Wearable Activity Monitors to Quantify Gait During Stroke Rehabilitation: Data from a Pilot Randomised Controlled Trial Examining Auditory Rhythmical Cueing
Abstract
1. Introduction
- To quantify the success rate of integrating wearable activity monitors to collect data within a pilot RCT of ARC training post-stroke.
- Use 48 DMOs to visualise how, relative to a control group, the gait mechanics of stroke survivors are impacted following a 6-week ARC intervention,
- Provide DMO reference data recorded with the use of wearable activity monitors for both a control group and following 6 weeks of an ARC intervention.
- Discuss our findings with the purpose of informing future intervention studies aiming to integrate wearable activity monitors and quantify intervention impact for stroke survivors.
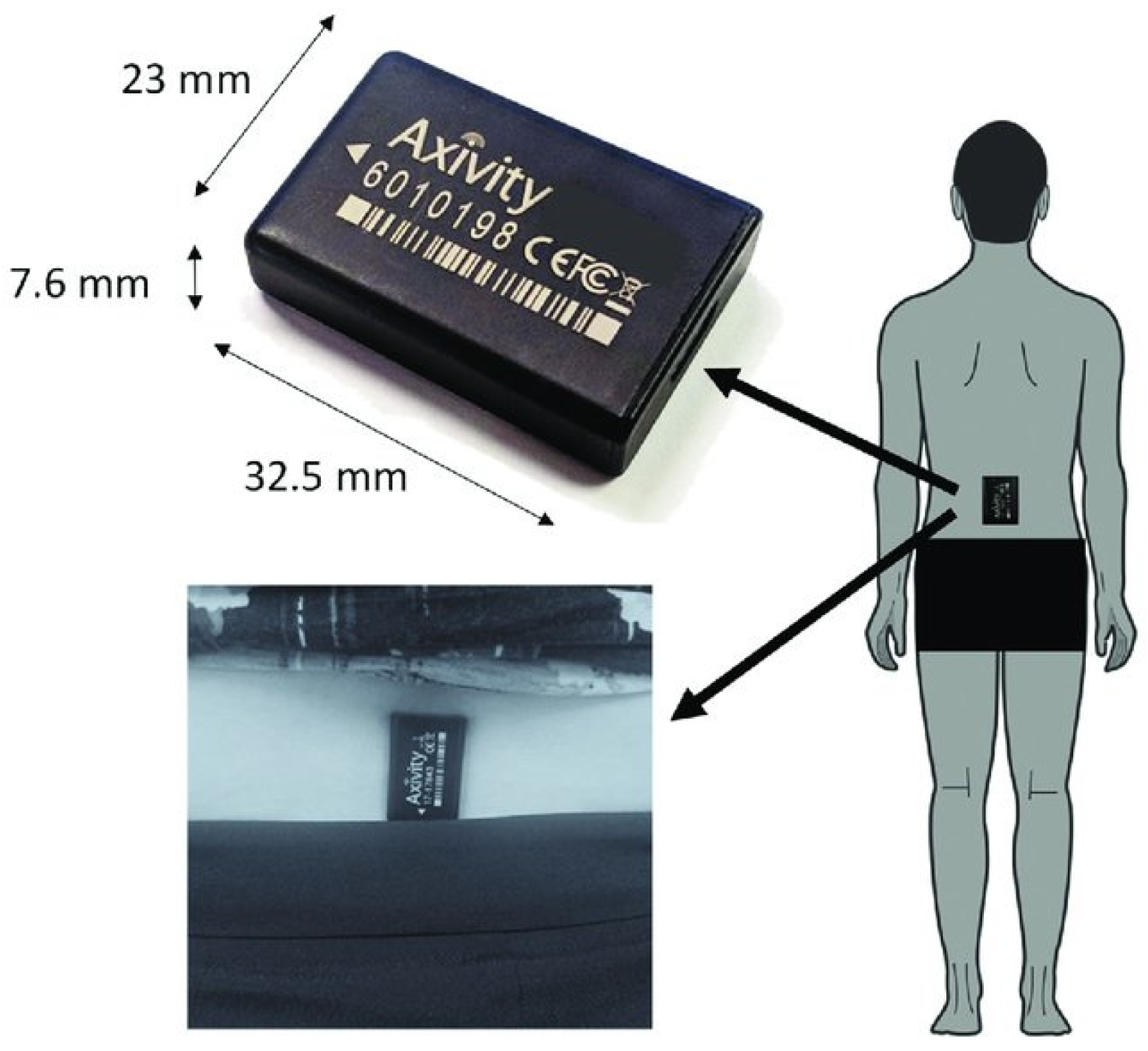
2. Materials and Methods
3. Results
3.1. Objective 1: Success Rates of Collecting Wearable Activity Monitor Gait Data
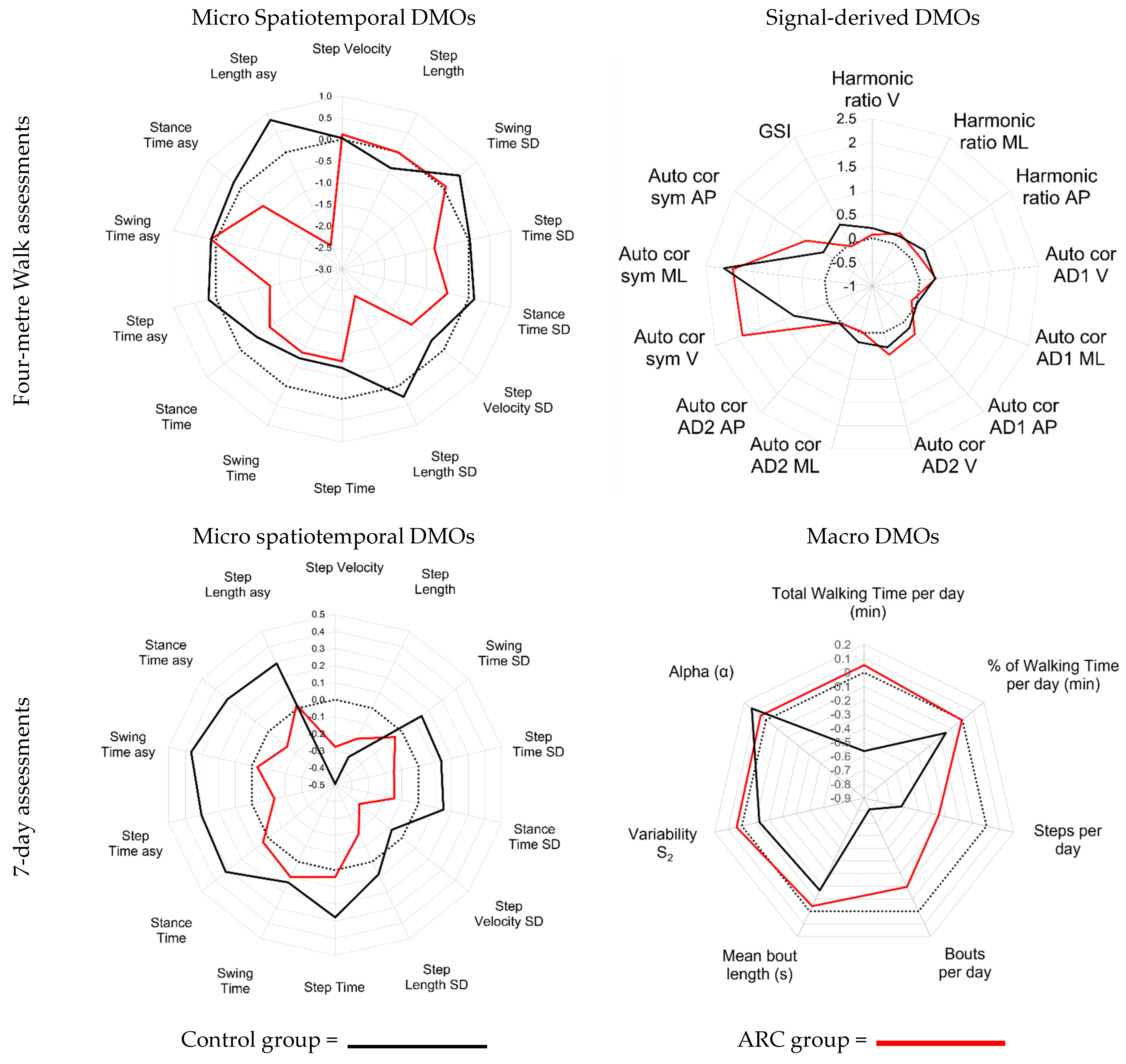
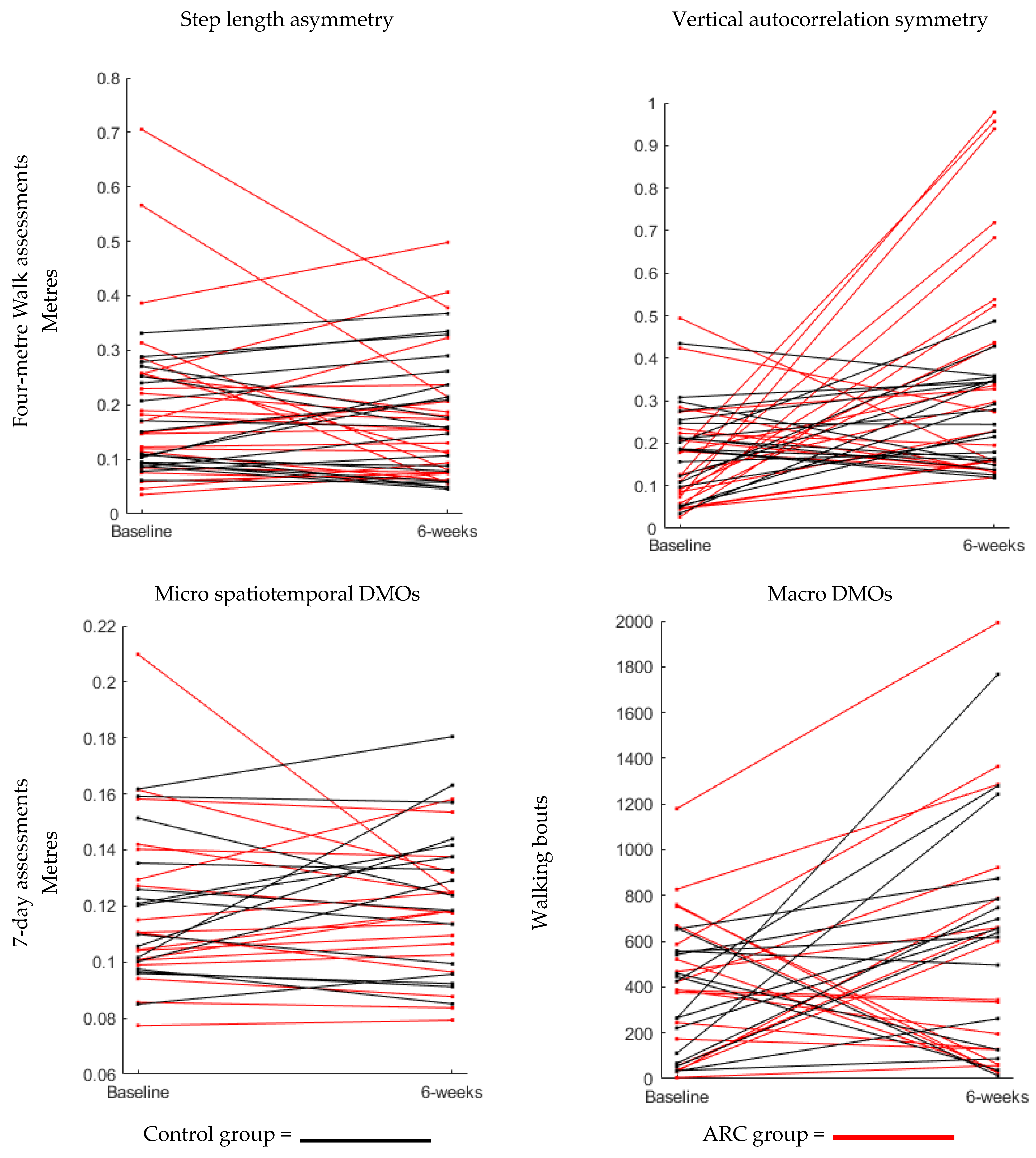
3.2. Objective 2: Visualisation of the Impact of Both Interventions on All DMOs
3.3. Objective 3: Reference Values Obtained for All DMOs Analysed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huizenga, D.; Rashford, L.; Darcy, B.; Lundin, E.; Medas, R.; Shultz, S.T.; DuBose, E.; Reed, K.B. Wearable Gait Device for Stroke Gait Rehabilitation at Home. Top. Stroke Rehabil. 2021, 28, 443–455. [Google Scholar] [CrossRef]
- Felius, R.A.W.; Geerars, M.; Bruijn, S.M.; van Dieën, J.H.; Wouda, N.C.; Punt, M. Reliability of IMU-Based Gait Assessment in Clinical Stroke Rehabilitation. Sensors 2022, 22, 908. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; St George, B.; Fenton, M.; Firkins, L. Top Ten Research Priorities Relating to Life after Stroke. Lancet Neurol. 2012, 11, 209. [Google Scholar] [CrossRef]
- Stretton, C.M.; Mudge, S.; Kayes, N.M.; McPherson, K.M. What Does Real-World Walking Mean to People with Stroke? An Interpretive Descriptive Study. Disabil. Rehabil. 2022, 44, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Algurén, B.; Lundgren-Nilsson, Å.; Sunnerhagen, K.S. Functioning of Stroke Survivors—A Validation of the ICF Core Set for Stroke in Sweden. Disabil. Rehabil. 2010, 32, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Khoo, I.; Marayong, P.; DeMars, K.; Cormack, J. Gait Training in Chronic Stroke Using Walk-Even Feedback Device: A Pilot Study. Neurosci. J. 2016, 2016, 6808319. [Google Scholar] [CrossRef]
