A Robust Zn-Hydroxamate Metal–Organic Framework Constructed from an Unsymmetrical Ligand for Iodine Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Methyl 4-(Chlorocarbonyl)benzoate
2.2. Synthesis of 4-Methoxycarbonylphenylhydroxamic Acid
2.3. Synthesis of 4-Carboxyphenylhydroxamic Acid
2.4. Synthesis of SUM-13
2.5. Iodine Vapor Adsorption Measurement
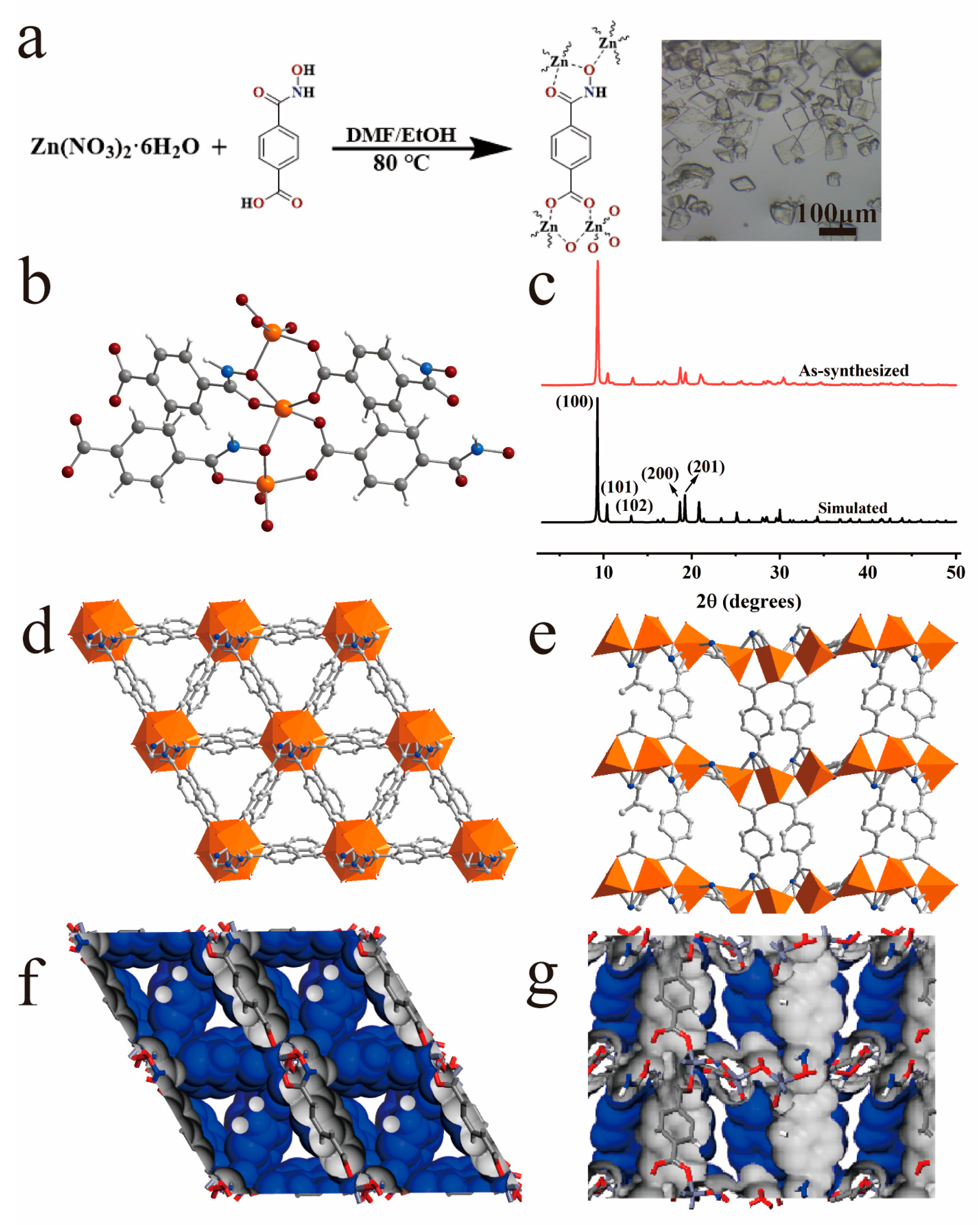
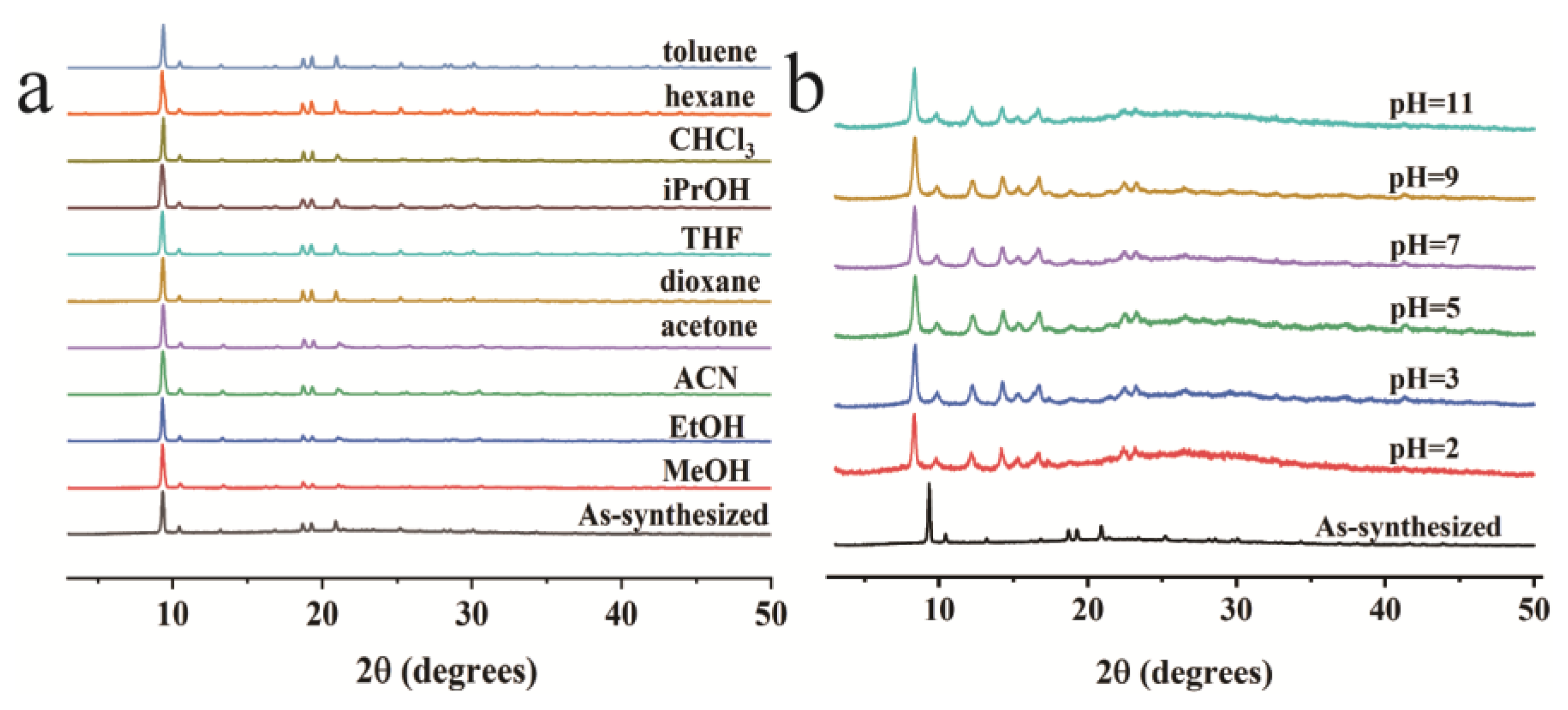
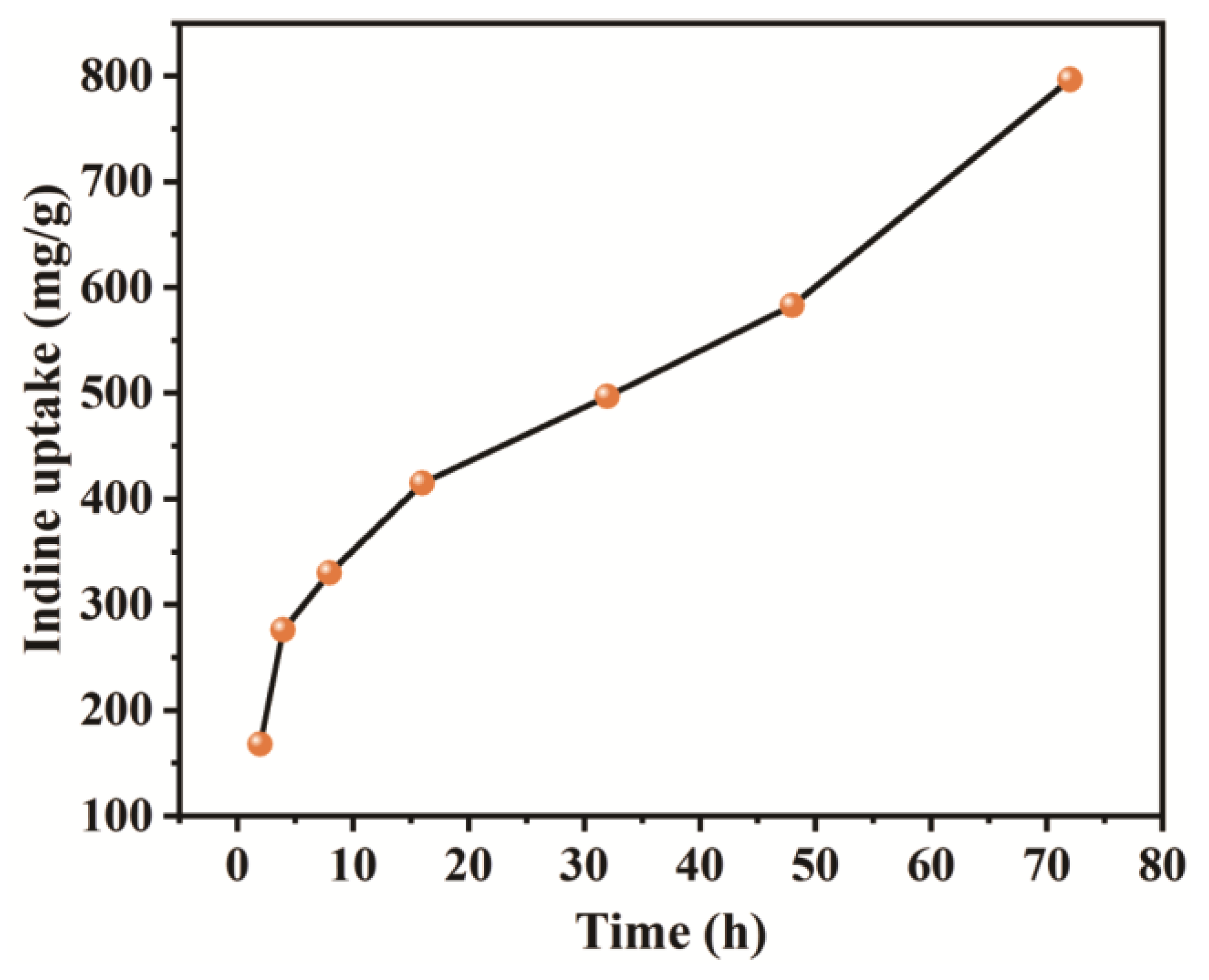
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, J.; Buongiorno, J.; Corradini, M.; Petti, D. A fresh look at nuclear energy. Science 2019, 363, 105. [Google Scholar] [CrossRef] [PubMed]
- Sovacool, B.K.; Schmid, P.; Stirling, A.; Walter, G.; MacKerron, G. Differences in carbon emissions reduction between countries pursuing renewable electricity versus nuclear power. Nat. Energy 2020, 5, 928–935. [Google Scholar] [CrossRef]
- Rodríguez-Penalonga, L.; Moratilla Soria, B.Y. A Review of the Nuclear Fuel Cycle Strategies and the Spent Nuclear Fuel Management Technologies. Energies 2017, 10, 1235. [Google Scholar] [CrossRef]
- Park, S.; Ewing, R.C. US Legal and Regulatory Framework for Nuclear Waste from Present and Future Reactors and Their Fuel Cycles. Annu. Rev. Environ. Resour. 2023, 48, 713–736. [Google Scholar] [CrossRef]
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef]
- Hertzsch, T.; Gervais, C.; Hulliger, J.; Jaeckel, B.; Guentay, S.; Bruchertseifer, H.; Neels, A. Open-Pore Organic Material for Retaining Radioactive I2 and CH3I. Adv. Funct. Mater. 2006, 16, 268–272. [Google Scholar] [CrossRef]
- Vienna, J.D.; Collins, E.D.; Crum, J.V.; Ebert, W.L.; Frank, S.M.; Garn, T.G.; Gombert, D.; Jones, R.; Jubin, R.T.; Maio, V.C.; et al. Closed Fuel Cycle Waste Treatment Strategy; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2015. [Google Scholar]
- Geng, T.; Zhu, Z.; Zhang, W.; Wang, Y. A nitrogen-rich fluorescent conjugated microporous polymer with triazine and triphenylamine units for high iodine capture and nitro aromatic compound detection. J. Mater. Chem. A 2017, 5, 7612–7617. [Google Scholar] [CrossRef]
