Abstract
The mining of metal ores generates both liquid and solid wastes, which are increasingly important to manage. In this paper, an attempt was made to use waste rocks produced in the mining of zinc and lead to neutralizing acid mine drainage and alkaline flotation wastewater. Waste rock is a quartz-feldspar rock of hydrothermal origin. It is composed of, besides quartz and potassium feldspar (orthoclase), phyllosilicates (chlorite and mica), and sulfides (chiefly pyrite). To determine its physicochemical parameters and their variability, acid mine water and flotation wastewater were monitored for 12 months. Acid mine drainage (AMD) is characterized by a low pH (~3), high zinc concentration (~750 mg·L−1), and high sulfate content (~6800 mg·L−1). On the other hand, the determinations made for flotation wastewater showed, among others, a pH of approximately 12 and ca. 780 mg·L−1 of sulfates. AMD and flotation wastewater neutralization by the waste rock was shown to be possible and efficient. However, in both cases, the final solution contained elevated concentrations of metals and sulfates. Premixing AMD with alkaline flotation wastewater in the first step and then neutralizing the obtained mixture with the waste rock was considered the best solution. The produced solution had a circumneutral pH. However, the obtained solution does not meet the legislative requirements but could be further treated by, for example, passive treatment systems. It is noteworthy that the proposed approach is low cost and does not require any chemical reagents.
1. Introduction
The advancement of the mining industry and its improved efficiency entail an increase in the generation of various types of waste. Lottermoser divided them into mining wastes (waste rocks, mine waters, mine drainage sludges), processing wastes (tailings), and metallurgical wastes (bauxite red mud, historical base metal smelting slags, phosphogypsum) [1,2]. The wastes most frequently produced by processing plants result from mineral extraction, physical and chemical processing of metal ores, physical and chemical processing of minerals other than metal ores, drilling muds, and other drilling wastes. Mine wastes are estimated to be one of the largest waste streams in the world [3,4]. Existing waste management technologies are still not efficient enough and/or require large financial outlays, therefore these solid, flotation wastewater or slurry are most often deposited at or near the mine, and since they usually contain high concentrations of elements and compounds, they can harm the environment. This can happen not only in the vicinity of the processing plant but also over a much larger area, e.g., contamination of rivers streams, and lakes, the devastation of the landscape [5,6,7,8,9]. It seems there is a pressing need to develop and apply solutions that are not only environmentally friendly and efficient but also economically viable [10].
AMD is an issue that mines have been struggling with for decades. Its origin can be twofold. In areas where minerals containing sulfides are present in the geological structure, the formation of AMD is the result of chemical, physical, and biological complex processes. As a consequence of the impact of water and air on the iron-bearing sulfides, mainly pyrite and pyrrhotite (oxidizing conditions), the resultant processes, often including microbial activity, lead to AMD formation [11,12]. Pyrite oxidation, ferrous sulfur, and sulfuric acid are formed first, followed by reddish-orange ferric oxyhydroxide with additional sulfuric acid. The resulting conditions allow for the further dissolution of other minerals containing hazardous and toxic elements including arsenic, cadmium, lead, and zinc [13,14,15]. Alongside pyrite and pyrrhotite, other sulfide-bearing minerals such as sphalerite, galena, arsenopyrite, gersdorffite, or chalcopyrite contribute to the formation of AMD [16,17]. Acid mine drainage can also be caused by human activity as a result of mining and processing works [18]. When the mine is in operation, it is necessary to maintain a sufficiently low level of groundwater. The cessation of mining activities causes the abandoned excavations to slowly fill up with water. High water oxygenation promotes the oxidation of sulfide minerals, especially pyrite. As a consequence of the reactions, sulfates are formed and metals are released. Commonly fine-grained waste rocks, which are exposed to the atmosphere, are stored in piles. Due to precipitation, geochemical weathering of metal-containing primary minerals takes place. The result is an outflow of watercourses containing significant loads of metals [17,19,20,21]. Over the years, many methods of AMD treatment have been developed [11,22,23,24,25,26,27]. Generally, they can be divided into two categories: passive and active types of acid mine drainage clean-up. One of the most well-studied and employed methods is based on chemical neutralization with the use of limestone [22], which is characterized by its low cost and wide availability. The result is a pH increase and, by precipitation, metal removal [17,28]. However, this method has some limitations due to its tendency to form an external coating caused by secondary mineral precipitation [29]. This may result in a significant reduction of the concentration of such elements, e.g., Cu, Ni, or Zn. In turn, in the presence of aqueous sulfate a gypsum sediment precipitates [30]. What is more, such a solution would be effective enough for AMD treatment only when the formation of AMD was temporary or short-term [15].
Problems with mine leachate treatment relate not only to AMD but also to other effluents generated during operation and deposit processing. Many mines also transform the ore into the final product. The most frequent ore dressing method is flotation. Briefly, pre-prepared rock material is subjected to chemical processes and air. In this manner, a particular metal concentrate is obtained (solid/solid separation). As usual, this type of process produces waste containing off-balance amounts of metals, including heavy metals with a strongly acidic or strongly alkaline reaction. In recent years, many attempts have been made to conduct post-flotation wastewater treatment [31,32,33,34]. Despite the many available solutions, due to the specific nature of flotation, it is necessary to adapt them to the type of waste being treated. Thus, storage in sedimentary tailings ponds is standard practice. Of course, there exist no longer used tailings containing waste that have been deposited for decades, whose treatment can be beneficial not only for the environment but also economically feasible. The technologies used in the past were less efficient, and, therefore, settling ponds contain significant amounts of metals that might be recycled [35,36]. Nonetheless, in the event of a breakdown or failure, a leak of hundreds of Mt of waste may pose an unimaginable risk to the environment, even over an extended period [35,37].
The zinc and lead mine Gradir in Sula, Montenegro, is an example of a site that still operates as a mine and which faces significant environmental issues. This mine has been operating for decades. Former and present activity contributes to the generation of four types of waste (AMD, flotation wastewater, and two types of waste rock). Traces of past activity can be seen in the form of closed, more or less collapsed adits. Most of them are filled with acid mine drainage. The presence of AMD is the result of both previous human activity and natural processes. As mentioned above, the formation of AMD is based on sulfide-containing minerals, and Montenegro is rich in them [38]. The production of lead and zinc concentrate is carried out by a flotation, the by-product of which is liquid waste. It is pumped into flotation tailings. To minimize waste emissions and energy consumption, the mine operates in a circular loop: that is, part of the leachate, after the suspension has settled, is reused in production activity. At present, mining is carried out using the open-pit method. A variety of rock material is produced during mining (blasting) operations. Based on the decision of the geologist the material is then separated. Limestone, which is the overburden of the deposit, is used both inside and outside the mine. The rock poor in the deposit are considered waste and are stored on the slopes of the mountain in the form of different size rock coarse and fine rock material. The material stored on the slopes is exposed to weather conditions, thus can contribute to the formation of AMD. The rock rich in Pb and Zn ore is crushed in mills, Appropriate chemical reagents are added and then hydro-transported to the hall where the flotation takes place. The final product is Pb and Zn concentrates. The wastewaters remaining after the process are discharged into the pond.
