Initial Stages of Gypsum Nucleation: The Role of “Nano/Microdust”
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Gypsum Nucleation Measurements
2.4. Chemical Speciations
3. Results
3.1. Stock Solutions Characterization
3.2. Gypsum Nucleation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radcliffe, T. Notes from the chemical laboratory of the University of North Carolina—Analysis of rock-salt from saltville, Washington co., va. J. Am. Chem. Soc. 1882, 4, 255–256. [Google Scholar] [CrossRef][Green Version]
- Van’T Hoff, J.H. Über Gips. Z. für Elektrochem. 1902, 8, 575–579. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Stawski, T.M.; Kellermeier, M. Calcium sulfate precipitation pathways in natural and engineered environments. Chem. Geol. 2019, 530, 119274. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Peacock, C.L.; Benning, L.G. Investigating the Effectiveness of Phosphonate Additives in Hindering the Calcium Sulfate Dihydrate Scale Formation. Ind. Eng. Chem. Res. 2020, 59, 14970–14980. [Google Scholar] [CrossRef]
- Schm, T.; Jungnickel, R.; Dariz, P. Insights into the CaSO4-H2O system: A Raman-spectroscopic study. Minerals 2020, 10, 115. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, T.; Cheng, Y.; Cohen, Y. Observed crystallization induction time in seeded gypsum crystallization. Ind. Eng. Chem. Res. 2019, 58, 23359–23365. [Google Scholar] [CrossRef]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- Mady, M.F.; Kelland, M.A. Review of nanotechnology impacts on oilfield scale management. ACS Appl. Nano Mater. 2020, 3, 7343–7364. [Google Scholar] [CrossRef]
- Smith, B.R.; Sweett, F. The crystallization of calcium sulfate dihydrate. J. Colloid Interface Sci. 1971, 37, 612–618. [Google Scholar] [CrossRef]
- Liu, S.T.; Nancollas, G.H. The kinetics of crystal growth of calcium sulfate dihydrate. J. Cryst. Growth 1970, 6, 281–289. [Google Scholar] [CrossRef]
- Liu, S.T.; Nancollas, G.H. Linear crystallization and induction period studies of the growth of calcium sulfate dihydrate crystals. Talanta 1973, 20, 211–216. [Google Scholar] [CrossRef]
- Liu, S.T.; Nancollas, G.H. A kinetics and morphological study of the seeded growth of calcium sulfate dihydrate in the presence of additives. J. Colloid Interface Sci. 1975, 52, 593–601. [Google Scholar] [CrossRef]
