Kaolinite-Magnesite or Kaolinite–Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Dispersions Preparation
2.3. Preparation of Green and Sintered Bodies
2.4. Characterization Techniques
2.4.1. Thermal Analysis
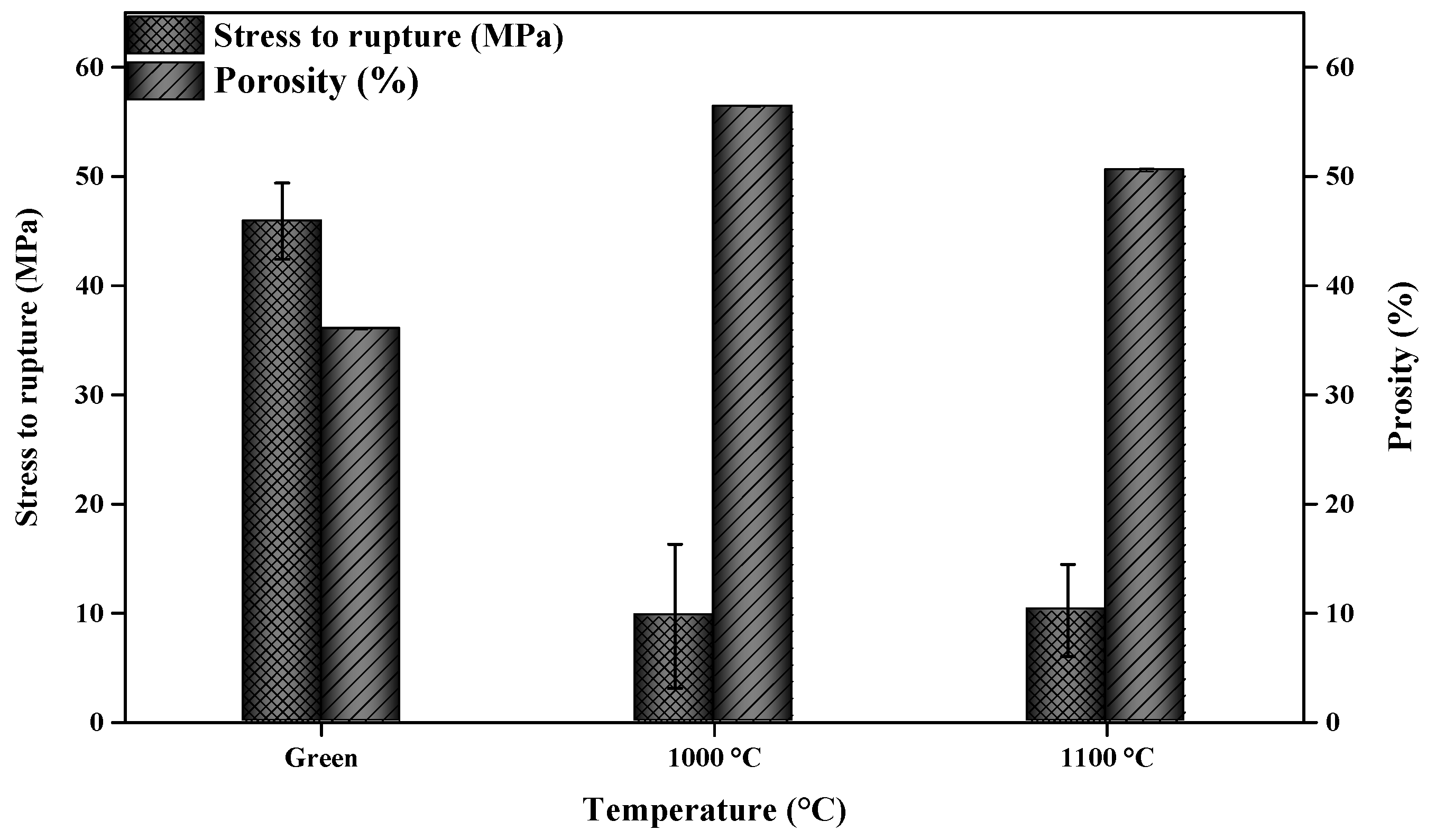
2.4.2. Apparent Porosity
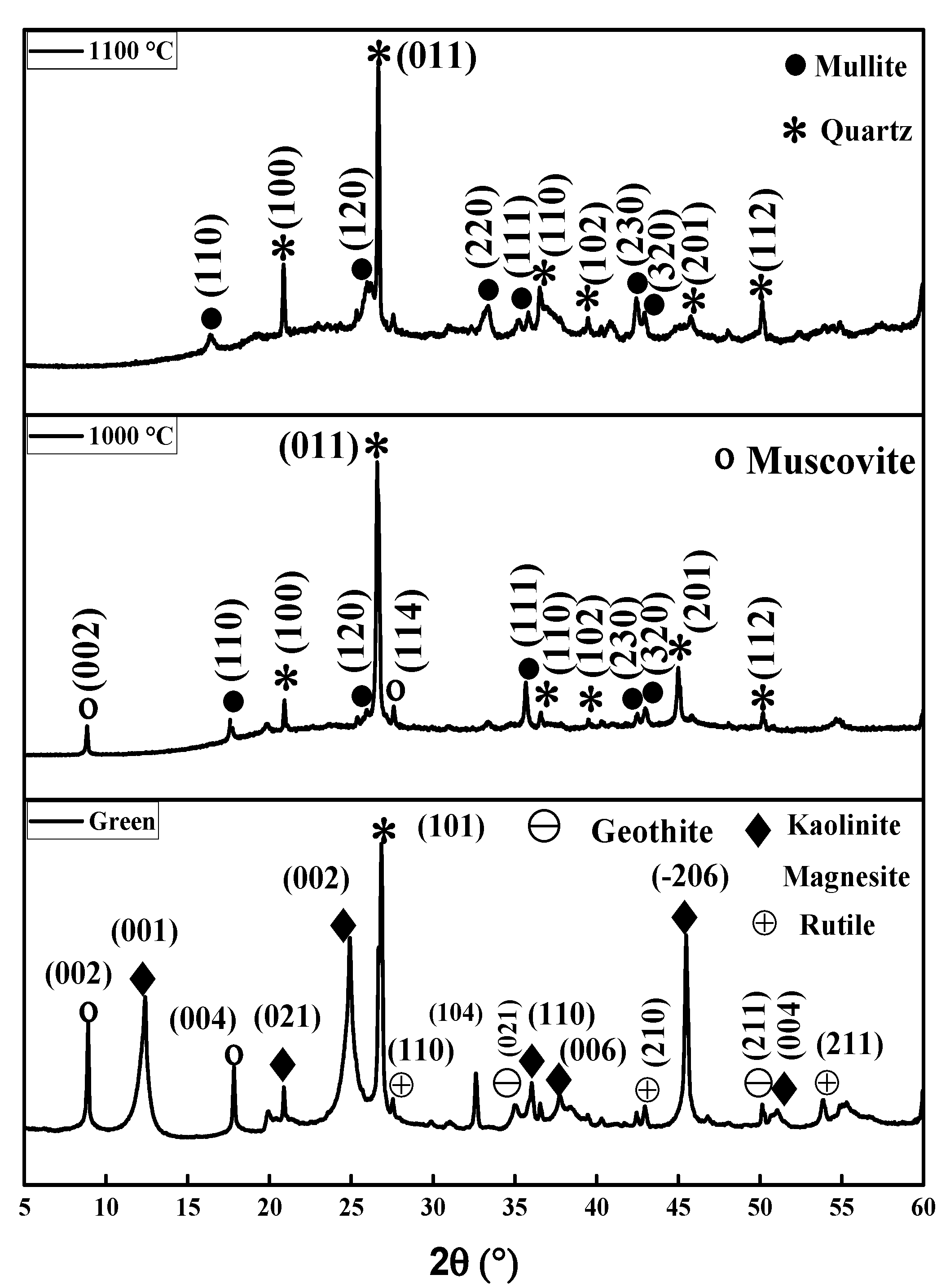
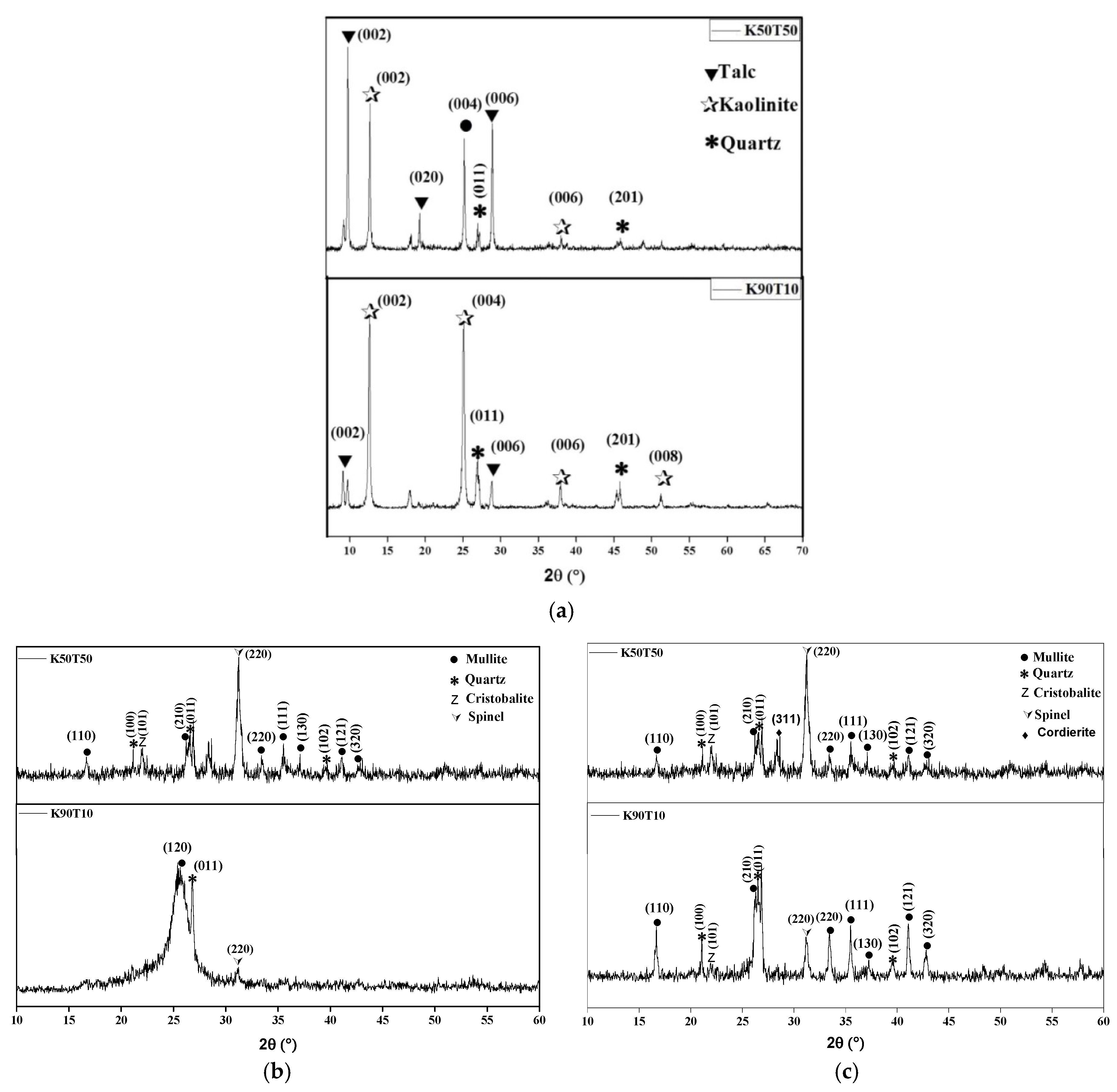
