Abstract
The electronic circular dichroism (CD)-silent 2,5-bis(biphen-2-yl)terephthalaldehyde has been used as a sensor (reporter) of chirality for primary amines. The through-space inductor–reporter interactions force a change in the chromophore conformation toward one of the diastereomeric forms. The structure of the reporter, with the terminal flipping biphenyl groups, led to generating Cotton effects in both lower- and higher-energy regions of the ECD spectrum. The induction of an optical activity in the chromophore was due to the cascade point-to-axial chirality transmission mechanism. The reporter system turned out to be sensitive to the subtle differences in the inductor structure. Despite the size of the chiral substituent, the molecular structure of the inductor–reporter systems in the solid-state showed many similarities. The most important one was the tendency of the core part of the molecules to adapt pseudocentrosymmetric conformation. Supported by a weak dispersion and Van der Waals interactions, the face-to-face and edge-to-face interactions between the π-electron systems present in the molecule were found to be responsible for the molecular arrangement in the crystal.
1. Introduction
Chirality and its demonstration, an optical activity of non-racemic compounds, is one of the most fascinating phenomena observed in nature. Without a doubt, chirality represents the most decisive factor that affects the functioning of living organisms. The tangible evidence of chirality is the so-called “asymmetry of life” that is manifested, for example, by the same configuration of amino acids that are building blocks of living organisms. Self-organization of bio-organic molecules, molecular recognition, and induction of chirality, which take place in living organisms, are fundamental processes of which chirality plays the first fiddle [1,2,3,4,5,6,7].
On the other hand, one of the convenient ways to acquire chiral compounds in the enantiomerically pure form relies on the process of chirality induction in prochiral substrate. The stereoselective synthesis is the leading aspect of contemporary synthetic organic chemistry [8,9,10,11].
The optical activity of chiral compounds manifests itself inter alia by their optical rotation (OR) and circular dichroism (CD), both vibrational (VCD) and electronic (ECD) [12,13]. The latter two spectroscopic methods are particularly useful for determining the structure of chiral compounds and their aggregates, however, the presence of suitable chromophore (or chromophores) in the molecule skeleton is compulsory. Thus, in the case of the compounds lacking chromophoric system(s), the proper functionalization, which means introducing the appropriate chromophore into the molecule, allows for structural studies using CD spectroscopy.
It is an axiom to say that the optically active compounds are characterized by the permanent chirality, i.e., they are non-changeable under standard conditions. There is a group of dynamically racemic compounds, usually characterized by strong electronic absorption in UV–VIS spectral region, which remain optically inactive (ECD-silent) due to the easily achieved equilibrium between the enantiomeric forms [14]. This equilibrium might be affected by the covalent or non-covalent attachment of permanently chiral “inducer” to such a stereodynamic chromophore. As a result of the adaptation of the structure of the chromophore to the chiral environment created by the inducer, the arising of Cotton effects (CEs) in the region of the absorption of the chromophore is observed in the ECD spectra [14].
The above-mentioned mode of action of stereodynamic chromophoric probes is in fact a foundation of chirality sensing process [15,16,17,18,19]. To date, a number of artificial probes have been introduced in stereochemical analysis to establish chirality of natural and man-made compounds. Among the probes, those based on exciton coupling between strong electric dipole transition moments (EDTMs) seem to be particularly useful for chirality sensing [20,21,22]. The direct correlation between the shape of the ECD spectrum (with particular emphasis on the spectral region where exciton couplet is appearing) and geometrical relationship between interacting EDTMs allows for determining the inducer’s chirality.
Among the permanently chiral molecules, the inducers having two or more groups prone to functionalization represent rather less demanding cases for stereochemical assignments. On the opposite pole are chiral molecules in which there is no more than one group available for functionalization. In such cases, the chromophoric probe needs to contain two aromatic parts twisting relatively to each other upon attaching the inducer [23,24,25]. As a result, the chromophoric system becomes optically active. The way of action of these probes relies on the point-to-axial chirality transfer mechanism and, usually, the efficiency of the probe is directly proportional to the differences between substituents flanking the stereogenic center(s) [26,27,28,29].
Recently, we have proven that the ECD-silent chromophoric probe, based on the 2,5-di(1-naphthyl)terephthalaldehyde skeleton, might be efficiently applied for chirality sensing of primary amines through the point-to-axial chirality transmission mechanism [30]. A feature that distinguishes this probe from others is unprecedentedly high sensitivity to subtle differences in the inductor structure. The generated exciton Cotton effects were observed in the region of the 1Bb electron transition in the naphthalene chromophore, which was more than enough for stereochemical studies of aliphatic amines. However, for the inducers with aromatic chromophores, the measured ECD spectra exhibited complex shapes, which made simple structure–spectrum correlations impossible.
To check the possibility of overcoming this problem, we decided to modify the structure of the receptor in such a way that the CEs were visible beyond the absorption range of the typical π-electron chromophores. However, the main goal was to develop a probe operating on the cascade mechanism of chirality transmission. Thus, the attachment of an additional flexible chromophore to the terephthalaldehyde core will lead to formation of the chirality sensor capable of sequential transferring of the structural information. The intention behind this idea is depicted in Figure 1a.
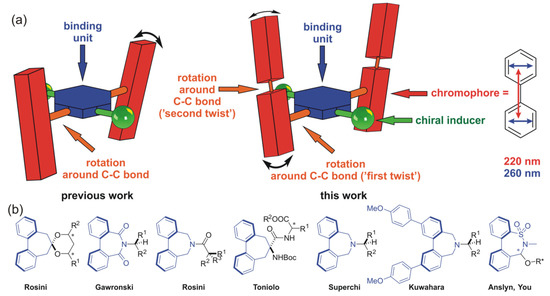
Figure 1.
