Abstract
Evidence derived from functional imaging and brain-lesion studies has shown a strong left lateralization for language, and a complementary right hemisphere dominance for visuospatial abilities. Nevertheless, the symmetrical functional division of the two hemispheres gives no reason for the complexity of the cognitive operations involved in carrying out a linguistic task. In fact, a growing number of neuroimaging and neurostimulation studies suggest a possible right hemisphere involvement in language processing. The objective of this work was to verify the contribution of the left and right parietal areas in a phonological task. We applied anodal transcranial direct current stimulation (tDCS) to the right or left inferior parietal lobe, during a syllabic reordering task. After having learnt a combination of images of real objects and trisyllabic pseudowords with a simple consonant–vowel (CV) syllabic structure (e.g., tu-ru-cu), participants were shown the same images paired to two different pseudowords: one correct but with transposed syllables, and one alternative, never before seen. The participant’s task was to orally produce the chosen pseudoword, after having rearranged the order of its syllables. Two types of error were considered: transposition (correct pseudoword but incorrectly reordered) and identity (incorrect pseudoword). The results showed that right anodal stimulation significantly reduced the number of transposition errors, whereas left anodal stimulation significantly reduced the number of identity errors. These results suggested that both left and right inferior parietal areas were differentially involved in a syllabic reordering task, and, crucially, they demonstrated that visuospatial processes served by the right inferior parietal area could be competent for establishing the correct syllabic order within a word.
1. Introduction
The possible role of the right hemisphere in linguistic tasks has long been studied, both in subjects with damage to the right and left hemispheres [1,2,3,4,5,6,7], and in healthy volunteers [8,9,10,11,12]. Neuroimaging and neurostimulation studies with healthy subjects suggested the involvement of the right hemisphere in phonological tasks [13,14,15,16,17,18]. For example, Raboyeau et al. [12] reported PET activation of the right inferior frontal areas in healthy subjects, who had to learn and then retrieve phonological representations of foreign words. Hartwigsen and collaborators [17] used high-frequency repetitive transcranial magnetic stimulation (rTMS) over the posterior inferior frontal gyrus (pIFG); meanwhile, participants had to decide if the presented word had two or three syllables. They found that both the left and right pIFG contributed to the improvement of accuracy and reaction times in visual and auditory phonological decision tasks, and they concluded that the right pIFG is necessary for accurate and efficient phonological decisions in the healthy brain. The same researchers [18] reported an increase in the number of errors and reaction times during a syllabic decision task using interfering rTMS on both left and right supramarginal gyri. No significant result was obtained during the administration of semantic and perceptive decision tasks, nor during interfering stimulation on angular gyri, demonstrating the functional and anatomical specificity of the stimulated regions. The authors argued that not only the left supramarginal gyrus, but also the right one, may be necessary for correct phonological processing, and they concluded that the right hemisphere may not intervene for compensatory purposes only, following lesions of the left hemisphere, but could play a role in phonological processing of words.
In this paper, we address the distinctive and different role of the right parietal area, compared to the corresponding area of the left hemisphere, in a syllabic order task. The involvement of this cerebral area in serial order tasks has been previously recognized [19]. Interestingly, the right parietal lobe is critical for spatial processing. This role was demonstrated by patient studies showing impaired spatial processing and right hemisphere damage [20]. Furthermore, neuroimaging and neurostimulation studies have confirmed the right parietal area to be a neural correlate of spatial processing in healthy subjects [21,22]. We attempt to test if the spatial competence of the right parietal area could represent the underpinning mechanism of the contribution of the right hemisphere in a phonological task, such as the reordering of syllables within a word.
Transcranial direct current stimulation (tDCS) is a non-invasive method of brain stimulation, capable of increasing with anodal polarity, or decreasing with cathodal polarity, the frequency of spontaneous discharge of neurons in the stimulated area [23,24]. Due to these characteristics, it has been successfully used to improve the performance in different cognitive tasks of healthy participants [25,26,27,28] and patients suffering from various neurological diseases [29,30,31]. Recent tDCS studies reported the involvement of the left parietal area in phonological tasks [32,33]. On the contrary, its right counterpart is known to have a major role in processing visuospatial information [34,35,36,37].
In the present study, anodal tDCS was applied to both the right and the left inferior parietal lobes during a syllabic reordering task. The experiment started with a learning phase, in which picture-pseudoword pairs were presented. In the second phase, the same pictures were presented along with two pseudowords: one correct but with a transposed syllabic order, and one alternative. Participants were asked to choose the correct pseudoword, and to produce it orally by re-ordering its syllables. In order to enhance task difficulty, both pictures and pseudowords have a simple consonant–vowel (CV) syllabic structure in the Italian language (i.e., the picture of a table, TA-VO-LO, matched with the pseudoword TU-RU-CU). In healthy speakers, ordering letters within a word is an automatic process, provided that they know the given words in that language. In the case of new words, these lexical processes cannot be used; instead, phonological post-lexical mechanisms such as the production of the identity and order of the individual letters that make up the new word are mainly involved [38]. We decided to use pseudowords in order to stress segmental processing.
