Polyazulene Based Materials for Heavy Metal Ions Detection. 4. Search for Conditions for Thiophen-Vinyl-Pyridine-Azulene Based CMEs Preparation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
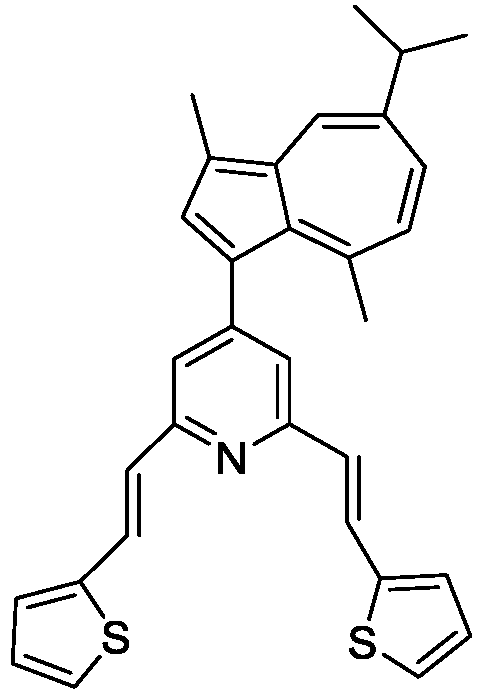
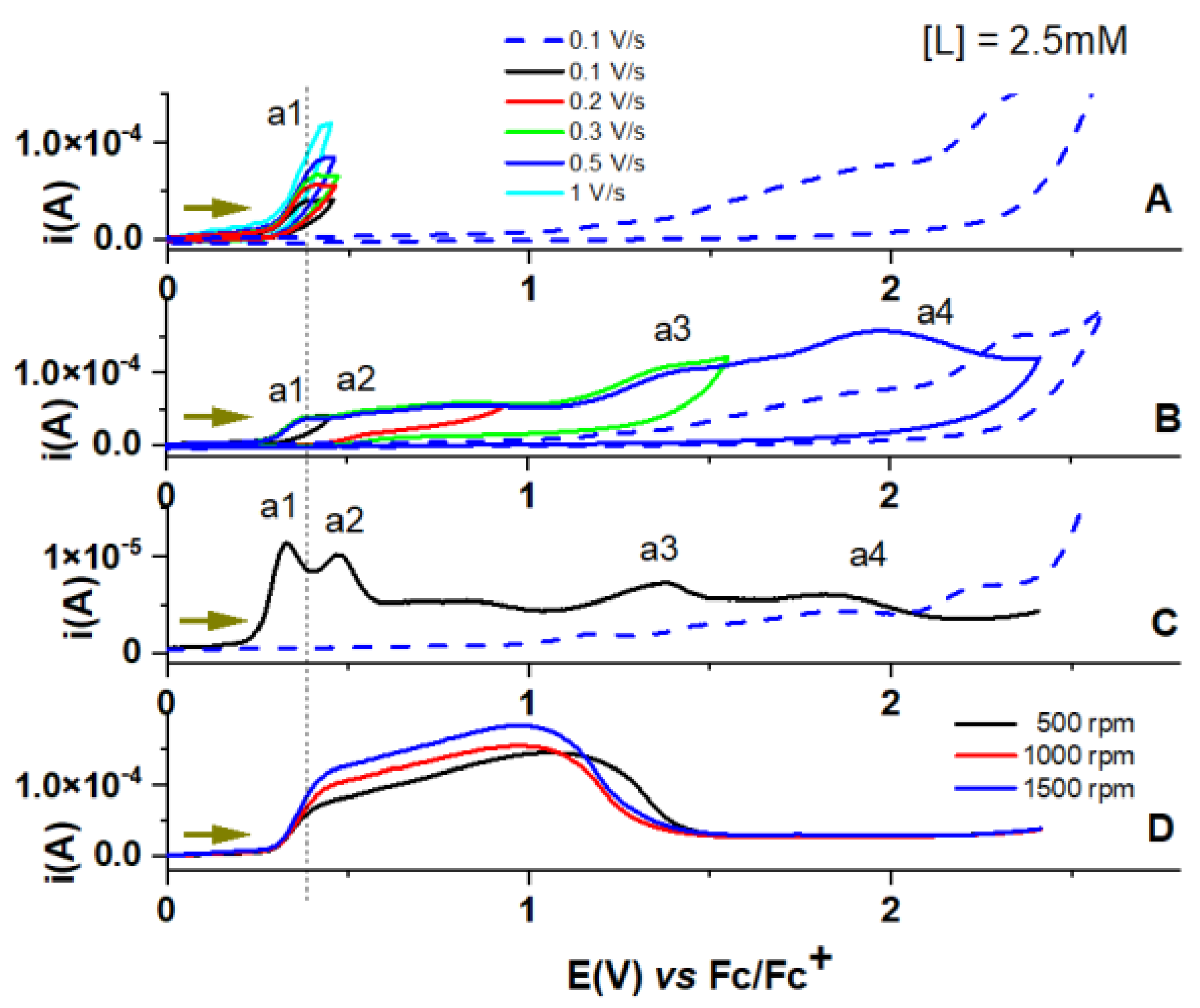
3.1. Electrochemical Oxidation of L
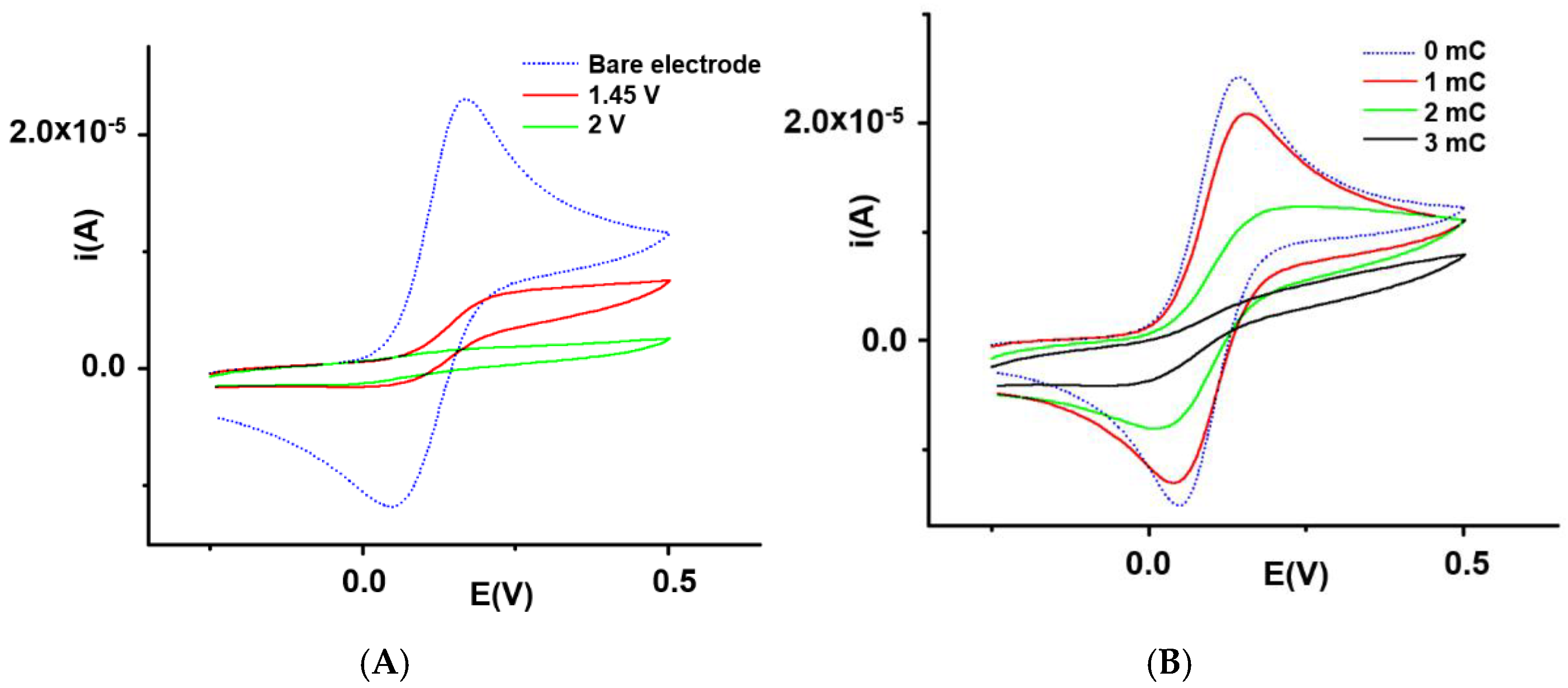
3.2. L-CMEs Preparation
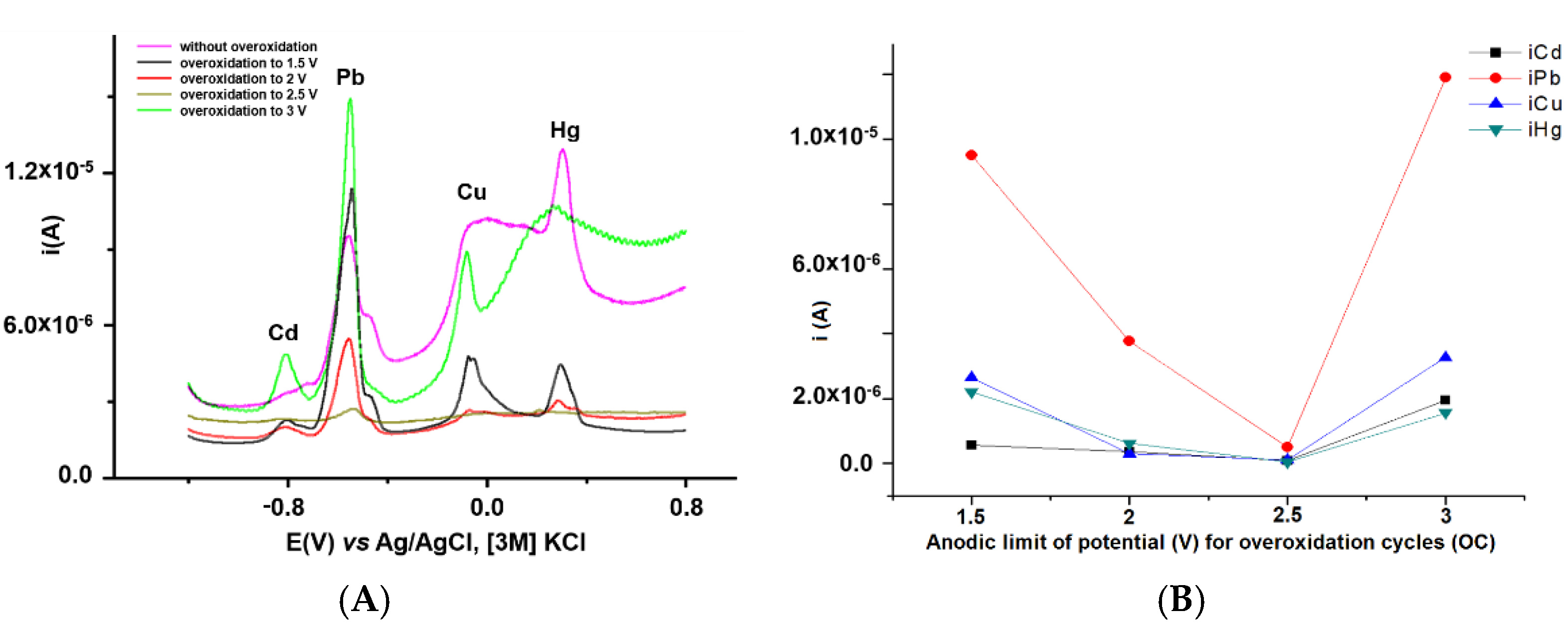
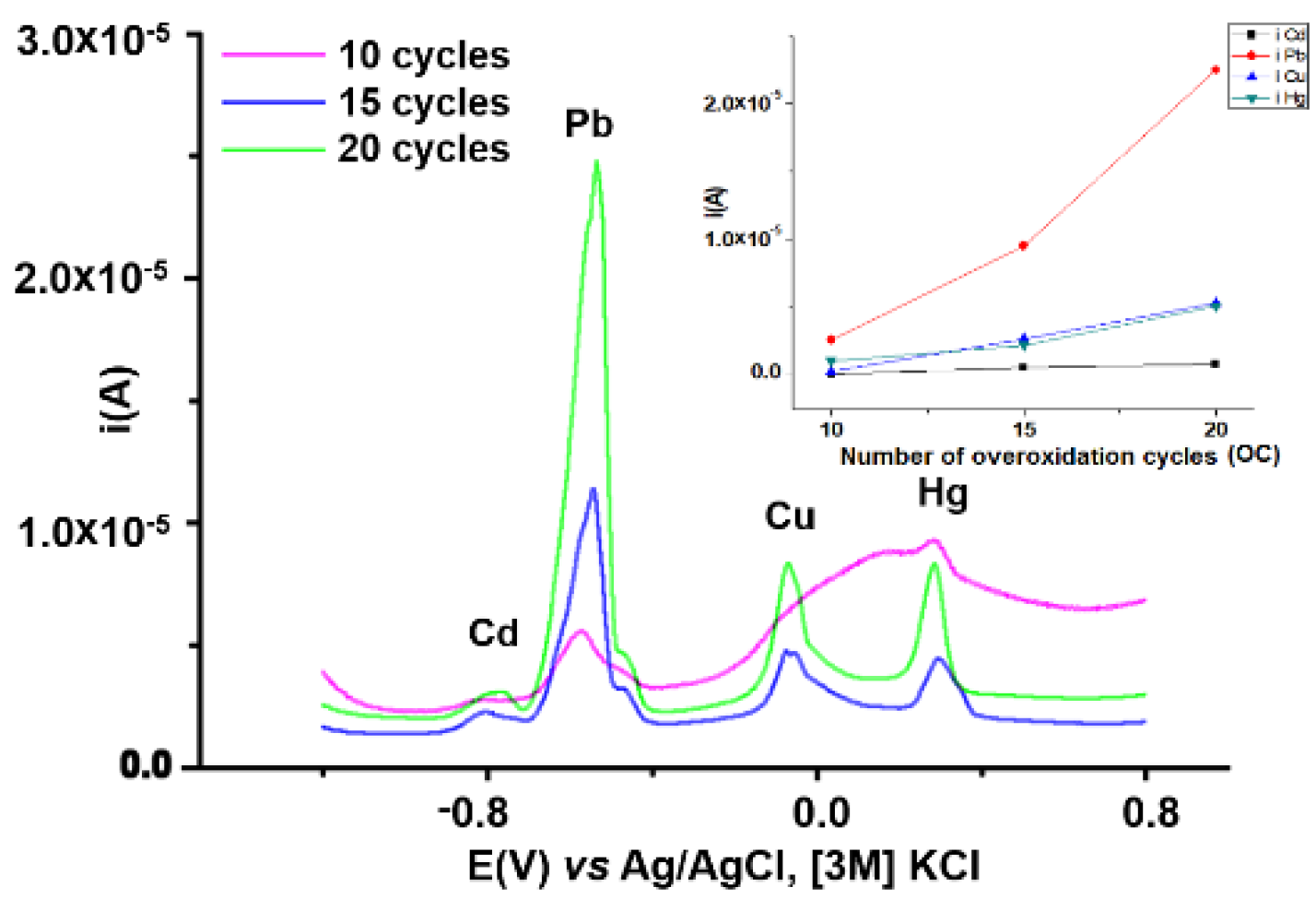
3.2.1. Influence of Overoxidation Conditions on HMs Analyses
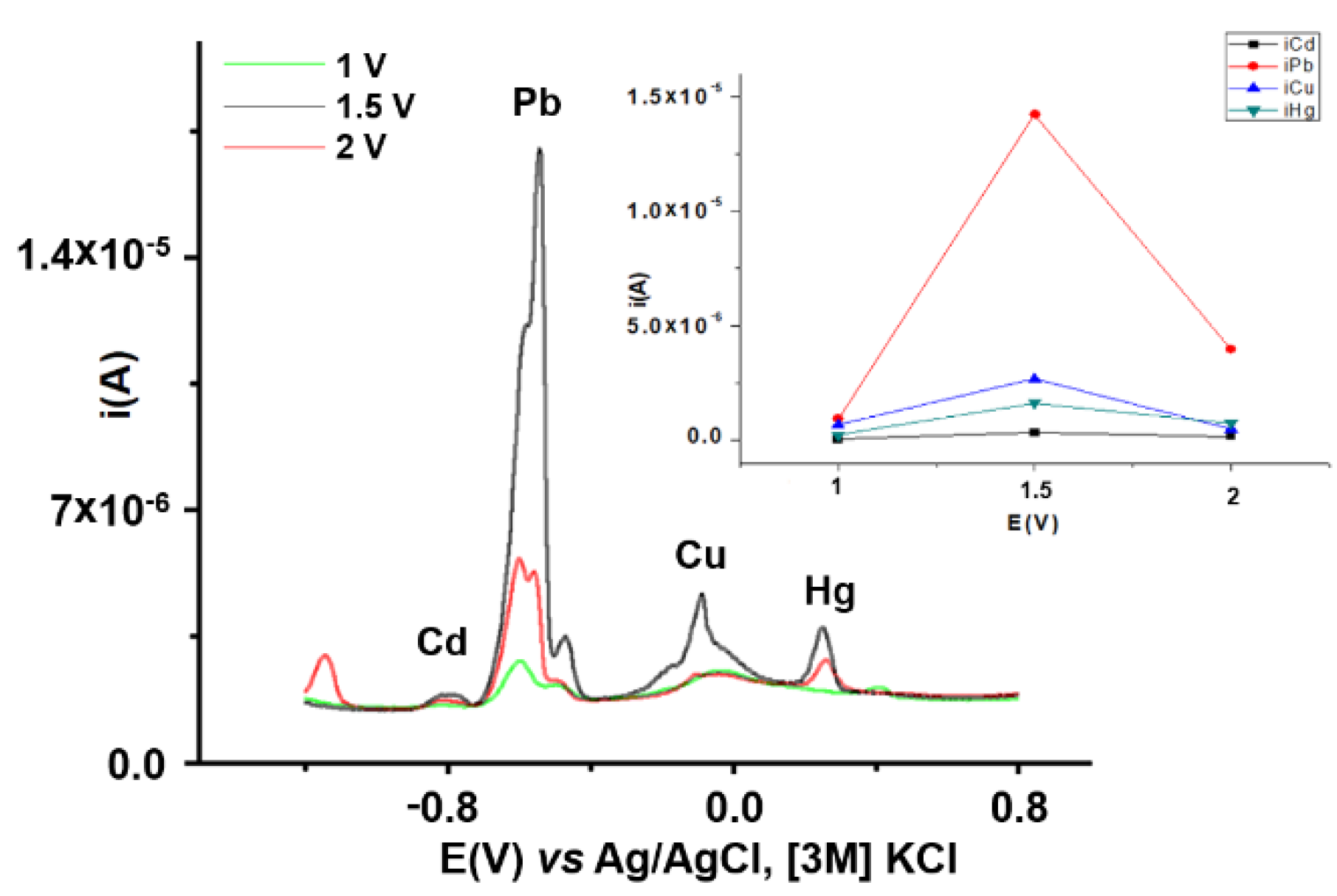
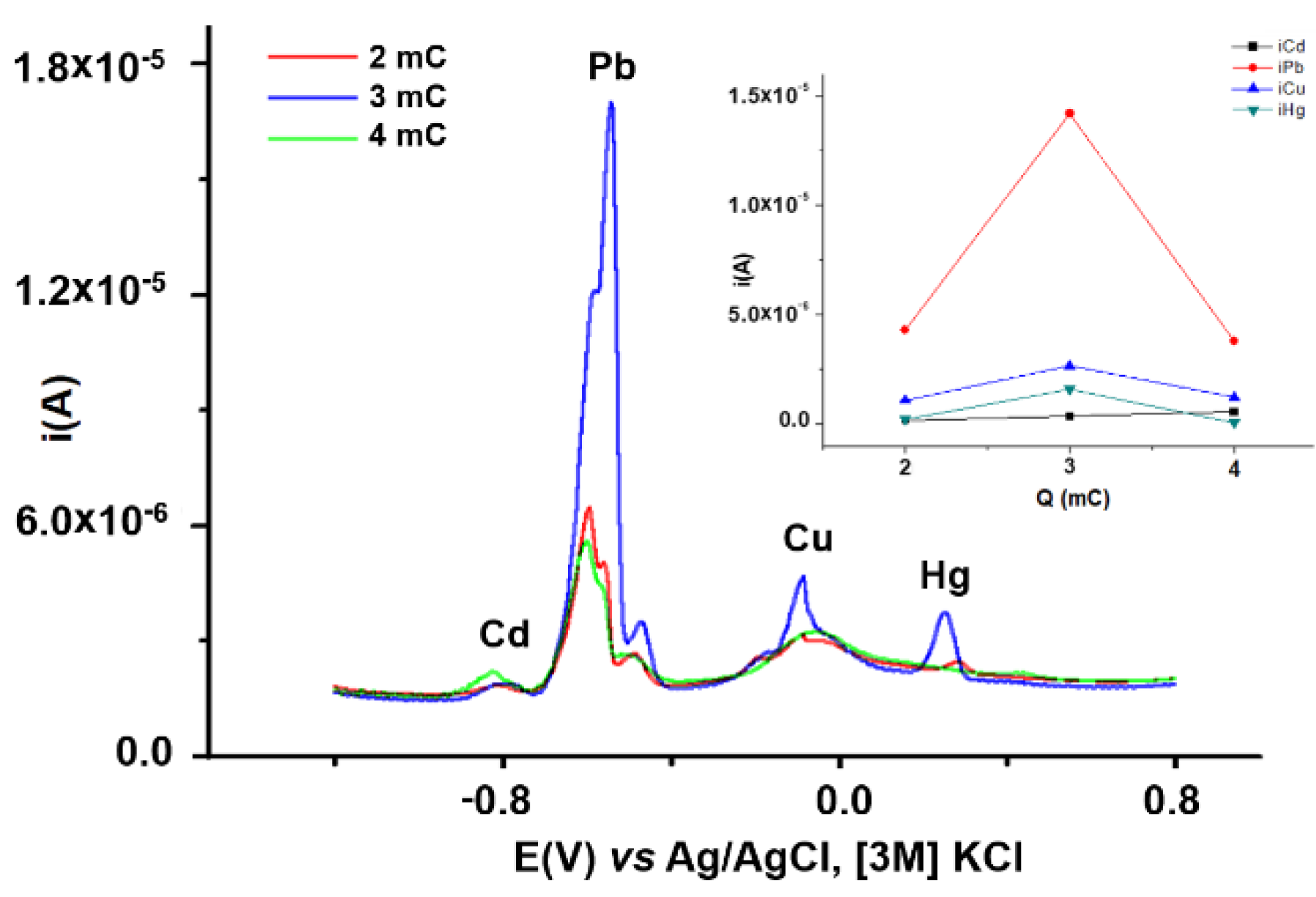
3.2.2. Influence of CPE Charge and Potential on HMs Analytical Signals
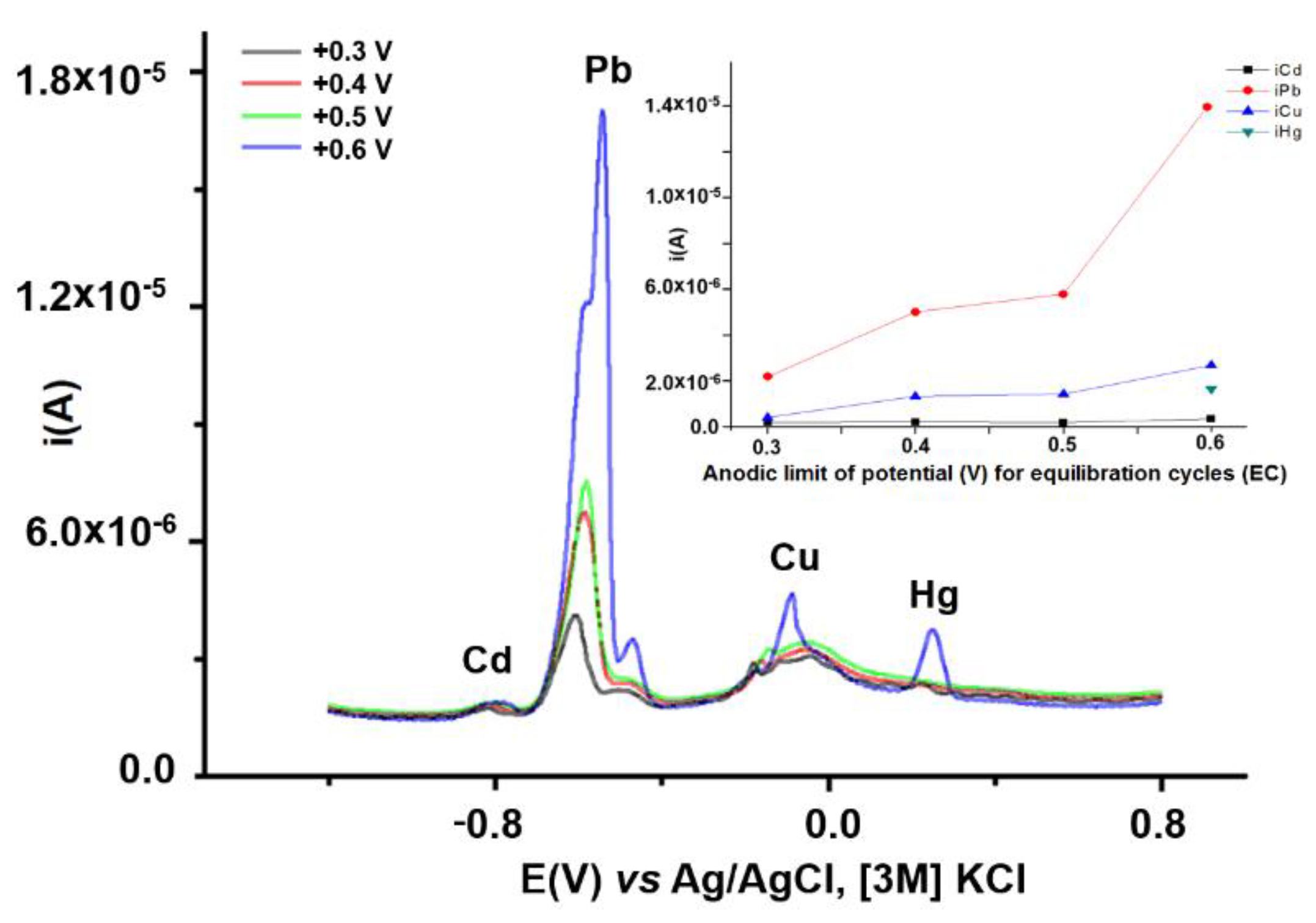
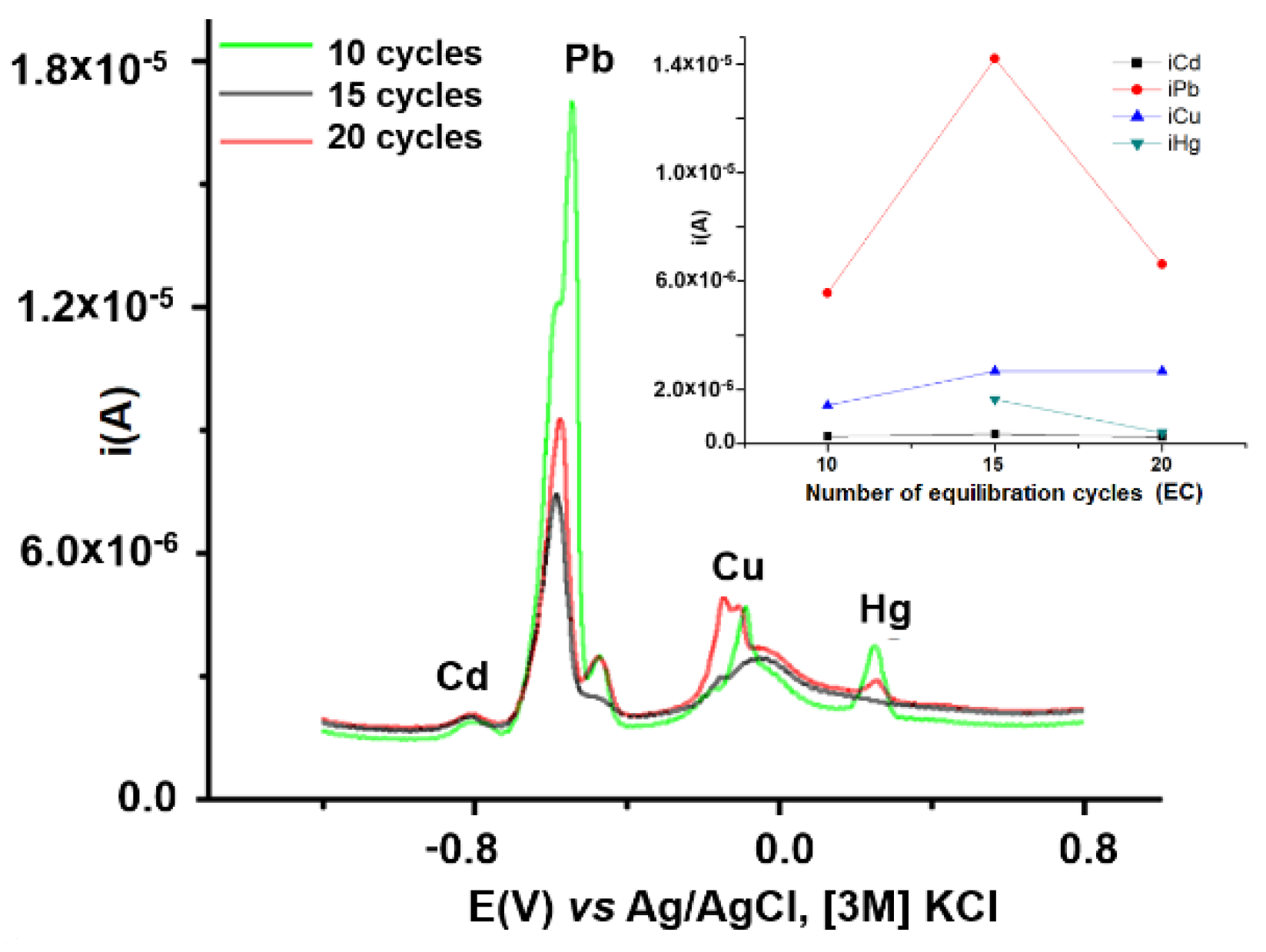
3.2.3. Influence of Equilibration Conditions on HMs Analytical Signals
3.3. Establishing Favourable Conditions for Pb Analysis
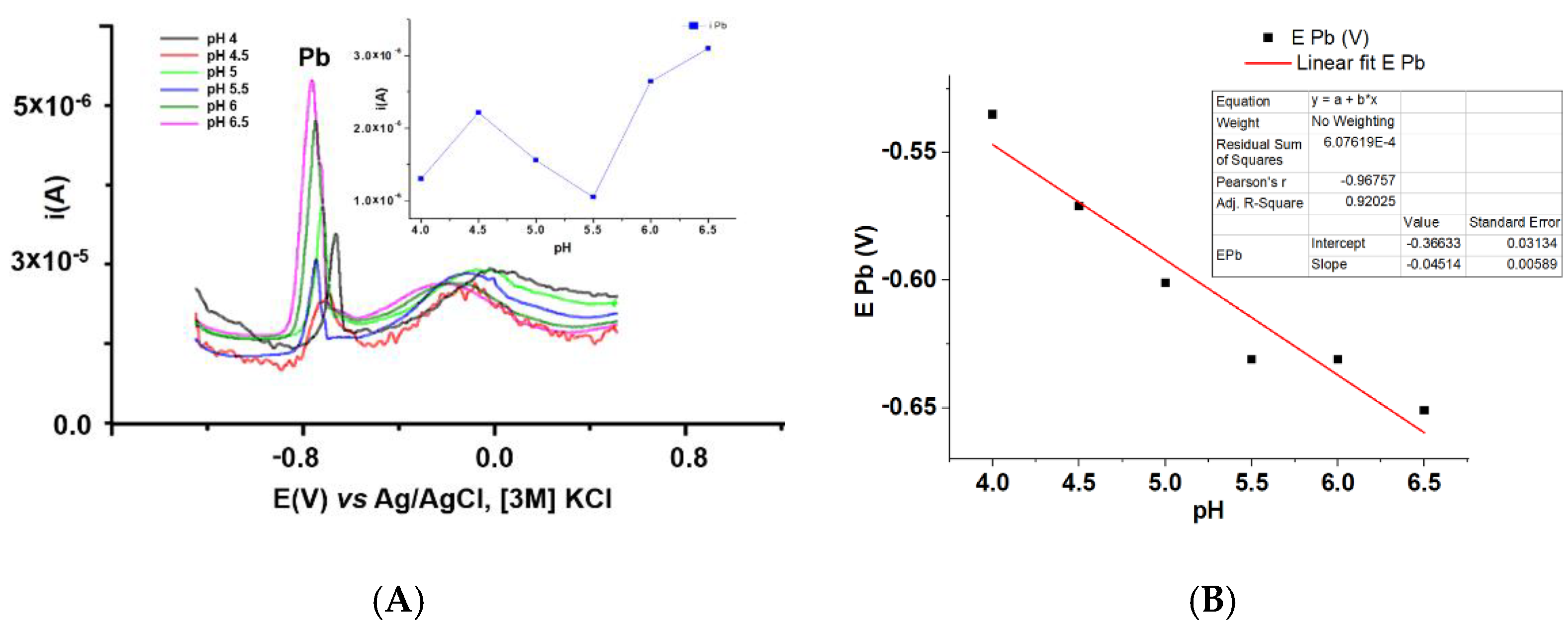