(a) Schematic representation of the designed stereodynamic probe. Arrows indicate polarizations of electron transition of the highest oscillator strengths within the biphenyl chromophore. (b) Examples of biphenyl-containing sterodynamic probes for chirality sensing. The biphenyl-based probe core is marked in blue.
From the point of view of the assumption made, biphenyl as a flexible chromophore has turned out to be a natural choice. This belief is based on the solid foundations. It was as early as at the turn of the century when Rossini “induced a preferred twist in a biphenyl core”, which allowed for determining the absolute configuration of chiral diols [31]. Shortly after, we and other research groups have proven the usefulness of biphenyl-based compounds for stereochemical studies [32,33,34,35,36,37,38]. Although these probes were different in the method of binding the inductor, the general mode of an action of the probes was the same. The through-space inductor-reporter interactions enforced the shift of the biphenyl P/M equilibrium (“twist” of the chromophore) toward one of the two diastereomeric forms. This resulted in the appearance of non-zero CEs in the region of the biphenyl UV-absorption, with the position and the amplitude of CD bands potentially being affected by proper functionalization of chromophore core [35].
Continuing our interests in the dynamic chirality induction phenomenon, we decided to put some efforts into designing and synthesizing the new sensitive stereodynamic reporter for primary amines operating on the basis of the point-to-axial chirality mechanism. The modular structure of the probe core would consist of the amine-binder, whereas the external flexible chromophore systems would be responsible for generating the CEs. We will point out that the efficiency in the chirality sensing, understood as the quantitative correlation between the size of the substituent(s) and the Cotton effect(s) amplitude, turned out to us to be less important than providing the evidence of chirogenesis taking place in such a cascaded probe. An opportunity to investigate the effect of a substituent on the chromophore structure in the solid state would be given by comparison of the structures of the inductor–reporter systems found in the crystal.
2. Materials and Methods
Detailed experimental procedures, details regarding X-ray diffraction studies, and theoretical calculations are given in the Supplementary Information file.
3. Results and Discussion
As it has been reported previously, the initial attempts to the modification of the 2,5-di(1-naphthyl)terephthalaldehyde probe by replacing the naphthalene with other chromophore (preferably anthracene) failed [30]. However, we successfully synthesized the derivative 1 of the modular structure by Suzuki coupling of 2,5-dibromoterephthalaldehyde with 2-biphenylboronic acid (all details regarding synthesis and full spectroscopic characterization of the compounds are contained in the Supplementary Information) [39].
In 1, the central dialdehyde part would bind the permanently chiral inducer (the primary amine), whereas the biphenyl units act as “double” switchable chromophores. We expected a possibility of rotation around the carbon–carbon bonds connecting the binding part with the chromophore (“the first twist”) and around those bonds, which connect the aromatic rings in the biphenyls (“the second twist”). Therefore, the structural information from the stereogenic center of the inducer to the external phenyl ring would be transferred sequentially. As the inducers, we chose primary amines, which varied in the size of the substituents flanking stereogenic center including the demanding case of 3-aminotetrahydrofurane. In such a particular case, the probe will have to distinguish the differences between oxygen atom and CH2 group in the inductor structure.
The diimine compounds 2a–2k (shown in Figure 2) were obtained quantitatively through a simple condensation of 1 with twofold excess of the respective amine. Further purification by crystallization allowed us to obtain analytically pure samples, which were further used for stereochemical studies. Having an opportunity for a deeper look into the structure of the compounds in the crystalline phase, we begin the discussion from the results of the X-ray diffraction studies.
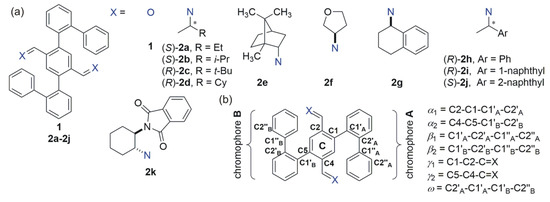
Figure 2.
(a) Structures of compounds under study. (b) Torsion angles that characterize a molecular conformation.
3.1. The Solid-State Molecular Structure and Molecular Organization in the Crystals of Diimines
The asymmetric unit of crystal 1 consists of half molecule of the aldehyde located on inversion center, which means that the molecule uses its own symmetry. The molecule of 1 does not contain any functional groups considered as potential donors of strong classical hydrogen bonds. However, it is possible to form C–H···O hydrogen bonds, and in the crystal structure of 1, we observed supramolecular chains made from the molecules of 1 (Figure 3). Additionally, despite the presence of the formyl group, the probe 1 represents a rich aromatic system. Therefore, the π-electron interactions constitute the main force “sticking” molecules together to form the 3D crystal structure.
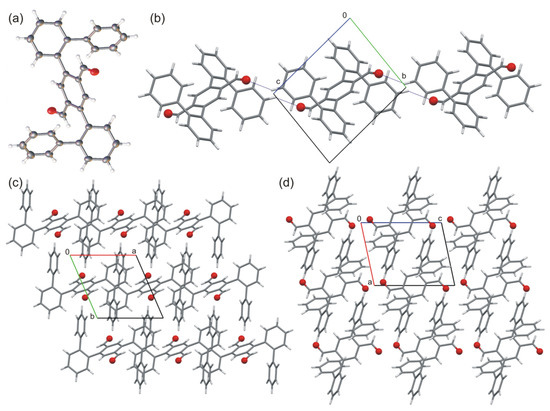
Figure 3.
(a) Molecular structure of 1. (b) Supramolecular chain via H-bonds in the crystal structure (view along a-axis). Molecular packing in the crystal structure of 1: (c) view along c-axis and (d) view along b-axis. Oxygen atoms are shown as red balls.