- Yin, Z.-J.; Xu, S.-Q.; Zhan, T.-G.; Qi, Q.-Y.; Wu, Z.-Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef]
- Huve, J.; Ryzhikov, A.; Nouali, H.; Lalia, V.; Augé, G.; Daou, T.J. Porous sorbents for the capture of radioactive iodine compounds: A review. RSC Adv. 2018, 8, 29248–29273. [Google Scholar] [CrossRef]
- Haefner, D. Methods of Gas Phase Capture of Iodine from Fuel Reprocessing Off-Gas: A Literature Survey; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2007.
- Nandanwar, S.U.; Coldsnow, K.; Utgikar, V.; Sabharwall, P.; Eric Aston, D. Capture of harmful radioactive contaminants from off-gas stream using porous solid sorbents for clean environment—A review. Chem. Eng. J. 2016, 306, 369–381. [Google Scholar] [CrossRef]
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal–Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Pourebrahimi, S.; Pirooz, M. Reversible iodine vapor capture using bipyridine-based covalent triazine framework: Experimental and computational investigations. Chem. Eng. J. Adv. 2021, 8, 100150. [Google Scholar] [CrossRef]
- Pourebrahimi, S.; Pirooz, M.; De Visscher, A.; Peslherbe, G.H. Highly efficient and reversible iodine capture utilizing amorphous conjugated covalent triazine-based porous polymers: Experimental and computational studies. J. Environ. Chem. Eng. 2022, 10, 107805. [Google Scholar] [CrossRef]
- He, L.; Chen, L.; Dong, X.; Zhang, S.; Zhang, M.; Dai, X.; Liu, X.; Lin, P.; Li, K.; Chen, C.; et al. A nitrogen-rich covalent organic framework for simultaneous dynamic capture of iodine and methyl iodide. Chem 2021, 7, 699–714. [Google Scholar] [CrossRef]
- Zhang, X.; Maddock, J.; Nenoff, T.M.; Denecke, M.A.; Yang, S.; Schröder, M. Adsorption of iodine in metal–organic framework materials. Chem. Soc. Rev. 2022, 51, 3243–3262. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Zia, H.; Arham Shamim, M.; Zeeshan, M.; Yasir Khan, M.; Shahid, M. Metal organic frameworks as a versatile platform for the radioactive iodine capture: State of the art developments and future prospects. Inorganica Chim. Acta 2022, 539, 121026. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal–Organic Frameworks against Toxic Chemicals. Chem. Rev. 2020, 120, 8130–8160. [Google Scholar] [CrossRef]
