Asymmetric Lateralization during Pain Processing
Abstract
1. Pain Principles
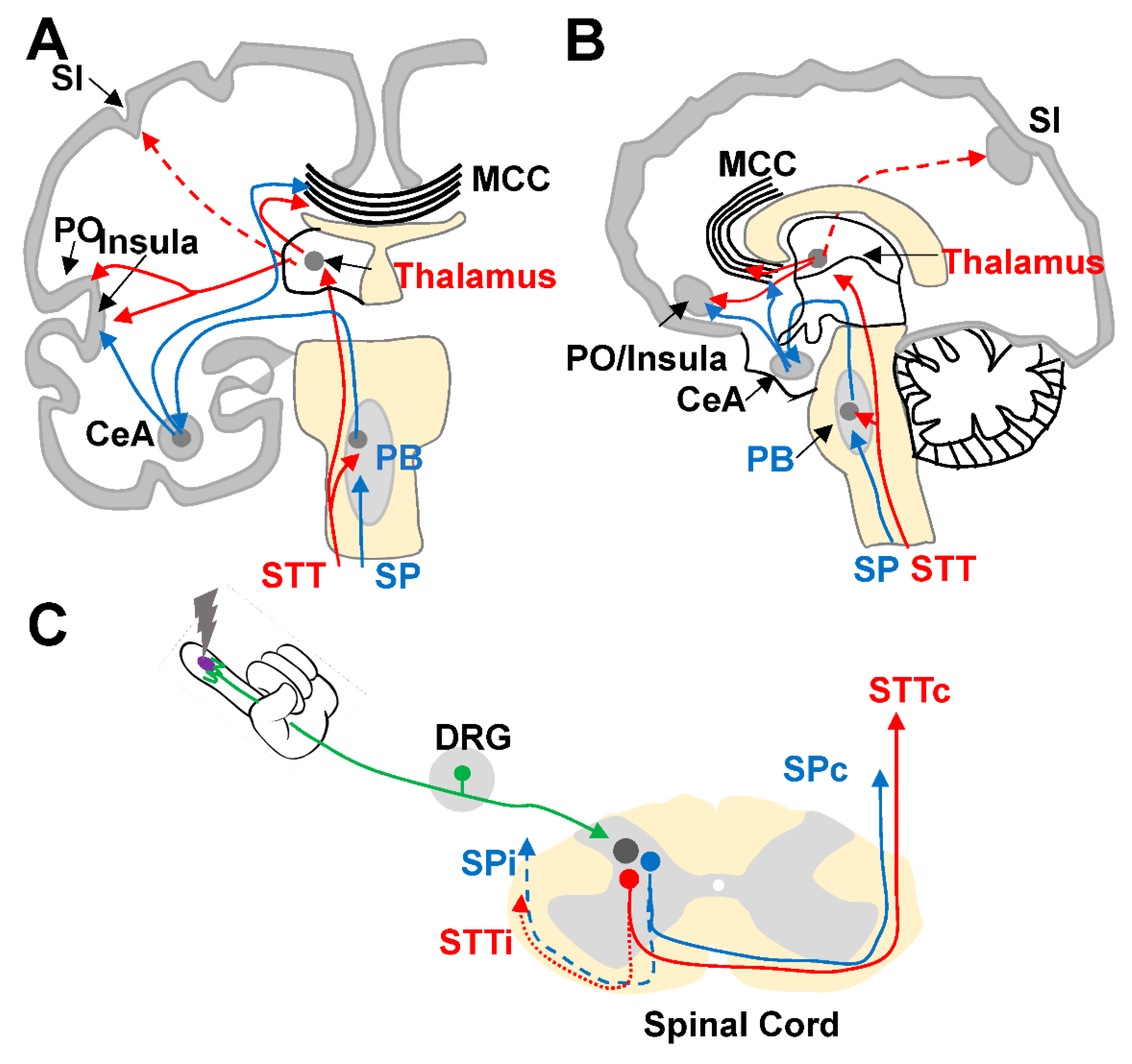
Neural Pathways and Brain Areas Involved in Pain Processing
2. Experimental Basis for Functional Lateralization during Pain Processing
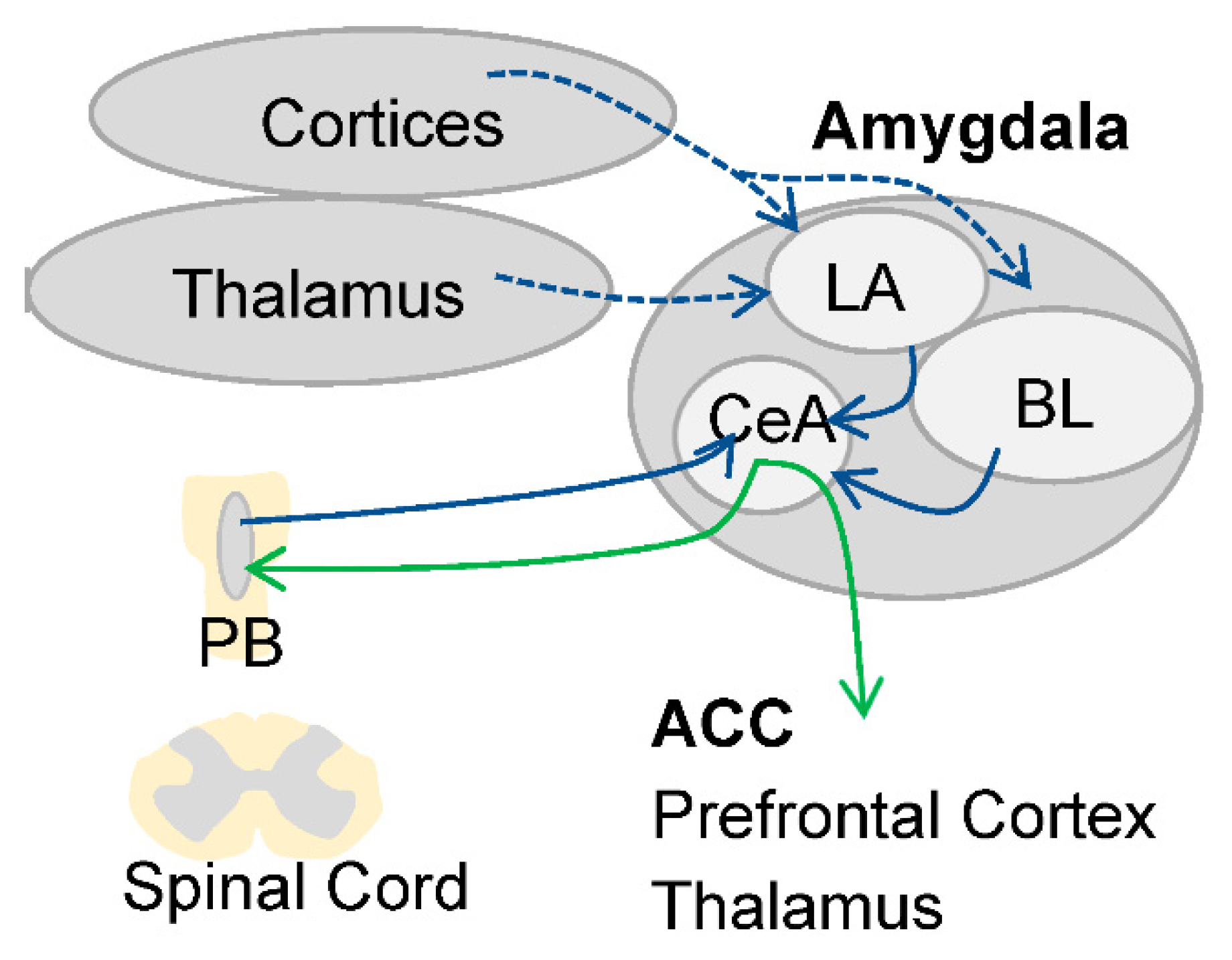
2.1. Asymmetric Lateralization during Pain Processing in the Amygdala
2.2. Signs of Asymmetric Lateralization during Pain Processing in Other Brain Areas: Non-Functional Studies in Humans
2.3. Asymmetric Lateralization of Pain Processing in Patients with Neuropathic Pain
3. Lateralization of Neurotransmitter Systems Involved in Pain Processing
Asymmetric Lateralization of Opioid Receptors in Areas Involved in Pain Processing
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Schon, K.R.; Parker, A.P.J.; Woods, C.G. Congenital Insensitivity to Pain Overview. In GeneReviews® 2020 Adam MP; Ardinger, H.H., Pagon, R.A., Eds.; University of Seattle: Seattle, WA, USA, 1993. [Google Scholar]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Borod, J.C.; Cicero, B.A.; Obler, L.K.; Welkowitz, J.; Erhan, H.M.; Santschi, C.; Grunwald, I.S.; Agosti, R.M.; Whalen, J.R. Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychologia 1998, 12, 446–458. [Google Scholar] [CrossRef]
- Ahern, G.L.; Schwartz, G.E. Differential lateralization for positive versus negative emotion. Neuropsychologia 1979, 17, 693–698. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Yurgelun-Todd, D.A. The right-hemisphere and valence hypotheses: Could they both be right (and sometimes left)? Soc. Cogn. Affect. Neurosci. 2007, 2, 240–250. [Google Scholar] [CrossRef]
- Palomero-Gallagher, N.; Amunts, K. A short review on emotion processing: A lateralized network of neuronal networks. Brain Struct. Funct. 2021, 1–12. [Google Scholar] [CrossRef]
- Peyron, R.; Faillenot, I.; Pomares, F.; Le Bars, D.; Garcia-Larrea, L.; Laurent, B. Mechanical allodynia in neuropathic pain. Where are the brain representations located? A positron emission tomography (PET) study. Eur. J. Pain 2013, 17, 1327–1337. [Google Scholar] [CrossRef]