Two types of errors appear to be crucial here: identity errors (i.e., choice of the alternative pseudoword), and transposition errors (i.e., choice of the correct pseudoword, but incorrect order of its syllables). The distinction between representation of identity and serial order at the different levels of the components of language (words, syllables, or letters) was supported by behavioral and neuropsychological studies [39,40]. The neural substrate of this distinction has been identified at the level of the left and right intraparietal sulcus [41,42]. Recently, Antoine and colleagues [43] reported that patients affected by hemispatial neglect with lesions to the right parietal posterior cortex produced more order errors than item errors in a verbal working memory task, compared to healthy controls.
We hypothesized that anodal tDCS may differentially affect the number and the type of participants’ errors when applied to the left or to the right inferior parietal lobe. Specifically, after left anodal stimulation, we expected a reduction in the number of times the wrong pseudoword was recognized as correct (i.e., less identity errors relative to Sham condition), thus confirming previous findings [31,44,45,46]. On the other hand, right anodal stimulation should reduce the number of errors in which the correct chosen string is incorrectly ordered (i.e., less transposition errors relative to Sham condition). In fact, spatial components served by the right inferior parietal lobe may be involved in the ordering of syllables [41,42]. Furthermore, some studies have demonstrated a functional link between spatial attention and serial order in verbal tasks [47,48]. Therefore, ordering letters within words could at least partly involve a spatial operation charged to, or in cooperation with, the right hemisphere, and could play an important role in language tasks.
2. Materials and Methods
Twenty-two (ten males and twelve females) healthy volunteers, right-handed, without a history of neurological or psychiatric illness, took part in the experiment. All participants were Italian native speakers, aged between 20 and 31 years (mean = 26.3 years, SD = 3.22), with 15 to 18 years of formal education (mean = 17.4, SD = 1.16). A set of 30 pictures belonging to different semantic categories (clothes, food, furniture, animals, tools, body parts) was used [49]. All of the pictures depicted objects with CV-CV-CV real nouns (e.g., ta-vo-lo, ‘table’). Forty CV-CV-CV pseudowords were also created and paired to the pictures. Each pseudoword consisted of three syllables with the same repeated vowel, but different consonants (i.e., PACADA; REVEZE; TILIPI; GOTOLO; FULUDU). Moreover, an additional 30 pseudowords were selected as alternatives in the second phase of the task. They were constructed following the same criteria as for the target pseudowords. Correct and alternative pseudowords belonging to the same trial always shared the same vowels, but never the same consonants (see Appendix A). The 30 pictures with their target and alternative associated pseudowords were divided into three blocks of equal numerosity (n = 10 per block). Each block was used in a different stimulation condition. Participants were invited to maintain a state of great relaxation and to sit in front of the screen, at a distance subjectively considered convenient for reading. All participants performed the same task. Each trial started with a fixation point lasting for 500 milliseconds, followed by the figure along with the pseudoword written in uppercase (e.g., TURUCU; see Figure 1a). The picture–pseudoword pair remained on the screen for 4 s. The inter-trial interval lasted between 300 and 500 milliseconds. Participants were asked to memorize each picture–pseudoword pair. Immediately after the end of this phase, each figure along with the correct and the alternative pseudowords was presented (see Figure 1b). All pseudowords appeared in uppercase and were divided into three syllables. Moreover, the syllables of the previously learned pseudowords were in a transposed order (for example, FULUDU: LU DU FU). The syllabic order of the target pseudowords was modified in a way that the first syllable was never maintained. The position of the target and alternative pseudowords (up versus down) was counterbalanced across items in each stimulation condition. Pictures and pseudowords remained on the screen for 4 s. Fixation point and inter-trial interval were the same as the previous phase.
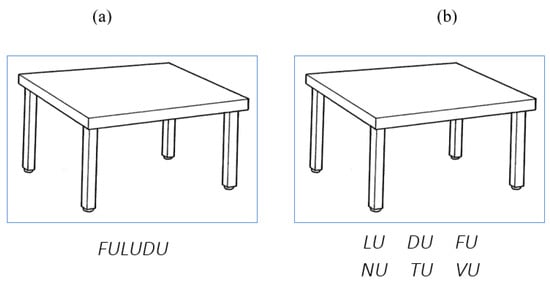
Figure 1.
(a) Example of a picture–pseudoword pair in the learning phase; (b) example of a picture with target and alternative pseudowords in the re-order phase.