- Yang, J.; Gao, M.; Zhang, M.; Zhang, Y.; Gao, M.; Wang, Z.; Xu, L.; Wang, X.; Shen, B. Advances in the adsorption and degradation of chemical warfare agents and simulants by Metal-organic frameworks. Coord. Chem. Rev. 2023, 493, 215289. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A critical review on recent developments in MOF adsorbents for the elimination of toxic heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Rieth, A.J.; Wright, A.M.; Dincă, M. Kinetic stability of metal–organic frameworks for corrosive and coordinating gas capture. Nat. Rev. Mater. 2019, 4, 708–725. [Google Scholar] [CrossRef]
- Mei, D.; Liu, L.; Yan, B. Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Z.; Wang, S.; Bi, K.; Hu, K.-q.; Dai, Z.; Dai, Y.; Liu, C.; Zhou, L.; Ji, X.; et al. A large-scale screening of metal-organic frameworks for iodine capture combining molecular simulation and machine learning. Front. Environ. Sci. Eng. 2023, 17, 148. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Li, R.; Wang, H.; Zhao, Y.; Pei, Y.; Wang, J. Highly efficient triiodide ion adsorption from water by ionic liquid hybrid metal–organic frameworks. J. Mol. Liq. 2023, 370, 121009. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Xie, L.-H.; Li, X.; Li, J.-R. Construction and application of base-stable MOFs: A critical review. Chem. Soc. Rev. 2022, 51, 6417–6441. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Alsalme, A.; Xiang, S.; Zhang, Z.; Chen, B. Design and applications of water-stable metal-organic frameworks: Status and challenges. Coord. Chem. Rev. 2020, 423, 213507. [Google Scholar] [CrossRef]
- He, T.; Kong, X.-J.; Li, J.-R. Chemically Stable Metal–Organic Frameworks: Rational Construction and Application Expansion. Acc. Chem. Res. 2021, 54, 3083–3094. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, Structure, and Metalation of Two New Highly Porous Zirconium Metal–Organic Frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.T.; Stair, P.C.; Snurr, R.Q.; et al. Vapor-Phase Metalation by Atomic Layer Deposition in a Metal–Organic Framework. J. Am. Chem. Soc. 2013, 135, 10294–10297. [Google Scholar] [CrossRef]