3.3.1. Influence of Conditioning Buffer pH on Pb Analysis
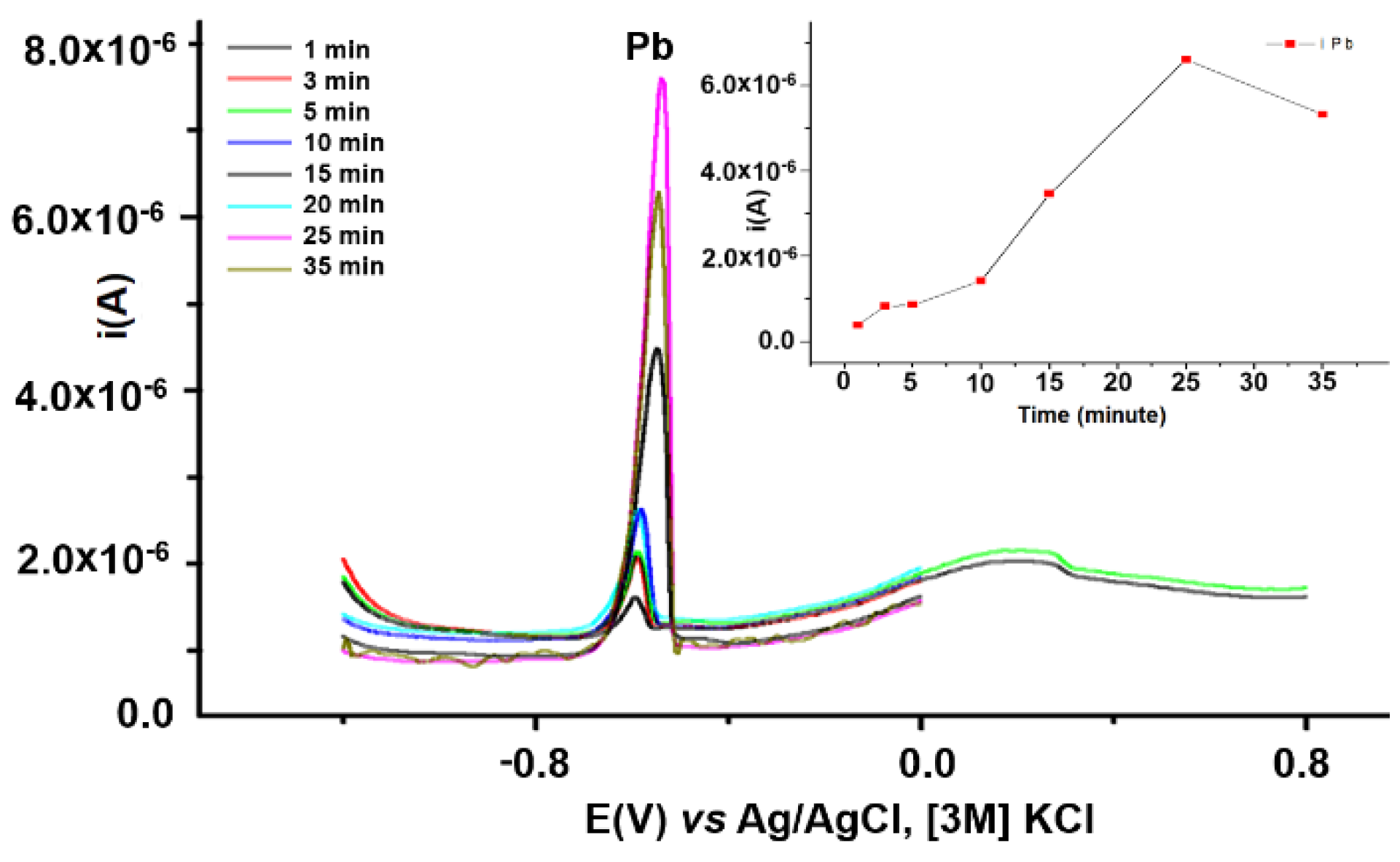
3.3.2. Influence of the Accumulation Time on Pb Analysis
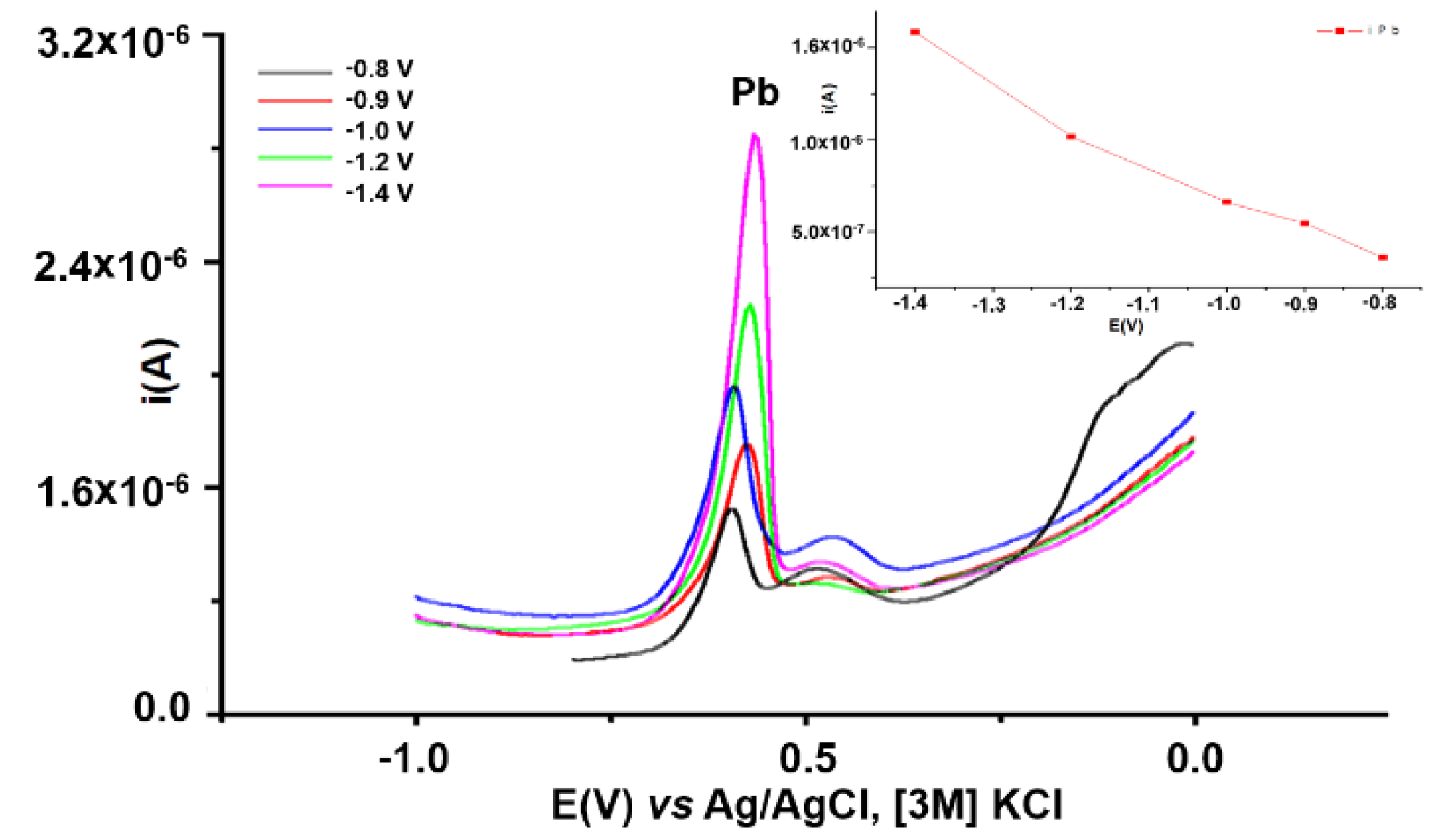
3.3.3. Influence of Reduction Potential on Pb Analysis
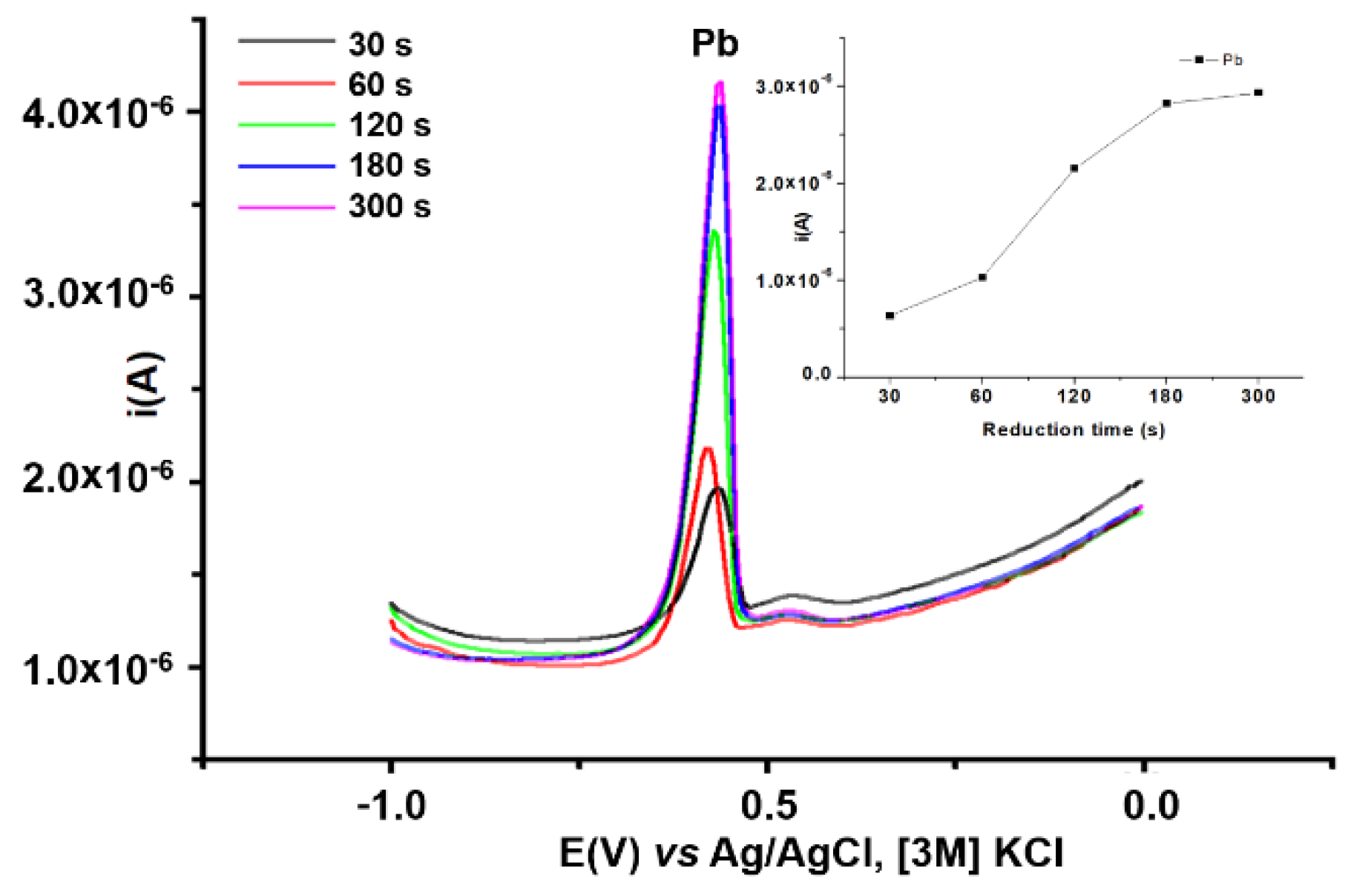
3.3.4. Influence of Reduction Time on Pb Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cristea, M.; Bîrzan, L.; Dumitrascu, F.; Enache, C.; Tecuceanu, V.; Hanganu, A.; Drăghici, C.; Deleanu, C.; Nicolescu, A.; Maganu, M.; et al. 1-vinylazulenes with oxazolonic ring-potential ligands for metal ion detectors; synthesis and products properties. Symmetry 2021, 13, 1209. [Google Scholar] [CrossRef]
- Essaidi, Z.; Niziol, J.; Sahraoui, B. Azo-azulene based compounds-nonlinear optical and photorefractive properties. Opt. Mater 2011, 33, 1387–1390. [Google Scholar] [CrossRef] [Green Version]
