Non-Selective Reduction of P-Stereogenic Phosphinoylacetic Acid Esters and 3-Phosphorylated Coumarins to Phosphino-Boranes: Discovery of Unexpected 2,3-Dihydrobenzofuran Derivative
Abstract
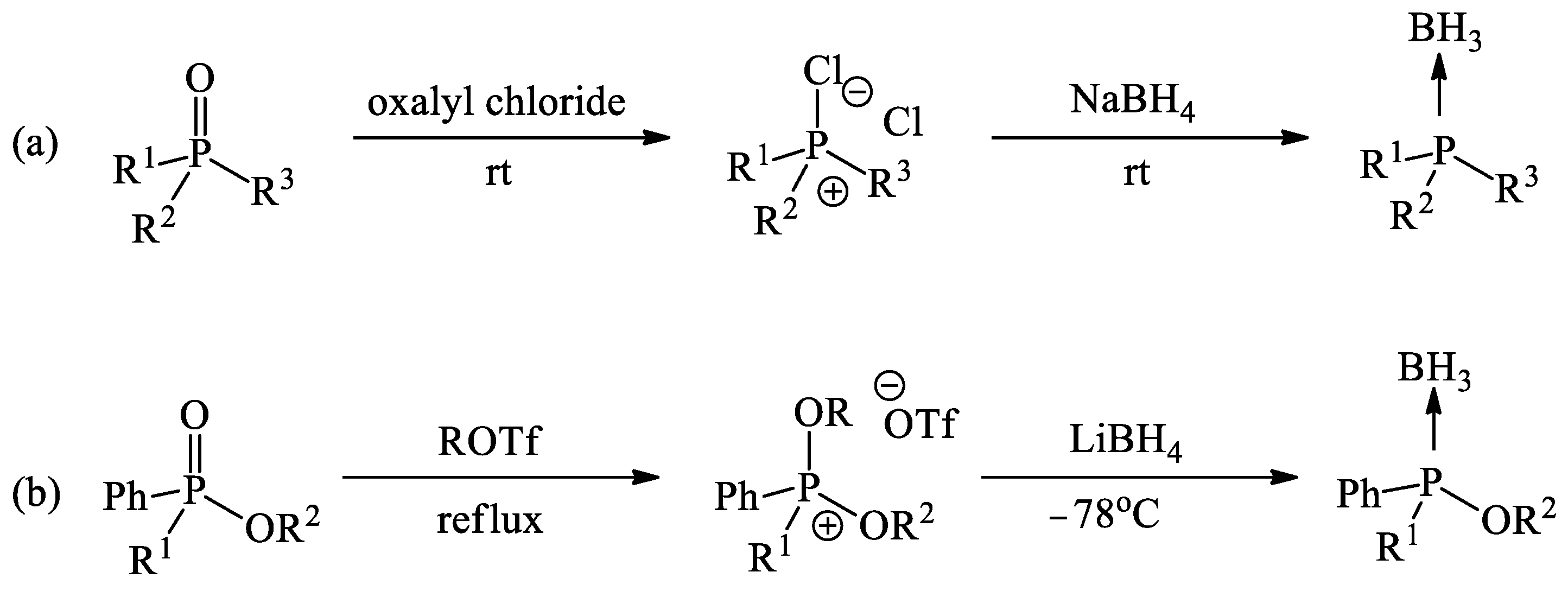
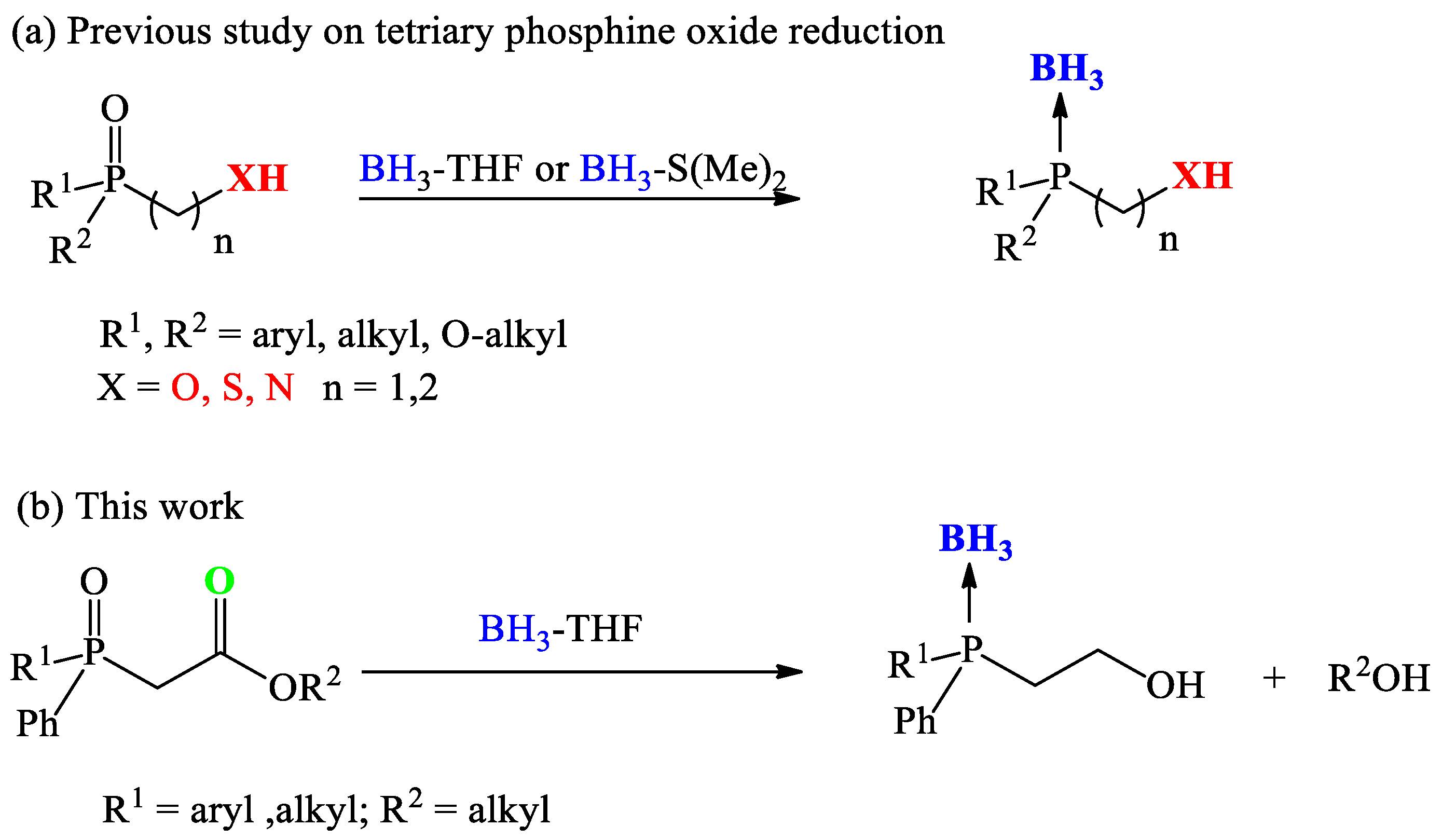
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. General
2.1.2. General Procedure for the Synthesis of Phosphinoylacetic Acid Ethyl Esters 1a–d
2.2. General Procedure for the Synthesis of Phosphinoylacetic Acid L-menthyl Esters 2a–c
2.3. General Procedure for Synthesis of Phosphinoylacetic Acids 3a–b
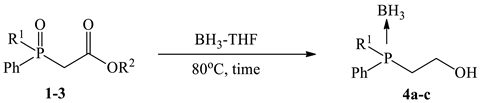
2.4. General Procedure for Synthesis of Phosphino-Borane Complexes 4a–d from Phosphinoylacetic Acid Ethyl Esters 1a–d
(2-Hydroxyethyl)(2-metoxyphenyl)phenylphosphino-Borane (4b)
2.5. General Procedure for Synthesis of Phosphino-Borane Complexes 4a–b from Phosphinoylacetic Acid L-menthyl Esters 2a–b
2.6. Procedure for Synthesis of 3-(Diphenylphosphinyl)-2H-Chromen-2-One (5)
3-(Diphenylphosphinyl)-2H-Chromen-2-One (5)
2.7. Procedure for Synthesis of (2-methyl-tetrahydro-[2]furyl)-diphenylphosphine-borane (6)
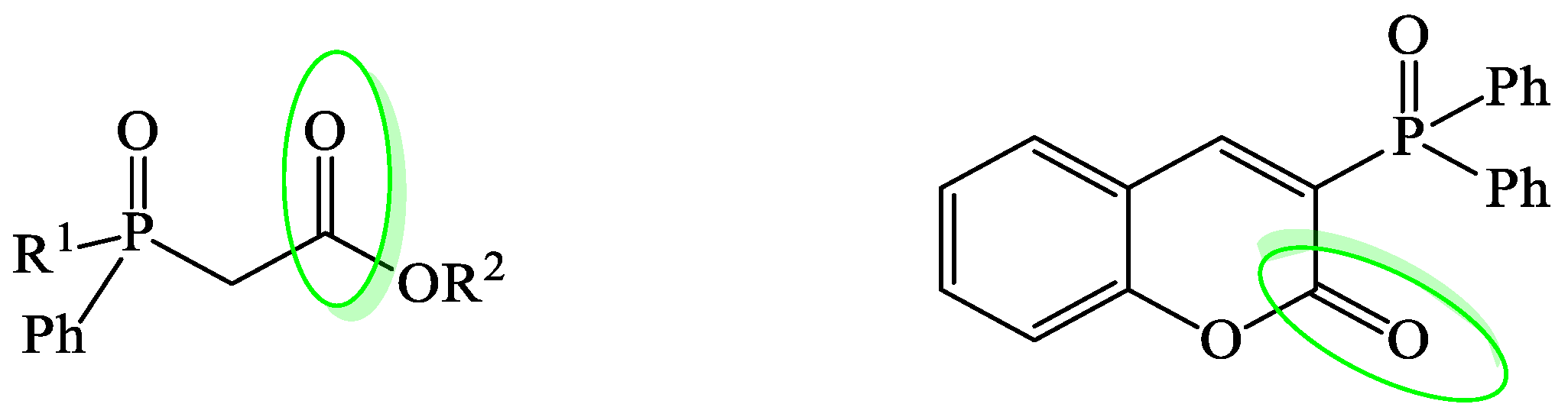
3. Results and Discussion
3.1. Synthesis and Borane Reduction of Phosphinoylacetic Esters and Acids
3.2. Study on the Reduction of 3-Phosphorylated Coumarin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Methot, J.L.; Roush, W.R. Nucleophilic Phosphine Organocatalysis. Adv. Synth. Catal. 2004, 346, 1035–1050. [Google Scholar] [CrossRef]
- Xie, C.; Smaligo, A.J.; Song, X.R.; Kwon, O. Phosphorus-Based Catalysis. ACS Cent. Sci. 2021, 7, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, P.; Zhou, H.C.; Madrahimov, S.T. Bridging Homogeneous and Heterogeneous Catalysis: Phosphine-Functionalized Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2024, 63, e202315075. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fan, Y.C.; Sun, Z.; Wu, Y.; Kwon, O. Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049–10293. [Google Scholar] [CrossRef] [PubMed]