Participants were instructed to choose the correct pseudoword, to re-order its syllables according to the string presented in the previous phase, and to name it orally. This type of linguistic task stressed the production of order errors. Vocal responses were recorded; all answers given after 4 s were excluded from the analysis. Instructions emphasized response speed and accuracy. The task was repeated five times. For each repetition, the order of the presentation of trials was differently randomized. The experiment was controlled by the software PsyScope X [50]. Each participant performed the task under three conditions: (1) tDCS over the right inferior parietal lobe; (2) tDCS over the left inferior parietal lobe; and (3) Sham tDCS over the right inferior parietal lobe.
Each condition was administered in a different session, for a total of three sessions with an interval of at least one week between each other. The order of sessions was counterbalanced across participants according to a Latin square design. A direct battery-powered current stimulator (Magstim Company Ltd., Whitland, Wales, UK) was used. The two electrodes (35 cm2 (7 × 5) size), covered with saline-soaked sponges, were applied to the scalp. Anodal tDCS was delivered at 2 mA for 25 min. The current increased in the first 8 s up to 2 mA, and then remained constant throughout the stimulation time. For the Sham tDCS, the 2 mA intensity was only given for 30 s, reproducing sensations provoked by the passage of current at the beginning of the stimulation. By using an electroencephalography (EEG) cap, according to the International 10–20 system for EEG electrode placement, the active anode was positioned over CP5 (left hemisphere) or CP6 (right hemisphere). In real and Sham tDCS sessions, the reference electrode (cathode) was placed over the contralateral supraorbital area (however, for a discussion of different montages, see [51,52,53]). The task began at the beginning of the stimulation, and was completed at the end of the stimulation. This research was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all volunteers, and the protocol was approved by the Ethics Committee of the University Hospital Paolo Giaccone of Palermo.
The number of errors was the dependent variable of interest. Two types of errors were considered:
Identity errors: production of the alternative pseudoword (e.g., NUTUVU instead of FULUDU) (see Figure 1);
Transposition errors: production of the correct pseudoword, but in a transposed order (e.g., FUDULU instead of FULUDU) (see Figure 1).
Two different repeated measure analyses of variance (ANOVA) were performed separately for identity and transposition errors, with repetition (one to five) and conditions (three levels: left tDCS, right tDCS, Sham) as within-subject factors. A p-value of 0.05 was considered statistically significant. Moreover, the Mauchly test was carried out in order to check the assumption of sphericity, and the Greenhouse–Geisser correction was used in the case of violations of the assumption. A post hoc pairwise comparison (adjusted to Bonferroni) was performed only for statistical significative effect, in order to verify between which groups there was a significant difference. No responses or other types of errors were excluded from the analysis.
3. Results
1.8% of the responses were excluded from the analyses for being longer than 4 s. The cutoff was selected on the basis of a brief pilot study, with six participants showing that average RTs augmented by two standard deviations were around 4 s. Indeed, in the experiment reported here, responses equal to or longer than 4 s were quite low (1.8%) and evenly distributed across conditions.
The ANOVAs on the identity and the transpositions errors both showed a main effect of repetition (identity: F(4,84) = 62.80, p = 0.0000; transposition: F(4,84) = 55.68, p = 0.0000), demonstrating that errors decrease as a function of the repetition. Moreover, a main effect of condition was shown (identity: F(2,42) = 11.54, p = 0.0001; transposition: F(2,42) = 8.48, p = 0.0008). Finally, a conditions*repetitions interaction was found only for transposition errors (F(8168) = 3.04, p = 0.0186), whereas no interaction emerged for identity errors: (F(8168) = 1.49, p = 0.193). The post hoc pairwise comparisons (adjusted to Bonferroni) showed that transposition errors were significantly reduced during the right anodal tDCS, compared to both the left anodal tDCS (p = 0.041) and Sham (p = 0.0006), whereas no significant difference emerged between the left anodal tDCS and Sham (p = 0.417). Concerning identity errors, the post hoc pairwise comparisons (adjusted to Bonferroni) showed that the left anodal tDCS was significantly different compared to right anodal tDCS (p = 0.043) and Sham (p = 0.00000), but no significant difference was found between the right anodal tDCS and Sham (p = 0.088). Figure 2 and Figure 3 report mean errors respectively for transposition and identity errors.
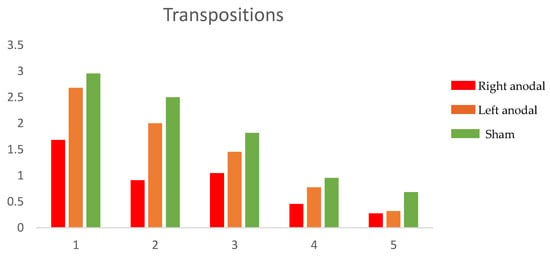
Figure 2.
Mean transposition errors in the five repetitions of the task for the three stimulation conditions.
