3.2. Drug Cytotoxicity
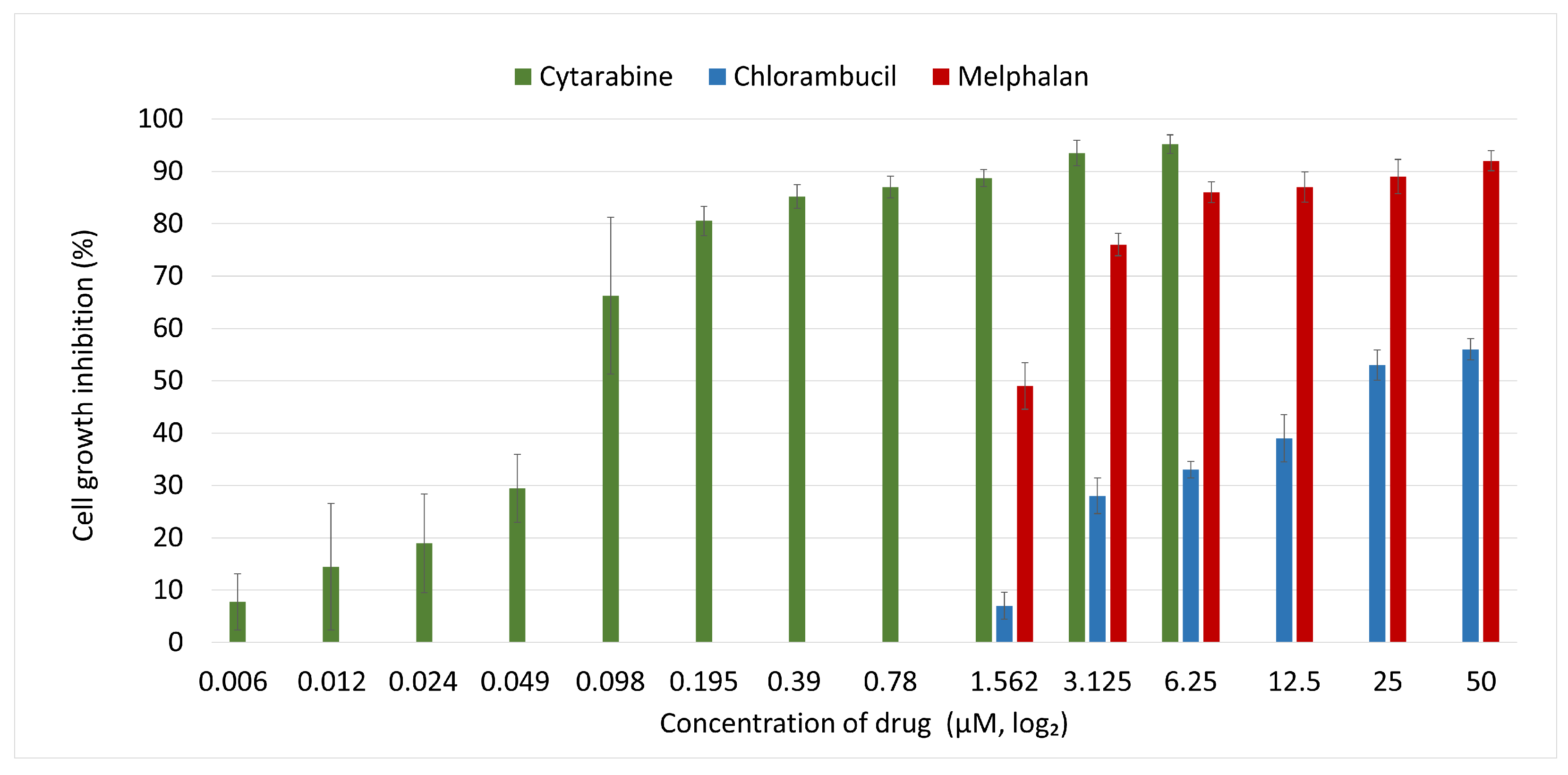
Cells were cultured for 72 h in fresh medium containing either Chl, Mel, or Cut, and the cellular metabolic status was determined. The results are shown in
Figure 3.