- Gallen, A.; Riera, A.; Verdaguer, X.; Grabulosa, A. Coordination chemistry and catalysis with secondary phosphine oxides. Catal. Sci. Technol. 2019, 9, 5504–5561. [Google Scholar] [CrossRef]
- Swor, C.D.; Tyler, D.R. Synthesis and coordination chemistry of macrocyclic phosphine ligands. Coord. Chem. Rev. 2011, 255, 2860–2881. [Google Scholar] [CrossRef]
- Fan, Y.C.; Kwon, O. Advances in nucleophilic phosphine catalysis of alkenes, allenes, alkynes, and MBHADs. Chem. Commun. 2013, 49, 11588–11619. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.A.; Gilheany, D.G. The modern interpretation of the Wittig reaction mechanism. Chem. Soc. Rev. 2013, 42, 6670–6696. [Google Scholar] [CrossRef] [PubMed]
- Appel, R. Tertiary Phosphane/Tetrachloromethane, a Versatile Reagent for Chlorination, Dehydration, and P-N Linkage. Angew. Chem. Int. Ed. 1975, 14, 801–811. [Google Scholar] [CrossRef]
- Del Rio Fuenzalida, N.M.; Alme, E.; Lundevall, F.J.; Bjørsvik, H.R. An environmentally benign and high-rate Appel type reaction. React. Chem. Eng. 2022, 7, 1650–1659. [Google Scholar] [CrossRef]
- Kumara Swamy, K.C.; Bhuvan Kumar, N.N.B.; Balaraman, E.; Pavan Kumar, K.V.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, O.; Yamada, M. Preparation of Esters of Carboxylic and Phosphoric Acid via Quaternary Phosphonium Salts. BCSJ 1967, 40, 2380–2382. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; Reitz, A.B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Hérault, D.; Nguyen, D.H.; Nuel, D.; Buono, G. Reduction of secondary and tertiary phosphine oxides to phosphines. Chem. Soc. Rev. 2015, 44, 2508–2528. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Tian, A.; Yang, Q.; Huang, N.; Wang, L.; Li, D. Organophosphorus catalytic reaction based on reduction of phosphine oxide. Green Synth. Catal. 2023, 4, 135–149. [Google Scholar] [CrossRef]
- Podyacheva, E.; Kuchuk, E.; Chusov, D. Reduction of phosphine oxides to phosphines. Tetrahedron Lett. 2019, 60, 575–582. [Google Scholar] [CrossRef]