- Osaka, T.; Naoi, K.; Hirabayashi, T. Application of electrochemically formed polyazulene to rechargeable lithium battery. Electrochem. Soc. 1987, 134, 2645–2649. [Google Scholar] [CrossRef]
- Szlachcic, P.; Danel, K.S.; Gryl, M.; Stadnicka, K.; Usatenko, Z.; Nosidlak, N.; Lewińska, G.; Sanetra, J.; Kuźnik, W. Organic light emitting diodes (OLED) based on helical structures containing 7- membered fused rings. Dyes Pigm. 2015, 114, 184–195. [Google Scholar] [CrossRef]
- Razus, A.C.; Birzan, L. Synthesis of azulenic compounds substituted in the 1-position with heterocycles. Monatsh Chem 2019, 150, 139–161. [Google Scholar] [CrossRef]
- Buica, G.-O.; Ungureanu, E.-M.; Birzan, L.; Razus, A.C.; Mandoc Popescu, L.-R. Voltammetric sensing of lead and cadmium using poly(4-azulen-1-yl-2,6-bis(2-thienyl)pyridine) complexing films. J. Electroanal. Chem. 2013, 693, 67–72. [Google Scholar] [CrossRef]
- Enache, L.-B.; Anăstăsoaie, V.; Lete, C.; Brotea, A.G.; Matica, O.-T.; Amarandei, C.-A.; Brandel, J.; Ungureanu, E.-M.; Enăchescu, M. Polyazulene-Based Materials for Heavy Metal Ion Detection. 3. (E)-5-((6-t-Butyl-4,8-dimethylazulen-1-yl) diazenyl)-1H-tetrazole-Based Modified Electrodes. Symmetry 2021, 13, 1642. [Google Scholar] [CrossRef]
- López-Alled, C.; Murfin, L.; Kociok-Köhn, G.; James, T.; Wenk, J.; Lewis, S. Colorimetric detection of Hg2+ with an azulene-containing chemodosimeter via dithioacetal hydrolysis. Analyst 2020, 145, 6262–6269. [Google Scholar] [CrossRef] [PubMed]
- Ugo, P.; Sperni, L.; Moretto, L. Ion-exchange voltammetry of trace mercury(II) at glassy carbon electrodes coated with a cationic polypyrrole derivative. Application To Pore-Waters Analysis. Electroanalysis 1997, 9, 1153–1158. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A.; Gros, P. Electrochemical sensors and devices for heavy metals assay in water: The french groups' contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes-Bayón, M.; DeNicola, K.; Caruso, J.A. Liquid chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2003, 1000, 457–476. [Google Scholar] [CrossRef]
- Welz, B.; Sperling, M. Atomic Absorption Spectrometry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1999; pp. 335–475. [Google Scholar] [CrossRef]
- Zhang, Y.; Adeloju, S.B. Coupling of non-selective adsorption with selective elution for novel in-line separation and detection of cadmium by vapour generation atomic absorption spectrometry. Talanta 2015, 137, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.H.; Day, J.A.; Palmer, C.D.; Price, W.J.; Smith, C.M.M.; Tyson, J.F. Atomic spectrometry update. Advances in atomic emission, absorption and fluorescence spectrometry, and related techniques. J. Anal. At. Spectrom. 2005, 20, 562–590. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Ahmadi, S.A.; Askari, M.B.; Di Bartolomeo, A. Screen-Printed Electrode Surface Modification with NiCo2O4/RGO Nanocomposite for Hydroxylamine Detection. Nanomaterials 2021, 11, 3208. [Google Scholar] [CrossRef] [PubMed]
- March, G.; Nguyen, T.D.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Păun, A.-M.; Matica, O.-T.; Anăstăsoaie, V.; Enache, L.-B.; Diacu, E.; Ungureanu, E.-M. Recognition of Heavy Metal Ions by Using E-5-((5-Isopropyl-3,8-Dimethylazulen-1-yl) Dyazenyl)-1H-Tetrazole Modified Electrodes. Symmetry 2021, 13, 644. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.C.; Tecuceanu, T.; Hanganu, A.M.; Ungureanu, E.-M.; Razus, A.C. 4-(Azulen-1-yl) six-membered heteroaromatics substituted in 2- and 6- positions with 2-(2-furyl)vinyl, 2-(2-thienyl)vinyl or 2-(3-thienyl)vinyl moieties. Tetrahedron 2017, 73, 2488–2500. [Google Scholar] [CrossRef]