In this paper, an attempt was made to use waste materials produced in the mining of zinc and lead in the treatment and neutralization of acid and alkaline wastewater before discharge into the environment. We investigated whether the waste material remaining after the extraction of zinc and lead ore can be used to neutralize and stabilize the pH of acid mine waters flowing out of ancient adits and alkaline wastewater generated by flotation of Zn and Pb ore. The study of wastewater treatment was preceded by a detailed geochemical description of the raw material in terms of an environmental risk assessment associated with the uncontrolled release of toxic elements. In turn, acid mine drainage and flotation wastewater were monitored for 12 months to analyze seasonal variations in pollutant concentrations.
2. Materials and Methods
2.1. Site Description
In 1948, at the site of the current Gradir Montenegro mine, the search for zinc- and lead-bearing veins began. During the four-year investigation, the presence of deposits was documented, allowing the underground mine to be exploited and 250,000 tons of ore to be floated. The dominant minerals were sphalerite and galena. Over 50,000 meters of underground adits were drilled. Mining ceased in 1987 for two reasons: low metal prices and a lack of reserves of the vein type of ore. The mine resumed operations in 1997, though as an open-pit mine. The war in the Balkans and the economic sanctions imposed once again resulted in the closure of the mine. The mine was shut down from 2000 to 2008. Current mining is based on the exploitation of the remaining massive sulfide ore veins and disseminated sulfide stockwerk mineralization. The outline of the geology of the area is presented in Figure 1. Postvolcanic sulfide ores are related to Triassic bimodal effusive volcanism including basalts and rhyolites and later dioritic intrusions which were the source of hydrothermal ore-bearing fluids. The host rock for the ores is highly altered andesite. The metasomatic alteration includes chloritization, sericitization, silification, and pyritisation processes. Annual production reaches 1,000,000 tons of ore, resulting in 350,000 tons of pre-concentrate after the flotation process (more than 17,000 t of Zn and 5000 t of Pb). The Gradir Montenegro mine is located in the NW part of Montenegro at an elevation of 1180–1470 meters above sea level. Winters are mostly snowy (November–March) with several degrees of frost. In summer, the average daily temperature is about 20°, although the maximum reaches 30°. Most rainfall occurs from October to November. However, April has the most days with rain, and July to September have the least.
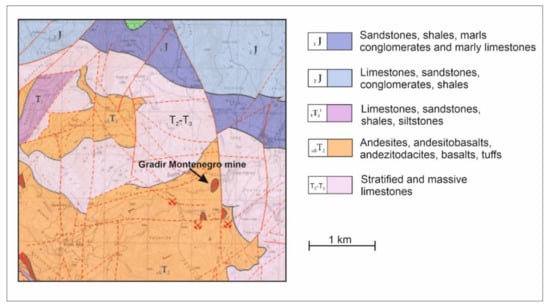
Figure 1.
Geological map of the exploration area with a marked Gradir Montenegro mine [39].
2.2. Waste Materials
2.2.1. Acid Mine Drainage
The cessation of the use of adits for mining works has caused their gradual destruction. Currently, most of them have collapsed. Water seeping through the rocks is not pumped out, and it fills the corridors and, as a result, flows out naturally. The conditions inside (air, temperature, microbial activity, etc.) are conducive to acid mine drainage formation [4,40,41]. For the purposes of this publication, AMD samples were taken from one of the adits. The initial characteristics observed confirmed the low pH and high content of sulfate ions.
2.2.2. Alkaline Flotation Wastewater
The main activity of the Gradir mine (Sula, Montenegro) is based on the production of lead and zinc concentrates. After crushing and initial enrichment, the excavated deposit is directed to the flotation. It is a multi-stage process, and during its course, various chemical reagents (collectors, frothers, depressants, activants, etc.) are dosed [42,43]. Apart from the final product, liquid waste is produced. Its composition depends on the chemical reagents used in the process. The highly alkaline wastewater that is generated, about 70 cubic meters per hour, is pumped into a tailings pond nearby the site. The pond itself is a geomembrane-lined basin with strengthened banks. As a result, potential leaks and environmental threats are minimized. Most of the waste is deposited, and some of it is returned to the mine to be used for additional processes that are carried out.
2.2.3. Mine Waste Rocks
Among the minerals of most interest in the opencast area are pyrite, sphalerite, galena, and chalcopyrite. Of course, they are accompanied by other minerals not used in the exploitation of zinc and lead ores. A variety of rock material is produced during mining (blasting) operations. Then, according to the assessment of the mining geologist, the material is separated. Limestone, which is the overburden of the deposit, is not treated as waste material as it is used both inside and outside the mine. Barren rocks are considered waste and are stored on the slopes of the mountain in the form of different size rock coarse and fine rock material. One use for the rocks is the reconstruction of the hill, which is destroyed as a result of the opencast mining of rock material. The material stored on the slopes is exposed to weather conditions. In this work, an attempt has been made to check whether there is any difference in the properties of waste material that has just been acquired and that has been stored for some time.
2.3. Sample Collection and Data Acquisition
Since February 2018, flotation wastewater and acid mine drainage content have been monitored in order to measure their quality and determine the seasonal variability of discharged effluents. Each month, samples (in triplicates) were collected. All were filtered with the use of a syringe filter (0.45 µm). For anions analysis, samples were filled to full capacity. Cation samples were preserved with nitric acid. Then, samples were transported (at 4 °C) to the laboratory. At the site, pH and conductivity were measured with the use of a portable pH/conductometer. During the research, the capacity of the settling pond was reached. A new one was launched. Therefore, subsequent samples were taken from it. AMD was taken from a partially collapsed adit. The tunnel is located near the flotation building and filled with water, which flows at an almost constant speed.
2.3.1. Chemical Analysis of Wastewaters (AMD and Flotation Wastewater)
A whole range of analyses was performed, beginning with pH and conductivity (portable pH/conductometer with temperature compensation, CPC-411, Elmetron, Zabrze, Poland). The metal composition was determined by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES, iCAP 6500 DUO, Thermo Fisher Scientific, Waltham, MA, USA), while anions concentration was assessed with chromatography (HPLC with conductivity and photodiode array detectors, Alliance, Waters). The of limits quantification of selected techniques are given in the tables below (Table 1 and Table 2). Changes in the samples matrix caused multiple dilutions, thus increasing the limits of quantification. These are given in brackets.

Table 1.
Limits of Quantification (LOQ) of the measurement elements by ICP-OES.

Table 2.
Limits of Quantification (LOQ) of the measurement anions by HPLC.
2.3.2. Geochemical Analysis of Waste Rocks
Chemical composition of the waste material was analyzed using X-ray fluorescence spectroscopy. The Rigaku ZSX Primus II WDS spectrometer (Tokyo, Japan) equipped with an Rh lamp was used. The mineral composition was characterized by scanning electron microscopy and X-ray diffractometry. Scanning electron microscopy observations were carried out in a low vacuum mode, using an FEI 200 Quanta FEG microscope equipped with an EDS/EDAX spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). XRD patterns were collected using a Rigaku SmartLab diffractometer equipped with a graphite monochromator and rotation Cu anode in a recording range of 2–75°2θ at a 0.05° step size, and a counting time of 1 s per step.