- Coghill, R.C. The Distributed Nociceptive System: A Framework for Understanding Pain. Trends Neurosci. 2020, 43, 780–794. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Dum, R.P.; Levinthal, D.; Strick, P.L. The Spinothalamic System Targets Motor and Sensory Areas in the Cerebral Cortex of Monkeys. J. Neurosci. 2009, 29, 14223–14235. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Duncan, G.H.; Hofbauer, R.K.; Ha, B.; Chen, J.-I.; Carrier, B. Pain perception: Is there a role for primary somatosensory cortex? Proc. Natl. Acad. Sci. USA 1999, 96, 7705–7709. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Peyron, R. Pain matrices and neuropathic pain matrices: A review. Pain 2013, 154, S29–S43. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Bastuji, H. Pain and consciousness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 87, 193–199. [Google Scholar] [CrossRef]
- Bastuji, H.; Frot, M.; Perchet, C.; Magnin, M.; Garcia-Larrea, L. Pain networks from the inside: Spatiotemporal analysis of brain responses leading from nociception to conscious perception. Hum. Brain Mapp. 2016, 37, 4301–4315. [Google Scholar] [CrossRef]
- Light, A.R. Spinoparabrachial Tract. In Encyclopedia of Pain; Gebhart, G.F., Schmidt, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; p. 2257. [Google Scholar] [CrossRef]
- Gauriau, C.; Bernard, J.-F. Pain Pathways and Parabrachial Circuits in the Rat. Exp. Physiol. 2002, 87, 251–258. [Google Scholar] [CrossRef]
- Bastuji, H.; Frot, M.; Perchet, C.; Hagiwara, K.; Garcia-Larrea, L. Convergence of sensory and limbic noxious input into the anterior insula and the emergence of pain from nociception. Sci. Rep. 2018, 8, 13360. [Google Scholar] [CrossRef]
- Hashmi, J.A.; Baliki, M.N.; Huang, L.; Baria, A.T.; Torbey, S.; Hermann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013, 136, 2751–2768. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.-D.; Zubieta, J.-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463. [Google Scholar] [CrossRef]
- Tracey, I. Imaging pain. Br. J. Anaesth. 2008, 101, 32–39. [Google Scholar] [CrossRef]
- Davis, K.D.; Flor, H.; Greely, H.T.; Iannetti, G.; Mackey, S.; Ploner, M.; Pustilnik, A.; Tracey, I.; Treede, R.-D.; Wager, T.D. Brain imaging tests for chronic pain: Medical, legal and ethical issues and recommendations. Nat. Rev. Neurol. 2017, 13, 624–638. [Google Scholar] [CrossRef]
- Coghill, R.C.; Sang, C.N.; Maisog, J.M.; Iadarola, M.J. Pain Intensity Processing Within the Human Brain: A Bilateral, Distributed Mechanism. J. Neurophysiol. 1999, 82, 1934–1943. [Google Scholar] [CrossRef]
