Co-Disposal of Coal Gangue and Red Mud for Prevention of Acid Mine Drainage Generation from Self-Heating Gangue Dumps
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
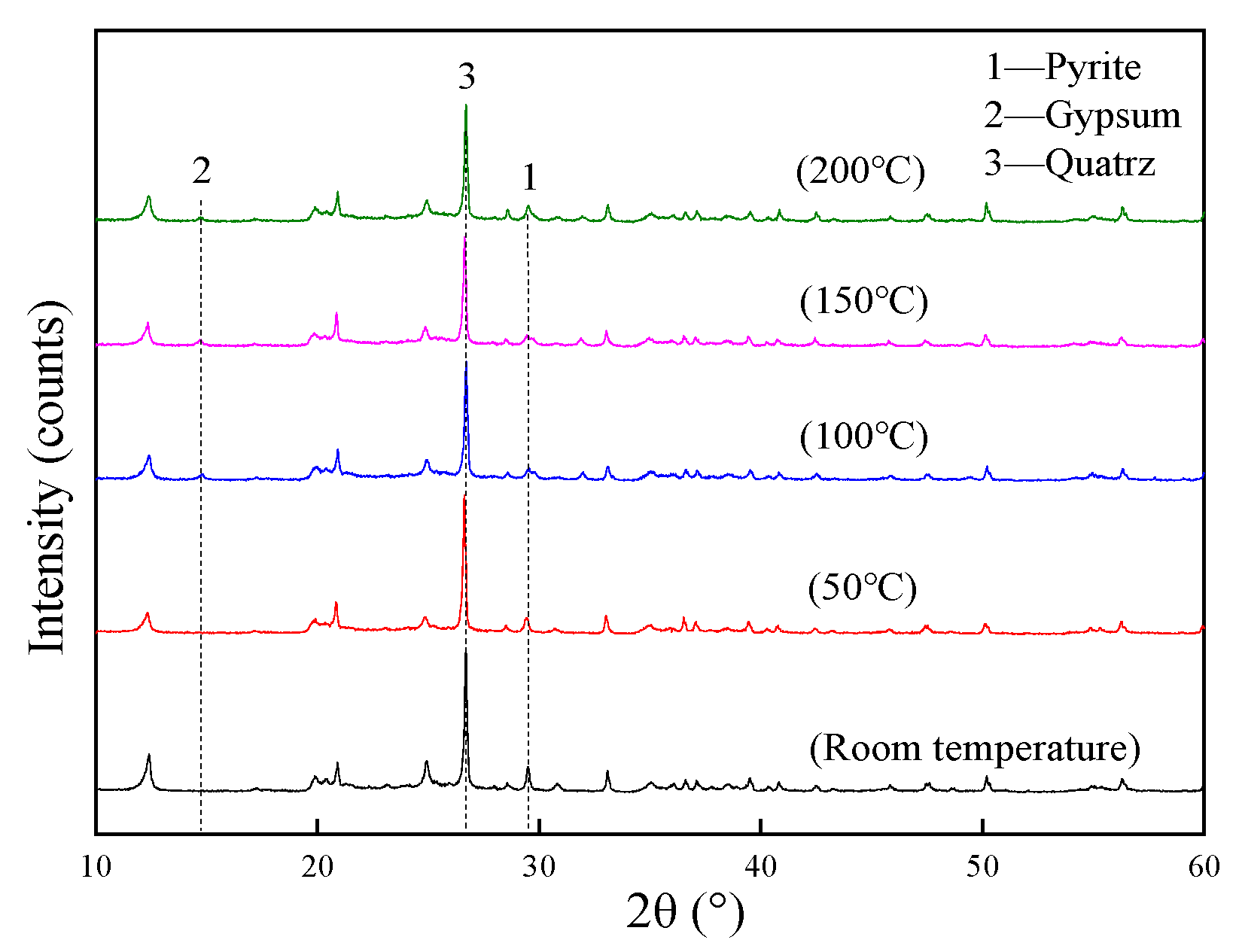
2.2. Mineralogical Determination
2.3. Leaching Experimental Device
2.4. Dynamic Heating and Leaching
3. Results and Discussion
3.1. Properties of Materials
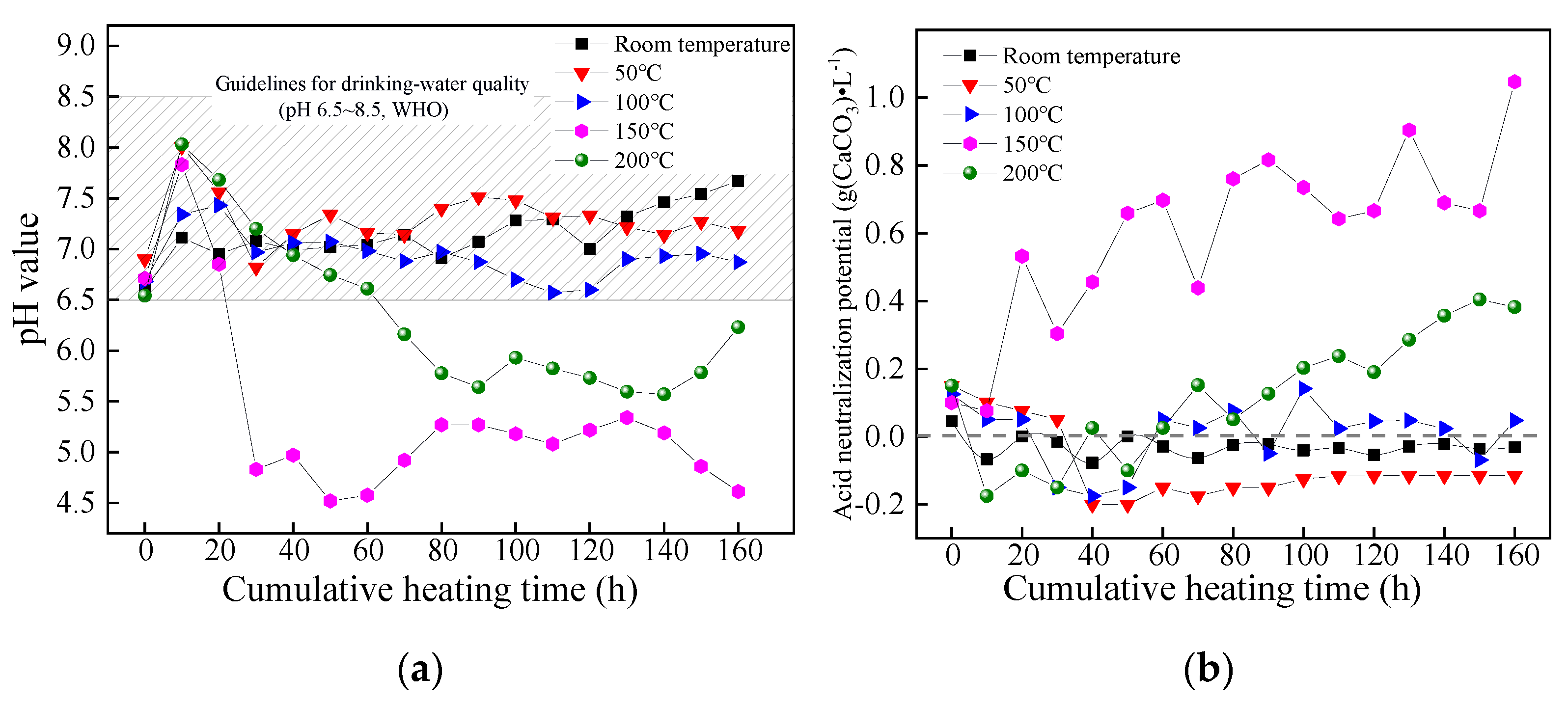
3.2. Release Characteristics of Acidic Contamination
3.3. Co-Disposal Prevention
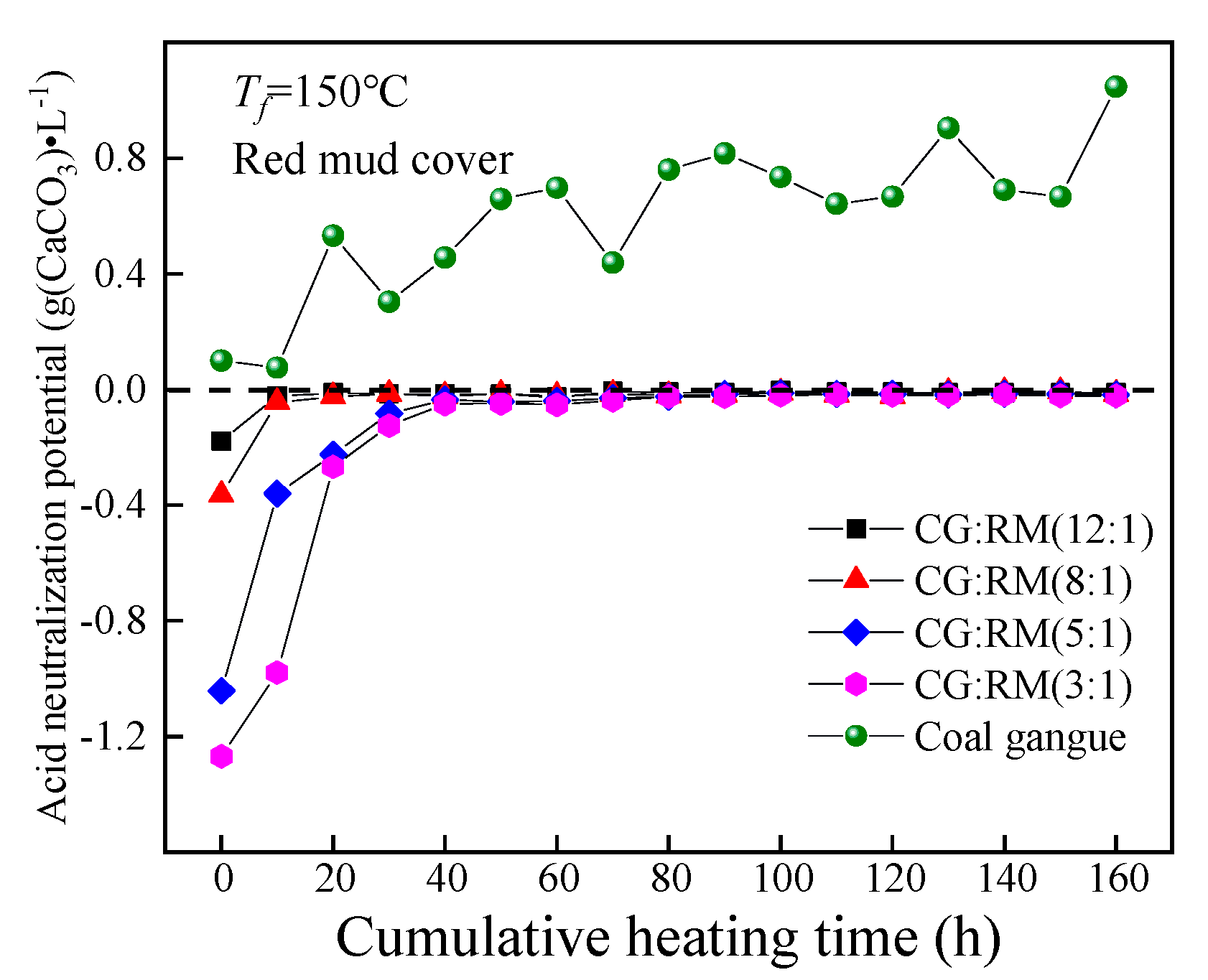
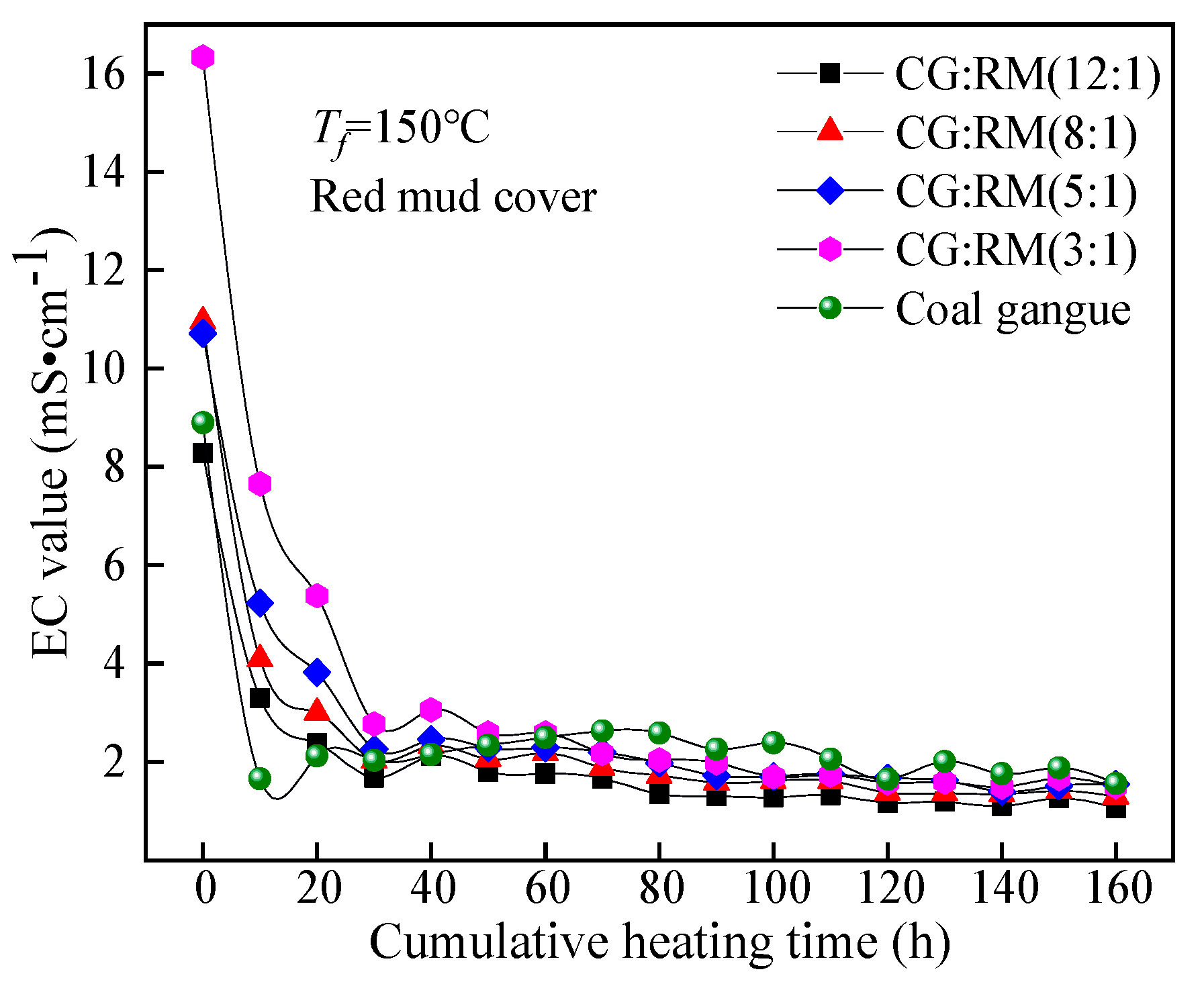
3.3.1. Mass Ratio
pH Value
ANP
EC Value
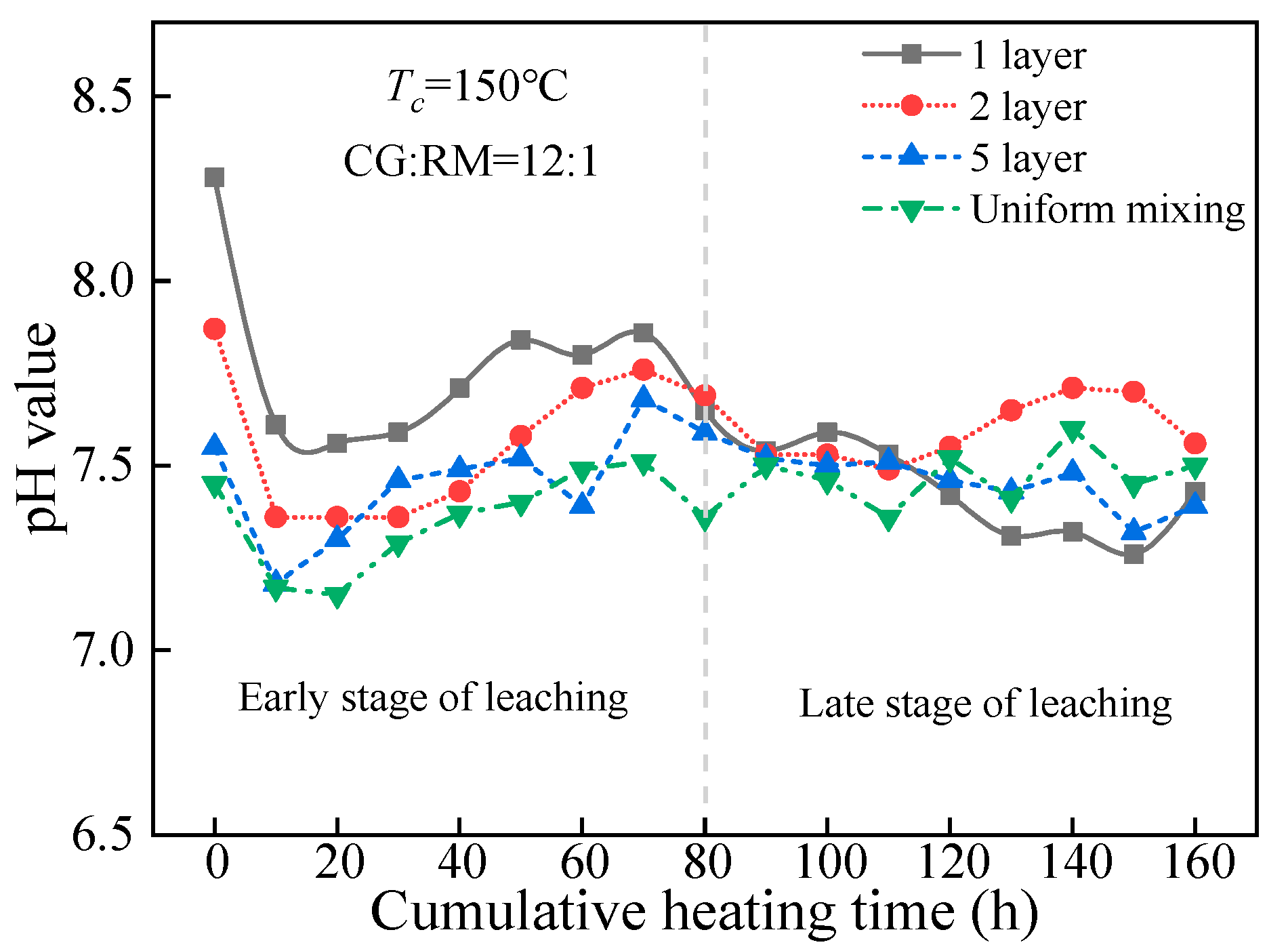
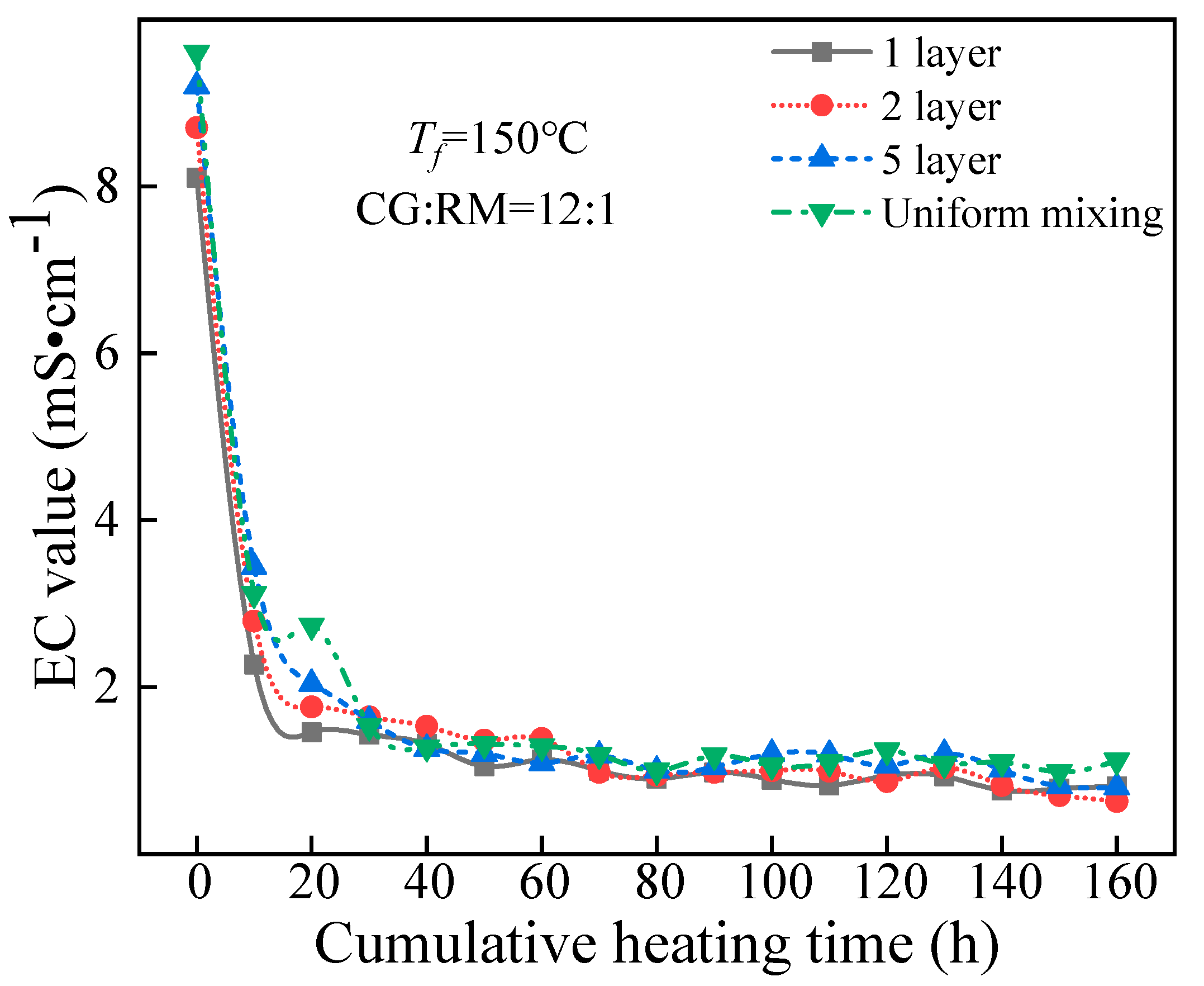
3.3.2. Storage Method
pH Value
ANP
EC Value
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Li, M.S. Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: A review of research and practice. Sci. Total Environ. 2006, 357, 38–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Pone, J.D.N.; Hein, K.A.A.; Stracher, G.B.; Annegarn, H.J.; Finkleman, R.B.; Blake, D.R.; McCormack, J.K.; Schroeder, P. The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int. J. Coal Geol. 2007, 72, 124–140. [Google Scholar] [CrossRef]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, S.; Wang, K.; Drebenstedt, C.; Zhao, J. Environment influences and extinguish technology of spontaneous combustion of coal gangue heap of Baijigou coal mine in China. Energy Procedia 2017, 136, 66–72. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, C.; Chen, C. Characteristics of polycyclic aromatic hydrocarbon release during spontaneous combustion of coal and gangue in the same coal seam. J. Loss Prev. Proc. 2018, 55, 392–399. [Google Scholar] [CrossRef]
- Fu, T.; Wu, Y.; Ou, L.; Yang, G.; Liang, T. Effects of thin covers on the release of coal gangue contaminants. Energy Procedia 2012, 16, 327–333. [Google Scholar] [CrossRef]
- Baker, B.J.; Banfield, J.F. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef]
- Campbell, R.N.; Lindsay, P.; Clemens, A.H. Acid generating potential of waste rock and coal ash in New Zealand coal mines. Int. J. Coal Geol. 2001, 45, 163–179. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: I. Batch leaching experiments and kinetic modeling calculations. Sci. Total Environ. 2017, 580, 687–698. [Google Scholar] [CrossRef]