2.3.3. Titration with Mine Wastewater
Acid mine drainage from the abandoned adit and the alkaline flotation wastewater was used for the research on neutralization. The samples were collected twice: in February and April. For the titration, 25 mL of flotation effluent was used, to which acidic mine drainage was added. During the experiment, the pH was constantly monitored. Titration was carried out in five independent series.
2.3.4. Mine Water Neutralization by Waste Rocks
Two types of experiments were conducted. For the first one, two types of waste rock were selected: short- (less than one month of storage) and long-term deposited (more than three months of storage). Water, acid mine drainage, and flotation wastewater were used as a medium. The quantities of the used materials were 1, 4, 6, and 10 g. The volume of the medium was 10 mL. The neutralization was performed in PP tubes shaken at 120 rpm for 84 h. Values of pH and conductivity were measured at selected times. For the second experiment, only long-term deposited waste rock was used. Solid material (20 g) was mixed with AMD, flotation wastewater, and water as a control. In this test, we also used AMD and flotation wastewater mixture (with a final pH of 9). Each time, 20 mL of medium was used. The test was carried out in the same manner, except it took 96 h.
3. Results
3.1. Characterization of Waste Rock
The analyzed waste material comes from the Zn-Pb pre-enrichment process and is a quartz-feldspar rock of likely hydrothermal origin. The X-ray diffraction pattern (Figure 2) shows that phyllosilicates (chlorite and mica) and sulfides (chiefly pyrite) constitute the rock besides quartz and potassium feldspar (orthoclase). The presence of minor dolomite-type carbonate, as well as traces of gypsum, are also apparent.
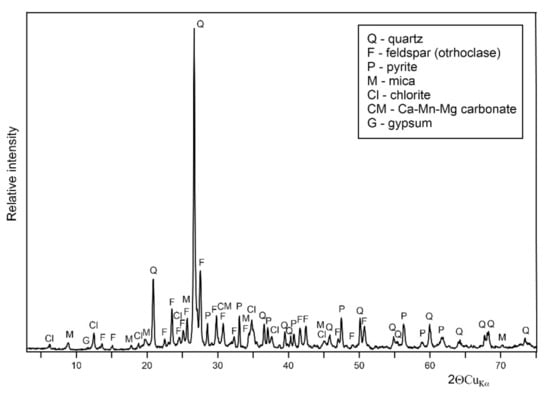
Figure 2.
X-ray diffraction pattern of the long-term stored waste rock.
The dominance of quartz and potassium feldspar is reflected by chemical composition, with SiO2, Al2O3, and K2O being the main constituents (Table 3). On the other hand, the presence of ore minerals results in high iron, sulfur, zinc, and lead contents. The sulfides are disseminated within the rock mass (Figure 3A) but may form more dense accumulations in places (Figure 3B). Euhedral or subhedral cubic pyrite crystals up to 0.3–0.4 mm in size do not show distinct weathering alterations. Minor sulfides (sphalerite and galena) usually form subhedral grains and vein infillings (Figure 3D), which are also apparently unaltered. Electron microscopic observations revealed the presence of accessory minerals: apatite, Ti oxides, dolomite, and Ca-Mn carbonate (Figure 3C), as well as manganese oxides. Traces of monazite were also encountered.

Table 3.
Chemical composition of the long-term stored waste rock used in the experiments.
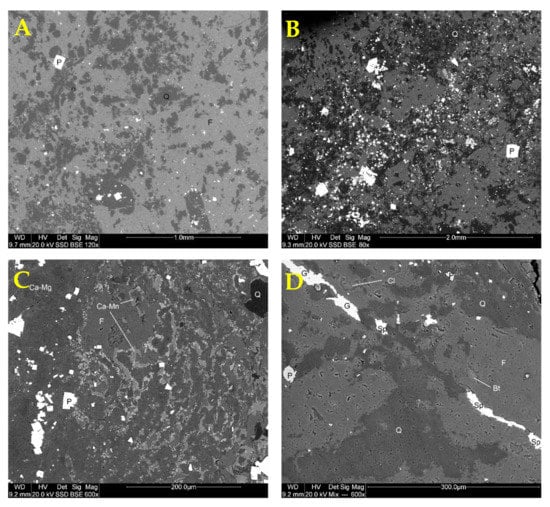
Figure 3.
Back-scattered electron images (BSE) of the waste rock. (A) structure of a typical pyrite-poor area, (B) structure of pyrite-rich area, (C) carbonate-rich area, (D) typical vein infilling with minor Zn and Pb sulfides. Explanations: Q—quartz, F—potassium feldspar, P—pyrite, Ca-Mg—calcium-magnesium carbonate (dolomite), Ca-Mn—calcium-manganese carbonate, Bt—biotite (mica), Cl—chlorite, Sp—sphalerite, G—galena.
3.2. Characterization of Mine Wastewater
In the area where the Gradir mine operates today, zinc and lead ores have been mined since the mid-20th century. Until the late 1990s, the deposit was mined through drilled tunnels. When mining ceased, the abandoned drifts began to be destroyed. Unused mining galleries began to be filled with water seeping through the rock mass. As a result of leaching rocks containing, among others, pyrite, the water has the character of acidic mine water (Table 4). Low pH, high concentrations of sulfate ions, and significant amounts of zinc, cadmium, or aluminum were recorded. It was observed that the outflow of water from the adit is almost constant during the year. It goes directly to the creek (Mjedenički), flowing through the mine area and then to the nearby river (Cehotina).

Table 4.
General characteristics of acid mine drainage (AMD) and flotation wastewater. Twelve-month average compared with the Standards for the industrial water discharge (Službeni list Crne Gore) [44].
As mentioned earlier, after extraction, crushing, and enrichment, zinc- and lead-bearing ore undergoes a flotation, which requires the use of significant amounts of various chemical compounds. The final product is a zinc and lead concentrate. The by-product is an alkaline flotation effluent. It contains off-balance amounts of zinc and lead and significant sulfate content (Table 4). The wastewater in the amount of 70 cubic meters per hour is discharged to a nearby pond where it is deposited. It is not subjected to further treatment.
The obtained results of samples taken each month over the year are shown in Tables S1 and S2. Due to heavy snowfall, it was not possible to take samples on the scheduled date in March 2018.
Changes in the pH of acid mine drainage were not significant. The minimum was 3.00, the maximum 3.26, and the average 3.11 (Figure 4). However, an upward trend is noticeable. The variation in conductivity was much greater. The maximum (9.64 mS·cm−1) was in April and the minimum (3.20 mS·cm−1) in December 2018. Throughout the year, it averages 6.22 mS·cm−1. Sulfate concentrations were changing. They reached their maximum (10,333 mg·L−1) at the beginning of the third quarter of 2018, then decreased in the following months until November (minimum 3700 mg·L−1). A similar trend can be seen in the case of most metals (Figure 4). Maximum concentrations or close to maximum concentrations were reached in July 2018. The following months brought about a decrease. From December 2018 onwards, the concentrations increased. The changes in the chemical composition of acid mine wastewater flowing from the abandoned mine adit are not expected to be a result of mine operations. The relationships of the determined parameters with average rainfall and average temperature were compared (Figure 4 and Figure S1). The amount of rainfall was observed to influence such parameters as conductivity and concentrations of sulfates and metal cations. As the amount of rainfall decreases, the mentioned above parameters also decrease, but with a one-, two-month delay. In the case of pH, this effect was not observed. Furthermore, in the temperature, its influence on pH is not observed, whereas the influence on other parameters is visible. However, the change in the values is seen about a month ahead of the temperature change. In June 2018, part of the tunnel collapsed. Unfortunately, the technical conditions of the adit did not allow for safe penetration. Undoubtedly, this incident could have affected the results obtained.