- Fulk, G.D.; He, Y.; Boyne, P.; Dunning, K. Predicting Home and Community Walking Activity Poststroke. Stroke 2017, 48, 406–411. [Google Scholar] [CrossRef]
- Wright, R.L.; Briony Brownless, S.; Pratt, D.; Sackley, C.M.; Wing, A.M. Stepping to the Beat: Feasibility and Potential Efficacy of a Home-Based Auditory-Cued Step Training Program in Chronic Stroke. Front. Neurol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Felius, R.A.W.; Wouda, N.C.; Geerars, M.; Bruijn, S.M.; van Dieën, J.H.; Punt, M. Beyond Gait Speed: Exploring the Added Value of Inertial Measurement Unit-Based Measurements of Gait in the Estimation of the Walking Ability in Daily Life. BMC Neurol. 2024, 24, 129. [Google Scholar] [CrossRef]
- Spencer, J.; Wolf, S.L.; Kesar, T.M. Biofeedback for Post-Stroke Gait Retraining: A Review of Current Evidence and Future Research Directions in the Context of Emerging Technologies. Front. Neurol. 2021, 12, 637199. [Google Scholar] [CrossRef]
- Schaefer, R.S. Auditory Rhythmic Cueing in Movement Rehabilitation: Findings and Possible Mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130402. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Pili, R.; Corona, F.; Sors, F.; Agostini, T.A.; Bernardis, P.; Casula, C.; Cossu, G.; Guicciardi, M.; Pau, M. The Use of Footstep Sounds as Rhythmic Auditory Stimulation for Gait Rehabilitation in Parkinson’s Disease: A Randomized Controlled Trial. Front. Neurol. 2018, 9, 348. [Google Scholar] [CrossRef]
- Nascimento, L.R.; de Oliveira, C.Q.; Ada, L.; Michaelsen, S.M.; Teixeira-Salmela, L.F. Walking Training with Cueing of Cadence Improves Walking Speed and Stride Length after Stroke More than Walking Training Alone: A Systematic Review. J. Physiother. 2015, 61, 10–15. [Google Scholar] [CrossRef]