- Symonds, L.L.; Gordon, N.S.; Bixby, J.C.; Mande, M.M. Right-Lateralized Pain Processing in the Human Cortex: An fMRI Study. J. Neurophysiol. 2006, 95, 3823–3830. [Google Scholar] [CrossRef]
- Bingel, U.; Quante, M.; Knab, R.; Bromm, B.; Weiller, C.; Büchel, C. Subcortical structures involved in pain processing: Evidence from single-trial fMRI. Pain 2002, 99, 313–321. [Google Scholar] [CrossRef]
- Brooks, J.C.; Nurmikko, T.J.; Bimson, W.E.; Singh, K.D.; Roberts, N. fMRI of Thermal Pain: Effects of Stimulus Laterality and Attention. NeuroImage 2002, 15, 293–301. [Google Scholar] [CrossRef]
- Coghill, R.C.; Gilron, I.; Iadarola, M.J. Hemispheric lateralization of somatosensory processing. J. Neurophysiol. 2001, 85, 2602–2612. [Google Scholar] [CrossRef]
- Stein, B.E.; Price, D.; Gazzaniga, M.S. Pain perception in a man with total corpus callosum transection. Pain 1989, 38, 51–56. [Google Scholar] [CrossRef]
- Duquette, M.; Rainville, P.; Alary, F.; Lassonde, M.; Lepore, F. Ipsilateral cortical representation of tactile and painful information in acallosal and callosotomized subjects. Neuropsychology 2008, 46, 2274–2279. [Google Scholar] [CrossRef]
- Fabri, M. Mechanical Noxious Stimuli Cause Bilateral Activation of Parietal Operculum in Callosotomized Subjects. Cereb. Cortex 2002, 12, 446–451. [Google Scholar] [CrossRef][Green Version]
- Peyron, R.; García-Larrea, L.; Grégoire, M.-C.; Costes, N.; Convers, P.; Lavenne, F.; Mauguière, F.; Michel, D.; Laurent, B. Haemodynamic brain responses to acute pain in humans. Brain 1999, 122, 1765–1780. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Petrini, L.; Arendt-Nielsen, L. Understanding Pain Catastrophizing: Putting Pieces Together. Front. Psychol. 2020, 11, 603420. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V. Amygdala physiology in pain. Handb. Object Nov. Recognit. 2020, 26, 101–113. [Google Scholar] [CrossRef]
- Allen, H.N.; Bobnar, H.J.; Kolber, B.J. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol. 2021, 196, 101891. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Woolf, C.J.; Andrews, N.A. Composite Pain Biomarker Signatures for Objective Assessment and Effective Treatment. Neuron 2019, 101, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Wu, Y.-T.; Yeh, T.-C.; Chen, L.-F.; Chang, F.-Y.; Lee, S.-D.; Ho, L.-T.; Hsieh, J.-C. Neuronal correlates of gastric pain induced by fundus distension: A 3T-fMRI study. Neurogastroenterol. Motil. 2004, 16, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The Amygdala and Persistent Pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef]