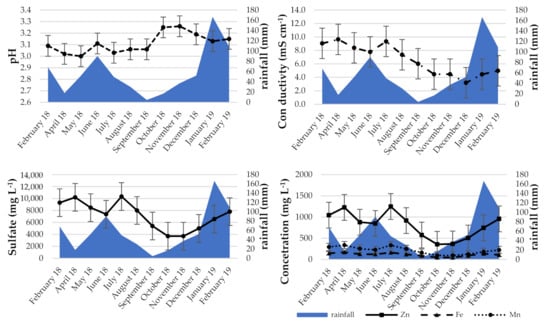
Figure 4.
Evolution of selected parameters in AMD with rainfall data. Metrological seasons: Spring: 1 March to 31 May; Summer: 1 June to 31 August; Autumn: 1 September to 30 November; Winter: 1 December to 28 February.
It can be noted that for flotation wastewater, the pH varies from 11.32 to 12.56, with an average of 12.10. There was no correlation observed between pH and conductivity (or other parameters), which ranged from 0.99 to 5.04 mS·cm−1 (average 2.69 mS·cm−1) (Figure 5). It can be assumed that the physicochemical parameters of flotation wastewater do not depend on seasonal variations. They could be dependent on the processes taking place in the tailing pond. High summer temperatures are conducive to the evaporation of water and consequently impact the concentration of the solution. On the other hand, rainfall and spring melting of snow can cause dilution. It is also possible that some of the elements, together with the sediment, are bound in the lower parts of the settling tank. However, it seems that changes in the flotation, such as the amount of reagent added, shutdowns, failures, etc., have the greatest impact on the chemical composition of flotation waters. The content of lead varied from 0.03 to 3.48 mg·L−1. The calculated average was 0.68 mg·L−1. Differences are visible in individual months. The maximum was determined in May 2018, and the minimum was observed in December 2018 and January 2019 when the new tailing pond was launched. The sulfate content also varied: the minimum amount was 440 and the maximum 1100 mg·L−1. The average concentration was determined at the level of 774 mg·L−1.
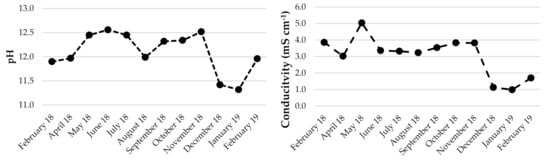
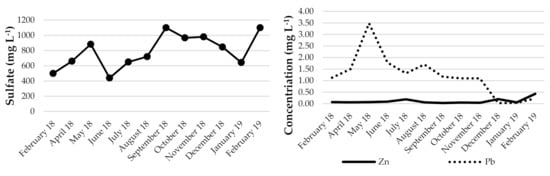
Figure 5.
Evolution of selected parameters in flotation wastewater.
3.3. Flotation Wastewater Neutralization by Acid Mine Drainage
The essence of this test was to determine the scope to neutralize flotation wastewater by acid mine drainage. As presented above, the flotation wastewater had a high pH of ~12, and AMD had a low pH of ~3, while the water discharged into the river should have a pH of 7–8. Thus, acid mine waters were used to neutralize alkaline flotation wastewater. For titration, 25 mL of post-flotation effluent was used, to which acidic mine waters were added, and pH was monitored with pH-meter. The titration was carried out in five independent series, and the averaged results are presented in Table S3 and Figure 6.
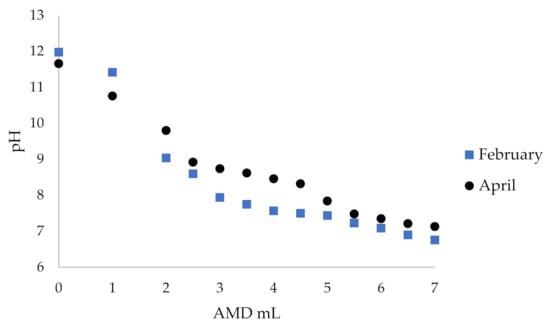
Figure 6.
Titration of flotation wastewater with acid mine drainage from the adit collected in February and April.
Based on the results obtained, it can be concluded that four times less acid mine drainage from the adit (1:0.24 v/v) should be used to neutralize the flotation wastewater water to pH 7. It is also possible to reduce sulfate content from circa 10,000 to less than 2500 mg·L−1 (Tables S1 and S3). The experiment was conducted to observe the influence of the aggregate on the physicochemical composition of the medium. When acidic water is mixed with alkaline water, a colloidal mixture is formed, which then settles down. The sediments precipitated at pH 7 had a creamy color. An attempt was made to assess the structure of the sediment. As can be seen on the XRD pattern below (Figure 7), the crystalline iron (both ferrous and ferric), zinc and manganese sulfate hydrates as well as calcium and magnesium carbonates dominated the precipitate composition with an admixture of aluminum hydroxide.
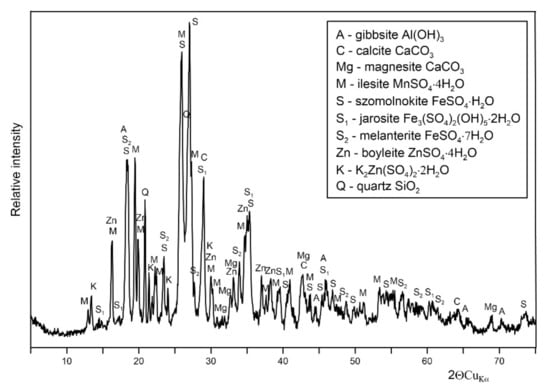
Figure 7.
X-ray diffraction pattern of the sediment formed after mixing AMD and flotation wastewater.
3.4. Mine Wastewater Neutralization by Waste Rock—Batch Test 1
In order to verify which solid material (short- or long-term stored) should be used for the neutralization of mine wastewater, an initial 84-h experiment was carried out. A batch adsorption test was conducted by mixing a known weight of long-term and short-term stored waste rock with 10 mL of water, flotation wastewater, and AMD. The mixture was shaken in a mechanical shaker for 84 h. After a specified time, the pH and conductivity were measured.
Water as a medium was used to verify how the length of storage of waste rock affects pH and conductivity. A pH increase could be observed, especially in the case of small weights (Table 6). While larger weights showed a slight increase in pH, when compared to pure water, conductivity rose significantly. On the other hand, small weights had a minor influence on the conductivity value. The pH of the water remained close to neutral with bigger weights in waste rock stored for a shorter period (Table 5). The conductivity, however, increased similarly when solid waste stored for a long time was used. In the case of small weights, an increase in both pH and conductivity was observed.