- McCue, P.; Shaw, L.; Del Din, S.; Hunter, H.; Lord, S.; Price, C.I.M.; Rodgers, H.; Rochester, L.; Moore, S.A. Acceptability and Deliverability of an Auditory Rhythmical Cueing (ARC) Training Programme for Use at Home and Outdoors to Improve Gait and Physical Activity Post-Stroke. Arch. Physiother. 2022, 1, 1. [Google Scholar] [CrossRef]
- Shaw, L.; McCue, P.; Brown, P.; Buckley, C.; Del Din, S.; Francis, R.; Hunter, H.; Lambert, A.; Lord, S.; Price, C.I.M.; et al. Auditory Rhythmical Cueing to Improve Gait in Community-Dwelling Stroke Survivors (ACTIVATE): A Pilot Randomised Controlled Trial. Pilot. Feasibility Stud. 2022, 8, 239. [Google Scholar] [CrossRef]
- Seo, M.; Shin, M.J.; Park, T.S.; Park, J.H. Clinometric Gait Analysis Using Smart Insoles in Patients with Hemiplegia after Stroke: Pilot Study. JMIR Mhealth Uhealth 2020, 8, e22208. [Google Scholar] [CrossRef]
- Moore, S.A.; Boyne, P.; Fulk, G.; Verheyden, G.; Fini, N.A. Walk the Talk: Current Evidence for Walking Recovery after Stroke, Future Pathways and a Mission for Research and Clinical Practice. Stroke 2022, 53, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of Gait Symmetry after Stroke: A Comparison of Current Methods and Recommendations for Standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Mansfield, A.; Biasin, L.; Brunton, K.; Inness, E.L.; McIlroy, W.E. Longitudinal Changes in Poststroke Spatiotemporal Gait Asymmetry over Inpatient Rehabilitation. Neurorehabil Neural Repair. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Fini, N.A.; Simpson, D.; Moore, S.A.; Mahendran, N.; Eng, J.J.; Borschmann, K.; Moulaee Conradsson, D.; Chastin, S.; Churilov, L.; English, C. How Should We Measure Physical Activity After Stroke? An International Consensus. Int. J. Stroke 2023, 18, 1132–1142. [Google Scholar] [CrossRef]
- Smith, B.A.; Lang, C.E. Sensor Measures of Symmetry Quantify Upper Limb Movement in the Natural Environment across the Lifespan. Arch. Phys. Med. Rehabil. 2019, 100, 1176. [Google Scholar] [CrossRef]
- Peters, D.M.; O’Brien, E.S.; Kamrud, K.E.; Roberts, S.M.; Rooney, T.A.; Thibodeau, K.P.; Balakrishnan, S.; Gell, N.; Mohapatra, S. Utilization of Wearable Technology to Assess Gait and Mobility Post-Stroke: A Systematic Review. J. Neuroeng. Rehabil. 2021, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Hickey, A.; Lord, S.; Del Din, S.; Godfrey, A.; Rochester, L. Comprehensive Measurement of Stroke Gait Characteristics with a Single Accelerometer in the Laboratory and Community: A Feasibility, Validity and Reliability Study. J. Neuroeng. Rehabil. 2017, 14, 130. [Google Scholar] [CrossRef]