- Yu, J.; Heo, B.; Choi, I.; Cho, J.; Chang, H. Apparent solubilities of schwertmannite and ferrihydrite in natural stream waters polluted by mine drainage. Geochim. Cosmochim. Acta 1999, 63, 3407–3416. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States—An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, A.H.; Puhakka, J.A. Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng. Life Sci. 2007, 7, 541–564. [Google Scholar] [CrossRef]
- Taylor, J.S.P.; Murphy, N. A Summary of Passive and Active Treatment Technologies for Acid and Metalliferous Drainage (AMD). In Proceedings of the 5th Australian Workshop on Acid Drainage, Fremantle, Australia, 29–31 August 2005. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Liu, B.; Tang, Z.; Dong, S.; Wang, L.; Liu, D. Vegetation recovery and groundwater pollution control of coal gangue field in a semi-arid area for a field application. Int. Biodeter. Biodegr. 2018, 128, 134–140. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, H. Development of a novel bentonite–acrylamide superabsorbent hydrogel for extinguishing gangue fire hazard. Powder Technol. 2018, 323, 486–494. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Hu, S.; Shao, H.; Liao, Q.; Fan, Y. Experimental study of the effects of stacking modes on the spontaneous combustion of coal gangue. Process. Saf. Evniron. 2019, 123, 39–47. [Google Scholar] [CrossRef]
- Demers, I.; Mbonimpa, M.; Benzaazoua, M.; Bouda, M.; Awoh, S.; Lortie, S.; Gagnon, M. Use of acid mine drainage treatment sludge by combination with a natural soil as an oxygen barrier cover for mine waste reclamation: Laboratory column tests and intermediate scale field tests. Miner. Eng. 2017, 107, 43–52. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Evniron. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, H.; Fang, D.; Liang, J.; Zhou, L. A novel approach to rapidly purify acid mine drainage through chemically forming schwertmannite followed by lime neutralization. Water Res. 2019, 151, 515–522. [Google Scholar] [CrossRef]
- Hong, S.; Cannon, F.S.; Hou, P.; Byrne, T.; Nieto-Delgado, C. Adsorptive removal of sulfate from acid mine drainage by polypyrrole modified activated carbons: Effects of polypyrrole deposition protocols and activated carbon source. Chemosphere 2017, 184, 429–437. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Millar, G.J. Performance of bauxite refinery residues for treating acid mine drainage. J. Water Process. Eng. 2018, 26, 28–37. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.I.; Mamba, B.B. Synthesis and application of hematite nanoparticles for acid mine drainage treatment. J. Evniron. Chem. Eng. 2018, 6, 1865–1874. [Google Scholar] [CrossRef]