- Packter, A. The precipitation of calcium sulphate dihydrate from aqueous solution: Induction periods, crystal numbers and final size. J. Cryst. Growth 1974, 21, 191–194. [Google Scholar] [CrossRef]
- He, S.; Oddo, J.E.; Tomson, M.B. The nucleation kinetics of calcium sulfate dihydrate in NaCl solutions up to 6 m and 90 C. J. Colloid Interface Sci. 1994, 162, 297–303. [Google Scholar] [CrossRef]
- Lancia, A.; Musmarra, D.; Prisciandaro, M. Measuring induction period for calcium sulfate dehydrate precipitation. AICHE J. 1999, 45, 390–397. [Google Scholar] [CrossRef]
- Klepetsanis, P.G.; Dalas, E.; Koutsoukos, P.G. Role of temperature in the spontaneous precipitation of calcium sulfate dehydrate. Langmuir 1999, 15, 1534–1540. [Google Scholar] [CrossRef]
- Hina, A.; Nancollas, G.H. Precipitation and dissolution of alkaline earth sulfates: Kinetics and surface energy. Rev. Mineral. Geochem. 2000, 40, 277–301. [Google Scholar] [CrossRef]
- Prisciandaro, M.; Lancia, A.; Musmarra, D. Gypsum nucleation into sodium chloride solutions. AICHE J. 2001, 47, 929–934. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Garcia-Ruiz, J.M.; Delgado-Lopez, J.M.; Sazaki, G. In situ observation of step dynamics on gypsum crystals. Cryst. Growth Des. 2010, 10, 3909–3916. [Google Scholar] [CrossRef]
- Stawski, T.M.; Van Driessche, A.E.; Ossorio, M.; Rodriguez-Blanco, J.D.; Besselink, R.; Benning, L.G. Formation of calcium sulfate through the aggregation of sub-3 nanometre primary species. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Stawski, T.M.; Van Driessche, A.E.S.; Besselink, R.; Byrne, E.H.; Raiteri, P.; Gale, J.D.; Benning, L.G. The structure of CaSO4 nanorods: The precursor of gypsum. J. Phys. Chem. C 2019, 123, 23151–23158. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallization. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Coelfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal nucleation in liquids: Open questions and future challenges in molecular dynamics simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef]
- Fan, C.; Pashley, R.M. The controlled growth of calcium sulfate dihydrate (gypsum) in aqueous solution using the inhibition effect of a bubble column evaporator. Chem. Eng. Sci. 2016, 142, 23–31. [Google Scholar] [CrossRef]