- Li, Z.-J.; Ju, Y.; Yu, B.; Wu, X.; Lu, H.; Li, Y.; Zhou, J.; Guo, X.; Zhang, Z.-H.; Lin, J.; et al. Modulated synthesis and isoreticular expansion of Th-MOFs with record high pore volume and surface area for iodine adsorption. Chem. Commun. 2020, 56, 6715–6718. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tang, W.; Bao, C.; Lai, Q.; Zhang, Z.; Feng, X.; Liu, C. An fcu Th-MOF Constructed from In Situ Coupling of Monovalent Ligands. Symmetry 2021, 13, 1332. [Google Scholar] [CrossRef]
- Chiong, J.A.; Zhu, J.; Bailey, J.B.; Kalaj, M.; Subramanian, R.H.; Xu, W.; Cohen, S.M.; Tezcan, F.A. An Exceptionally Stable Metal–Organic Framework Constructed from Chelate-Based Metal–Organic Polyhedra. J. Am. Chem. Soc. 2020, 142, 6907–6912. [Google Scholar] [CrossRef]
- Padial, N.M.; Castells-Gil, J.; Almora-Barrios, N.; Romero-Angel, M.; da Silva, I.; Barawi, M.; García-Sánchez, A.; de la Peña O’Shea, V.A.; Martí-Gastaldo, C. Hydroxamate Titanium–Organic Frameworks and the Effect of Siderophore-Type Linkers over Their Photocatalytic Activity. J. Am. Chem. Soc. 2019, 141, 13124–13133. [Google Scholar] [CrossRef]
- Lai, Q.; Chu, Z.-Q.; Xiao, X.; Dai, D.; Song, T.; Luo, T.-Y.; Tang, W.; Feng, X.; Zhang, Z.; Li, T.; et al. Two-dimensional Zr/Hf-hydroxamate metal–organic frameworks. Chem. Commun. 2022, 58, 3601–3604. [Google Scholar] [CrossRef]
- Feng, X.; Qin, Z.; Lai, Q.; Zhang, Z.; Shao, Z.-W.; Tang, W.; Wu, W.; Dai, Z.; Liu, C. Mixed-matrix membranes based on novel hydroxamate metal–organic frameworks with two-dimensional layers for CO2/N2 separation. Sep. Purif. Technol. 2023, 305, 122476. [Google Scholar] [CrossRef]
- Feng, X.; Li, W.; Yang, L.; Song, T.; Xia, Z.; Lai, Q.; Zhou, X.; Xiao, H.; Liu, C. Ce-hydroxamate metal–organic frameworks for photocatalytic H2 generation. Chem. Commun. 2022, 58, 13503–13506. [Google Scholar] [CrossRef]
- Tang, W.; Shao, Z.-W.; Xiong, L.; Zhang, Z.; Tan, K.; Feng, X.; Wu, W.; Liu, C. A siderophore-inspired two-dimensional Fe–hydroxamate metal–organic framework. CrystEngComm 2023, 25, 1462–1466. [Google Scholar] [CrossRef]