- Kovács, T.; Keglevich, G. 9. Deoxygenation of phosphine oxides. In Organophosphorus Chemistry; Walter de Gruyter GmbH: Berlin/Munich, Germany, 2018; pp. 179–198. [Google Scholar] [CrossRef]
- Henson, P.D.; Naumann, K.; Mislow, K. Stereomutation of phosphine oxides by lithium aluminum hydride. J. Am. Chem. Soc. 1969, 91, 5645–5646. [Google Scholar] [CrossRef]
- Kovacs, T.; Keglevich, G. The Reduction of Tertiary Phosphine Oxides by Silanes. Curr. Org. Chem. 2017, 21, 569–585. [Google Scholar] [CrossRef]
- Fritzsche, H.; Hasserodt, U.; Korte, F. Reduktion organischer Verbindungen des fünfwertigen Phosphors zu Phosphinen, II. Reduktion tertiärer Phosphinoxyde zu tertiären Phosphinen mit Trichlorsilan. Chem. Ber. 1965, 98, 171–174. [Google Scholar] [CrossRef]
- Wu, H.C.; Yu, J.Q.; Spencer, J.B. Stereospecific Deoxygenation of Phosphine Oxides with Retention of Configuration Using Triphenylphosphine or Triethyl Phosphite as an Oxygen Acceptor. Org. Lett. 2004, 6, 4675–4678. [Google Scholar] [CrossRef]
- Kapuśniak, Ł.; Plessow, P.N.; Trzybiński, D.; Woźniak, K.; Hofmann, P.; Jolly, P.I. A Mild One-Pot Reduction of Phosphine(V) Oxides Affording Phosphines(III) and Their Metal Catalysts. Organometallics 2021, 40, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Naumann, K.; Zon, G.; Mislow, K. Use of hexachlorodisilane as a reducing agent. Stereospecific deoxygenation of acyclic phosphine oxides. J. Am. Chem. Soc. 1969, 91, 7012–7023. [Google Scholar] [CrossRef]
- Gevorgyan, A.; Mkrtchyan, S.; Grigoryan, T.; Iaroshenko, V.O. Disilanes as oxygen scavengers and surrogates of hydrosilanes suitable for selective reduction of nitroarenes, phosphine oxides and other valuable substrates. Org. Chem. Front. 2017, 4, 2437–2444. [Google Scholar] [CrossRef]
- Coumbe, T.; Lawrence, N.J.; Muhammad, F. Titanium (IV) catalysis in the reduction of phosphine oxides. Tetrahedron Lett. 1994, 35, 625–628. [Google Scholar] [CrossRef]