Similarly to the parent compound 1, the possibility of forming classic strong hydrogen bonds in crystal structure is limited in the case of the imines 2a–2f, 2h, and 2k. The addition of the chiral substituent to the probe 1 core disrupted the inversion symmetry. However, for most of the obtained derivatives, this did not cause a significant change of the geometry of the core of the molecule. For compounds 1, 2a–2e, and 2h, the conformation of the molecule in crystal phase was practically the same (Table S4). It is worth emphasizing that the core part of the molecules remained pseudocetrosymmetric. The comparison of the geometry of the selected molecules is shown in Figure 4.
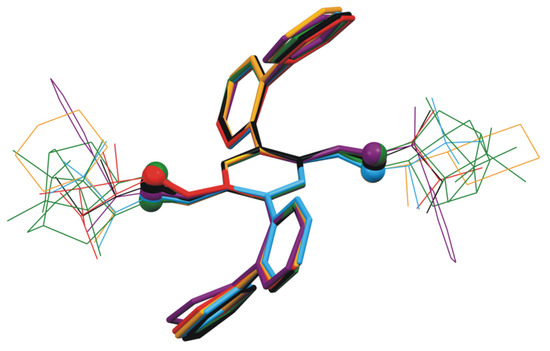
Figure 4.
Overlying of molecular structures of compounds 1, 2a–2e, and 2h.
Interestingly, comparing the unit cell parameters found for the compounds 2a–2e and 2h showed that they are very similar (see Table S5). Moreover, the crystal packing turned out to be quite repeatable in the case of various derivatives.
The crystal structure is glued by interactions of π-electron systems. For the exemplary case of 2c, two molecules interact with each other through face-to-face interactions between the aromatic rings of biphenyl substituents. Such a discrete stack is obscured on both sides by edge-to-face interactions with the next two molecules (Figure 5a). The crystal structure is composed of layers stabilized by a series of aromatic interactions, supported in some cases by weak C–H···N interactions. The 3D structure is stabilized by weak Van der Waals interactions and dispersion interactions (Figure 5b). The visible differences in crystal packing of individual derivatives result from volume and shape differences between the chiral substituents attached to the nitrogen atoms. It can be concluded that the geometry of the molecule determines the packing of the molecules in the crystal structure. Being strict, we must admit that the type of the substituent, its size, and its shape causes slight differences in the packing of the molecules in the crystal phase. This results from the steric fit of the molecules and does not disturb the general patterns observed for most compounds under study.
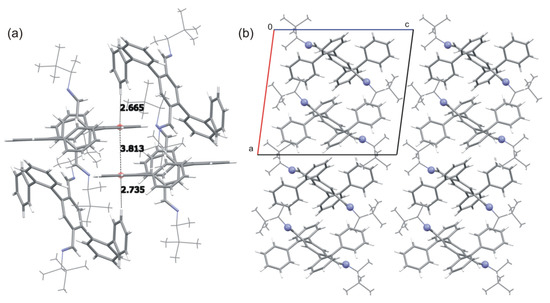
Figure 5.
(a) Supramolecular interactions in the crystal structure of 2c. For clarity, the aliphatic substituents are shown with thinner lines whereas intermolecular interactions are shown as dashed lines. Distances are in angstroms. (b) Molecular packing in the crystal of 2c viewed along the b-axis (nitrogen atoms are shown as balls).
A significant change in the geometry of the molecule can be observed in the case of compounds 2f and 2k, where both biphenyl wings are on the same side of the molecule. Interestingly, in both cases, the crystals contain solvent molecules. In the crystals of 2f, the solvent molecules are located in voids, which constitute about 9.5% of the unit cell volume (Figure 6a). For the imine 2k, the solvent is located in the channels occupying 20% of the unit cell volume (Figure 6b). The crystals of both compounds left in the air, apart from the solvent, are unstable and destroyed after some time (several hours for 2f and two days for 2k). Similarly to the previously mentioned cases, for these two compounds, the main force that is responsible for the arrangement of the molecules in the 3D structure are the interactions between the aromatic systems supported by weak dispersion and Van der Waals interactions.
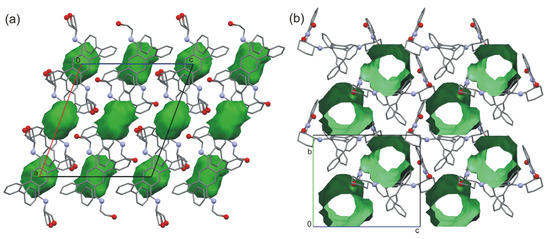
Figure 6.
(a) Structural voids in the crystal of compounds 2f and (b) channels in the crystal structure of 2k.
3.2. Chirogenesis in Imines 2a–2k
The crystallographic studies did not provide any ultimate prediction regarding the possibility of induction of an optical activity in the compounds under study. What is more, the observed solid-state molecular behavior of the studied compounds calls the usefulness of probe 1 as the chirality sensor into question. On the other hand, the structure of a given compound in the crystal is determined mostly by the way of packing and strong intermolecular interactions, whereas in the diluted solution, more subtle intramolecular interactions can influence the conformation.
The initial attempts to measure ECD spectra of imines failed due to very limited solubility or even insolubility of these compounds in polar solvents. However, the imines 2a–2k turned out to be unexpectedly well soluble in non-polar solvents, i.e., cyclohexane; therefore, both UV and ECD spectra were measured in this solvent. The numerical UV and ECD data are juxtaposed in Table 1, and in Figure 7, example ECD spectra of imines 2a, 2c, 2f, and 2h are shown. For the sake of comparison, in Figure 7, we additionally show the ECD spectra of the corresponding imines obtained from 2,5-di(1-naphthyl)terephthalaldehyde and (R)-2-aminebutane, (R)-2-amine-3,3-dimethylbutane, (R)-3-aminetetrahydrofurane, and (R)-1-phenylethylamine (data taken from [30]).