2.4.3. X-Ray Diffraction
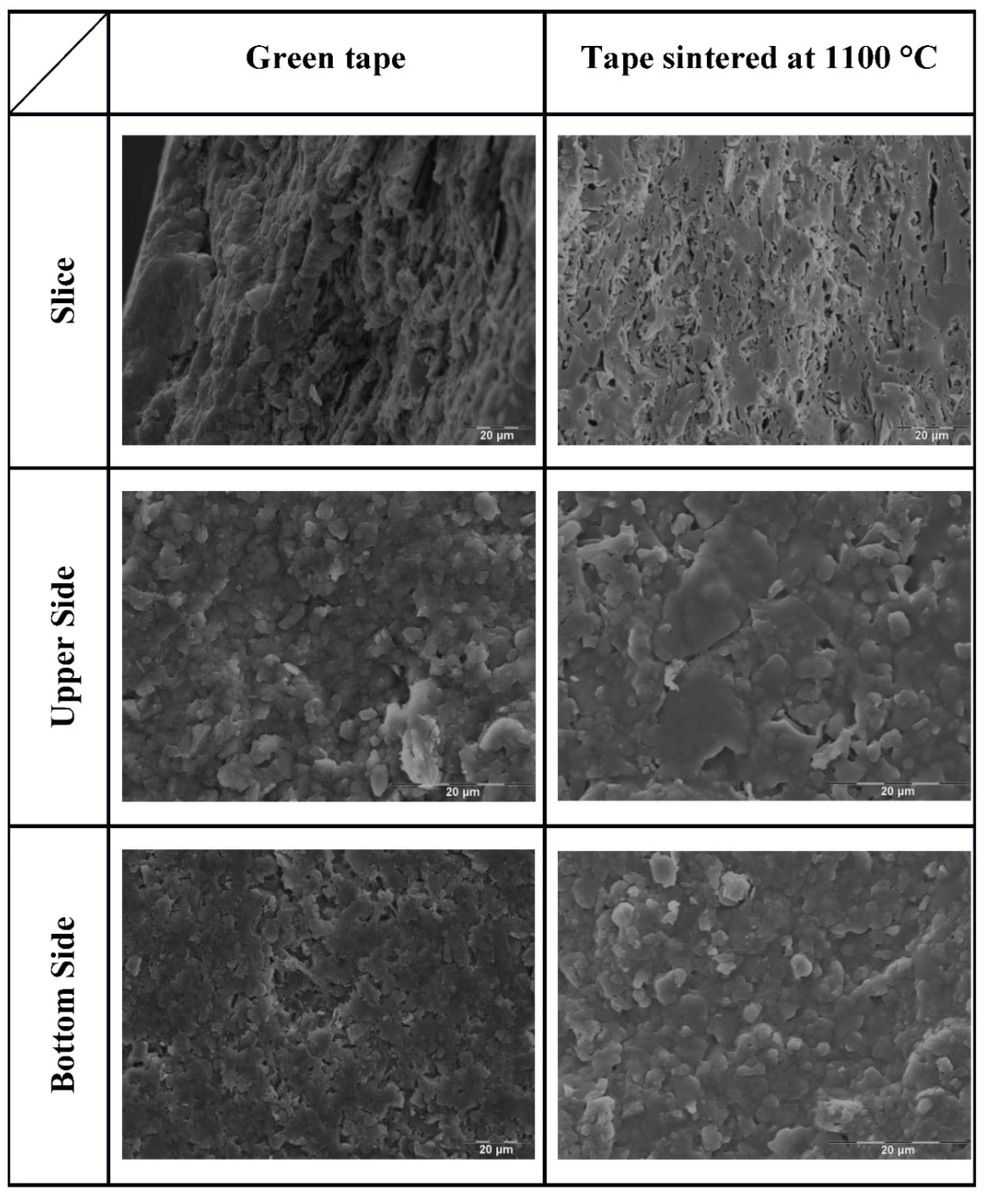
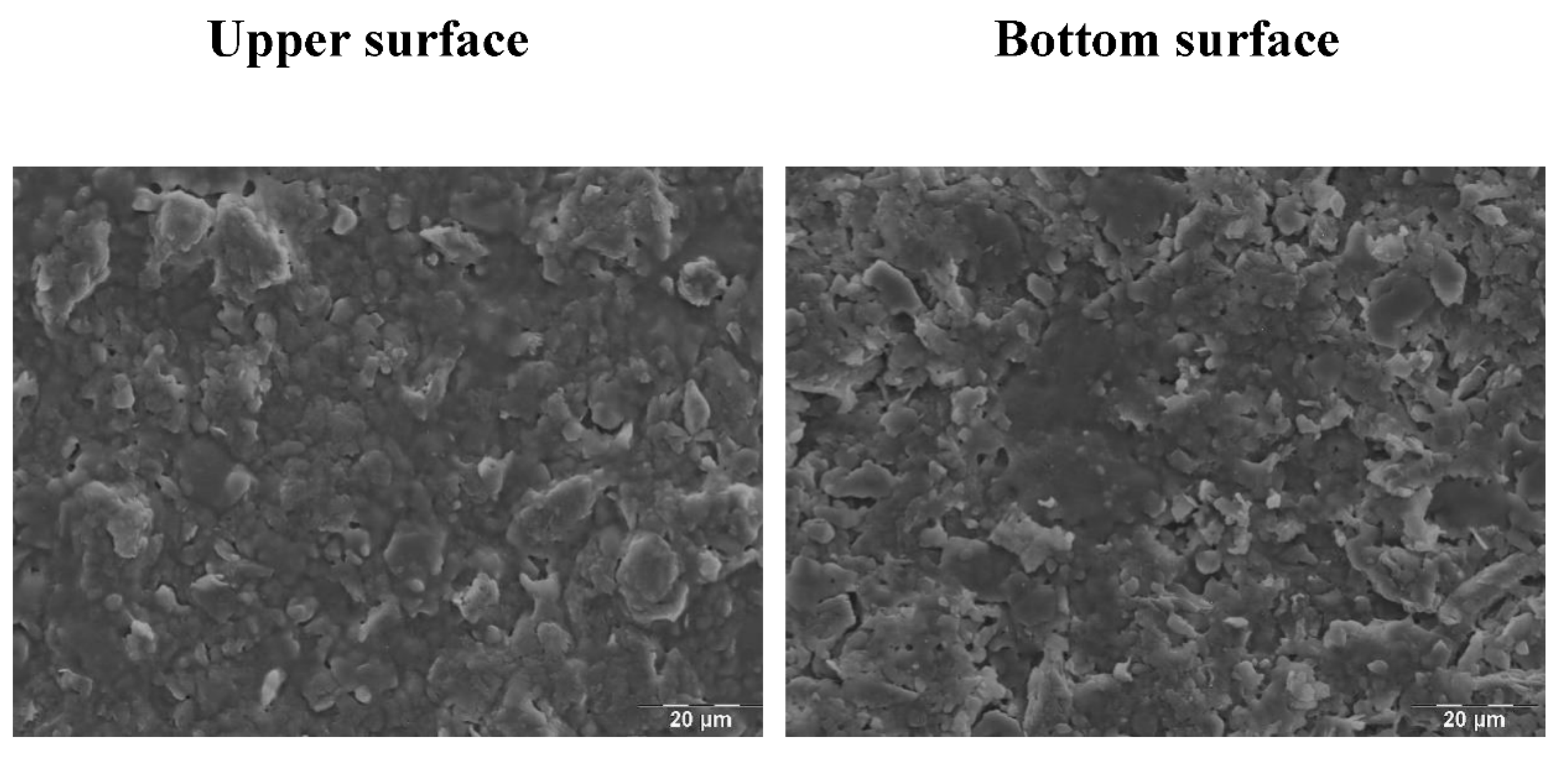
2.4.4. Scanning Electron Microscopy
2.4.5. Biaxial Flexural Strength
3. Results and Discussion
3.1. XRD, Thermal Behavior and Microstructure of Green and Sintered Specimens
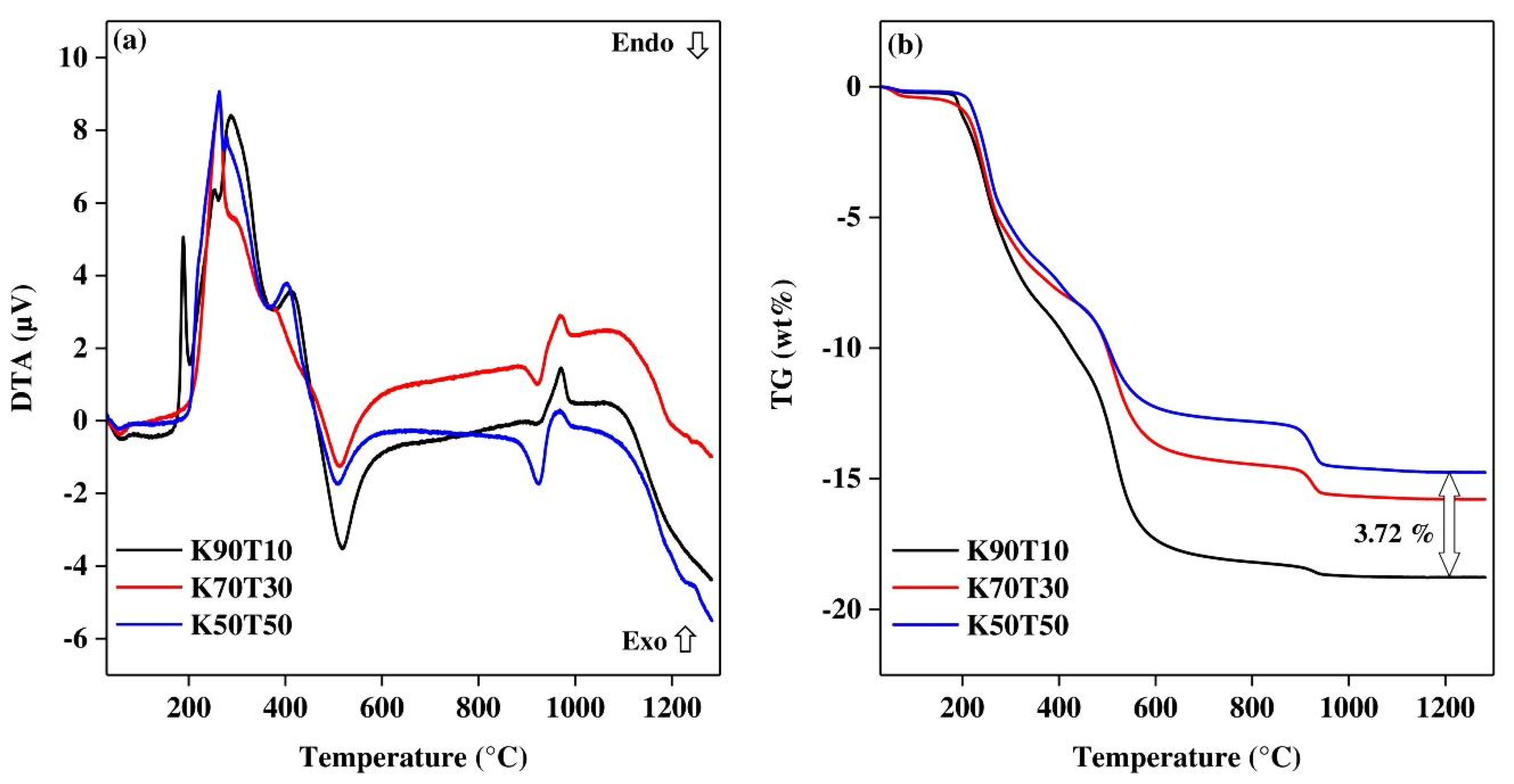
- The dehydration of the green tapes in the region between 70 and 120 °C (endothermic peak);
- The burning of organics used as binders and plasticizer in the range 150 to 400 °C (consecutive exothermic peaks associated with significant mass loss);
- The dehydroxylation of kaolinite between 400 and 650 °C, characterized by an endothermic peak which appeared proportional to the kaolin content in the mixture (also the related mass loss);
- The dehydroxylation of talc in the range 850 to 1000 °C, characterized by an endothermic peak (proportional to the talc content as the mass loss) very close to the exothermic peak associated to the structural reorganization of the metakaolinite.