Table 5.
Waste rocks stored for a short and long amount of time treated with water, flotation wastewater, and AMD. The pH and conductivity were measured after 84 h of mixing.
In order to neutralize flotation wastewater, it is necessary to use bigger weights. A significant drop in pH was visible for a 10-gram weighting. For the long-term stored waste rock, values close to neutral were obtained (Table 5). The short-term stored waste rock showed instability, which was expressed by a definite difference in conductivity. Short-term stored waste rocks are unoxidized and are highly reactive; its properties change significantly in a short time. This effect was also observed in the case of the long-term stored material, where the values for the respective weightings were similar (1.71–1.82 mS·cm−1).
The influence of short- and long-term storage of waste rock on the changes in acid mine drainage parameters was also tested within 84 h. The use of 10 grams of long-term stored solid waste was also much more effective here. In this case, it was possible to increase the pH from 2.83 up to 6.24 (5.70 in the case of the short-term stored solid waste) (Table 5). Longer contact time may encourage a further decrease in pH. Short-term stored solid waste did not demonstrate any significant changes in acid mine drainage conductivity. The use of long-term stored solid waste caused only a slight reduction in conductivity: from 5.21 to 4.15.
It can be concluded from the conducted studies that long-term storage of waste rock is more effective in neutralizing both alkaline and acidic leachate. It is worth noting that the storage time of the waste rock may be critical due to the influence of atmospheric conditions.
3.5. Mine Wastewater Neutralization by Waste Rock—Batch Test 2
Long-term stored waste rock and four liquids were selected for the study. This time, a mixture of AMD and flotation wastewater, alongside AMD and flotation wastewater, was used. Distilled water was used as a control. As noted in previous experiments, it is possible to neutralize flotation wastewater using AMD. Therefore, considering that waste rock can lower the pH, AMD and flotation wastewater were mixed to reach a pH of 9 only. An appropriate weight (20 g) was placed in vessels with 20 mL of each medium. Then, the whole sample was shaken for 96 h. After a selected time, pH and conductivity were measured. At the beginning and end of the experiment, the sulfate concentration was determined. The results are given in Table 6.

Table 6.
Neutralization of four selected liquids by long-term stored waste rocks 20 g of the waste rocks was mixed with 20 mL of the liquid.
Analyzing the results, it can be concluded that by using long-term stored waste rock, it is possible to neutralize AMD, flotation wastewater, and their mixtures. As shown in Table 6, a sharp change in pH occurred after 24 h. If the contact time is extended, the reaction was stabilized and the measurements in the following days brought similar results (respectively). Furthermore, as the control test showed, a 96 h contact time did not fundamentally change the pH of the water. The use of long-term stored waste rock in leachate treatment poses the risk of increasing the load of undesired components. As a result of the processes and reactions occurring on the surface of rocks, elements released from the used aggregate may be released into solution. The chemical results of the tests carried out after the 96-h experiment are presented in Table 7. They are compared with the initial results for AMD and flotasation wastewater used in the experiment. In the case of AMD neutralization with waste rock, a slight increase in lead concentration was observed. However, an essential decrease in zinc concentration was visible. Furthermore, the sulfate content decreased by over 1000 mg·L−1. Using waste rock for flotation wastewater neutralization had the opposite effect. Both zinc and sulfate concentrations increased. As the results of the experiment show, to utilize AMD, flotation wastewater, and waste rock, it seems optimal to initially lower the flotation wastewater pH by mixing with AMD and then adding waste rock. Such a solution leads to pH neutralization and a slight increase in conductivity. It must be admitted that the concentration of Zn and sulfates increased, though in a range similar to other variants, and even less than in the use of AMD to neutralize flotation wastewater.

Table 7.
Comparison of the selected results of physicochemical parameters of flotation wastewater, AMD, and mixtures of long-term stored waste rocks, and proposed medium.
4. Discussion
Regardless of the many efforts, the majority of the mining waste generated still goes to waste disposal sites [2,45]. Increasing emphasis on protection against climate change and concern for environmental protection have resulted in the mining industry beginning to change its approach to waste management [46]. In the presented case, the waste includes AMD flowing from abandoned adits, slurry from the processing of zinc and lead ore stored in the tailings storage facility, and finally, waste rocks stored on the slopes of the mountain. The XRD analysis shows that alongside quartz and potassium feldspar, the rock material contains phyllosilicates as well as sulfides iron, zinc, lead. What is characteristic for rocks containing sulfide minerals where besides pyrite, there are also other sulfide minerals containing valuable metals such as gold, silver, copper, lead, and zinc [14,47]. As Lefebvre [48] noted, oxidation of sulfide minerals occurs gradually. Once the pyrite close to the surface of the mineral is oxidized, the oxidant has to penetrate deep into the rock. This is following the observations made during the experiments. The rock material deposited no more than a month (short-term stored) reacted more strongly than that which has been longer (long-term stored) in contact with the atmospheric conditions. Mining waste containing sulfate-bearing minerals exposed to atmospheric oxygen and water causes the uncontrolled formation of AMD [49]. Moreover, the accurate prediction of the volume of released AMD is difficult not only due to the mentioned gradual oxidation but also dependent on co-occurring minerals. The presence, among others, of carbonates and silicates may cause neutralization of the leachate [36,50,51].
The solubility of carbonates, depending on the pH, increases the amount of carbonate ions in the solution, thus increasing the potential to neutralize the solution. As it progresses, the pH increases and the calcite may precipitate as a secondary mineral. The dissolution of silicates is much slower than carbonates. The feldspar weathering process depends mainly on pH, silica concentration, Na, K, and Ca. It results in the formation of secondary minerals such as muscovite, kaolinite, and gibbsite [52]. The experiments carried out have shown that waste rocks are very reactive. The scope to change the pH values of the leaching medium, even in a short time, is significant. By using acid mine wastewater as a leaching solution after 24 h, a pH above 6 was obtained (at the beginning, 2.85). However, there is a danger that the acidification of the solution may occur again over time. Still, this solution brings a 31% reduction in sulfate and a 79% reduction in zinc concentration. This is probably due to the precipitation of sulfate hydrates typical of the AMD environment. Unfortunately, a 2.5 increase in lead concentration was observed. The effect of using solid waste to neutralize flotation wastewater appeared to be unsuccessful. Admittedly, a sharp drop in pH and lead but with the simultaneous growth of zinc and sulfate concentrations.