- Lee, G.; Cui, M.; Yoon, Y.; Khim, J.; Jang, M. Passive treatment of arsenic and heavy metals contaminated circumneutral mine drainage using granular polyurethane impregnated by coal mine drainage sludge. J. Clean. Prod. 2018, 186, 282–292. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, M.W.; Tutu, H.; DeBeer, M. Efficiency of ball milled South African bentonite clay for remediation of acid mine drainage. J. Water Process Eng. 2015, 8, 227–240. [Google Scholar] [CrossRef]
- Masindi, V.; Madzivire, G.; Tekere, M. Reclamation of water and the synthesis of gypsum and limestone from acid mine drainage treatment process using a combination of pre-treated magnesite nanosheets, lime, and CO2 bubbling. Water Resour. Ind. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Rodrigues, C.; Lapolli, F.R.; Lobo-Recio, M.Á. Adsorption of heavy metals from coal acid mine drainage by shrimp shell waste: Isotherm and continuous-flow studies. J. Environ. Chem. Eng. 2019, 7, 102787. [Google Scholar] [CrossRef]
- Gibert, O.; de Pablo, J.; Cortina, J.L.; Ayora, C. Treatment of acid mine drainage by sulphate-reducing bacteria using permeable reactive barriers: A review from laboratory to full-scale experiments. Rev. Environ. Sci. Biotechnol. 2002, 1, 327–333. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Prior, H. Bioremediation of acid mine drainage: An introduction to the Wheal Jane wetlands project. Sci. Total Evniron. 2005, 338, 15–21. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Evniron. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Wadekar, S.S.; Hayes, T.; Lokare, O.R.; Mittal, D.; Vidic, R.D. Laboratory and Pilot-Scale Nanofiltration Treatment of Abandoned Mine Drainage for the Recovery of Products Suitable for Industrial Reuse. Ind. Eng. Chem. Res. 2017, 56, 7355–7364. [Google Scholar] [CrossRef]
- Doye, I.; Duchesne, J. Neutralisation of acid mine drainage with alkaline industrial residues: Laboratory investigation using batch-leaching tests. Appl. Geochem. 2003, 18, 1197–1213. [Google Scholar] [CrossRef]
- Bois, D.; Poirier, P.; Benzaazoua, M.; Bussière, B. A feasibility study on the use of desulphurized tailings to control acid mine drainage. CIM Bull. 2004, 98, 361–380. [Google Scholar]