3.2. Mechanical Properties and Porosity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hammas, A.; Lecomte-Nana, G.; Daou, I.; Zibouche, F. Sintering and final properties of kaolinite-magnesite tapes for the manufacture of cordierite-mullite ceramics. Int. J. Appl. Ceram. Technol. 2020, 17, 2265–2276. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Ten, J.G.; Orts, M.J.; Saburit, A.; Silva, G. Thermal conductivity of traditional ceramics. Part I: Influence of bulk density and firing temperature. Ceram. Int. 2010, 36, 1951–1959. [Google Scholar]
- Manfredini, T.; Hanuskova, M. Natural raw materials in “Traditional” ceramic manufacturing. J. Univ. Chem. Technol. Metall. 2012, 47, 465–470. [Google Scholar]
- Tarvornpanich, T.; Souza, G.P.; Lee, W.E. Microstructural Evolution in Clay-Based Ceramics II: Ternary and Quaternary Mixtures of Clay, Flux, and Quartz Filler. J. Am. Ceram. Soc. 2008, 91, 2272–2280. [Google Scholar] [CrossRef]
- Pontikes, Y.; Nikolopoulos, P.; Angelopoulos, G.N. Thermal behaviour of clay mixtures with bauxite residue for the production of heavy-clay ceramics. J. Eur. Ceram. Soc. 2007, 27, 1645–1649. [Google Scholar] [CrossRef]
- Zbik, M.; Smart, R.S.C. Influence of dry grinding on talc and kaolinite morphology: Inhibition of nano-bubble formation and improved dispersion. Miner. Eng. 2005, 18, 969–976. [Google Scholar] [CrossRef]
- de Brito, I.P.; de Almeida, E.P.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; da Silva, V.J.; de Lima Santana, L.N. Development of cordierite/mullite composites using industrial wastes. Int. J. Appl. Ceram. Technol. 2020. [Google Scholar] [CrossRef]
- Naga, S.M.; Sayed, M.; El-Omla, M.M.; Wassel, A.R.; El-Mehalawy, N. Processing of electric ceramic insulators from slate rocks and MgO. Mater. Manuf. Process. 2020, 35, 893–900. [Google Scholar] [CrossRef]
- Sittiakkaranon, S. Thermal Shock Resistance of Mullite-Cordierite Ceramics from Kaolin, Talc and Alumina Raw Materials. Mater. Today Proc. 2019, 17, 1864–1871. [Google Scholar] [CrossRef]