- Polhemus, A.; Ortiz, L.D.; Brittain, G.; Chynkiamis, N.; Salis, F.; Gaßner, H.; Gross, M.; Kirk, C.; Rossanigo, R.; Taraldsen, K.; et al. Walking on Common Ground: A Cross-Disciplinary Scoping Review on the Clinical Utility of Digital Mobility Outcomes. NPJ Digit. Med. 2021, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Lanotte, F.; Okita, S.; O’Brien, M.K.; Jayaraman, A. Enhanced Gait Tracking Measures for Individuals with Stroke Using Leg-Worn Inertial Sensors. J. Neuroeng. Rehabil. 2024, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A.; Munoz-Novoa, M.; Heremans, C.; Branscheidt, M.; Cabanas-Valdés, R.; Engelter, S.T.; Kruuse, C.; Kwakkel, G.; Lakičević, S.; Lampropoulou, S.; et al. European Stroke Organisation (ESO) Guideline on Motor Rehabilitation. Eur. Stroke J. 2025, 23969873251338142. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Heremans, C.; Burridge, J.; Deutsch, J.E.; Hammerbeck, U.; Hollands, K.; Karthikbabu, S.; Mehrholz, J.; Moore, J.L.; Salbach, N.M.; et al. Standardized Measurement of Balance and Mobility Post-Stroke: Consensus-Based Core Recommendations from the Third Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2024, 19, 158–168. [Google Scholar] [CrossRef]
- Del Din, S.; Lewis, E.G.; Gray, W.K.; Collin, H.; Kissima, J.; Rochester, L.; Dotchin, C.; Urasa, S.; Walker, R. Monitoring Walking Activity with Wearable Technology in Rural-Dwelling Older Adults in Tanzania: A Feasibility Study Nested within a Frailty Prevalence Study. Exp. Aging Res. 2020, 46, 367–381. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J. Gerontol.—Ser. A Biomed. Sci. Med. Sci. 2013, 68, 820–827. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use. IEEE J. Biomed. Health Inf. 2016, 20, 838–847. [Google Scholar] [CrossRef]