- Kazadi, M.C.; Harrison, S.T.L.; Franzidis, J.P.; Broadhurst, J.L. Mitigating Acid Rock Drainage Risks while Recovering Low-Sulfur Coal from Ultrafine Colliery Wastes Using Froth Flotation. Miner. Eng. 2012, 29, 13–21. [Google Scholar] [CrossRef]
- Jha, R.K.T.; Satur, J.; Hiroyoshi, N.; Ito, M.; Tsunekawa, M. Suppression of floatability of pyrite in coal processing by carrier microencapsulation. Fuel Process. Technol. 2011, 92, 1032–1036. [Google Scholar] [CrossRef][Green Version]
- Yaşar, Ö.; Uslu, T.; Şahinoğlu, E. Fine coal recovery from washery tailings in Turkey by oil agglomeration. Powder Technol. 2018, 327, 29–42. [Google Scholar] [CrossRef]
- Sahinoglu, E.; Uslu, T. Effect of particle size on cleaning of high-sulphur fine coal by oil agglomeration. Fuel Process Technol. 2014, 128, 211–219. [Google Scholar] [CrossRef]
- Beauchemin, S.; Clemente, J.S.; Thibault, Y.; Langley, S.; Gregorich, E.G.; Tisch, B. Geochemical stability of acid-generating pyrrhotite tailings 4 to 5 years after addition of oxygen-consuming organic covers. Sci. Total Evniron. 2018, 645, 1643–1655. [Google Scholar] [CrossRef]
- Querol, X.; Zhuang, X.; Font, O.; Izquierdo, M.; Alastuey, A.; Castro, I.; van Drooge, B.L.; Moreno, T.; Grimalt, J.O.; Elvira, J.; et al. Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: A review and new experimental data. Int. J. Coal Geol. 2011, 85, 2–22. [Google Scholar] [CrossRef]
- Kotsiopoulos, A.; Harrison, S.T.L. Application of fine desulfurised coal tailings as neutralising barriers in the prevention of acid rock drainage. Hydrometallurgy 2017, 168, 159–166. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.; Hiroyoshi, N.; Tabelin, C.B.; Taketsugu, T.; Ito, M. Suppression of pyrite oxidation by ferric-catecholate complexes: An electrochemical study. Miner. Eng. 2019, 138, 226–237. [Google Scholar] [CrossRef]
- Li, X.; Hiroyoshi, N.; Tabelin, C.B.; Naruwa, K.; Harada, C.; Ito, M. Suppressive effects of ferric-catecholate complexes on pyrite oxidation. Chemosphere 2019, 214, 70–78. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Hu, Z.; Zhang, M.; Ma, B.; Wang, P.; Kang, J. Fly ash for control pollution of acid and heavy metals from coal refuse. Meitan Xuebao/J. China Coal Soc. 2009, 34, 79–83. [Google Scholar]