- Lecomte-Nana, G.L.; Lebdioua, K.; Laffort, M.; Houta, N.; Tessier-Doyen, N.; Abouliatim, Y.; Peyratout, C. Effect of phyllosilicate type on the microstructure and properties of kaolin-based ceramic tapes. Ceram. Eng. Sci. Proc. 2017, 37, 47–601. [Google Scholar]
- Tufar, W. Talc. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Wattanasiriwech, S.; Wattanasiriwech, D. Roles of talc-illite on phase transformation, vitrification and physical properties of a triaxial porcelain body. J. Ceram. Process. Res. 2019, 20, 643–648. [Google Scholar] [CrossRef]
- Lao, X.; Xu, X.; Jiang, W.; Liang, J.; Miao, L.; Wu, Q. Influences of impurities and mineralogical structure of different kaolin minerals on thermal properties of cordierite ceramics for high-temperature thermal storage. Appl. Clay Sci. 2020, 187, 105485. [Google Scholar] [CrossRef]
- Chakraborty, A.K. Phase Transformation of Kaolinite Clay; Springer: New Delhi, India, 2014; ISBN 9788132211549. [Google Scholar]
- Scholtzová, E.; Tunega, D. Prediction of mechanical properties of grafted kaolinite—A DFT study. Appl. Clay Sci. 2020, 193, 105692. [Google Scholar] [CrossRef]
- Hammas, A.; Lecomte-Nana, G.; Azril, N.; Daou, I.; Peyratout, C.; Zibouche, F. Kaolinite-Magnesite Based Ceramics. Part I: Surface Charge and Rheological Properties Optimization of the Suspensions for the Processing of Cordierite-Mullite Tapes. Minerals 2019, 9, 757. [Google Scholar] [CrossRef]
- Tunega, D.; Zaoui, A. Mechanical and Bonding Behaviors Behind the Bending Mechanism of Kaolinite Clay Layers. J. Phys. Chem. C 2020, 124, 7432–7440. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, W.; Zhou, J.; Chen, J. Interactions between kaolinite AlOH surface and sodium hexametaphosphate. Appl. Surf. Sci. 2016, 387, 759–765. [Google Scholar] [CrossRef]