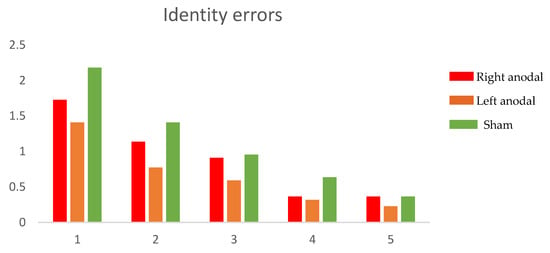
Figure 3.
Mean identity errors in the five repetitions of the task for the three stimulation conditions.
4. Discussion
The aim of this work was to verify the effect of anodal tDCS when applied to the right or left inferior parietal cortex in a syllabic reordering task. The results showed that transposition and identity errors significantly decreased during respectively right and left anodal tDCS. On the other hand, right anodal stimulation had no effect on identity errors, whilst left anodal stimulation had no effect on transposition errors.
Furthermore, the post hoc analysis highlighted that the specific effects are based on the direct comparison of the lateralized tDCS with both Sham and real tDCS of the opposite hemisphere. In other words, the reduction in transposition errors was statistically significant versus both Sham stimulation and anodal stimulation of the left hemisphere, and the reduction in identity errors was statistically significant both versus Sham stimulation and right anodal stimulation. We discuss these results separately.
4.1. Reduction of Identity Errors during Left Hemisphere Stimulation
The decrease in identity errors during anodal tDCS over the left parietal cortex could be explained by the well-known involvement of the left perisylvian areas in verbal tasks [31,44,45,46]. However, the literature data were not univocal in this regard. In a recent study, Fiori and collaborators [54] explored the impact of unilateral and bilateral tDCS on verbal learning in young and elderly subjects. The authors did not find differences in young subjects (like ours) in the three stimulation conditions (unilateral with anode on the left, bilateral with cathode on the right and anode on the left, and Sham) on an area adjacent to the one stimulated in our experiment (CP5), whereas the elderly subjects’ performance improved after bilateral stimulation. In order to explain the discrepancy with respect to previous findings [31,44,45,46], the authors speculated that the stimuli in their set were not sufficiently difficult for an effect to emerge. Their stimuli were bisyllabic pseudowords coupled with images of various semantic categories, matched by frequency. They hypothesized that, with a more difficult task, for example, increasing the number and the complexity of the stimuli, performance could have improved, even for young participants.
However, we found a significant effect of the left anodal stimulation on young adult participants with a task analogous to that used in Fiori et al. [54]. One possibility is that our linguistic task was in fact more difficult due to the syllabic structure of the pseudowords and the pictures selected. In Fiori et al. [54], however, the syllabic structure of the actual pictured noun may be the same as or different from the syllabic structure of the paired pseudoword; in the present study, all of the pseudowords used shared the syllabic structure with the actual nouns of the pictures to which they were paired. Possibly, during both the learning and the re-order phase of the pseudowords, the actual lexical representation of the pictured noun remained continuously active and available to the participant. According to one accredited model of speech production [55], the phonological and the lexical level are linked bidirectionally: that is to say that, despite each level of representation being autonomous and defining some specific characteristics, the two levels influence each other in language production. The lexical level is assumed to specify word features such as grammatical class and frequency of occurrence, whereas the phonological level includes representations relative to linear order and syllabic structure. Therefore, one could imagine a sort of “syllabic interference” between the pictured noun and the paired pseudoword that may make the task more difficult. This effect could be assimilated to the well-known phonological similarity effect [56,57,58]. This effect refers to the phenomenon according to which learning words with a similar sound (e.g., “tana”, burrow, “lana” wool, “rana”, frog) is more difficult than learning words with dissimilar sounds (e.g., “cane”, dog, “vaso”, jar, “luna”, moon). In the same vein, a syllabic similarity effect would predict more difficulty in learning a word–pseudoword pair with analogous versus different syllabic structures. Indeed, many studies have documented the psychological relevance of the syllabic structure on both speech-related behaviors, as the temporal dynamics of speech articulation [59], and on phoneme perception and production, even in second language acquisition [60].
4.2. Reduction of Transposition Errors during Right Anodal Stimulation
According to the most recent functional imaging [10,12] and neurostimulation studies [17,18], our results suggested an involvement of the right hemisphere for some phonological aspects of language, such as the order of letters within a word. It has been shown that the ability to represent the order of the letters within a word is not a specifically linguistic mechanism, but a domain-general principle that could also be shared by different domains [61]. From a neural point of view, a possible consequence of this idea is that the two cerebral hemispheres collaborate, each with its own specificity, to linguistic elaboration.