- Mylona, E.; Xenidis, A.; Paspaliaris, I. Inhibition of acid generation from sulphidic wastes by the addition of small amounts of limestone. Miner. Eng. 2000, 13, 1161–1175. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Utilization of Bactericide Technology for Pollution Control of Acidic Coal Mine Waste. In Proceedings of the 2017 6th International Conference on Energy, Environment and Sustainable Development, Zhuhai, China, 11–12 March 2017. [Google Scholar]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in red mud utilization—A review. Min. Proc. Ext. Met. Rev. 2005, 26, 1–29. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef]
- Jones, B.E.H.; Haynes, R.J.; Phillips, I.R. Addition of an organic amendment and/or residue mud to bauxite residue sand in order to improve its properties as a growth medium. J. Evniron. Manag. 2012, 95, 29–38. [Google Scholar] [CrossRef]
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Jones, B.E.H.; Haynes, R.J. Bauxite processing residue: A critical review of its formation, properties, storage, and revegetation. Crit. Rev. Environ. Sci. Technol. 2011, 44, 271–315. [Google Scholar] [CrossRef]
- Li, X.; Ye, Y.; Xue, S.; Jiang, J.; Wu, C.; Kong, X.; Hartley, W.; LI, Y. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue. Trans. Nonferrous Met. Soc. 2018, 28, 1248–1255. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization processes of bauxite residue: A comprehensive review. J. Hazard. Mater. 2021, 403, 123671. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Zhu, F.; Hartley, W.; Liao, J.; An, W.; Xue, S.; Jiang, J. Variation on leaching behavior of caustic compounds in bauxite residue during dealkalization process. J. Evniron. Sci.-China 2020, 92, 141–150. [Google Scholar] [CrossRef]
- Xue, S.; Kong, X.; Zhu, F.; Hartley, W.; Li, X.; Li, Y. Proposal for management and alkalinity transformation of bauxite residue in China. Evniron. Sci. Pollut. Res. 2016, 23, 12822–12834. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Liu, C.; Gao, Y.; Qi, Y. Properties of red mud blended with magnesium phosphate cement paste: Feasibility of grouting material preparation. Constr. Build. Mater. 2020, 260, 119704. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Liu, L.; Wang, X.; Zhang, Z. Environmental investigation on co-combustion of sewage sludge and coal gangue: SO2, NOx and trace elements emissions. Waste Manag. 2016, 50, 213–221. [Google Scholar] [CrossRef]
- Sobek, A.A.; Shuller, W.A.; Freeman, J.R.; Smith, R.M. Field and Laboratory Methods Applicable to Overburdens and Minesoils; EPA 600/2-78-054; U.S. Environmental Protection Agency: Washington, DC, USA, 1978.