- Oprisanu, A.; Lazar, G.I.; Pop, M.D.; Ungureanu, E.-M.; Isopescu, R.; Birzan, L. Polyazulenes based materials for heavy metal ions detection. Rev. Chim. 2017, 68, 2215–2218. [Google Scholar] [CrossRef]
- Zejli, H.; Sharrock, P.; Hidalgo-Hidalgo de Cisneros, J.L.; Naranjo-Rodriguez, I.; Temsamani, K. Voltammetric determination of Trace mercury at a sonogel–carbon electrode modified with poly-3-methylthiophene. Talanta 2005, 68, 79–85. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, E.-M.; Anăstăsoaie, V.; Bujduveanu, M.-R.; Brotea, A.-G.; Isopescu, R.; Stanciu, G. Polyazulene Based Materials for Heavy Metal Ions Detection. 4. Search for Conditions for Thiophen-Vinyl-Pyridine-Azulene Based CMEs Preparation. Symmetry 2022, 14, 225. https://doi.org/10.3390/sym14020225
Ungureanu E-M, Anăstăsoaie V, Bujduveanu M-R, Brotea A-G, Isopescu R, Stanciu G. Polyazulene Based Materials for Heavy Metal Ions Detection. 4. Search for Conditions for Thiophen-Vinyl-Pyridine-Azulene Based CMEs Preparation. Symmetry. 2022; 14(2):225. https://doi.org/10.3390/sym14020225
Chicago/Turabian StyleUngureanu, Eleonora-Mihaela, Veronica Anăstăsoaie, Magdalena-Rodica Bujduveanu, Alina-Giorgiana Brotea, Raluca Isopescu, and Gabriela Stanciu. 2022. "Polyazulene Based Materials for Heavy Metal Ions Detection. 4. Search for Conditions for Thiophen-Vinyl-Pyridine-Azulene Based CMEs Preparation" Symmetry 14, no. 2: 225. https://doi.org/10.3390/sym14020225
APA StyleUngureanu, E.-M., Anăstăsoaie, V., Bujduveanu, M.-R., Brotea, A.-G., Isopescu, R., & Stanciu, G. (2022). Polyazulene Based Materials for Heavy Metal Ions Detection. 4. Search for Conditions for Thiophen-Vinyl-Pyridine-Azulene Based CMEs Preparation. Symmetry, 14(2), 225. https://doi.org/10.3390/sym14020225