- Katircioglu-Bayel, D. Impact of Operating Parameters on the Breakage Process of Talc. Mining Metall. Explor. 2020, 37, 1717–1727. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Chu, J.; Wang, X.; Zhang, M. Crystalline structure variation within phlogopite, muscovite and talc under 0–1000 kGy γ ray irradiation: A clear dependence on intrinsic characteristic. Appl. Clay Sci. 2020, 187, 105475. [Google Scholar] [CrossRef]
- Kirstein, A.F.; Woolley, R.M. Symmetrical bending of thin circular elastic plates on equally spaced point supports. J. Res. Natl. Bur. Stand. C 1967, 71, 1–10. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lan, G.S.; Tuan, W.H. Microstructural evolution of mullite during the sintering of kaolin powder compacts. Ceram. Int. 2000, 26, 715–720. [Google Scholar] [CrossRef]
- Benhammou, A.; El Hafiane, Y.; Abourriche, A.; Abouliatim, Y.; Nibou, L.; Yaacoubi, A.; Tessier-Doyen, N.; Smith, A.; Tanouti, B. Influence of sintering temperature on the microstructural and mechanical properties of cordierite synthesized from andalusite and talc. Mater. Lett. 2016, 172, 198–201. [Google Scholar] [CrossRef]
- Souto, P.M.D.; Menezes, R.R.; Kiminami, R.H.G.A. Evaluation of the Influence of MgO and La2O3 on the Fast Sintering of Mullite. Mater. Res. 2015, 18, 42–53. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, Z.; Sun, W.; Hou, J.; He, B.; Dong, Y. Cost-effective utilization of mineral-based raw materials for preparation of porous mullite ceramic membranes via in-situ reaction method. Appl. Clay Sci. 2016, 120, 135–141. [Google Scholar] [CrossRef]
- Hurt, S.M.; Wolf, A.S. Anomalous structure of MgCO3 liquid and the buoyancy of carbonatite melts. Earth Planet. Sci. Lett. 2020, 531, 115927. [Google Scholar] [CrossRef]
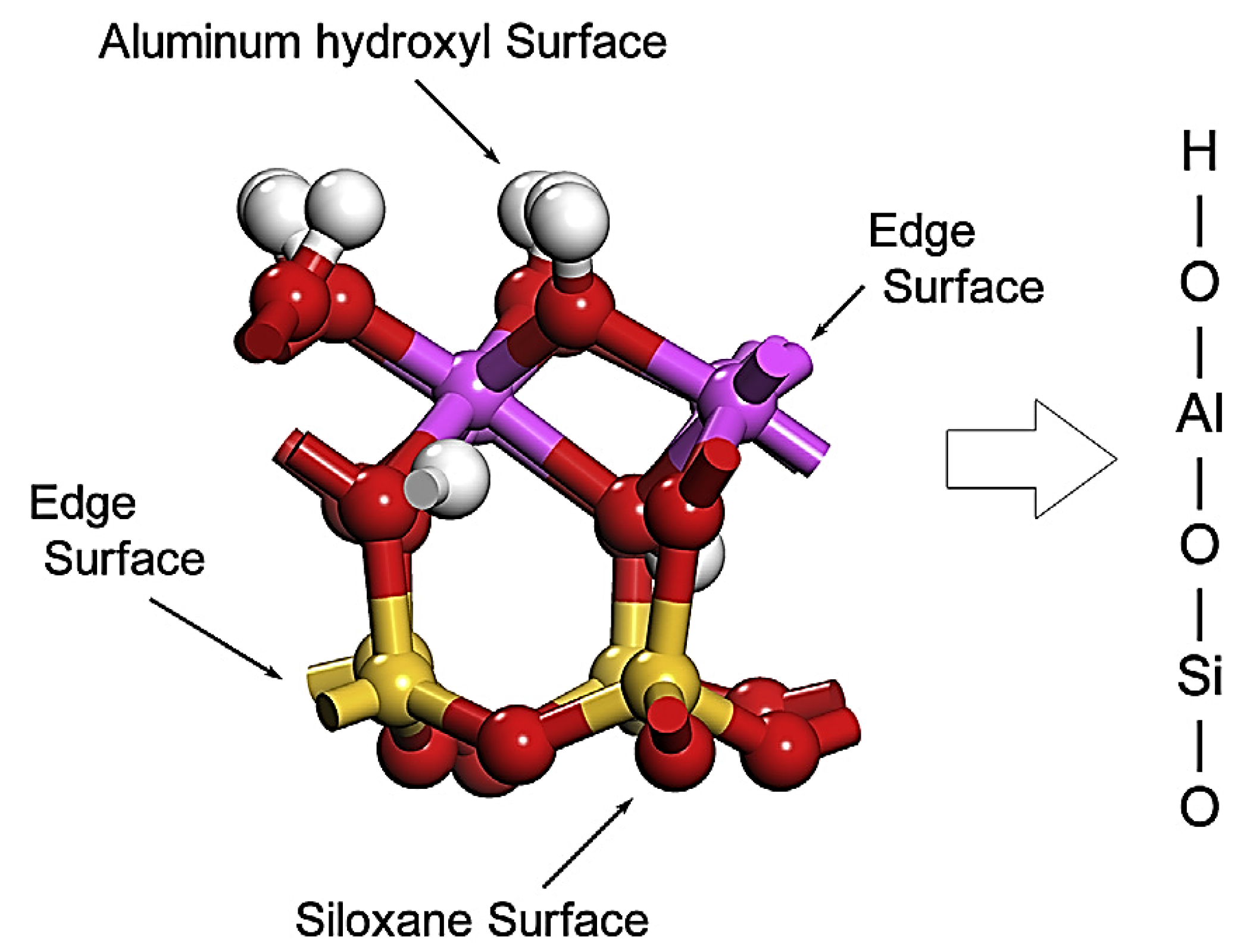
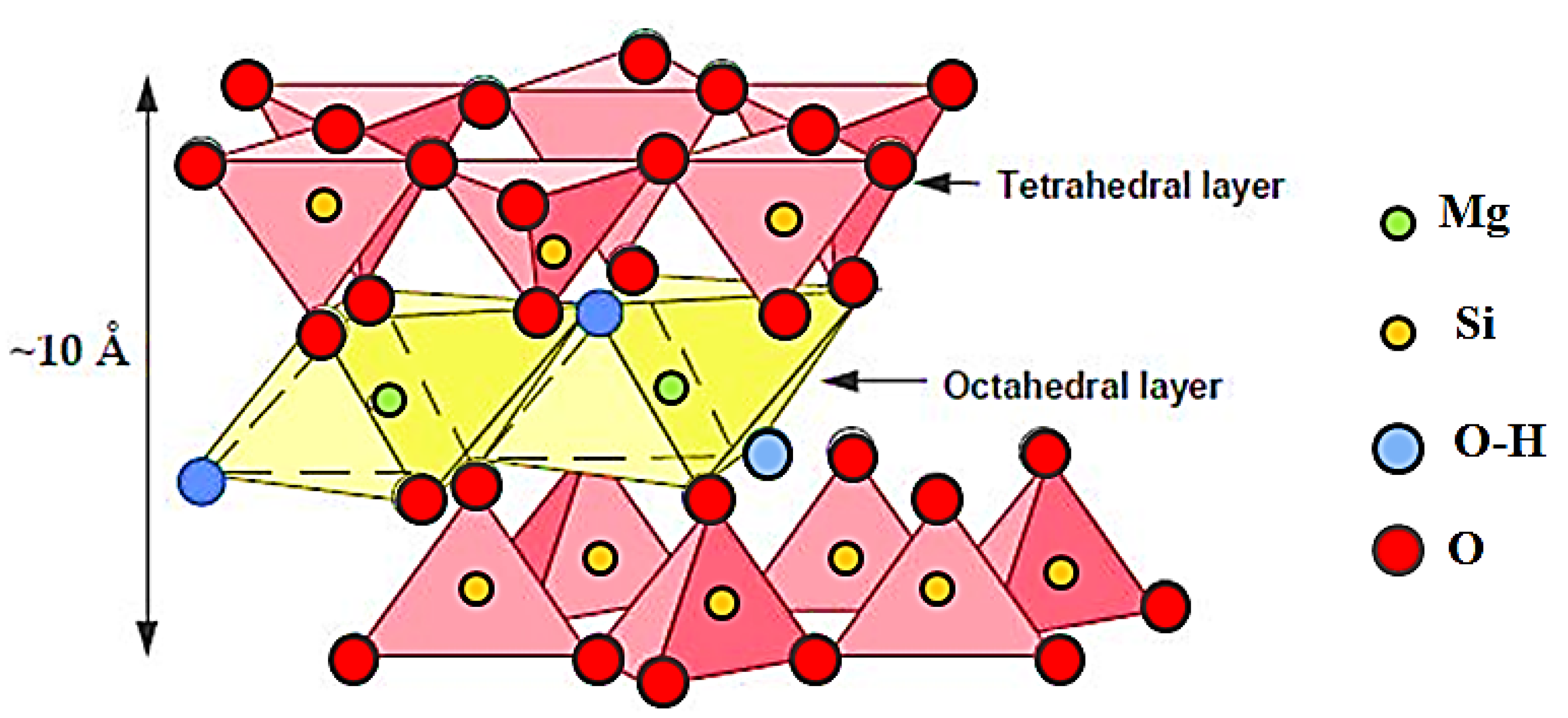
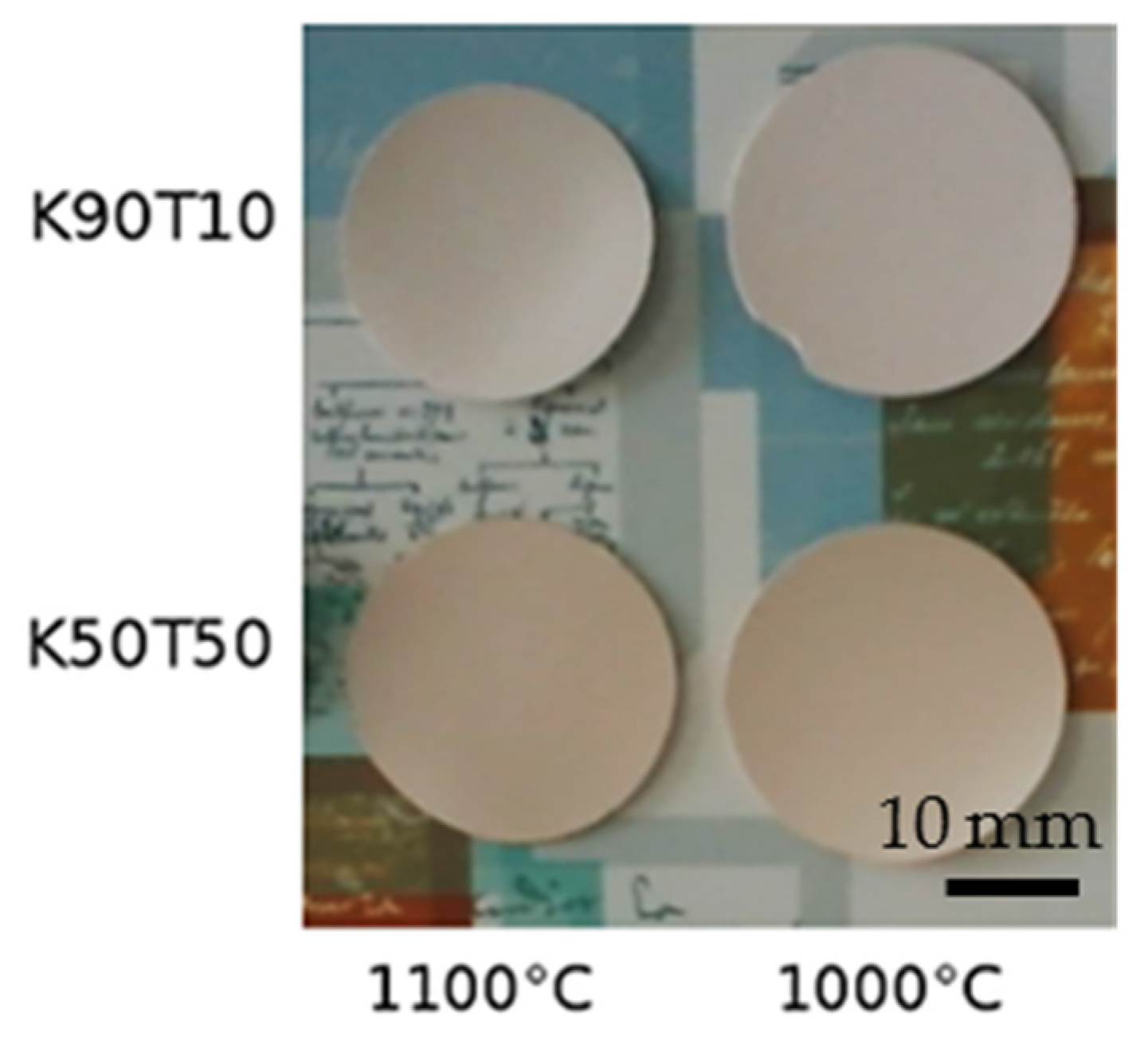

| Raw Material | SSA (BET) (±0.1 m2·g−1) | Density (g·cm−3) | Chemical Composition (mass%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | Fe2O3 | TiO2 | Li2O | CaO | Na2O | K2O | LOI | |||