Table 1.
The UV (ε, in dm3·mol−1·cm−1) and electronic circular dichroism (ECD) (Δε, in dm3·mol−1·cm−1) data for imines 2a–2k [a].
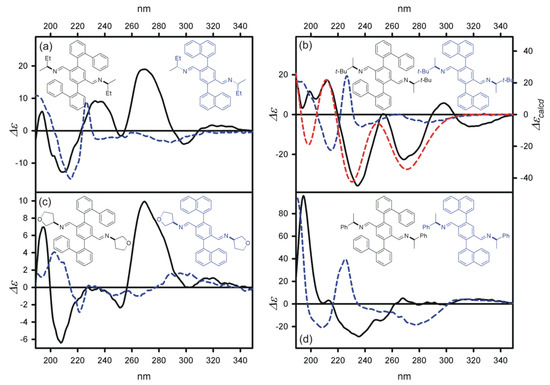
Figure 7.
The ECD spectra of (a) 2a, (b) 2c, (c) 2f, and (d) 2h (solid black lines) and the ECD spectra of their counterparts obtained from 2,5-di(1-naphthyl)terephthalaldehyde and (a) (R)-2-aminebutane, (b) (R)-2-amine-3,3-dimethylbutane, (c) (R)-3-aminetetrahydrofurane, and (d) (R)-1-phenylethylamine (blue dashed lines, data taken from [30]); all spectra were measured in cyclohexane. Insets show structures of the respective compounds. (d) The ECD spectrum of 2c (red dashed line) calculated at the TD-CAM-B3LYP/6-311++G(d,p) level and Boltzmann averaged. The calculated spectrum was wavelength-corrected to match the UV maximum.
The UV spectra of 2a–2k exhibited a few absorption bands. The number, intensity, and the position of the UV absorption bands depended on the type and structure of the compound. Apart from the higher-energy maximum appearing at around 190 nm, the remaining UV bands were usually not well-distinguished from each other. An attempt to generalize led to the conclusion that it is possible in the UV spectra to distinguish at least four areas in which the absorption maximums or curve inflection points appeared. For example, in the simplest case of 2a, the first lowest energy band appeared at 319 nm and the position of the higher-energy bands were 237 and 196 nm, whereas in the region of 270 nm, there was an inflection point of the UV curve rather than an actual maximum. A subtle change in the structure of the inductor (2b) resulted in better visibility of the 270-nm absorption band, however, in the lower-energy region, the absorption bands were found to not be well separated from each other.
On the contrary, the ECD spectra showed a clearer picture, although for all the imines 2a–2k, the ECD spectra were rich in CEs. Among the CEs visible in each individual spectrum, those that appeared at around 270, 230, and 210 were the most intense, whereas in the lower- and the higher-energy regions, the amplitudes of respective CEs were smaller. The exceptions were imines 2i and 2j, additionally containing naphthalene chromophore in each of the inducer moieties. In these cases, the ECD spectra were dominated by the strong exciton couplets appearing in the region of the 1Bb electron transition in the naphthalene chromophores. The amplitudes were equal to −43 and 295, respectively, for 2i and 2j. For the remaining cases, the CEs observed in ECD spectra originated from dynamically induced optical activity in the chromophore. However, while the CEs appearing at ≈260–280 nm were not surprising for biphenyl derivatives, the origin of the lower-energy CEs appearing at around 310–320 nm remained unclear.
Even the cursory reading data collected in Table 1 led to the conclusion that for fully aliphatic imines 2a–2d, which are characterized by the same structural type, the sequence of CEs reflected the chirality of stereogenic center. The amplitudes of respective CEs might be (to some extent) correlated with the volume of substituents flanking the stereogenic center. Thus, the sequence of CEs found for imines of S absolute configuration at stereogenic centers is as follows: +/−/+/−/+/−/+, and for imines of opposite absolute configuration, the CEs sequence is mirrored. Other results obtained from the analysis suggest that the relative bulkiness of the substituents in similar imines 2a–2d rose as follows: t-Bu > i-Pr > Cy > Et > Me. Unfortunately, for other compounds under study, this analysis is not straightforward and is limited to the closely related inducers (for example, 2g and 2h).
Abstracting from a quantitative structure–spectra correlation, we argue that the aspect that should be first of all paid attention to is unprecedently high sensitivity of the probe to the chirality of the inducer. The best example confirming these words is the direct comparison of the ECD spectra measured for imines containing the probe 1 skeleton and for those obtained from the 2,5-di(1-naphthyl)terephthalaldehyde-based probe (see Figure 7). For the most demanding case of 3-aminetetrahydrofurane, the CEs, observed for 2f, turned out to be of three to six times higher amplitude than the CEs measured for the corresponding diimine of 2,5-di(1-naphthyl)terephthalaldehyde.
Having confirmed the efficiency in chirality sensing, we carried out some computational studies on the structure–chiroptical property relationships for the arbitrary chosen representative example 2c. We assumed that this would cast some light on the problem of the dynamic chirality induction in such a complex. Unexpectedly, this task turned out to be more complicated than we thought. Among several methods tested for structure and ECD calculations, the “classical” hybrid functional B3LYP including empirical dispersion correction (GD3BJ), used for geometry optimization and newer CAM-B3LYP hybrid functional for excited states calculations (both in conjunction with enhanced 6-311++G(d,p) basis set), gave the most satisfying results (see Figure 7c) [40,41,42,43,44].