Abandoned underground mines are quite a challenging task. Mining shafts very often are collapsed and filled with water. Access to them is very difficult and it is usually impossible to reach the precise plans [53]. The Gradir Montenegro mine is no different. Most of the over 50,000 meters of underground adits are already inundated and destroyed. Flooding out water is characterized by very low pH values (avg. 3.11) and substantial sulfate content (avg. 6769 mg·L−1). It was observed that the chemical composition depended on weather conditions. As the temperature and the amount of rainfall decreased, the concentrations of sulfates and metal cations also decreased. Considering the significantly elevated content of metals, including heavy metals, the acidic leachate may have an irreversible impact on the environment [54,55,56,57,58]. Alkaline substances are often used to neutralize AMD [18,59,60,61]. Their use allows to reduce the metal content almost completely with a 60% reduction of sulfates [62]. Cost estimates and limitations of the use of alkaline substances were undertaken by Taylor et al. [63]. One of the most well studied and employed methods is based on chemical neutralization with the use of limestone [22], which is characterized by its low cost and wide availability. The result is a pH increase and, by precipitation, metal removal [17,28]. However, this method has some limitations due to its tendency to form an external coating caused by secondary mineral precipitation [29]. In turn, in the presence of aqueous sulfate a gypsum sediment precipitates [30]. What is more, such a solution would be effective enough for AMD treatment only when the formation of AMD was temporary or short-term [15]. The process creates significant amounts of metal hydroxide sediment. As described by Sibrell and Watten [64], the neutralization of 50 metric tons of AMD generated 450 metric tons of sludge, the disposal of which was costly. In the case of the mine described here, it is estimated that from only one abandoned adit, about 30 cubic meters per hour flows out. Thus, almost 263,000 cubic meters are required to be neutralized during the year.
The extraction of metals from sulfide ores results in significant amounts of waste. The amount of waste generated by the mines might be close to the volume of processed raw material [65]. As it has been proven, the tailing content is related to a specific process, its modifications, failures, etc. used in the mine. Still, the most common method is depositing it into sedimentary ponds without any treatment [33,66,67]. Tailings no longer used are protected by a layer of soil. However, without a protective plant cover, wind and water erosion is possible, resulting in the release of pollutants into the environment [68,69,70]. Some solutions have been proposed using different plants or microorganisms not only for stabilization but also for pond remediation [32,71,72,73,74]. As mentioned, these are only applicable to tailings no longer in use. Attempts have been made to treatment alkaline wastewater from an operating mine by O’Sulivan et al. [75] using Surface-Flow Wetlands. The pilot-scale installation has been in operation for two years. The chemical composition of the slurry was quite similar to that of the one being treated in this paper—sulfates 900 mg·L−1, lead 0.15 mg·L−1, and zinc 2.0 mg·L−1. Unfortunately, the authors do not mention a pH value. At the flow of 1.5 L min−1, it was possible to reduce sulfate and lead concentrations by about 30% and 74% of zinc.
The authors propose the use of acidic mine drainage to neutralize alkaline flotation leachate. The conducted experiments indicated that it is possible to reduce the pH from ~12 to values close to neutral by using a volume of acidic mine wastewater four times smaller than that of flotation wastewater. After mixing both streams, colloidal sediment was formed, constituting about 1/6 of the volume. Besides the iron, zinc, and manganese sulfate hydrates calcium and magnesium carbonates prevailed in the precipitate. As expected, gibbsite was observed among the secondary minerals. The final solution contained four times less sulfate than the flotation wastewater and AMD combined. However, the value of the mentioned parameter does not allow for safe discharge into the environment. Still, it is worth considering the use of the AMD flowing from the abandoned adit for the neutralization of all flotation wastewater (~70 cubic meters per hour) instead of deposition in the tailing pond. The received solution could then be treated using a passive treatment system, for example, constructed wetlands.
The experiences described above have led to the proposal to use three waste materials for mutual treatment. In the first step, mixing the flotation wastewater with AMD to pH 9 causes fewer secondary minerals to be precipitated and thus formation less sludge. Simultaneously, the sulfate concentration decreased. In the next step, mixing the resulting mixture with the rock material results in neutralization of the pH, reduction of metal and sulfate concentrations (compared to a total of three waste materials). The management of the three waste materials produced in the mining of zinc and lead does not require the use of any additional reagents (e.g., limestone) and although the parameters of the obtained solution do not meet the legislative requirements, their further treatment seemed to be relatively easy.
5. Conclusions
The rock waste material stored on the hillsides is a quartz-feldspar rock still containing certain amounts of zinc and lead. When exposed to weather conditions, it can be a potential source of acidic mine water. The flotation wastewater stored in the sedimentary tailings is characterized by a high pH (about 12), elevated sulfate, and low metal concentrations. Acid mine drainage filling abandoned adits have an acidic pH (about 3). They also have a significant amount of sulfates and metals. The chemical composition of these two solutions appear to change over time. In the case of flotation wastewater, these changes result from changes in the flotation. However, AMD has a rather seasonal background. The performed experiments proved that AMD can be used to neutralize flotation wastewater. Positive results indicate that four times less AMD is sufficient for the pH neutralization of the final solution. Furthermore, the possibility of using both liquid and waste rocks was proven. Premixing alkaline flotation wastewater with AMD and then flowing through waste rock neutralized the pH and reduced the total charge of metals and sulfates. The proposed solution does not require the use of additional chemical reagents and minimizes the sediments needed for storage.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/10/12/1061/s1, Figure S1: Evolution of selected parameters in acid mine drainage (AMD) with temperature data. Table S1: Monitoring results of AMD, Table S2: Monitoring results of tailings, Table S3: Titration of tailings using acid mine drainage (collected in February and April) from the adit.