| Kaolinitic clay KT2 | 25.0 | 2.6 | 47.8 | 32.9 | 0.6 | 3.5 | 0.5 | - | 0.1 | 0.1 | 2.9 | 11.5 |
| Kaolin BIP | 11.7 | 2.6 | 48.1 | 39.9 | 0.17 | 0.26 | <0.25 | 0.27 | <0.2 | <0.2 | 1.9 | 10 |
| Talc | 11.0 | 2.7 | 63 | 0.2 | 30.5 | 1.4 | <0.1 | - | 0.25 | 0.03 | 0.01 | 4.7 |
| Magnesite | 11.7 | 2.9 | 3.2 | 0.1 | 42.7 | 0.4 | - | - | 3.2 | - | - | 50.4 |
| K94M6 | 45.0 | 30.9 | 3.1 | 3.3 | 0.4 | - | 0.3 | 0.1 | 2.7 | 13.8 | ||
| K90T10 | 49.6 | 35.9 | 3.2 | 0.4 | <0.23 | 0.24 | 0.2 | 0.18 | 1.7 | 9.5 | ||
| K80T20 | 51.1 | 31.9 | 6.2 | 0.5 | <0.2 | 0.22 | 0.2 | 0.16 | 1.5 | 8.9 | ||
| K70T30 | 52.6 | 28 | 9.3 | 0.6 | <0.17 | 0.2 | 0.2 | 0.14 | 1.3 | 8.4 | ||
| K60T40 | 54.1 | 24 | 12.3 | 0.7 | <0.15 | 0.16 | 0.2 | 0.12 | 1.14 | 7.9 | ||
| K50T50 | 55.6 | 20.1 | 15.3 | 0.8 | 0.18 | 0.13 | 0.2 | 0.1 | 0.95 | 7.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammas, A.; Lecomte-Nana, G.; Daou, I.; Tessier-Doyen, N.; Peyratout, C.; Zibouche, F. Kaolinite-Magnesite or Kaolinite–Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes. Minerals 2020, 10, 1080. https://doi.org/10.3390/min10121080
Hammas A, Lecomte-Nana G, Daou I, Tessier-Doyen N, Peyratout C, Zibouche F. Kaolinite-Magnesite or Kaolinite–Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes. Minerals. 2020; 10(12):1080. https://doi.org/10.3390/min10121080
Chicago/Turabian StyleHammas, Aghiles, Gisèle Lecomte-Nana, Imane Daou, Nicolas Tessier-Doyen, Claire Peyratout, and Fatima Zibouche. 2020. "Kaolinite-Magnesite or Kaolinite–Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes" Minerals 10, no. 12: 1080. https://doi.org/10.3390/min10121080
APA StyleHammas, A., Lecomte-Nana, G., Daou, I., Tessier-Doyen, N., Peyratout, C., & Zibouche, F. (2020). Kaolinite-Magnesite or Kaolinite–Talc-Based Ceramics. Part II: Microstructure and the Final Properties Related Sintered Tapes. Minerals, 10(12), 1080. https://doi.org/10.3390/min10121080