- Hu, G.; Lyu, F.; Khoso, S.A.; Zeng, H.; Sun, W.; Tang, H.; Wang, L. Staged leaching behavior of red mud during dealkalization with mild acid. Hydrometallurgy 2020, 196, 105422. [Google Scholar] [CrossRef]
- Alam, S.; Das, S.K.; Rao, B.H. Characterization of coarse fraction of red mud as a civil engineering construction material. J. Clean. Prod. 2017, 168, 679–691. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Wang, H.; Dowd, P.A.; Xu, C. A reaction rate model for pyrite oxidation considering the influence of water content and temperature. Miner. Eng. 2019, 134, 345–355. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Long, H.; Dixon, D.G. Pressure oxidation of pyrite in sulfuric acid media: A kinetic study. Hydrometallurgy 2004, 73, 335–349. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Kishida, M.; Harato, T.; Tokoro, C.; Owada, S. In situ remediation of bauxite residue by sulfuric acid leaching and bipolar-membrane electrodialysis. Hydrometallurgy 2017, 170, 58–67. [Google Scholar] [CrossRef]
- McCleskey, R.B.; Nordstrom, D.K.; Ryan, J.N. Comparison of electrical conductivity calculation methods for natural waters. Limnol. Oceanogr. Methods 2012, 10, 952–967. [Google Scholar] [CrossRef]
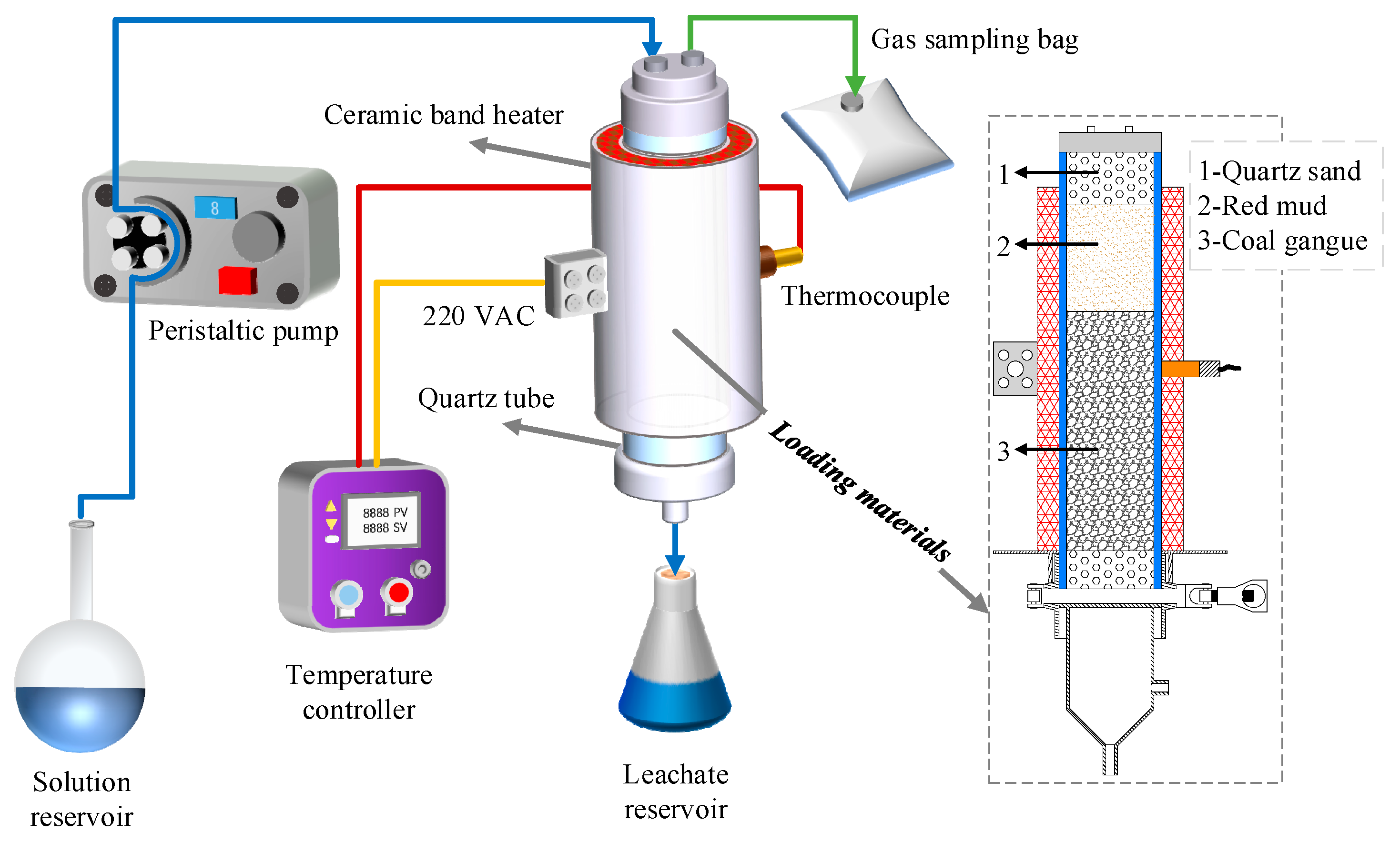
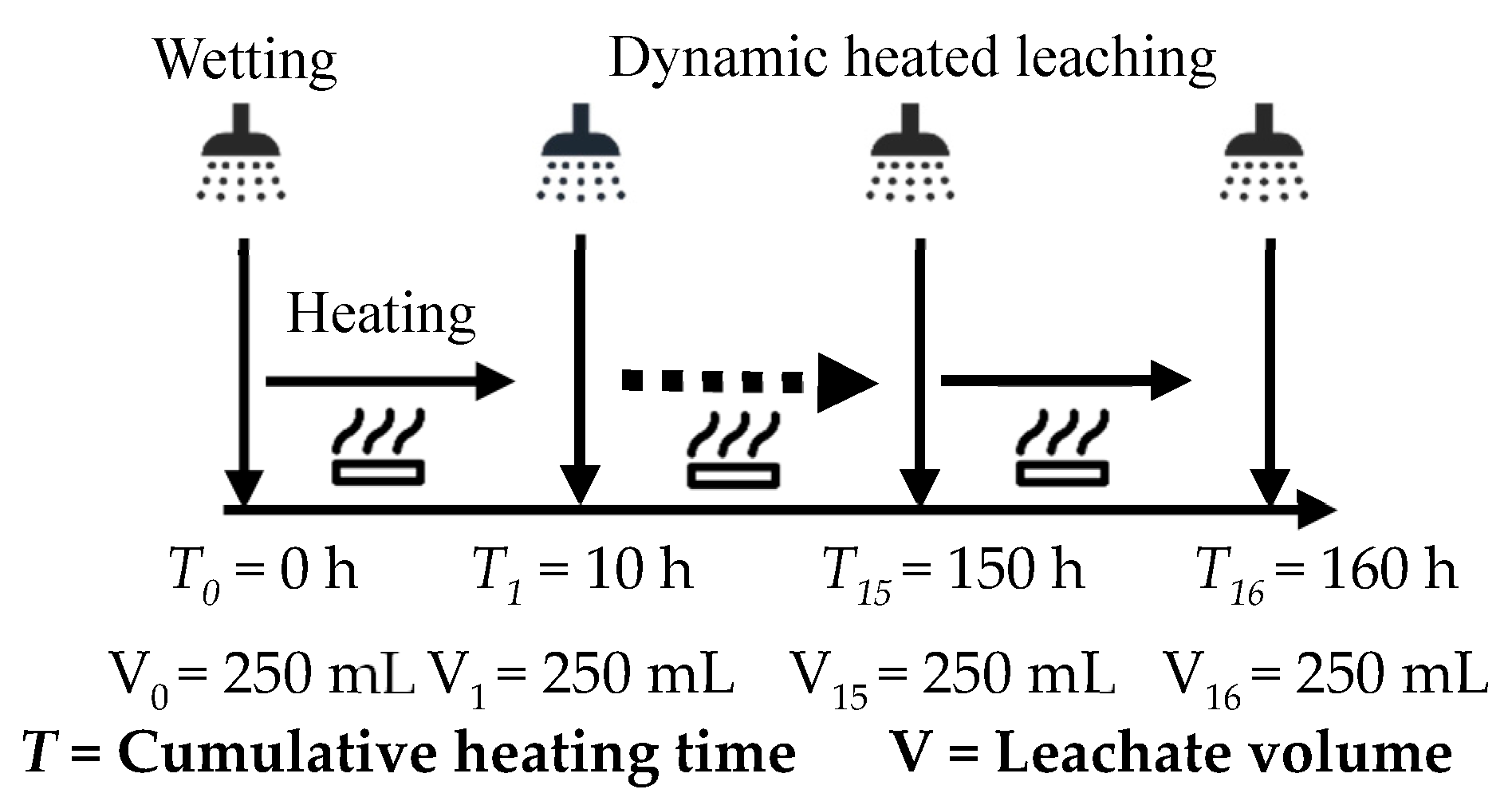
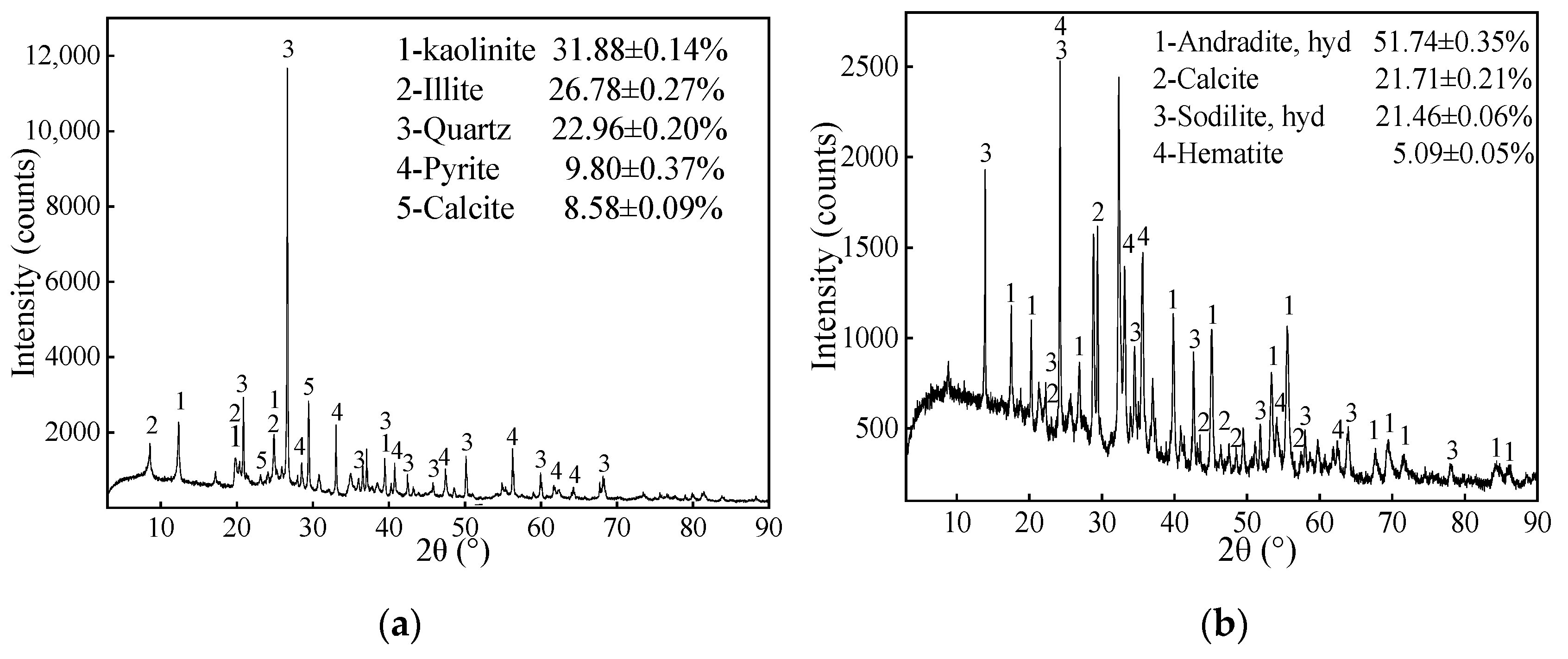