Author Contributions
Conceptualization, J.R., and L.D.; methodology, J.R., G.R., T.B.; validation, J.R., T.B., G.R.; formal analysis, J.R., L.D. investigation, J.R., G.R., T.B.; writing—original draft preparation, J.R.; writing—review and editing, L.D.; visualization, J.R., T.B., G.R.; project administration, L.D.; funding acquisition, L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Team Tech programme of Foundation for Polish Science No. POIR.04.04-00-00-2053/16 (TEAM TECH 2016-2/9) as a part of Measure 4.4 of the 2014–2020 Smart Growth Operational Programme, EU.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Recycling, reuse and rehabilitation of mine wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B.G. Mine Wastes: Past, Present, Future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Bian, Z.; Miao, X.; Lei, S.; Chen, S.; Wang, W.; Struthers, S. The Challenges of Reusing Mining and Mineral-Processing Wastes. Science 2012, 337, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, A.M.; DelValls, A.; Nieto, J.M.; Salamanca, M.J.; Carballo, M.A. Toxicity and potential risk assessment of a river polluted by acid mine drainage in the Iberian Pyrite Belt (SW Spain). Sci. Total Environ. 2001, 409, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.A.; Santisteban, M.; de la Torre, M.L.; Davila, J.M.; Perez-Ostale, E. Map of impact by acid mine drainage in the river network of The Iberian Pyrite Belt (Sw Spain). Chemosphere 2018, 199, 269–277. [Google Scholar] [CrossRef]
- Byrne, P.; Wood, P.J.; Reid, I. The impairment of river systems by metal mine contamination: A review including remediation options. Crit. Rev. Environ Sci. Technol. 2012, 42, 2017–2077. [Google Scholar] [CrossRef]
- Iribar, V.; Izco, F.; Tames, P.; Antiguedad, I.; da Silva, A. Water contamination and remedial measures at the Troya abandoned Pb-Zn mine (The Basque Country, Northern Spain). Environ. Geol. 2000, 39, 800–806. [Google Scholar] [CrossRef]
- Lebre, E.; Corder, G.D.; Golev, A. Sustainable practices in the management of mining waste: A focus on the mineral resource. Miner. Eng. 2017, 107, 34–42. [Google Scholar] [CrossRef]
- Durucan, S.; Korre, A.; Munoz-Melendez, G. Mining life cycle modelling: A cradle-to-gate approach to environmental management in the minerals industry. J. Clean. Prod. 2006, 14, 1057–1070. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Yuniati, M.D.; Kitagawa, K.; Hirajima, T.; Miki, H.; Okibe, N.; Sasaki, K. Suppression of pyrite oxidation in acid mine drainage by carrier microencapsulation using liquid product of hydrothermal treatment of low-rank coal, and electrochemical behavior of resultant encapsulating coatings. Hydrometallurgy 2015, 158, 83–93. [Google Scholar] [CrossRef]
- Li, X.; Hiroyoshi, N.; Tabelin, C.B.; Naruwa, K.; Harada, C.; Ito, M. Suppressive effects of ferric-catecholate complexes on pyrite oxidation. Chemosphere 2019, 214, 70–78. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, P.S.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef]
- Chopard, A.; Benzaazoua, M.; Bouzahzah, H.; Plante, B.; Marion, P. A contribution to improve the calculation of the acid generating potential of mining wastes. Chemosphere 2017, 175, 97–107. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Schaider, L.A.; Senn, D.B.; Estes, E.R.; Brabander, D.J.; Shine, J.P. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total Environ. 2014, 490, 456–466. [Google Scholar] [CrossRef]
- Soni, A.K.; Mishra, B.; Singh, S. Pit lakes as an end use of mining: A review. J. Min. Environ. 2014, 5, 99–111. [Google Scholar] [CrossRef]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Li, H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Pyrbot, W.; Shabong, L.; Singh, O.P. Neutralization of acid mine drainage contaminated water and ecorestoration of stream in a coal mining area of east Jaintia Hills, Meghalaya. Mine Water Environ. 2019, 38, 551–555. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Mittal, H.; Waanders, F.; Ray, S.S. Thermodynamic properties and adsorption behavior of hydrogel nanocomposites for cadmium removal from mine effluents. J. Ind. Eng. Chem. 2017, 48, 151–161. [Google Scholar] [CrossRef]
- Kaartinen, T.; Laine-Ylijoki, J.; Ahoranta, S.; Korhonen, T.; Neitola, R. Arsenic removal from mine waters with sorption techniques. Mine Water Environ. 2017, 36, 199–208. [Google Scholar] [CrossRef]
- Garcia, V.; Hayrynen, P.; Landaburu-Aguirre, J.; Pirila, M.; Keiski, R.L.; Urtiaga, A. Purification techniques for the recovery of valuable compounds from acid mine drainage and cyanide tailings: Application of green engineering principles. J. Chem. Technol. Biotechnol. 2013, 89, 803–813. [Google Scholar] [CrossRef]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Jacobs, J.A.; Lehr, J.H.; Testa, S.M. (Eds.) Acid Mine Drainage, Rock Drainage, and Acid Sulfate Soils. Causes, Assessment, Prediction, Prevention, and Remediation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Offeddu, F.G.; Cama, J.; Solera, J.M.; Dávila, G.; McDowell, A.; Craciunescu, T.; Tiseanu, I. Processes affecting the efficiency of limestone in passive treatments for AMD: Column experiments. J. Environ. Chem. Eng. 2015, 3, 304–316. [Google Scholar] [CrossRef]
- Othmani, M.A.; Souissi, F.; Bouzahzah, H.; Bussiere, B.; Silva, E.F.; Benzaazoua, M. The flotation tailings of the former Pb-Zn mine of Touiref (NW Tunisia): Mineralogy, mine drainage prediction, base-metal speciation assessment and geochemical modeling. Environ. Sci. Pollut. Res. 2015, 22, 2877–2890. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E.; Gambus, F.; Muszynska, E.; Czech, T. Phytostabilization of Zn-Pb ore flotation tailings with Dianthus carthusianorum and Biscutella laevigata after amending with mineral fertilizers or sewage sludge. J. Environ. Manag. 2017, 189, 75–83. [Google Scholar] [CrossRef]
- Stankovic, V.; Milosevic, V.; Milicevic, D.; Gorgievski, M.; Bogdanovic, G. Reprocessing of the old flotation tailings deposited on the RTB Bor tailings pond—A case study. Chem. Ind. Chem. Eng. Q. 2018, 24. [Google Scholar] [CrossRef]
- Khalil, A.; Argane, R.; Benzaazoua, M.; Bouzahzah, H.; Taha, Y.; Hakkou, R. Pb–Zn mine tailings reprocessing using centrifugal dense media separation. Miner. Eng. 2019, 131, 28–37. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Dimitrijevic, M.D.; Stevanovic, Z.O.; Serbula, S.M.; Bogdanovic, G.D. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 158, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.; Meima, J.A. Characterization and Economic Potential of Historic Tailings from Gravity Separation: Implications from a Mine Waste Dump (Pb-Ag) in the Harz Mountains Mining District, Germany. Minerals 2019, 9, 303. [Google Scholar] [CrossRef]
- Shadrunova, I.V.; Orekhova, N.N. A Process for Advanced Recycling of Water Originating from Mining Operations, with Metal Recovery. Mine Water Environ. 2015, 34, 478–484. [Google Scholar] [CrossRef]
- Wolkersdorfer, C.; Bowell, R. Contemporary Reviews of Mine Water Studies in Europe, Part 2. Mine Water Environ. 2005, 24, 2–37. [Google Scholar] [CrossRef]
- Geological Map of the Exploration Area. Available online: http://videolectures.net/outbursts_herlec_sshm/ (accessed on 17 October 2020).
- Gutierrez, M.; Mickus, K.; Camacho, L.M. Abandoned Pb-Zn mining wastes and their mobility as proxy to toxicity: A review. Sci. Total Environ. 2016, 565, 393–400. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef]
- Jennett, J.C.; Wixson, B.G. Problems in lead mining waste control. J. Water Pollut. Control Fed. 1972, 44, 2103–2110. [Google Scholar]
- Wang, L.K.; Shammas, N.K.; Selke, W.A.; Aulenbach, D.B. (Eds.) Flotation Technology; Humana Press: London, UK, 2010. [Google Scholar] [CrossRef]
- Službeni List Crne Gore, broj 26/2012 od 24.05.2012. Available online: http://www.sluzbenilist.me/pregled-dokumenta/?id={E16B3C65-E56B-4812-A15D-022796224105} (accessed on 17 October 2020).
