1.898(6) | 1.910(6) |
| Avg. | 1.909(6) | 1.907(6) | 1.918(6) | 1.909(6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Xu, H. Using Complementary Methods of Synchrotron Radiation Powder Diffraction and Pair Distribution Function to Refine Crystal Structures with High Quality Parameters—A Review. Minerals 2020, 10, 124. https://doi.org/10.3390/min10020124
Lee S, Xu H. Using Complementary Methods of Synchrotron Radiation Powder Diffraction and Pair Distribution Function to Refine Crystal Structures with High Quality Parameters—A Review. Minerals. 2020; 10(2):124. https://doi.org/10.3390/min10020124
Chicago/Turabian StyleLee, Seungyeol, and Huifang Xu. 2020. "Using Complementary Methods of Synchrotron Radiation Powder Diffraction and Pair Distribution Function to Refine Crystal Structures with High Quality Parameters—A Review" Minerals 10, no. 2: 124. https://doi.org/10.3390/min10020124
APA StyleLee, S., & Xu, H. (2020). Using Complementary Methods of Synchrotron Radiation Powder Diffraction and Pair Distribution Function to Refine Crystal Structures with High Quality Parameters—A Review. Minerals, 10(2), 124. https://doi.org/10.3390/min10020124