- Popov, K.; Oshchepkov, M.; Kovalenko, A.; Redchuk, A.; Dikareva, J.; Pochitalkina, I. The Scale nucleation natural precursors: A case study of “micro/nanodust” impurities nature in laboratory aqueous samples, obtained from Moscow tap water. Int. J. Corros. Scale Inhib. 2020, 9, 1097–1112. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Butterworth-Heinemann: Woburn, MA, USA, 2001. [Google Scholar]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Marshall, W.L.; Slusher, R. Thermodynamics of Calcium Sulfate Dihydrate in Aqueous Sodium Chloride Solutions, 0-1 10°112. J. Phys. Chem. 1966, 70, 4015–4027. [Google Scholar] [CrossRef]
- Raju, K.; Atkinson, G.J. The thermodynamics of «scale» mineral solubilities. 3. Calcium sulfate in aqueous sodium chloride. Chem. Eng. Data 1990, 35, 361–367. [Google Scholar] [CrossRef]
- Powell, K.J.; Pettit, L.D.; Town, R.M.; Popov, K.I. SolEq: Tools and tutorials for studying solution equilibria. Univ. Chem. Educ. 2000, 4, 7–11. [Google Scholar]
- SPECIES in Solution Equilibria: Principles and Applications [Windows 95, 98]; Powell, K.J., Ed.; Release 1.04; Academic Software: Timble, UK, 2000. [Google Scholar]
- Stability Constants Database and Mini-SCDatabase; IUPAC and Academic Software, Version 5.3; Sourby Old Farm: Timble, UK; Otley, UK; Yorks, UK, 2011.
- Craggs, A.; Moody, G.J.; Thomas, J.D.R. Calcium ion-selective electrode-measurements in the presence of complexing ligands. Analyst 1979, 104, 961–972. [Google Scholar] [CrossRef]
- International standard ISO 13321. Methods for Determination of Particle Size Distribution; Part 8: Photon correlation spectroscopy; International Organization for Standartisation (ISO): Geneva, Switzerland, 1996. [Google Scholar]
- Dahneke, B.E. (Ed.) Measurement of Suspended Particles by Quasi-Elastic Light Scattering; John Wiley Sons: Hoboken, NJ, USA, 1983. [Google Scholar]
- Abdel-Aal, E.A.; Abdel-Ghafar, H.M.; El-Sayed, D.; El-Shazly, A.N.; Hoinkis, J. Crystallization study of reverse osmosis desalination scales at low salinity with and without inhibitor. Part. Sci. Technol. 2017, 35, 749–754. [Google Scholar] [CrossRef]
- Ziegenheim, S.; Peintler, G.; Palinko, I.; Sipos, P. The kinetics of the precipitation of gypsum, CaSO4·2H2O, over a wide range of reactant concentrations. React. Kinet. Mech. Catal. 2020, 131, 75–88. [Google Scholar] [CrossRef]