The role of the right parietal areas in linguistic tasks could be linked to the ability of the right hemisphere to process spatial information, wherever the tasks require them, such as the ordering of syllables within a word. Therefore, a decrease in transposition errors during anodal tDCS over the right parietal cortex could be attributed mainly to a spatial operation, linked to the functions of the right hemisphere. Indeed, this kind of error is related to the choice of the spatial position of a letter relative to the others within a string. Previously, the right intraparietal sulcus was suggested as a neural correlate with the ability to represent the serial order of linguistic elements [41,42]. Lesion studies seem to confirm the involvement of the right parietal cortex in a verbal working memory task, in which the serial order of memorized consonants had to be recognized [43].
In the literature, as already mentioned, there are no studies in which anodal tDCS over the right hemisphere was used to verify its involvement in syllabic order. Fiori et al. [62] stimulated the right occipital-parietal cortex as a site of control of the left temporo-parietal cortex during a retrieval phase of pseudowords coupled with images of known objects; they did not find significant results, probably because the right occipital-parietal cortex was not specifically involved in verbal tasks. Recently, Manuel and Schnider [63] used tDCS over the left and right prefrontal and parietal cortices in a continuous and delayed recognition task with verbal (pseudowords) and visual (abstract geometrical figures) stimuli. The authors reported an improvement in the visual but not in the verbal task with anodal tDCS over both posterior left and right parietal cortices, thus supporting the hemispheric non-specificity for visual material. However, no spatial processing was prompted for their verbal task, contrary to our experiment, in which the subjects had to re-order the syllables within a word previously presented.
Under normal conditions, the two hemispheres divide the competencies, and do not interfere with one another. The fact that they do not interfere, however, does not exclude that they collaborate for the success of certain tasks. Our data suggested that visuospatial abilities served by the right parietal cortex could contribute to establishing the correct syllabic order within a word. In the present study, we set out to overcome the schematism of the verbal/non-verbal dichotomy of the two cerebral hemispheres, and to fairly evaluate the complexity of hemispheric interactions in language production.
Further studies are needed in order to investigate the role of the right parietal cortex in phonological order tasks. Firstly, considering the size of the electrodes used in this experiment, the effect of the tDCS could have also reached, in addition to the inferior parietal cortex, to a lesser extent, to the adjacent areas of the right and left hemisphere. In order to achieve more focal stimulation of specific cortical targets, future research may use multichannel set-ups. Secondly, it is necessary to carry out further experiments with a more representative sample, and it would be interesting to examine the syllabic order ability in patients with damage to the right inferior parietal cortex. Finally, future works with non-invasive methods of brain stimulation could assist in examining the neural systems underlying the perception and production of syllables, informed by current models of brain dynamics for language, according to which the syllabic elaboration would follow a neural oscillatory pattern (for a recent review, see [64]).
Author Contributions
Conceptualization, G.G., G.C. and F.B.; methodology, F.B.; software, G.G.; validation, V.C., S.T. and G.G.; formal analysis, V.C. and S.T.; investigation, S.T. and G.C.; resources, V.C., S.T. and G.G.; data curation, V.C., S.T., G.G. and F.B.; writing—original draft preparation, V.C.; writing—review and editing, S.T., C.F., B.F. and F.B.; supervision, C.F. and B.F.; project administration, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Universitary Hospital “Paolo Giaccone” of Palermo (protocol code number 1; 9 January 2013 data of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be freely available from the Open Science Framework platform.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Target and alternative pseudowords paired to the pictures (n = 30 each), 10 per stimulation condition.
Table A1.
Target and alternative pseudowords paired to the pictures (n = 30 each), 10 per stimulation condition.