- Hickey, A.; Del Din, S.; Rochester, L.; Godfrey, A. Detecting Free-Living Steps and Walking Bouts: Validating an Algorithm for Macro Gait Analysis. Physiol. Meas. 2017, 38, N1–N15. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Chastin, S.F.M.; McInnes, L.; Little, L.; Briggs, P.; Rochester, L. Exploring Patterns of Daily Physical and Sedentary Behaviour in Community-Dwelling Older Adults. Age Ageing 2011, 40, 205–210. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Baker, K.; Jones, D.; Burn, D.; Granat, M.H.; Rochester, L. The Pattern of Habitual Sedentary Behavior Is Different in Advanced Parkinson’s Disease. Mov. Disord. 2010, 25, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.; Chastin, S.F.M.; Lord, S.; Baker, K.; Burn, D.J. Understanding the Impact of Deep Brain Stimulation on Ambulatory Activity in Advanced Parkinson’s Disease. J. Neurol. 2012, 259, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Micó-Amigo, M.E.; Dunne-Willows, M.; Godfrey, A.; Hickey, A.; Lord, S.; Rochester, L.; Del Din, S.; Moore, S.A. Gait Asymmetry Post-Stroke: Determining Valid and Reliable Methods Using a Single Accelerometer Located on the Trunk. Sensors 2019, 20, 37. [Google Scholar] [CrossRef]
- Rehman, R.Z.U.R.; Buckley, C.; Micó-Amigo, M.E.; Kirk, C.; Dunne-Willows, M.; Mazzà, C.; Shi, J.Q.; Alcock, L.; Rochester, L.; Del Din, S. Accelerometry-Based Digital Gait Characteristics for Classification of Parkinson’s Disease: What Counts? IEEE Open J. Eng. Med. Biol. 2020, 1, 65–73. [Google Scholar] [CrossRef]
- Bellanca, J.L.; Lowry, K.A.; VanSwearingen, J.M.; Brach, J.S.; Redfern, M.S. Harmonic Ratios: A Quantification of Step to Step Symmetry. J. Biomech. 2013, 46, 828–831. [Google Scholar] [CrossRef]
- Moe-Nilssen, R.; Helbostad, J.L. Estimation of Gait Cycle Characteristics by Trunk Accelerometry. J. Biomech. 2004, 37, 121–126. [Google Scholar] [CrossRef]
- Zhang, W.; Smuck, M.; Legault, C.; Ith, M.A.; Muaremi, A.; Aminian, K. Gait Symmetry Assessment with a Low Back 3D Accelerometer in Post-Stroke Patients. Sensors 2018, 18, 3322. [Google Scholar] [CrossRef]
- Stockley, R.C.; Graham, I.S. The Importance of Embracing Complexity in Rehabilitation. J. Eval. Clin. Pr. 2023, 29, 657–661. [Google Scholar] [CrossRef]
- Hulleck, A.A.; Menoth Mohan, D.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and Future of Gait Assessment in Clinical Practice: Towards the Application of Novel Trends and Technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef]