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste through an Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef]
- Guerin, T.F. Heavy equipment maintenance wastes and environmental management in the mining industry. J. Environ. Manag. 2002, 66, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bonnissel-Gissinger, P.; Alnot, M.; Ehrhardt, J.J.; Behera, P. Surface Oxidation of Pyrite as a Function of pH. Environ. Sci. Technol. 1998, 32, 2839–2845. [Google Scholar] [CrossRef]
- Lefebvre, R.; Gelinas, P.J. Numerical modeling of AMD production in waste rock dumps. In Proceedings of the Sudbury ’95, Conference on Mining and the Environment, Sudbury, ON, Canada, 28 May–1 June 1995. [Google Scholar]
- Jamieson, H.E. Geochemistry and Mineralogy of Solid Mine Waste: Essential Knowledge for Predicting Environmental Impact. Elements 2011, 7, 381–386. [Google Scholar] [CrossRef]
- Becker, M.; Dyantyi, N.; Broadhurst, J.L.; Harrison, S.T.L.; Franzidis, J.P. A mineralogical approach to evaluating laboratory scale acid rock drainage characterisation tests. Miner. Eng. 2015, 80, 33–36. [Google Scholar] [CrossRef]
- Karlsson, T.; Raisanen, M.L.; Lehtonen, M.; Alakangas, L. Comparison of static and mineralogical ARD prediction methods in the Nordic environment. Environ. Monit. Assess. 2018, 190, 719. [Google Scholar] [CrossRef]
- Dold, B. Basic concepts in environmental geochemistry of sulfidic mine-waste management. In Waste Management; Kumar, S.E., Ed.; IntechOpen: London, UK, 2010; ISBN 978-953-7619-84-8. [Google Scholar]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Rodriguez, L.; Ruiz, E.; Alonso-Azcarate, J.; Rincon, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Casiot, C.; Egal, M.; Elbaz-Poulichet, F.; Bruneel, O.; Bancon-Montigny, C.; Cordier, M.-A.; Gomez, E.; Aliaume, C. Hydrological and geochemical control of metals and arsenic in a Mediterranean river contaminated by acid mine drainage (the Amous River, France); preliminary assessment of impacts on fish (Leuciscus cephalus). Appl. Geochem. 2009, 24, 787–799. [Google Scholar] [CrossRef]
- Strosnider, W.H.J.; Llanos Lopez, F.S.; Nairn, R.W. Acid mine drainage at Cerro Rico de Potosı’ II: Severe degradation of the Upper Rio Pilcomayo watershed. Environ. Earth Sci. 2011, 64, 911–923. [Google Scholar] [CrossRef]
- Cherry, D.S.; Currie, R.J.; Soucel, D.J.; Latimer, H.A.; Trent, G.C. An integrative assessment of a watershed impacted by abandoned mined land discharges. Environ. Pollut. 2001, 111, 377–388. [Google Scholar] [CrossRef]
- Uster, B.; O’Sullivan, A.D.; Ko, S.Y.; Evans, A.; Pope, J.; Trumm, D.; Caruso, B. The use of mussel shells in upward-flow sulfate-reducing bioreactors treating acid mine drainage. Mine Water Environ. 2015, 34, 442–454. [Google Scholar] [CrossRef]
- Doye, I.; Duchesne, J. Neutralisation of acid mine drainage with alkaline industrial residues: Laboratory investigation using batch-leaching tests. Appl. Geochem. 2003, 18, 1197–1213. [Google Scholar] [CrossRef]
- Iakovleva, E.; Makila, E.; Salonen, J.; Sitarz, M.; Wang, S.; Sillanpaa, M. Acid mine drainage (AMD) treatment: Neutralization and toxic elements removal with unmodified and modified limestone. Ecol. Eng. 2015, 81, 30–40. [Google Scholar] [CrossRef]
- Garcia-Valero, A.; Martinez-Martinez, S.; Faz, A.; Rivera, J.; Acosta, J.A. Environmentally sustainable acid mine drainage remediation: Use of natural alkaline material. J. Water Process. Eng. 2020, 33, 101064. [Google Scholar] [CrossRef]
- Tolonen, E.T.; Sarpola, A.; Hu, T.; Ramo, J.; Lassi, U. Acid mine drainage treatment using by-products from quicklime manufacturing as neutralization chemicals. Chemosphere 2014, 117, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Pape, S.; Murphy, N. A Summary of Passive and Active Treatment Technologies for Acid and Metalliferous Drainage (AMD). In Proceedings of the Fifth Australian Workshop on Acid Drainage, Fremantle, Australia, 29–31 August 2005. [Google Scholar]
- Sibrell, P.L.; Watten, B.J. Evaluation of sludge produced by limestone neutralization of AMD at the Friendship Hill National Historic Site. In Proceedings of the 2003 National Meeting of the American Society of Mining and Reclamation and the 9th Billings Land Reclamation Symposium Billings MT, ASMR, Lexington, KY, USA, 3–6 June 2003; pp. 1151–1169. [Google Scholar] [CrossRef]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Harrison, J.; Heijnis, H.; Caprarelli, G. Historical pollution variability from abandoned mine sites, Greater Blue Mountains World Heritage Area, New South Wales, Australia. Environ. Geol. 2003, 43, 680–687. [Google Scholar] [CrossRef]
- Bauerek, A.; Cabala, J.; Smieja-Krol, B. Mineralogical alterations of Zn-Pb flotation wastes of the Mississippi Valley type ores (Southern Poland) and their impact on contamination of rain water runoff. Pol. J. Environ. Stud. 2009, 18, 781–788. [Google Scholar]
- Zhang, S.; Li, T.; Huang, H.; Zou, T.; Zhang, X.; Yu, H.; Zheng, Z.; Wang, Y. Cd accumulation and phytostabilization potential of dominant plants surrounding mining tailings. Environ. Sci. Pollut. Res. 2012, 19, 3879–3888. [Google Scholar] [CrossRef]
- Lekovski, R.; Mikic, M.; Krzanovic, D. Impact of the flotation tailing dumps on the living environment of Bor and protective measures. Min. Metall. Eng. Bor 2013, 2, 97–116. [Google Scholar] [CrossRef]
- Pajak, M.; Gasiorek, M.; Cygan, A.; Wanic, T. Concentrations of Cd, Pb and Zn in the top layer of soil and needles of Scots Pine (Pinus Sylvestris L.); A case study of two extremely different conditions of the forest environment in Poland. Fresenius Environ. Bull. 2015, 24, 71–76. [Google Scholar]
- Krzaklewski, W.; Pietrzykowski, M. Selected physico-chemical properties of zinc and lead ore tailings and their biological stabilization. Water Air Soil Pollut. 2002, 141, 125–142. [Google Scholar] [CrossRef]
- Parraga-Aguado, I.; Querejeta, J.-I.; Gonzalez-Alcaraz, M.-N.; Jimenez-Carceles, F.J.; Conesa, H.M. Usefulness of pioneer vegetation for the phytomanagement of metal(loid)s enriched tailings: Grasses vs. shrubs vs. trees. J. Environ. Manag. 2014, 133, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Wong, J.W.C.; Wong, M.H.; Baker, A.J.M.; Shu, W.S.; Lan, C.Y. Revegetation of Pb/Zn Mine Tailings, Guangdong Province, China. Restor. Ecol. 2001, 8, 87–92. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huan, S.; Zhong, Y. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef]
- O’Sullivan, A.D.; Murray, D.A.; Otte, M.L. Removal of Sulfate, Zinc, and Lead from Alkaline Mine Wastewater Using Pilot-scale Surface-Flow Wetlands at Tara Mines, Ireland. Mine Water Environ. 2004, 23, 58–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).