| Correct Pseudowords Alternative Pseudowords Pictures | ||
|---|---|---|
| CABATA | VAGADA | LIMONE |
| CEZEBE | GEREPE | PALLONE |
| GIVIMI | FIBICI | CAROTA |
| GULUMU | NUCUFU | DIVANO |
| PACADA | GAMATA | BOTTONE |
| PUFUDU | RUTUBU | SEDANO |
| SIMIGI | LIRICI | CAMMELLO |
| SOLOTO | ZONODO | CATENA |
| TELENE | DERESE | GIRAFFA |
| ZOSODO | RONOTO | PATTINO |
| BELESE | PEZERE | CAPELLI |
| BUGUZU | MUCURU | BARILE |
| DELENE | TEREVE | BANANA |
| DOVOCO | POTOGO | CORONA |
| FOLOZO | BONORO | GORILLA |
| LINIVI | GIMITI | LATTUGA |
| GUMUFU | CUBUVU | PAVONE |
| LABACA | PAGARA | SIGARO |
| MAPASA | BAFALA | COLLANA |
| NIRIMI | LISIVI | MATITA |
| BASARA | FAZAVA | NUVOLA |
| DALAZA | TANARA | CAVALLO |
| FELEME | REBEVE | CIPOLLA |
| FULUDU | NUTUVU | TAVOLO |
| GIPIVI | CISIFI | PECORA |
| LINIVI | ZIRIFI | BULLONE |
| MOGOSO | BOCORO | DITALE |
| PONOLO | ROFOSO | MULINO |
| REVEZE | LEBESE | POLLICE |
| TUBURU | DUMUSU | PADELLA |
References
- Tompkins, C.A.; Lehman-Blake, C.; Baumgaertner, A.; Fassbinder, W. Mechanisms of discourse comprehension impairment after right hemisphere brain damage: Suppression in inferential ambiguity resolution. J. Speech Lang. Hear. Res. 2001, 44, 400–415. [Google Scholar] [CrossRef]
- Minga, J.; Fromm, D.; DeVane-Williams, C.; MacWhinney, B. Question use in adults with right-hemisphere brain damage. J. Speech Lang. Hear. Res. 2020, 63, 738–748. [Google Scholar] [CrossRef]
- Calvert, G.; Brammer, M.; Morris, R.; Williams, S.; King, N.; Matthews, P. Using fMRI to study recovery from acquired dysphasia. Brain Lang. 2000, 71, 391–399. [Google Scholar] [CrossRef]
- Crosson, B.; Moore, A.; Gopinath, K.; White, K.; Wierwnga, C.; Gaiefsky, M.; Rohti, L.J.G. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J. Cogn. Neurosci. 2005, 17, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, B.; Cardebat, D.; Demonet, J.; Joseph, P.; Mazaux, J.; Barat, M.; Alland, M. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke 2004, 35, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Vitali, P.; Abutalebi, J.; Tettamanti, M.; Danna, M.; Ansaldo, A.L.; Perani, D.; Joanette, Y.; Cappa, S.F. Training-induced brain remapping in chronic aphasia: A pilot study. Neurorehabilit. Neural Repair 2007, 21, 152–160. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Coslett, H.B.; Thomas, A.L.; Faseyitan, O.; Benson, J.; Norise, C.; Hamilton, R.H. The right hemisphere is not unitary in its role in aphasia recovery. Cortex 2012, 48, 1179–1186. [Google Scholar] [CrossRef]
- Démonet, J.F.; Price, C.; Wise, R.; Frankowiak, R.S. Differential activation of right and left posterior sylvian regions by semantic and phonological taks: A positron-emission tomography study in normal human subjects. Neurosci. Lett. 1994, 182, 25–28. [Google Scholar] [CrossRef]
- Van Ettinger-Veenstra, H.M.; Ragnehed, M.; Hällgren, M.; Karlsson, T.; Landtblom, A.; Lundberg, P.; Engström, M. Right-hemispheric brain activation correlates to language performance. Neuroimagine 2010, 49, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Van Ettinger-Veenstra, H.; Ragnehed, M.; McAllister, A.; Lundberg, P.; Engström, M. Right-hemispheric cortical contributions to language ability in healthy adults. Brain Lang. 2012, 120, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Noriko Tanigawa, N.; Ringel, F.; Zimmer, C.; Meyer, B.; Krieg, S. Language and Its Right-Hemispheric Distribution in Healthy Brains: An Investigation by Repetitive Transcranial Magnetic Stimulation. Neuroimage 2014, 102, 776–788. [Google Scholar] [CrossRef]
- Raboyeau, G.; De Boissezon, X.; Marie, N.; Balduyck, S.; Puel, M.; Bézy, C.; Démonet, J.F.; Cardebat, D. Right hemisphere activation in recovery from aphasia: Lesion effect or function recruitment? Neurology 2008, 70, 290–298. [Google Scholar] [CrossRef]
- Chee, M.W.; O’Craven, K.M.; Bergida, R.; Rosen, B.R.; Savoy, R.L. Auditory and visual word processing studied with fMRI. Hum. Brain Mapp. 1999, 7, 15–28. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Wagner, A.D.; Prull, M.W.; Desmond, J.E.; Glover, G.H.; Gabrieli, J.D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 1999, 10, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, N. Picture-name priming in the cerebral hemispheres: Evidence for phonological access in the right hemisphere. Percept. Mot. Ski. 2004, 99, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, T.; Monetta, L.; Joanette, Y. Phonological processing of words in right- and left-handers. Brain Cogn. 2004, 55, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Hartwigsen, G.; Price, C.J.; Baumgaertner, A.; Geiss, G.; Koehnke, M.; Ulmer, S.; Siebner, H.R. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: Evidence from dual-site TMS. Neuropsychologia 2010, 48, 3155–3163. [Google Scholar] [CrossRef]