- Trojaniello, D.; Ravaschio, A.; Hausdorff, J.M.; Cereatti, A. Comparative Assessment of Different Methods for the Estimation of Gait Temporal Parameters Using a Single Inertial Sensor: Application to Elderly, Post-Stroke, Parkinson’s Disease and Huntington’s Disease Subjects. Gait Posture 2015, 42, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuruvithadam, K.; Schaer, A.; Stoneham, R.; Chatzipirpiridis, G.; Easthope, C.A.; Barry, G.; Martin, J.; Pané, S.; Nelson, B.J.; et al. An Intelligent In-Shoe System for Gait Monitoring and Analysis with Optimized Sampling and Real-Time Visualization Capabilities. Sensors 2021, 21, 2869. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Sugiarto, T.; Liao, Y.-Y.; Lin, Y.-J.; Yang, F.-C.; Hueng, D.-Y.; Sun, C.-T.; Chou, K.-N.; Liao, Y.-Y.; Lin, Y.-J.; et al. Can Trunk Acceleration Differentiate Stroke Patient Gait Patterns Using Time- and Frequency-Domain Features? Appl. Sci. 2021, 11, 1541. [Google Scholar] [CrossRef]
| ARC | Control | |||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 6 Week | Baseline | 6 Week | ||
| Mean SD | Mean SD | Mean SD | Mean SD | |||
| Four-metre assessments | Micro | Step Time | 0.71 ± 0.14 | 0.69 ± 0.12 | 0.68 ± 0.09 | 0.66 ± 0.11 |
| Stance Time | 0.85 ± 0.14 | 0.83 ± 0.10 | 0.81 ± 0.09 | 0.80 ± 0.10 | ||
| Swing Time | 0.54 ± 0.09 | 0.52 ± 0.14 | 0.53 ± 0.08 | 0.51 ± 0.09 | ||
| Step Length | 0.57 ± 0.09 | 0.56 ± 0.11 | 0.58 ± 0.10 | 0.55 ± 0.09 | ||
| Step Velocity | 0.84 ± 0.11 | 0.85 ± 0.16 | 0.87 ± 0.14 | 0.87 ± 0.18 | ||
| Step Time SD | 0.17 ± 0.13 | 0.15 ± 0.12 | 0.14 ± 0.12 | 0.14 ± 0.11 | ||
| Stance Time SD | 0.16 ± 0.11 | 0.14 ± 0.11 | 0.14 ± 0.11 | 0.14 ± 0.10 | ||
| Swing Time SD | 0.10 ± 0.07 | 0.10 ± 0.06 | 0.10 ± 0.05 | 0.11 ± 0.07 | ||
| Step Length SD | 0.13 ± 0.06 | 0.11 ± 0.06 | 0.11 ± 0.05 | 0.12 ± 0.07 | ||
| Step Velocity SD | 0.20 ± 0.08 | 0.17 ± 0.09 | 0.18 ± 0.07 | 0.16 ± 0.07 | ||
| Step Time asy | 0.15 ± 0.18 | 0.12 ± 0.11 | 0.11 ± 0.09 | 0.11 ± 0.10 | ||
| Stance Time asy | 0.14 ± 0.15 | 0.12 ± 0.10 | 0.12 ± 0.09 | 0.12 ± 0.08 | ||
| Swing Time asy | 0.10 ± 0.13 | 0.11 ± 0.09 | 0.11 ± 0.09 | 0.11 ± 0.08 | ||