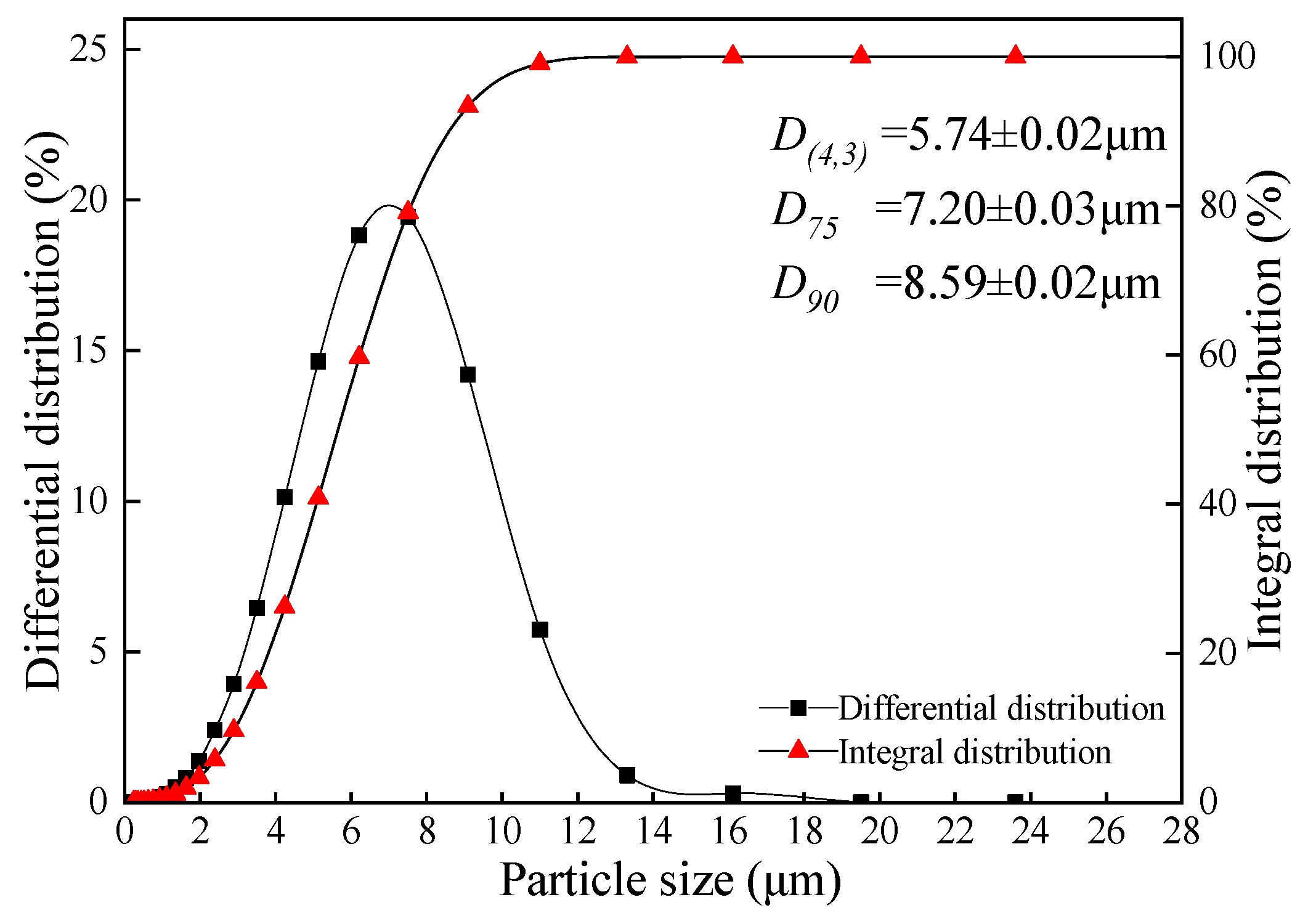


| Parameter | Apparatus and Methods |
|---|---|
| pH | FE28-standard pH meter (Mettler Toledo, Küsnacht, Switzerland) |
| EC | DDS-11A electrical conductivity meter (Lei Ci, Shanghai, China) |
| SO42− | Water quality-Determination of sulfate-Gravimetric method (GB11899-89) |
| ORP | Orion 3 Star mV Detector (Thermo Scientific, Waltham, MA, USA) |
| TDS | TDS-5 m (Greensky, Hangzhou, China) |
| ANP | Acid-base neutralization titration |
| Mineral composition | D/max-2550 X-ray diffractometer (Rigaku Corporation, Tokyo, Japan) |
| Proximate Analysis | Ultimate Analysis | ||
|---|---|---|---|
| Moisture, Mad 1 | 1.07 ± 0.02 | Carbon, Cad | 17.60 ± 0.06 |
| Ash, Aad | 72.70 ± 0.5 | Hydrogen, Had | 1.36 ± 0.05 |
| Volatile matter, VMad | 9.25 ± 0.02 | Nitrogen, Nad | 0.41 ± 0.03 |
| Fixed carbon, FCad | 16.98 ± 0.41 | Sulfur, Sad | 4.50 ± 0.16 |
| Material | Oxides | Loss | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | Na2O | Cr2O3 | NiO | CuO | ZnO | PbO | ||
| Coal gangue | 49.81 ± 0.06 | 24.82 ± 0.13 | 9.82 ± 0.05 | 6.39 ± 0.04 | 5.52 ± 0.03 | 0.45 ± 0.01 | 0.03 ± 0.002 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 27.2 ± 0.12 |
| Red mud | 20.43 ± 0.16 | 25.92 ± 0.09 | 14.62 ± 0.18 | 17.22 ± 0.08 | 2.61 ± 0.02 | 12.05 ± 0.07 | 0.06 ± 0.003 | 0.08 ± 0.002 | 0.009 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 7.10 ± 0.16 |