- Hartwigsen, G.; Baumgaertner, A.; Price, C.J.; Koehnke, M.; Ulmer, S.; Siebner, H.R. Phonological decisions require both the left and right supramarginal gyri. Proc. Natl. Acad. Sci. USA 2010, 107, 16494–16499. [Google Scholar] [CrossRef]
- Abrahamse, E.; Van Dijck, J.P.; Majerus, S.; Fias, W. Finding the answer in space: The mental whiteboard hypothesis on serial order in working memory. Front. Hum. Neurosci. 2014, 8, 932. [Google Scholar] [CrossRef] [PubMed]
- Karnath, H.O.; Fruhmann Berger, M.; Küker, W.; Rorden, C. The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cereb. Cortex. 2004, 14, 1164–1172. [Google Scholar] [CrossRef]
- Fierro, B.; Brighina, F.; Oliveri, M.; Piazza, A.; La Bua, V.; Buffa, D.; Bisiach, E. Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport 2000, 11, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.; Jackson, M.; van Koningsbruggen, M.G.; Shapiro, K.L.; Linden, D. Frontal and parietal theta burst TMS impairs working memory for visual-spatial conjunctions. Brain Stim. 2013, 6, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Chi, R.P.; Fregni, F.; Snyder, A.W. Visual memory improved by non-invasive brain stimulation. Brain Res. 2010, 1353, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Luber, B.; Lisanby, S.H. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 2014, 85, 961–970. [Google Scholar] [CrossRef]
- Sandrini, M.; Fertonani, A.; Cohen, L.G.; Miniussi, C. Double dissociation of working memory load effects induced by bilateral parietal modulation. Neuropsychologia 2012, 50, 396–402. [Google Scholar] [CrossRef][Green Version]
- Shin, Y.I.; Foerster, Á.; Nitsche, M.A. Transcranial direct current stimulation (tDCS)—Application in neuropsychology. Neuropsychologia 2015, 74, 74–95. [Google Scholar] [CrossRef]
- Miniussi, C.; Cappa, S.; Cohen, L.; Floel, A.; Fregni, F.; Nitsche, M.; Oliveri, M.; Pascual-Leone, A.; Paulus, W.; Priori, A.; et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008, 1, 326–336. [Google Scholar] [CrossRef]
- Shah, P.P.; Szaflarski, J.P.; Allendorfer, J.; Hamilton, R.H. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front. Hum. Neurosci. 2013, 7, 888. [Google Scholar] [CrossRef]
- Sparing, R.; Dafotakis, M.; Meister, I.G.; Thirugnanasambandam, N.; Fink, G.R. Enhancing language performance with non-invasive brain stimulation-a transcranial direct current stimulation study in healthy humans. Neuropsychologia 2008, 46, 261–268. [Google Scholar] [CrossRef]
- Pisoni, A.; Cerciello, M.; Cattaneo, Z.; Papagno, C. Phonological facilitation in picture naming: When and where? A tDCS study. Neuroscience 2017, 352, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Savill, N.; Ashton, J.; Gugliuzza, J.; Poole, C.; Sim, Z.; Ellis, A.W.; Jefferies, E. tDCS to temporoparietal cortex during familiarization coherence of nonwords in immediate serial recall. Cortex 2015, 63, 132–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burgess, N. Spatial cognition and the brain. Ann. N. Y. Acad Sci. 2008, 1124, 77–97. [Google Scholar] [CrossRef]
- Duhamel, J.R.; Bremmer, F.; Ben Hamed, S.; Graf, W. Spatial invariance of visual receptive fields in parietal cortex neurons. Nature 1997, 389, 845–848. [Google Scholar] [CrossRef]
- Schweid, L.; Rushmore, R.J.; Valero-Cabré, A. Cathodal transcranial direct current stimulation on posterior parietal cortex disrupts visuo-spatial processing in the contralateral visual field. Exp. Brain Res. 2008, 186, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Heimrath, K.; Sandmann, P.; Becke, A.; Müller, N.G.; Zaehle, T. Behavioral and electrophysiological effects of transcranial direct current stimulation of the parietal cortex in a visuo-spatial working memory task. Front. Psychiatry. 2012, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Goldrick, M.; Rapp, B. Lexical and post-lexical phonological representations in spoken production. Cognition 2007, 102, 219–260. [Google Scholar] [CrossRef]
- Majerus, S. Verbal short-term memory and temporary activation of language representations: The importance of distinguishing item and order information. In Interactions between Short-Term and Long-Term Memory in the Verbal Domain; Thorn, A., Ed.; Psychology Press: New York, NY, USA, 2009; pp. 244–276. [Google Scholar]
- Kalm, K.; Norris, D. The representation of order information in auditory-verbal short-term memory. J. Neurosci. 2014, 14, 6879–6886. [Google Scholar] [CrossRef]