- Alden, M.; Besson, J.-M. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: Aphaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J. Comp. Neurol. 1993, 329, 201–229. [Google Scholar] [CrossRef]
- Carrasquillo, Y.; Gereau, R.W., 4th. Activation of the Extracellular Signal-Regulated Kinase in the Amygdala Modulates Pain Perception. J. Neurosci. 2007, 27, 1543–1551. [Google Scholar] [CrossRef]
- Carrasquillo, Y.; Gereau, R.W. Hemispheric Lateralization of a Molecular Signal for Pain Modulation in the Amygdala. Mol. Pain 2008, 4, 24. [Google Scholar] [CrossRef]
- Kolber, B.; Montana, M.C.; Carrasquillo, Y.; Xu, J.; Heinemann, S.F.; Muglia, L.J.; Gereau, R.W. Activation of Metabotropic Glutamate Receptor 5 in the Amygdala Modulates Pain-Like Behavior. J. Neurosci. 2010, 30, 8203–8213. [Google Scholar] [CrossRef]
- Ji, G.; Neugebauer, V. Hemispheric Lateralization of Pain Processing by Amygdala Neurons. J. Neurophysiol. 2009, 102, 2253–2264. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Takahashi, Y.; Watabe, A.M.; Kato, F. Predominant synaptic potentiation and activation in the right central amygdala are independent of bilateral parabrachial activation in the hemilateral trigeminal inflammatory pain model of rats. Mol. Pain 2018, 14, 1744806918807102. [Google Scholar] [CrossRef]
- Crock, L.W.; Kolber, B.J.; Morgan, C.D.; Sadler, K.E.; Vogt, S.K.; Bruchas, M.R.; Gereau, R.W. Central Amygdala Metabotropic Glutamate Receptor 5 in the Modulation of Visceral Pain. J. Neurosci. 2012, 32, 14217–14226. [Google Scholar] [CrossRef]
- Sadler, K.E.; McQuaid, N.A.; Cox, A.C.; Behun, M.N.; Trouten, A.M.; Kolber, B.J. Divergent functions of the left and right central amygdala in visceral nociception. Pain 2017, 158, 747–759. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Saver, J.L. Pain after thalarnic stroke: Right diencephalic predominance and clinical features in 180 patients. Neurology 1997, 48, 1196–1199. [Google Scholar] [CrossRef]
- Geha, P.; Waxman, S.G. Pain Perception. JAMA Neurol. 2016, 73, 628–630. [Google Scholar] [CrossRef]
- De Courcy, J.; Liedgens, H.; Obradovic, M.; Holbrook, T.; Jakubanis, R. A burden of illness study for neuropathic pain in Europe. Clin. Outcomes Res. 2016, 8, 113–126. [Google Scholar] [CrossRef]