- Azaza, H.; Doggaz, A.; Mechi, L.; Optasanu, V.; Tlili, M.; Ben Amor, M. Synthesis of intermediate crystal Ba1-xCaxSO4 system via co-precipitation of BaSO4-CaSO4 and partial hindrance of gypsum formation. Desalin. Water Treat. 2017, 66, 80–87. [Google Scholar] [CrossRef]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Wang, L.T.; Ge, H.H.; Han, Y.T.; Wan, C.; Sha, J.Y.; Sheng, K. Effects of Al2O3 nanoparticles on the formation of inorganic scale on heat exchange surface with and without scale inhibitor. Appl. Therm. Eng. 2019, 151, 1–10. [Google Scholar] [CrossRef]
- Gromov, S.L. Deposit formation in spiral-wound reverse osmosis and nanofiltration elements and ways of preventing it. Therm. Eng. 2014, 61, 433–441. [Google Scholar] [CrossRef]
- Nielsen, A.E.; Toft, J.M. Electrolyte crystal growth kinetics. J. Cryst. Growth 1984, 67, 278–288. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Tkachenko, S.; Redchuk, A.; Rönkkömäki, H.; Rudakova, G.; Pervov, A.; Popov, K. Visualization of a novel fluorescent-tagged bisphosphonate in reverse osmosis facility during gypsum brine desalination. Sep. Sci. Technol. 2021, 225, 117382. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Redchuk, A.; Tkachenko, S.; Pervov, A.; Popov, K. Gypsum crystallization during reverse osmosis desalination of water with high sulfate content in presence of a novel fluorescent-tagged polyacrylate. Crystals 2020, 10, 309. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Kamagurov, S.; Tkachenko, S.; Popov, K.; Ryabova, A. An insight into the mechanisms of the scale inhibition. A case study of a novel task-specific fluorescent-tagged scale inhibitor location on gypsum crystals. ChemNanoMat 2019, 5, 586–592. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Frolova, S.; Tkachenko, S.; Kamagurov, S.; Rudakova, G.; Popov, K. Synthesis and visualization of a novel fluorescent-tagged polymeric antiscalant during gypsum crystallization in combination with bisphosphonate fluorophore. Crystals 2020, 10, 992. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Popov, K.; Ryabova, A.; Redchuk, A.; Tkachenko, S.; Dikareva, J.; Koltinova, E. Barite crystallization in presence of novel fluorescent-tagged antiscalants. Int. J. Corros. Scale Inhib. 2019, 8, 998–1021. [Google Scholar] [CrossRef]