| Step Length asy | 0.22 ± 0.16 | 0.18 ± 0.12 | 0.16 ± 0.09 | 0.17 ± 0.11 | ||
| Signal derived variables | Harmonic ratio V | 1.59 ± 0.67 | 1.64 ± 0.63 | 1.50 ± 0.57 | 1.62 ± 0.52 | |
| Harmonic ratio ML | 1.63 ± 0.36 | 1.72 ± 0.45 | 1.59 ± 0.38 | 1.66 ± 0.51 | ||
| Harmonic ratio AP | 1.38 ± 0.57 | 1.47 ± 0.63 | 1.20 ± 0.51 | 1.37 ± 0.50 | ||
| Auto cor AD1 V | 0.38 ± 0.27 | 0.47 ± 0.27 | 0.34 ± 0.18 | 0.40 ± 0.19 | ||
| Auto cor AD1 ML | 0.46 ± 0.17 | 0.43 ± 0.17 | 0.46 ± 0.15 | 0.46 ± 0.16 | ||
| Auto cor AD1 AP | 0.38 ± 0.23 | 0.46 ± 0.22 | 0.36 ± 0.30 | 0.41 ± 0.23 | ||
| Auto cor AD2 V | 0.47 ± 0.24 | 0.59 ± 0.28 | 0.48 ± 0.21 | 0.54 ± 0.18 | ||
| Auto cor AD2 ML | 0.48 ± 0.21 | 0.48 ± 0.22 | 0.50 ± 0.24 | 0.55 ± 0.24 | ||
| Auto cor AD2 AP | 0.54 ± 0.21 | 0.55 ± 0.19 | 0.58 ± 0.31 | 0.59 ± 0.16 | ||
| Auto cor sym V | 0.16 ± 0.12 | 0.39 ± 0.29 | 0.19 ± 0.10 | 0.27 ± 0.11 | ||
| Auto cor sym ML | 0.12 ± 0.09 | 0.29 ± 0.21 | 0.12 ± 0.11 | 0.34 ± 0.27 | ||
| Auto cor sym AP | 0.28 ± 0.17 | 0.39 ± 0.24 | 0.35 ± 0.20 | 0.40 ± 0.20 | ||
| Gait symmetry index | 0.45 ± 0.19 | 0.44 ± 0.18 | 0.43 ± 0.13 | 0.49 ± 0.20 | ||
| 7-day assessments | Micro | Step Time | 0.62 ± 0.03 | 0.62 ± 0.03 | 0.62 ± 0.02 | 0.62 ± 0.04 |
| Stance Time | 0.77 ± 0.03 | 0.77 ± 0.03 | 0.77 ± 0.03 | 0.77 ± 0.04 | ||
| Swing Time | 0.47 ± 0.03 | 0.47 ± 0.03 | 0.47 ± 0.03 | 0.47 ± 0.04 | ||
| Step Length | 0.55 ± 0.04 | 0.54 ± 0.04 | 0.58 ± 0.06 | 0.56 ± 0.07 | ||
| Step Velocity | 0.96 ± 0.08 | 0.94 ± 0.10 | 1.01 ± 0.13 | 0.95 ± 0.15 | ||
| Step Time SD | 0.20 ± 0.03 | 0.19 ± 0.03 | 0.19 ± 0.03 | 0.19 ± 0.03 | ||
| Stance Time SD | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.21 ± 0.03 | ||
| Swing Time SD | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.16 ± 0.02 | 0.16 ± 0.02 | ||
| Step Length SD | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | ||
| Step Velocity SD | 0.37 ± 0.04 | 0.36 ± 0.04 | 0.36 ± 0.06 | 0.35 ± 0.04 | ||
| Step Time asy | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.03 | ||
| Stance Time asy | 0.12 ± 0.03 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.03 | ||
| Swing Time asy | 0.11 ± 0.03 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.03 | ||