- Marsi, K.L. Phenylsilane reduction of phosphine oxides with complete stereospecificity. J. Org. Chem. 1974, 39, 265–267. [Google Scholar] [CrossRef]
- Nicolas, E.; Guerriero, A.; Lyaskovskyy, V.; Peruzzini, M.; Lammertsma, K.; Gonsalvi, L.; Slootweg, J.C. Metal-Free Reduction of Phosphine Oxides Using Polymethylhydrosiloxane. Inorganics 2016, 4, 34. [Google Scholar] [CrossRef]
- Keglevich, G.; Kovács, T.; Csatlós, F. The Deoxygenation of Phosphine Oxides under Green Chemical Conditions. Heteroatom Chem. 2015, 26, 199–205. [Google Scholar] [CrossRef]
- Bouhadir, G.; Amgoune, A.; Bourissou, D. Phosphine-Boranes and Related Ambiphilic Compounds: Synthesis, Structure, and Coordination to Transition Metals. Adv. Organomet. Chem. 2010, 58, 1–107. [Google Scholar] [CrossRef]
- Pellon, P. Phosphine-boranes in synthesis. Borane as an efficient protecting group in the preparation of functionalized phosphines. Tetrahedron Lett. 1992, 33, 4451–4452. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.; Sloan, M.E.; Manners, I. Amine− and Phosphine–Borane Adducts: New Interest in Old Molecules. Chem. Rev. 2010, 110, 4023–4078. [Google Scholar] [CrossRef]
- Ohff, M. Borane Complexes of Trivalent Organophosphorus Compounds. Versatile Precursors for the Synthesis of Chiral Phosphine Ligands for Asymmetric Catalysis. Synthesis 1998, 1998, 1391–1415. [Google Scholar] [CrossRef]
- Rajendran, K.V.; Gilheany, D.G. Simple unprecedented conversion of phosphine oxides and sulfides to phosphine boranes using sodium borohydride. Chem. Commun. 2012, 48, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Kenny, N.P.; Rajendran, K.V.; Gilheany, D.G. Chemoselective reduction of the phosphoryl bond of O-alkyl phosphinates and related compounds: An apparently impossible transformation. Chem. Commun. 2015, 51, 16561–16564. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Fekete, M.; Chuluunbaatar, T.; Dobó, A.; Böcskei, Z.; Töke, L. Convenient Method for the Reduction of the Double-Bond of Cyclic Vinylphosphine Oxides Using Borane. Synth. Commun. 2000, 30, 4221–4231. [Google Scholar] [CrossRef]