Each low-energy conformer of 2c might be characterized by at least four torsion angles (see Figure 2b for definition of torsion angles, and Table 2 for some energetic and structural data that characterize individual low-energy conformer). The twist α1 and α2 angles (defined here as α1 = C2-C1-C1′A-C2′A, α2 = C4-C5-C1′B-C2′B) define the relative orientation of biphenyl moieties A and B to the central terephthalaldehyde unit C. The computational study clearly indicates that the low-energy conformers are characterized by the values of the α angles ranging from ±60° to ±120°. As it was in the case described previously, the low-energy conformers of 2c, by analogy to the B-A-B-type triads, can be considered of C or S-type [45,46,47]. Conformers characterized by the opposite signs of the α1 and α2 angles (S-type conformers) were of higher energy than C-type structures characterized by the same signs (not necessarily the values) of the α angles. However, even among the low-energy C-type conformers of 2c, some structural preferences were visible. The prevailing conformers are characterized by symmetry and the total population of C2-symmetrical conformers no. 1, 4, and 13 of 2c are equal to 72%. The lowest-energy conformer no. 4, which was characterized by the highest abundance in the equilibrium (41%), had the greatest impact on the overall ECD spectrum as well. The difference between the lowest energy conformer no. 4 of 2c and the structure of the molecule found in the solid state (see Figure 8a) was noticeable.

Table 2.
Total (E, in Hartree) and relative (ΔE, in kcal mol−1) energies; percentage populations (Pop); and torsion angles α, β, γ, and ω (in degrees) calculated at the B3LYP-GD3BJ/6-311++G(d,p) level for individual low-energy conformer of diimine 2c and found experimentally for the molecule presents in the crystal of 2c.
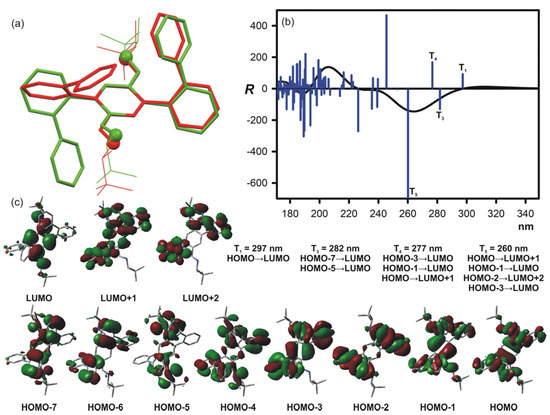
Figure 8.
(a) Overlays of X-ray diffraction determined solid-state structure of 2c (green) and the lowest energy conformer no. 4 of 2c, calculated at the B3LYP-GD3BJ/6-311++G(d,p) level (red). (b) The ECD spectrum calculated at the CAM-B3LYP/6-311++G(d,p) level for the lowest energy conformer no. 4 of 2c. Vertical bars represent calculated rotatory strengths. Wavelength was not corrected. (c) The main molecular orbitals involved in the low-energy electron transitions in the low-energy conformer no. 4 of 2c.
The values of the angles β1 and β2 describe “the second twist” of the chromophore. Strictly speaking, the angles β1 and β2 determine the helicities of the biphenyl units (from the two possibilities, the lower, in the absolute sense, value of the angle β was taken for each biphenyl moiety). Thus, the helicities of biphenyls may be consistent (either M,M or P,P) or opposite (M,P). The lowest-energy conformer no. 4 of 2c is characterized by P,P helicity of the biphenyls.
With the exception of the high-energy conformers no. 47, 69, and 77, conformation of both γ1 and γ2 angles remained antiperiplanar.
Conformation of the chromophore was reflected in generated rotatory strengths. In general, the computational analysis was to show the correlation between CEs calculated for individual low-energy conformers of 2c and the ω angle describing the spatial relationship between EDTMs polarized along the long axis of biphenyl chromophore. Unfortunately, the analysis was not as straightforward as what may be expected, and the computational results showed a rather complex spectroscopic pattern of the calculated ECD spectra.
Referring again to the lowest energy conformer no. 4 as the representative example, we looked into orbitals involved in the electron transitions. We were interested in to what extent such an analysis would allow us to identify the origin of the CEs, especially those observed in the low-energy spectral region. In Figure 8b,c, we show the ECD spectrum and main molecular orbitals involved in the low-energy electron transitions calculated for conformer no. 4. We took into consideration only those low-energy electron transitions that generate significant rotatory strengths. Thus, from the point of view of a chiroptical output, the most important electron transitions appeared at the calculated ECD spectrum at 297, 281, 277, and 260 nm. The first of these is responsible for positive low-energy CE observed in experimental ECD spectrum at around 300 nm, whereas superposition of the remaining electron transitions resulted in the negative CE observed at around 270 nm.
The calculated lowest energy electron transitions engaging orbitals came from both the central imine unit C as well as from lateral biphenyl chromophores. In other words, the HOMO–LUMO transition contributed the most to the positive rotatory strength calculated at 297 nm, involving orbitals delocalized to the whole molecular system. It is worth considering the fact that the twist of the biphenyl systems relative to the central unit as well as aromatic rings in biphenyl chromophores did not completely block the possibilities of electron delocalization. Therefore, the biphenyl moieties cannot be considered as “isolated” chromophores capable of generating exciton-type couplets.
Going to the higher energies—the remaining above-mentioned rotatory strengths originated mainly from the orbitals centered in the biphenyl units (Figure 8c). It should be noted that some contribution to the overall rotatory strengths were also established from electron transitions involving HOMO and HOMO-4 orbitals and LUMO, LUMO+1, and LUMO+2 orbitals delocalized either to the chromophore or centered solely at biphenyl units.