- Witting, N.; Kupers, R.; Svensson, P.; Jensen, T.S. A PET activation study of brush-evoked allodynia in patientswith nerve injury pain. Pain 2006, 120, 145–154. [Google Scholar] [CrossRef]
- Peyron, R.; Schneider, F.; Faillenot, I.; Convers, P.; Barral, F.-G.; Garcia-Larrea, L.; Laurent, B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology 2004, 63, 1838–1846. [Google Scholar] [CrossRef]
- Ducreux, D.; Attal, N.; Parker, F.; Bouhassira, D. Mechanisms of central neuropathic pain: A combined psychophysical and fMRI study in syringomyelia. Brain 2006, 129, 963–976. [Google Scholar] [CrossRef]
- Kimmey, B.A.; McCall, N.M.; Wooldridge, L.M.; Satterthwaite, T.D.; Corder, G. Engaging endogenous opioid circuits in pain affective processes. J. Neurosci. Res. 2020. [Google Scholar] [CrossRef]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef]
- Watanabe, H.; Fitting, S.; Hussain, M.Z.; Kononenko, O.; Iatsyshyna, A.; Yoshitake, T.; Kehr, J.; Alkass, K.; Druid, H.; Wadensten, H.; et al. Asymmetry of the Endogenous Opioid System in the Human Anterior Cingulate: A Putative Molecular Basis for Lateralization of Emotions and Pain. Cereb. Cortex 2013, 25, 97–108. [Google Scholar] [CrossRef]
- Mansour, A.; Fox, C.A.; Burke, S.; Akil, H.; Watson, S.J. Immunohistochemical localization of the cloned μ opioid receptor in the rat CNS. J. Chem. Neuroanat. 1995, 8, 283–305. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Kononenko, O.; Galatenko, V.; Andersson, M.; Bazov, I.; Watanabe, H.; Zhou, X.W.; Iatsyshyna, A.; Mityakina, I.; Yakovleva, T.; Sarkisyan, D.; et al. Intra- and interregional coregulation of opioid genes: Broken symmetry in spinal circuits. FASEB J. 2017, 31, 1953–1963. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roza, C.; Martinez-Padilla, A. Asymmetric Lateralization during Pain Processing. Symmetry 2021, 13, 2416. https://doi.org/10.3390/sym13122416
Roza C, Martinez-Padilla A. Asymmetric Lateralization during Pain Processing. Symmetry. 2021; 13(12):2416. https://doi.org/10.3390/sym13122416
Chicago/Turabian StyleRoza, Carolina, and Anabel Martinez-Padilla. 2021. "Asymmetric Lateralization during Pain Processing" Symmetry 13, no. 12: 2416. https://doi.org/10.3390/sym13122416
APA StyleRoza, C., & Martinez-Padilla, A. (2021). Asymmetric Lateralization during Pain Processing. Symmetry, 13(12), 2416. https://doi.org/10.3390/sym13122416