- Wu, W.; Tang, W.; Shao, Z.-W.; Feng, X.; Xiong, L.; Xiong, C.; Lai, Q.; Liu, C. Sacrificial-Hydroxamate-Enabled Sizable Crystallization of Scandium Carboxylate Metal–Organic Frameworks. Inorg. Chem. 2024, 63, 1720–1724. [Google Scholar] [CrossRef]
- Wu, W.; Qin, Z.; Duan, X.; Qiu, Y.; Tang, W.; Xiong, C.; Shao, Z.-W.; Xiong, L.; Dai, Z.; Liu, C. Structural Diversity in Ga/In-Hydroxamate Metal–Organic Materials. Inorg. Chem. 2024, 63, 10414–10422. [Google Scholar] [CrossRef]
- Sugamata, K.; Takagi, C.; Awano, K.; Iihama, T.; Minoura, M. Structural analysis of and selective CO2 adsorption in mixed-ligand hydroxamate-based metal–organic frameworks. Dalton Trans. 2020, 49, 9948–9952. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Rong, Z.; Iu-Fan Chen, O.; Yaghi, O.M. Metal-Organic Frameworks with Rod Yttrium Secondary Building Units. Isr. J. Chem. 2023, 63, e202300017. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, N.-N.; Fuhrmann, D.; Merker, S.; Krautscheid, H. A stable lanthanum hydroxamate metal–organic framework with radical character and electrical conductivity. Dalton Trans. 2022, 51, 15946–15953. [Google Scholar] [CrossRef]
- Sugamata, K.; Zhang, Y.; Amanokura, N.; Shirai, A.; Minoura, M. Alkoxy-Functionalized Hydroxamate/Zinc Metal–Organic Frameworks and the Effects of Substituents and Acid Addition on Their Structures. Inorg. Chem. 2024, 63, 2454–2459. [Google Scholar] [CrossRef]
- Parshad, M.; Kumar, D.; Verma, V. A mini review on applications of pyrazole ligands in coordination compounds and metal organic frameworks. Inorganica Chim. Acta 2024, 560, 121789. [Google Scholar] [CrossRef]
- Wang, K.; Lv, X.-L.; Feng, D.; Li, J.; Chen, S.; Sun, J.; Song, L.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Pyrazolate-Based Porphyrinic Metal–Organic Framework with Extraordinary Base-Resistance. J. Am. Chem. Soc. 2016, 138, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, T.; Yin, Q.; Li, L.; Liu, T.-F.; Huang, X.-S.; Cao, R. Tuning the Structure and Hydrolysis Stability of Calcium Metal–Organic Frameworks through Integrating Carboxylic/Phosphinic/Phosphonic Groups in Building Blocks. Cryst. Growth Des. 2020, 20, 8021–8027. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zhang, N.-N.; Tian, J.-Y.; Liu, C.