4. Conclusions
To conclude, we have proven the utility of 2,5-bis(biphen-2-yl)terephthalaldehyde as chirality sensor for primary amines. The amines act as inductors of an optical activity in the stereodynamic multichromophoric system. The measured ECD spectra are richer in CEs and therefore more difficult to interpret. Consequently, the structure–spectra correlations seemed to not be as straightforward as in the cases of respective 2,5-di(1-naphthyl)-terephthalaldehyde-based imines. The main advantage of the system described here relies on its unprecedent sensitivity, exceeding sensitivity of to-date described chromophoric probes. For structurally similar inductor systems, it is possible to draw some qualitative and quantitative correlations binding the absolute configuration, size of the substituents flanking stereogenic center with signs, and amplitudes of Cotton effects.
The computational analysis carried out for the representative example 2c indicated the preference to the C-type conformers in conformational equilibrium over conformers of the S-type higher in energy. While for 2,5-di(1-naphthyl)terephthalaldehyde-based imines the observed exciton CEs originated from interactions between EDTMs within naphthalene chromophores, the biphenyl-containing probe 1 represented a more complex chromophoric system engaging orbitals delocalized to the whole molecule. Therefore, the biphenyl chromophore did not contribute independently and solely to the observed low-energy Cotton effects.
The maximalization of dispersive interactions between aromatic rings forced an adaptation of pseudocentrosymmetric structure of the imines in the solid state. The mechanism of molecular packing was found to be irrelevant to the size and absolute configuration of the inductor part of the molecule.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-8994/13/2/325/s1: Experimental details, Calculation details, X-ray diffraction study details, copies of 1H and 13C NMR spectra. Figure S1: Calculated at the B3LYP-GD3BJ/6-311++G(d,p) level structures of thermally accessible conformers of compound 2c. Figure S2: ECD spectra of low-energy conformers of imine 2c calculated at TD-CAM-B3LYP/6-311++g(d,p) level. Wavelengths have not been corrected. Figure S3: Measured in cyclohexane (solid blue line) and calculated (dashed green line) at TD-CAM-B3LYP/6-311++g(d,p) level spectra of diimine 2c. Wavelengths have been corrected to match experimental UV maxima. Figure S4: (a) Molecular structure of compound 1 (numbering scheme shown for asymmetric part for clarity) and (b) C-H···O interactions in crystal structure (O-atoms shown as balls). Figure S5: (a) Molecular structure of 2a and atoms numbering scheme. The disorder model shown in the box (minor occupancy fragment shown as a balls with thinner bonds). Crystal packing (b) view along a axis and (c) view along b axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S6: (a) Molecular structure of 2b and atoms numbering scheme. The disorder model shown in the box (minor occupancy fragment shown as a balls with thinner bonds). Crystal packing (b) view along b axis and (c) view along c axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S7: (a) Molecular structure of 2c and atoms numbering scheme. Crystal packing (b) view along b axis and (c) view along c axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S8: (a) Molecular structure of 2d and atoms numbering scheme. Crystal packing (b) view along b axis and (c) view along c axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S9: (a) Molecular structure of 2e and atoms numbering scheme. Crystal packing (b) view along b axis and (c) view along c axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S10: (a) Molecular structure of one of the independent molecules in crystal structure of 2f and atoms numbering scheme (shown for asymmetric part), (b) voids in crystal structure, view along b axis and (c) molecular packing, view along a axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S11: (a) Molecular structure of 2h and atoms numbering scheme. Crystal packing (b) view along b axis and (c) view along c axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S12: (a) Molecular structure of 2k and atoms numbering scheme, (b) structural channels in crystal structure, view along a axis and (c) molecular packing, view along c axis. N-atoms shown as balls and hydrogen atoms are omitted for clarity. Figure S13: Copy of 1H NMR spectrum of studied dialdehyde 1 measured in CDCl3. Figure S14: Copy of 13C NMR spectrum of studied dialdehyde 1 measured in CDCl3. Figure S15: Copy of 1H NMR spectrum of studied diimine 2a measured in CDCl3. Figure S16: Copy of 13C NMR spectrum of studied diimine 2a measured in CDCl3. Figure S17: Copy of 1H NMR spectrum of studied diimine 2b measured in CDCl3. Figure S18: Copy of 13C NMR spectrum of studied diimine 2b measured in CDCl3. Figure S19: Copy of 1H NMR spectrum of studied diimine 2c measured in CDCl3. Figure S20: Copy of 13C NMR spectrum of studied diimine 2c measured in CDCl3. Figure S21: Copy of 1H NMR spectrum of studied diimine 2d measured in CDCl3. Figure S22: Copy of 13C NMR spectrum of studied diimine 2d measured in CDCl3. Figure S23: Copy of 1H NMR spectrum of studied diimine 2e measured in CDCl3. Figure S24: Copy of 13C NMR spectrum of studied diimine 2e measured in CDCl3. Figure S25: Copy of 1H NMR spectrum of studied diimine 2f measured in CDCl3. Figure S26: Copy of 13C NMR spectrum of studied diimine 2f measured in CDCl3. Figure S27: Copy of 1H NMR spectrum of studied diimine 2g measured in CDCl3. Figure S28: Copy of 13C NMR spectrum of studied diimine 2g measured in CDCl3. Figure S29: Copy of 1H NMR spectrum of studied diimine 2h measured in CDCl3. Figure S30: Copy of 13C NMR spectrum of studied diimine 2h measured in