- Wu, W.; Nancollas, G.H. Determination of interfacial tension from crystallization and dissolution data: A comparison with other methods. Adv. Colloid Interface Sci. 1999, 79, 229–279. [Google Scholar] [CrossRef]
- Ben Ahmed, S.; Tlili, M.M.; Ben Amor, M. Influence of a polyacrylate antiscalant on gypsum nucleation and growth. Cryst. Res. Technol. 2008, 43, 935–942. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Abdel-Ghafar, H.M.; El Anadouli, B.E. New findings about nucleation and crystal growth of reverse osmosis desalination scales with and without inhibitor. Cryst. Growth Des. 2015, 15, 5133–5137. [Google Scholar] [CrossRef]
- ISO 14644-1. Part 1: Classification of air cleanliness by particle concentration. In Cleanrooms and Associated Controlled Environments, 2nd ed.; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Knotter, D.M.; Wolters, S.A.M.; Kasanrokijat, M.A. Particle concentration measurements in process liquids using light-scattering techniques. Part. Sci. Technol. 2007, 25, 435–447. [Google Scholar] [CrossRef]
- Peuchot, C.; Lynch, G.J.; Petillon, N. Filter testing: Automatic particle counters and fluid contamination monitors. Filtr. Sep. 2011, 48, 29–33. [Google Scholar] [CrossRef]
- Knotter, D.M.; Wali, F. Particles in semiconductor processing. Dev. Surf. Contam. Clean 2010, 2, 81–120. [Google Scholar] [CrossRef]
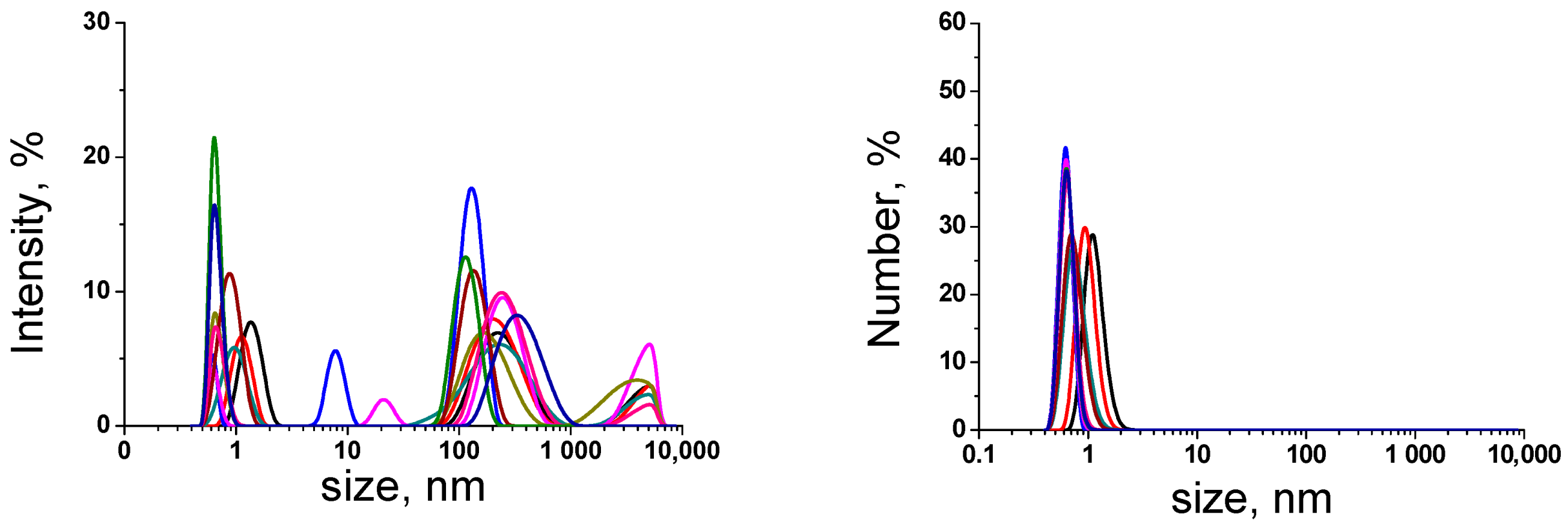
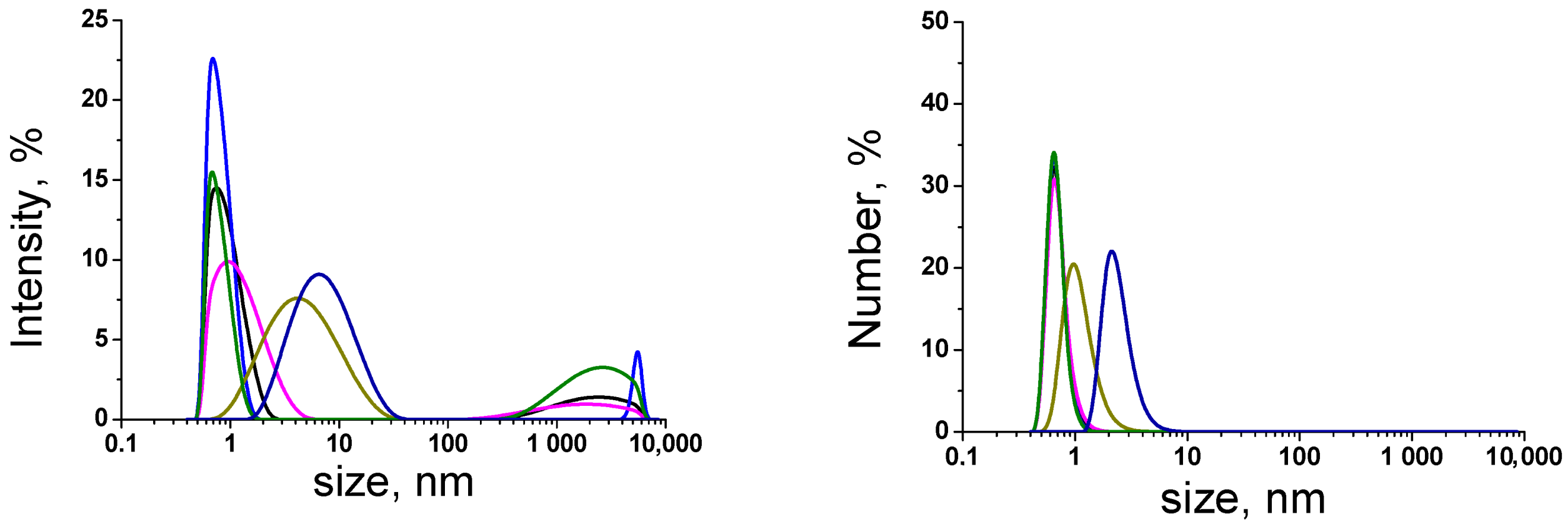


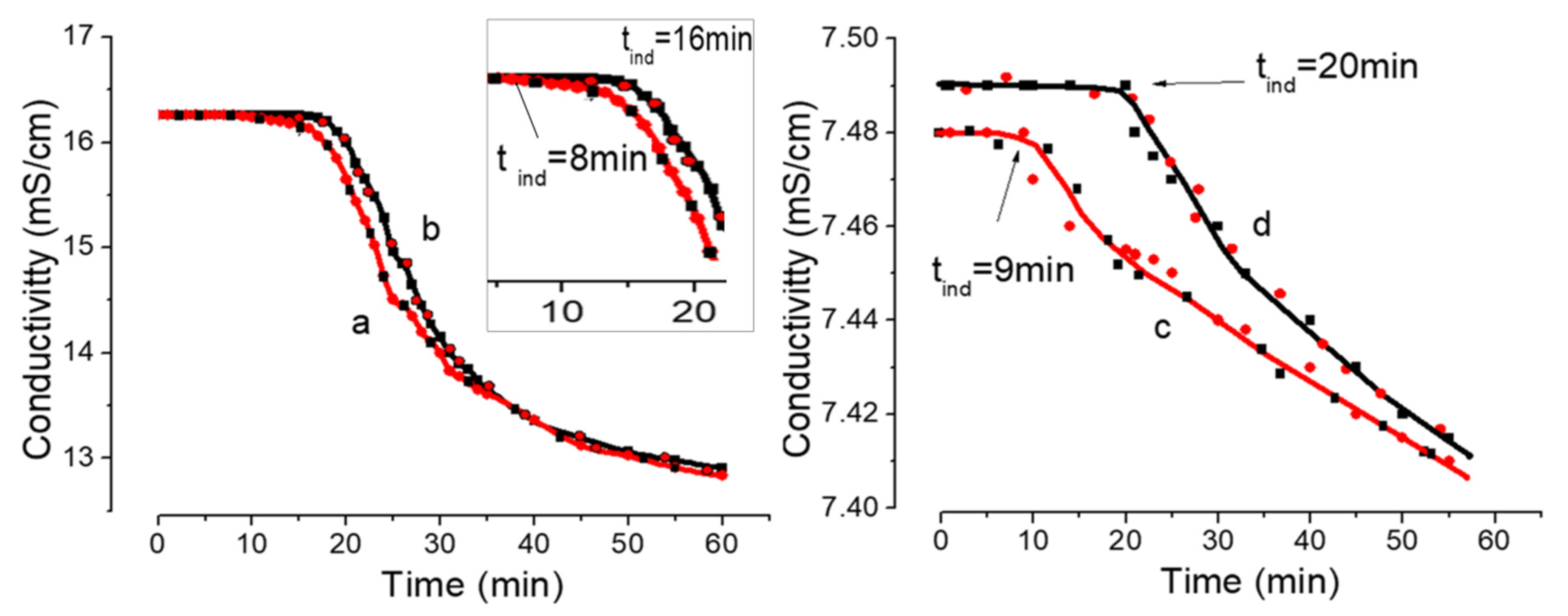
| Sample | Conductivity 25 °C; μS/cm | pH | Cumulative Number of Foreign Particles in 1 mL | Ref. | |||
|---|---|---|---|---|---|---|---|
| ≥100 nm | ≥200 nm | ≥300 nm | ≥500 nm | ||||
| In-house deionized water | 0.056 | 6.3 | 320 ± 10 | 60 ± 10 | 23 ± 8 | 9 ± 3 | Present work |
| Water for ion chromatography, Cat. #00612; Sigma-Aldrich | 1.8 | 5.6 | 3040 ± 20 | 630 ± 10 | 170 ± 20 | 130 ± 10 | [26] |
| In-house distilled water | <2.0 | 5.7 | Particles are not detected due to the limitations of a particle counter used in this work | 14,000 | [25] | ||
| Element | Element Content, ppm | |
|---|---|---|
| In-House Deionized Water; Present Study | Water for Ion Chromatography, Cat.#00612; Sigma-Aldrich [26] | |
| Al | 0.0011 ± 0.0001 | 0.0010 ± 0.0001 |