-S.; Du, M. Pore modulation of metal–organic frameworks towards enhanced hydrothermal stability and acetylene uptake via incorporation of different functional brackets. J. Mater. Chem. A 2017, 5, 4861–4867. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.-S.; Hu, H.-S.; Wang, S.-B.; Liu, J.-C.; Cheng, P.; Kaltsoyannis, N.; Li, J.; Zhao, B. High Uptake of ReO4− and CO2 Conversion by a Radiation-Resistant Thorium–Nickle [Th48Ni6] Nanocage-Based Metal–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 6022–6027. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Moharramnejad, M.; Ehsani, A.; salmani, S.; shahi, M.; Malekshah, R.E.; Robatjazi, Z.S.; Parsimehr, H. Zinc-based metal-organic frameworks: Synthesis and recent progress in biomedical application. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3339–3354. [Google Scholar] [CrossRef]
- Fu, J.; Liu, J.-Y.; Zhang, G.-H.; Zhu, Q.-H.; Wang, S.-L.; Qin, S.; He, L.; Tao, G.-H. Boost of Gas Adsorption Kinetics of Covalent Organic Frameworks via Ionic Liquid Solution Process. Small 2023, 19, 2302570. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Zhang, M.; Cao, K.; Tian, Y.; Qi, Y.; Li, S.; Li, K.; Yu, X.; Ma, L. Colyliform Crystalline 2D Covalent Organic Frameworks (COFs) with Quasi-3D Topologies for Rapid I2 Adsorption. Angew. Chem. Int. Ed. 2020, 59, 22697–22705. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zhang, L.; Fu, J.; Wang, S.-L.; Zhou, Y.-R.; Wang, Y.-H.; Qin, S.; Tao, G.-H.; He, L. Mobile hydrogen-bonding donor in covalent organic framework for efficient iodine capture. Sep. Purif. Technol. 2024, 331, 125664. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Shang, C.; Gautier, R.; Jiang, T.; Faulques, E.; Latouche, C.; Paris, M.; Cario, L.; Bujoli-Doeuff, M.; Jobic, S. A p-Type Zinc-Based Metal–Organic Framework. Inorg. Chem. 2017, 56, 6208–6213. [Google Scholar] [CrossRef] [PubMed]
- Omkaramurthy, B.M.; Krishnamurthy, G. Synthesis, characterization, crystal structure, and electrochemical study of zinc(II) metal-organic framework. Inorg. Nano-Met. Chem. 2019, 49, 375–384. [Google Scholar] [CrossRef]
- Gordy, W. Dependence of Bond Order and of Bond Energy Upon Bond Length. J. Chem. Phys. 1947, 15, 305–310. [Google Scholar] [CrossRef]