| Step Length asy | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.02 | ||
| Macro | Mean bout length (s) | 14.02 ± 3.28 | 13.88 ± 4.31 | 15.96 ± 3.05 | 15.45 ± 2.89 | |
| Variability s2 | 0.79 ± 0.10 | 0.79 ± 0.11 | 0.83 ± 0.07 | 0.82 ± 0.09 | ||
| Alpha | 1.67 ± 0.08 | 1.67 ± 0.08 | 1.63 ± 0.04 | 1.63 ± 0.04 | ||
| Total walk time per day (min) | 116.85 ± 66.02 | 120.38 ± 71.47 | 132.96 ± 56.57 | 101.10 ± 64.02 | ||
| Steps per day | 8154 ± 5274 | 6305 ± 6570 | 9066 ± 3821 | 6669 ± 3786 | ||
| Bouts per day | 486.19 ± 248.57 | 438.10 ± 321.98 | 506.69 ± 212.47 | 334.48 ± 228.90 | ||
| % of Walking Time per day | 8.11 ± 4.58 | 8.10 ± 4.88 | 9.23 ± 3.93 | 8.64 ± 3.52 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, C.; Shaw, L.; McCue, P.; Brown, P.; Del Din, S.; Francis, R.; Hunter, H.; Lambert, A.; Rochester, L.; Moore, S.A. Wearable Activity Monitors to Quantify Gait During Stroke Rehabilitation: Data from a Pilot Randomised Controlled Trial Examining Auditory Rhythmical Cueing. Symmetry 2025, 17, 1640. https://doi.org/10.3390/sym17101640
Buckley C, Shaw L, McCue P, Brown P, Del Din S, Francis R, Hunter H, Lambert A, Rochester L, Moore SA. Wearable Activity Monitors to Quantify Gait During Stroke Rehabilitation: Data from a Pilot Randomised Controlled Trial Examining Auditory Rhythmical Cueing. Symmetry. 2025; 17(10):1640. https://doi.org/10.3390/sym17101640
Chicago/Turabian StyleBuckley, Christopher, Lisa Shaw, Patricia McCue, Philip Brown, Silvia Del Din, Richard Francis, Heather Hunter, Allen Lambert, Lynn Rochester, and Sarah A. Moore. 2025. "Wearable Activity Monitors to Quantify Gait During Stroke Rehabilitation: Data from a Pilot Randomised Controlled Trial Examining Auditory Rhythmical Cueing" Symmetry 17, no. 10: 1640. https://doi.org/10.3390/sym17101640
APA StyleBuckley, C., Shaw, L., McCue, P., Brown, P., Del Din, S., Francis, R., Hunter, H., Lambert, A., Rochester, L., & Moore, S. A. (2025). Wearable Activity Monitors to Quantify Gait During Stroke Rehabilitation: Data from a Pilot Randomised Controlled Trial Examining Auditory Rhythmical Cueing. Symmetry, 17(10), 1640. https://doi.org/10.3390/sym17101640