- Keglevich, G.; Chuluunbaatar, T.; Ludányi, K.; Tőke, L. Phosphine-Boranes Based on the 7-Phosphanorbornene Framework: A Regioselective Approach to the Monoboranes of the Dimers of Phospholes. Tetrahedron 2000, 56, 1–6. [Google Scholar] [CrossRef]
- Keglevich, G.; Fekete, M.; Chuluunbaatar, T.; Dobó, A.; Harmat, V.; Tőke, L. One-pot transformation of cyclic phosphine oxides to phosphine–boranes by dimethyl sulfide–borane. J. Chem. Soc. Perkin Trans. 1 2000, 4451–4455. [Google Scholar] [CrossRef]
- Stankevič, M.; Pietrusiewicz, K.M. An Expedient Reduction of sec-Phosphine Oxides to sec-Phosphine-boranes by BH3·SMe2. Synlett 2003, 2003, 1012–1016. [Google Scholar] [CrossRef]
- Stankevič, M.; Andrijewski, G.; Pietrusiewicz, K.M. Direct Conversion of sec-Phosphine Oxides into Phosphinous Acid-Boranes. Synlett 2004, 2004, 311–315. [Google Scholar] [CrossRef]
- Sowa, S.; Stankevič, M.; Szmigielska, A.; Małuszyńska, H.; Kozioł, A.E.; Pietrusiewicz, K.M. Reduction of Functionalized Tertiary Phosphine Oxides with BH3. J. Org. Chem. 2015, 80, 1672–1688. [Google Scholar] [CrossRef]
- Sowa, S.; Stankevič, M.; Flis, A.; Pietrusiewicz, K.M. Reduction of Tertiary Phosphine Oxides by BH3, Assisted by Neighboring Activating Groups. Synthesis 2018, 50, 2106–21181. [Google Scholar] [CrossRef]
- Sowa, S.; Pietrusiewicz, K.M. Chemoselective Reduction of the P=O Bond in the presence of P-O and P-N Bonds in Phosphonate and Phosphinate Derivatives. Eur. J.Org. Chem. 2019, 5, 923–938. [Google Scholar] [CrossRef]
- Ou, Y.; Huang, Y.; Liu, Y.; Huo, Y.; Gao, Y.; Li, X.; Chen, Q. Iron-Catalyzed and Air-Mediated C(sp3)–H Phosphorylation of 1,3-Dicarbonyl Compounds Involving C–C Bond Cleavage. Adv. Synth. Catal. 2020, 362, 5783–5787. [Google Scholar] [CrossRef]
- Bredikhin, A.A.; Eliseenkova, R.M.; Tarasova, R.I.; Voskresenskaya, O.V.; Balandina, A.A.; Dobrynin, A.B.; Latypov, S.K.; Litvinov, I.A.; Sharafutdinova, D.R.; Efremov, Y.Y. Nonracemic menthyl phosphorylacetates. Russ. Chem. Bull. Int. Ed. 2007, 56, 290–297. [Google Scholar] [CrossRef]