- Henson, R.N.A.; Burgess, N.; Frith, C.D. Recoding, storage, rehearsal and grouping in verbal short-term memory: An fMRI study. Neuropsychologia 2000, 38, 426–440. [Google Scholar] [CrossRef]
- Majerus, S.; Poncelet, M.; Van der Linden, M.; Albouy, G.; Salmon, E.; Sterpenich, V.; Vandewalle, G.; Collette, F.; Maquet, P. The left intraparietal sulcus and verbal short-term memory: Focus of attention or serial order? NeuroImage 2006, 32, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Antoine, S.; Ranzini, M.; van Dijck, J.P.; Slama, H.; Bonato, M.; Tousch, A.; Dewulf, M.; Bier, J.C.; Gevers, W. Hemispatial neglect and serial order in verbal working memory. J. Neuropsychol. 2019, 13, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Floel, A.; Rosser, N.; MichkA, O.; Knecht, S.; Breitenstein, C. Noninvasive Brain Stimulation Improves Language learning. J. Cogn. Neurosci. 2008, 20, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.; Lindenberg, R.; Antonenko, D.; Flaisch, T.; Flöel, A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013, 33, 12470–12478. [Google Scholar] [CrossRef]
- Meinzer, M.; Jähnigen, S.; Copland, D.A.; Darkow, R.; Grittner, U.; Avirame, K.; Rodriguez, A.D.; Lindenberg, R.; Flöel, A. Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex 2014, 50, 137–147. [Google Scholar] [CrossRef]
- Van Dijck, J.P.; Abrahamse, E.L.; Majerus, S.; Fias, W. Spatial attention interacts with serial-order retrieval from verbal working memory. Psychol Sci. 2013, 24, 1854–1859. [Google Scholar] [CrossRef]
- Huttermann, S.; Memmert, D. Moderate movement, more vision: Effects of physical exercise on inattentional blindness. Perception 2012, 41, 963–975. [Google Scholar] [CrossRef]
- Snodgrass, J.G.; Vanderwart, M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. 1980, 6, 174–215. [Google Scholar] [CrossRef]
- Cohen, J.D.; MacWhinney, B.; Flatt, M.; Provost, J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behav. Res. Methods Instrum. Comput. 1993, 25, 257–271. [Google Scholar] [CrossRef]
- Moliadze, V.; Antal, A.; Paulus, W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin. Neurophysiol. 2010, 121, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Sadleir, R.J.; Vannorsdall, T.D.; Schretlen, D.J.; Gordon, B. Target optimization in transcranial direct current stimulation. Front. Psychiatry 2012, 17. [Google Scholar] [CrossRef]
- Faria, P.; Hallett, M.; Cavaleiro Miranda, P. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J. Neural Eng. 2011, 8. [Google Scholar] [CrossRef]
- Fiori, V.; Nitsche, M.; Iasevoli, L.; Cucuzza, G.; Caltagirone, C.; Marangolo, P. Differential effects of bihemispheric and unihemispheric transcranialdirect current stimulation in young and elderly adults in verbal learning. Behav. Brain Res. 2017, 15, 170–175. [Google Scholar] [CrossRef]
- Goldrick, M. Phonological processing: The retrieval and encoding of word form information in speech production. In The Oxford Handbook of Language Production; Goldrick, M., Ferreira, V., Miozzo, M., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 228–244. [Google Scholar]
- Baddeley, A.D. Short-term memory for word sequences as a function of acoustic, semantic and formal similarity. Q. J. Exp. Psychol. 1966, 18, 362–365. [Google Scholar] [CrossRef]
- Conrad, R. Order errors in immediate recall of sequences. J. Verbal Learn. Verbal Behav. 1965, 4, 161–169. [Google Scholar] [CrossRef]
- Hintzman, D.L. Classification and aural coding in short-term memory. Psychon. Sci. 1965, 3, 161–162. [Google Scholar] [CrossRef][Green Version]
- Shaw, J.A.; Gafos, A.I. Stochastic Time Models of Syllable Structure. PLoS ONE 2015, 10, e0124714. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, Y. Syllable Structure Universals and Native Language Interference in Second Language Perception and Production: Positional Asymmetry and Perceptual Links to Accentedness. Front. Psychol. 2015, 6, 1801. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Baum, S. General principles of serial order representation. Procedia Soc. Behav. Sci. 2013, 94, 216–217. [Google Scholar] [CrossRef][Green Version]
- Fiori, V.; Coccia, M.; Marinelli, C.V.; Vecchi, V.; Bonifazi, S.; Ceravolo, M.G.; Provinciali, L.; Tomaiuolo, F.; Marangolo, P. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasiac subjects. J. Cogn. Neurosci. 2011, 23, 2309–2323. [Google Scholar] [CrossRef]
- Manuel, A.L.; Schnider, A. Effect of prefrontal and parietal tDCS on learning and recognition of verbal and non-verbal material. Clin. Neurophysiol. 2016, 127, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, D.; Assaneo, M.F. Speech rhythms and their neural foundations. Nat. Rev. Neurosci. 2020, 21, 322–334. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