CDCl3. Figure S31: Copy of 1H NMR spectrum of studied diimine 2i measured in CDCl3. Figure S32: Copy of 13C NMR spectrum of studied diimine 2i measured in CDCl3. Figure S33: Copy of 1H NMR spectrum of studied diimine 2j measured in CDCl3. Figure S34: Copy of 13C NMR spectrum of studied diimine 2j measured in CDCl3. Figure S35: Copy of 1H NMR spectrum of studied diimine 2k measured in CDCl3. Figure S36: Copy of 13C NMR spectrum of studied diimine 2k measured in CDCl3. Figure S37: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2a measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S38: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2b measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S39: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2c measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S40: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2d measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S41: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2e measured in cyclohexane (solid blue line) Sample was insoluble in acetonitrile. Figure S42: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2f measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S43: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2g measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S44: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2h measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S45: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2i measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S46: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2j measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Figure S47: Copy of UV (upper chart) and ECD (bottom chart) spectra of studied diamine 2k measured in cyclohexane (solid blue line) and acetonitrile (dashed red line). Table S1: Total and Gibbs free energies (E, ΔG, in Hartree), relative energies (ΔE, ΔΔG, in kcal mol 1), ΔE and ΔΔG-based percentage populations (% ΔE, % ΔΔG) and numbers of imaginary frequencies (#Imfreq) calculated at B3LYP-GD3BJ/6-311++G(d,p) level for individual conformers of diimine 2c; Table S2: Dihedral angles α, β, γ, ω (in degrees) of calculated at the B3LYP-GD3BJ/6-311++G(d,p) level for each low-energy conformer of diimine 2c. Table S3: Steric energies (ESE, kcal mol-1), relative steric energies (ΔESE, kcal mol-1) and percentage populations (% ΔESE) calculated for low-energy conformers of imine 2c at the molecular mechanics level. Table S4: Selected crystal data and structure refinement details for 1, 2a – 2f, 2h and 2k. Table S5: Selected dihedral angles α, β, γ, and ω (in degrees) observed in the crystal structures of compounds 1, 2a–2f, 2h and 2k.
Author Contributions
Conceptualization, T.M., M.K., and A.C.; methodology, T.M., M.K., and A.C.; formal analysis, M.K. and A.C.; investigation, T.M. and A.C.; resources, T.M.; writing—original draft preparation, A.C. and M.K.; writing—review and editing, M.K. and A.C.; visualization, A.C. and M.K.; supervision, A.C. and M.K.; project administration, T.M.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Preludium program (2018/29/N/ST4/00567) from the National Science Centre, Poland (T.M.). All calculations were performed in Poznan Supercomputing and Networking Center.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available in the article and Supplementary Material. CCDC 2056869-2056877 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif; by emailing data_request@ccdc.cam.ac.uk; or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W.H. Freeman & Co: New York, NY, USA, 2010. [Google Scholar]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Testa, B.; Caldwell, J.; Kisakürek, M.V. (Eds.) Organic Stereochemistry. Guiding Principles and Biomedical Relevance; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. Stereochemistry of Organic Compounds; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Crossley, R. The relevance of chirality to the study of biological activity. Tetrahedron 1992, 48, 8155–8178. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The Significance of Chirality in Drug Design and Development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Mannschreck, A.; Kiesswetter, R.; von Angerer, E. Unequal Activities of Enantiomers via Biological Receptors: Examples of Chiral Drug, Pesticide, and Fragrance Molecules. J. Chem. Educ. 2007, 84, 2012–2018. [Google Scholar] [CrossRef]
- Todd, M. (Ed.) Separation of Enantiomers; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Pellissier, H. Chirality from Dynamic Kinetic Resolution; RSC: Cambridge, UK, 2011. [Google Scholar]
- Helmchen, G.; Hoffmann, R.W.; Mulzer, J.; Schaumann, E. (Eds.) Stereoselective Synthesis; G. Thieme: Stuttgart, Germany, 1996. [Google Scholar]
- Jacobsen, E.N.; Pfalz, A.; Yamamoto, H. (Eds.) Comprehensive Asymmetric Catalysis; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R.W. (Eds.) Comprehensive Chiroptical Spectroscopy (Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules); Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rodger, A.; Nordén, B. Circular Dichroism & Linear Dichroism; Oxford University Press Inc.: New York, NY, USA, 1997. [Google Scholar]
- Wolf, C. Dynamic Stereochemistry of Chiral Compounds: Principles and Applications; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Wolf, C.; Bentley, K.W. Chirality sensing using stereodynamic probes with distinct electronic circular dichroism output. Chem. Soc. Rev. 2013, 42, 5408–5424. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Hembury, G.A.; Inoue, Y. Origin, Control, and Application of Supramolecular Chirogenesis in Bisporphyrin-Based Systems. Acc. Chem. Res. 2004, 37, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, A.; Pereira-Cameselle, R.; Poklar Ulrih, N.; Petrovic, A.G.; Alonso-Gómez, J.L. Chiroptical Sensing: A Conceptual Introduction. Sensors 2020, 20, 974. [Google Scholar] [CrossRef]