- Dziuba, K.; Lubańska, M.; Pietrusiewicz, K.M. Enantiodivergent Synthesis of Both PAMPO Enantiomers Using l-Menthyl Chloroacetate and Stereomutation at P in Classical Quaternisation Reactions. Synthesis 2020, 52, 909–916. [Google Scholar] [CrossRef]
- Dziuba, K.; Szwaczko, K.; Frynas, S. Knoevenagel Condensation of Phosphinoylacetic Acids with Aldehydes: An Efficient One-Pot Strategy for the Synthesis of P-Functionalized Alkenyl Compounds. Synthesis 2021, 53, 2142–2154. [Google Scholar] [CrossRef]
- Dziuba, K.F.; Frynas, S.; Kozioł, A.E.; Szwaczko, K. Synthesis and Structural Elucidation of P-stereogenic Coumarins. Symmetry 2024, 16, 73. [Google Scholar] [CrossRef]
- Tsvetkov, E.N.; Bondarenko, A.N.; Malakhova, I.G.; Kabachnik, M.I. A Simple Synthesis and Some Synthetic Applications of Substituted Phosphide and Phosphinite Anions. Synthesis 1986, 1986, 198–208. [Google Scholar] [CrossRef]
- Arnott, G.E. Reduction of Carboxylic Acids and their Derivatives to Alcohols, Ethers, and Amines. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 368–409. [Google Scholar] [CrossRef]
- Ohashi, A.; Imamoto, T. Highly Enantioselective Hydrogenation of α-Dehydroamino Acids by Rhodium Complexes with New Unsymmetric P–Chirogenic Bisphosphine Ligands. Org. Lett. 2001, 3, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, A.; Kikuchi, S.-I.; Yasutake, M.; Imamoto, T. Unsymmetrical P-Chirogenic Bis(phosphane) Ligands: Their Preparation and Use in Rhodium-Catalyzed Asymmetric Hydrogenation. Eur. J. Org. Chem. 2002, 15, 2535–2546. [Google Scholar] [CrossRef]
- Morisaki, Y.; Imoto, H.; Hirano, K.; Hayashi, T.; Chujo, Y. Synthesis of Enantiomerically Pure P-Stereogenic Diphosphacrowns and Their Palladium Complexes. J. Org. Chem. 2011, 76, 1795–1803. [Google Scholar] [CrossRef]




 | |||||
| Entry | R1 | R2 | BH₃-THF (equiv.) | Time, h | Conv., % [a] (Yield), % [b] |
| 1 | Ph | Et | 5 | 24 | 4a; 40 (32) |