- Nimmermark, A.; Öhrström, L.; Reedijk, J. Metal-ligand bond lengths and strengths: Are they correlated? A detailed CSD analysis. Z. Für Krist. Cryst. Mater. 2013, 228, 311–317. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, M.; Xiao, X.; Bi, K.; Song, T.; Hu, K.-q.; Dai, Y.; Zhou, L.; Liu, C.; Ji, X.; et al. Machine-Learning-Guided Identification of Coordination Polymer Ligands for Crystallizing Separation of Cs/Sr. ACS Appl. Mater. Interfaces 2022, 14, 33076–33084. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Dong, Y.; Qiu, Y.; Ren, J.; Bi, K.; Ji, X.; Liu, C.; Zhou, L.; Dai, Y. Machine-Learning-Enabled Ligand Screening for Cs/Sr Crystallizing Separation. Inorg. Chem. 2023, 62, 13293–13303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, Y.; Qiu, Y.; Bi, K.; Hu, K.-Q.; Dai, Y.; Zhou, L.; Liu, C.; Ji, X.; Shi, W.-Q. Deep-Learning-Guided High-Throughput Evaluation of Ligands for Selective Sr/Cs Coordination. J. Nucl. Radiochem. 2023, 45, 456–465. [Google Scholar]
- Wang, D.; Guo, W.; Hu, J.; Liu, F.; Chen, L.; Du, S.; Tang, Z. Estimating Atomic Sizes with Raman Spectroscopy. Sci. Rep. 2013, 3, 1486. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Zhu, Y.; Li, Z.; Mei, Z.; Shao, Z.-W.; Liu, C. A Robust Zn-Hydroxamate Metal–Organic Framework Constructed from an Unsymmetrical Ligand for Iodine Capture. Symmetry 2024, 16, 1049. https://doi.org/10.3390/sym16081049
Song T, Zhu Y, Li Z, Mei Z, Shao Z-W, Liu C. A Robust Zn-Hydroxamate Metal–Organic Framework Constructed from an Unsymmetrical Ligand for Iodine Capture. Symmetry. 2024; 16(8):1049. https://doi.org/10.3390/sym16081049
Chicago/Turabian StyleSong, Ting, Yinning Zhu, Zhehao Li, Zhewei Mei, Zhen-Wu Shao, and Chong Liu. 2024. "A Robust Zn-Hydroxamate Metal–Organic Framework Constructed from an Unsymmetrical Ligand for Iodine Capture" Symmetry 16, no. 8: 1049. https://doi.org/10.3390/sym16081049
APA StyleSong, T., Zhu, Y., Li, Z., Mei, Z., Shao, Z.-W., & Liu, C. (2024). A Robust Zn-Hydroxamate Metal–Organic Framework Constructed from an Unsymmetrical Ligand for Iodine Capture. Symmetry, 16(8), 1049. https://doi.org/10.3390/sym16081049