| Fe | 0.0012 ± 0.0001 | 0.0011 ± 0.0001 |
| Ca | Not found | 0.0005 ± 0.0001 |
| Na | Not found | 0.0005 ± 0.0001 |
| K | Not found | 0.0026 ± 0.0002 |
| Zn | 0.0006 ± 0.0001 | 0.0005 ± 0.0001 |
| Sample * | Concentration, mol·L‒1 | Sample Type | pH | Cumulative Number of Foreign Particles in 1 mL | |||
|---|---|---|---|---|---|---|---|
| ≥ 100 nm | ≥ 200 nm | ≥ 300 nm | ≥ 500 nm | ||||
| Na2SO4 solution | 0.10 | A | 6.7 | (370 ± 60)·103 | (92 ± 20)·103 | (30 ± 6)·103 | (13 ± 2)·103 |
| B | 6.6 | (40 ± 8) ·102 | 54 ± 10 | 13 ± 3 | 4 ± 2 | ||
| 0.06 | A | 6.6 | (300 ± 60)·103 | (80 ± 20)·103 | (20 ± 5)·103 | (8 ± 1)·103 | |
| B | 6.6 | (30 ± 6)·102 | 35 ± 8 | 10 ± 3 | 4 ± 2 | ||
| CaCl2 solution | 0.10 | A | 9.8 | (480 ± 70)·103 | (180 ± 40)·103 | (81 ± 20)·103 | (40 ± 8)·103 |
| B | 9.8 | (90 ± 3)·102 | 23 ± 4 | 9 ± 3 | 4 ± 2 | ||
| 0.06 | A | 9.7 | (330 ± 60)·103 | (50 ± 10)·103 | (20 ± 5)·103 | (9 ± 2)·103 | |
| B | 9.7 | (15 ± 3)·102 | 50 ± 10 | 20 ± 4 | 10 ± 3 | ||
| Gypsum Initial Conc., mol·L‒1 | % of [CaSO4]o Complexes * | Gypsum Solution Type | Cumulative Number of Foreign Particles (≥100 nm) in 1 mL | Induction Time, min. |
|---|---|---|---|---|
| 0.06 | 42 | A | (430 ± 70)·103 | 8 ± 1 |
| B | (30 ± 7)·102 | 16 ± 1 | ||
| 0.03 | 33 | A | (300 ± 60)·103 | 9 ± 1 |
| B | (20 ± 6)·102 | 20 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshchepkov, M.; Popov, K.; Kovalenko, A.; Redchuk, A.; Dikareva, J.; Pochitalkina, I. Initial Stages of Gypsum Nucleation: The Role of “Nano/Microdust”. Minerals 2020, 10, 1083. https://doi.org/10.3390/min10121083
Oshchepkov M, Popov K, Kovalenko A, Redchuk A, Dikareva J, Pochitalkina I. Initial Stages of Gypsum Nucleation: The Role of “Nano/Microdust”. Minerals. 2020; 10(12):1083. https://doi.org/10.3390/min10121083
Chicago/Turabian StyleOshchepkov, Maxim, Konstantin Popov, Anna Kovalenko, Anatoly Redchuk, Julia Dikareva, and Irina Pochitalkina. 2020. "Initial Stages of Gypsum Nucleation: The Role of “Nano/Microdust”" Minerals 10, no. 12: 1083. https://doi.org/10.3390/min10121083
APA StyleOshchepkov, M., Popov, K., Kovalenko, A., Redchuk, A., Dikareva, J., & Pochitalkina, I. (2020). Initial Stages of Gypsum Nucleation: The Role of “Nano/Microdust”. Minerals, 10(12), 1083. https://doi.org/10.3390/min10121083