| 2 | Ph | Et | 10 | 72 | 4a; 98 (91) |
| 3 | o-An | Et | 10 | 24 | 4b; 44 |
| 4 | o-An | Et | 10 | 72 | 4b; 90 (85) |
| 5 | Bn | Et | 10 | 72 | 4c; 76 (68) |
| 6 | 1-Naphthyl | Et | 10 | 72 | 4d; 55 (49) |
| 7 | Ph | Menthyl | 10 | 72 | 4a; 66 (58) |
| 8 | o-An | Menthyl | 10 | 72 | 4b; 79 (73) |
| 9 | 1-Naphthyl | Menthyl | 10 | 72 | ND |
| 10 [c] | 1-Naphthyl | Menthyl | 10 | 72 | ND |
| 11 | Ph | H | 10 | 72 | 4a; 66 (60) |
| 12 | o-An | H | 10 | 72 | 4b; 64 (54) |
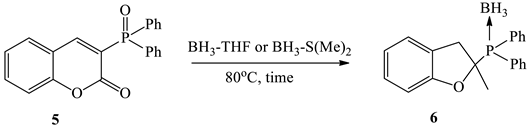 | ||||
| Entry | Borane Complexes (equiv.) | Time, h | Conv., % [a] | Yield, % [b] |
| 1 | BH3-THF (5.0) | 24 | 35 | complex mixture |
| 2 | BH3-THF (10.0) | 24 | 70 | 62 |
| 3 | BH3-THF (10.0) | 72 | 100 | 89 |
| 4 | BH₃-S(Me)₂ (5.0) | 24 | 100 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziuba, K.; Walczak, N.; Szwaczko, K. Non-Selective Reduction of P-Stereogenic Phosphinoylacetic Acid Esters and 3-Phosphorylated Coumarins to Phosphino-Boranes: Discovery of Unexpected 2,3-Dihydrobenzofuran Derivative. Symmetry 2024, 16, 976. https://doi.org/10.3390/sym16080976
Dziuba K, Walczak N, Szwaczko K. Non-Selective Reduction of P-Stereogenic Phosphinoylacetic Acid Esters and 3-Phosphorylated Coumarins to Phosphino-Boranes: Discovery of Unexpected 2,3-Dihydrobenzofuran Derivative. Symmetry. 2024; 16(8):976. https://doi.org/10.3390/sym16080976
Chicago/Turabian StyleDziuba, Kamil, Natalia Walczak, and Katarzyna Szwaczko. 2024. "Non-Selective Reduction of P-Stereogenic Phosphinoylacetic Acid Esters and 3-Phosphorylated Coumarins to Phosphino-Boranes: Discovery of Unexpected 2,3-Dihydrobenzofuran Derivative" Symmetry 16, no. 8: 976. https://doi.org/10.3390/sym16080976
APA StyleDziuba, K., Walczak, N., & Szwaczko, K. (2024). Non-Selective Reduction of P-Stereogenic Phosphinoylacetic Acid Esters and 3-Phosphorylated Coumarins to Phosphino-Boranes: Discovery of Unexpected 2,3-Dihydrobenzofuran Derivative. Symmetry, 16(8), 976. https://doi.org/10.3390/sym16080976