- Herrera, B.T.; Pilicer, S.L.; Anslyn, E.V.; Joyce, L.A.; Wolf, C. Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc. 2018, 140, 10385–10401. [Google Scholar] [CrossRef] [PubMed]
- Pasini, D.; Nitti, A. Recent Advances in Sensing Using Atropoisomeric Molecular Receptors. Chirality 2016, 28, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Pescitelli, G.; Petrovic, A.G.; Proni, G. Probing molecular chirality by CD-sensitive dimeric metalloporphyrin hosts. Chem. Commun. 2009, 5958–5998. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.F.; El-Bayoumi, S.A. The Exciton Model in Molecular Spectroscopy. Pure Appl. Chem. 1965, 11, 371. [Google Scholar]
- Harada, N.; Nakanishi, K. Circular Dichroism Spectroscopy: Exciton Coupling in Organic Stereochemistry; University Science Books: Mill Valley, CA, USA, 1983. [Google Scholar]
- Gawroński, J.; Kwit, M.; Gawrońska, K. Helicity Induction in a Bichromophore: A Sensitive and Practical Chiroptical Method for the Absolute Configuration Determination of Aliphatic Alcohols. Org. Lett. 2002, 4, 4185–4188. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, J.; Grajewski, J. A superior molecular bichromophore for the determination of absolute configuration of primary amines. Tetrahedron Asymmetry 2004, 15, 1527–1530. [Google Scholar] [CrossRef]
- Carmo dos Santos, N.A.; Badetti, E.; Licini, G.; Abbate, S.; Longhi, G.; Zonta, C. A stereodynamic fluorescent probe for amino acids. Circular dichroism and circularly polarized luminescence analysis. Chirality 2018, 30, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, D.P.; Wolf, C. A Stereodynamic Probe Providing a Chiroptical Response to Substrate-Controlled Induction of an Axially Chiral Arylacetylene Framework. J. Am. Chem. Soc. 2011, 133, 2414–2417. [Google Scholar] [CrossRef] [PubMed]
- Anyika, M.; Gholami, H.; Ashtekar, K.D.; Acho, R.; Borhan, B. Point-to-axial chirality transfer: A new probe for “sensing” the absolute configurations of monoamines. J. Am. Chem. Soc. 2014, 136, 550–553. [Google Scholar] [CrossRef]
- Huang, X.; Rickman, B.H.; Borhan, B.; Berova, N.; Nakanishi, K. Zinc porphyrin tweezer in host-guest complexation: Determination of absolute configurations of diamines, amino acids, and amino alcohols by circular dichroism. J. Am. Chem. Soc. 1998, 120, 6185–6186. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Meng, F.; Dai, C.; Cheng, Y.; Zhu, C. Central-to-Axial Chirality Transfer-Induced CD Sensor for Chiral Recognition and ee Value Detection of 1,2-DACH Enantiomers. Macromol. Chem. Phys. 2015, 216, 1925–1929. [Google Scholar] [CrossRef]
- Mądry, T.; Czapik, A.; Kwit, M. Point-to-axial chirality transmission—A highly sensitive triaryl chirality probe for stereochemical assignments of amines. J. Org. Chem. 2020, 85, 10413–10431. [Google Scholar] [CrossRef]
- Superchi, S.; Casarini, D.; Laurita, A.; Bavoso, A.; Rosini, C. Induction of a preferred twist in a biphenyl core by stereogenic centers: A novel approach to the absolute configuration of 1, 2-and 1, 3-diols. Angew. Chem. Int. Ed. 2001, 40, 451–454. [Google Scholar] [CrossRef]
- Superchi, S.; Bisaccia, R.; Casarini, D.; Laurita, A.; Rosini, C. Flexible Biphenyl Chromophore as a Circular Dichroism Probe for Assignment of the Absolute Configuration of Carboxylic Acids. J. Am. Chem. Soc. 2006, 128, 6893–6902. [Google Scholar] [CrossRef]
- Kwit, M.; Rychlewska, U.; Gawroński, J. Induced Homohelicity of Diphenimide Bis-propellers. New J. Chem. 2002, 26, 1714–1717. [Google Scholar] [CrossRef]
- Vergura, S.; Pisani, L.; Scafato, P.; Casarini, D.; Superchi, S. Central-to-axial chirality induction in biphenyl chiroptical probes for the stereochemical characterization of chiral primary amines. Org. Biomol. Chem. 2018, 16, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, S.; Nakamura, M.; Yamaguchi, A.; Ikeda, M.; Habata, Y. Combination of a New Chiroptical Probe and Theoretical Calculations for Chirality Detection of Primary Amines. Org. Lett. 2013, 15, 5738–5741. [Google Scholar] [CrossRef] [PubMed]
- Dutot, L.; Wright, K.; Gaucher, A.; Wakselman, M.; Mazaleyrat, J.-P.; De Zotti, M.; Peggion, C.; Formaggio, F.; Toniolo, C. The Bip Method, Based on the Induced Circular Dichroism of a Flexible Biphenyl Probe in Terminally Protected -Bip-Xaa*-Dipeptides, for Assignment of the Absolute Configuration of β-Amino Acids. J. Am. Chem. Soc. 2008, 130, 5986–5992. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zha, D.; Ye, H.; Hai, Y.; Zhou, Y.; Anslyn, E.V.; You, L. Dynamic Covalent Chemistry within Biphenyl Scaffolds: Reversible Covalent Bonding, Control of Selectivity, and Chirality Sensing with a Single System. Angew. Chem. Int. Ed. 2018, 57, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Bentley, K.W.; Joyce, L.A.; Sherer, E.C.; Sheng, H.; Wolf, C.; Welch, C.J. Antenna Biphenols: Development of Extended Wavelength Chiroptical Reporters. J. Org. Chem. 2016, 81, 1185–1191. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. TD-CAM-B3LYP/6-311++G(d,p)//B3LYP-GD3BJ/6-311++G(d,p) Method as Implemented in Gaussian Software: Gaussian 09; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Shimizu, K.D.; Dewey, T.M.; Rebek, J. Synthetic and structural studies of large and rigid molecular clefts. J. Am. Chem. Soc. 1994, 116, 5145–5149. [Google Scholar] [CrossRef]
- Degenhardt, C.; Shortell, D.B.; Adams, R.D.; Shimizu, K.D. Synthesis and structural characterization of adaptable shape-persistent building blocks. Chem. Commun. 2000, 929–930. [Google Scholar] [CrossRef]
- Gawroński, J.; Gawrońska, K.; Kacprzak, K. Chiral C and S Conformers of Aromatic Diimide Triads. Chirality 2001, 13, 322–328. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).