| Parameter | Unit | Material | |
|---|---|---|---|
| Coal Gangue | Red Mud | ||
| pH | 7.25 ± 0.04 | 11.11 ± 0.05 | |
| EC | mS·cm−1 | 0.715 ± 0.009 | 0.852 ± 0.006 |
| ORP | mV | 141.3 ± 4.2 | 8.5 ± 0.6 |
| TDS | ppm | 581 ± 5 | 509 ± 4 |
| ANP | g(CaCO3)·L−1 | −0.071 ± 0.003 * | −0.52 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, Z.; Pan, Y.; Liu, W. Co-Disposal of Coal Gangue and Red Mud for Prevention of Acid Mine Drainage Generation from Self-Heating Gangue Dumps. Minerals 2020, 10, 1081. https://doi.org/10.3390/min10121081
Ran Z, Pan Y, Liu W. Co-Disposal of Coal Gangue and Red Mud for Prevention of Acid Mine Drainage Generation from Self-Heating Gangue Dumps. Minerals. 2020; 10(12):1081. https://doi.org/10.3390/min10121081
Chicago/Turabian StyleRan, Zhou, Yongtai Pan, and Wenli Liu. 2020. "Co-Disposal of Coal Gangue and Red Mud for Prevention of Acid Mine Drainage Generation from Self-Heating Gangue Dumps" Minerals 10, no. 12: 1081. https://doi.org/10.3390/min10121081
APA StyleRan, Z., Pan, Y., & Liu, W. (2020). Co-Disposal of Coal Gangue and Red Mud for Prevention of Acid Mine Drainage Generation from Self-Heating Gangue Dumps. Minerals, 10(12), 1081. https://doi.org/10.3390/min10121081