As can be seen above (
Figure 3), Chl was the least effective of the three drugs, producing only 56% cell growth inhibition at the maximum concentration used in the experiment, 50
M, while Mel induced 92% inhibition at this dose. At the lowest dose of 1.56
M, Chl induced only 7% growth inhibition, while Mel induced 49% inhibition. On the other hand, Cyt maintained a high plateau level of growth inhibition of between 96% and 92% inhibition down to 3.125
M.
These experiments provided the basis for the development of our dynamic model.
3.3. Formulation of the Model
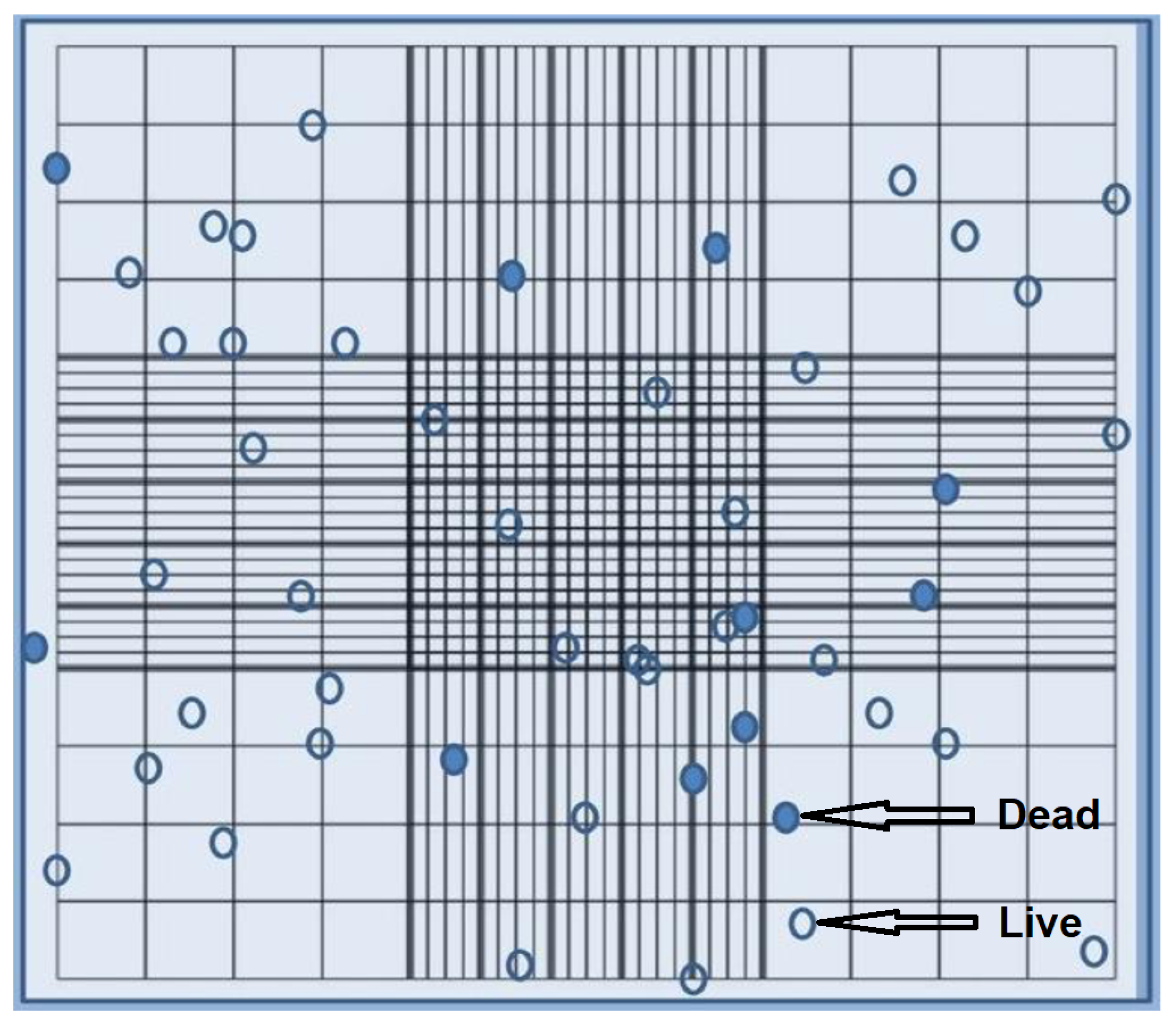
Based on cell counts with a hemocytometer (
Figure 1 and
Figure 2), A20 cells can be divided into two groups: live cells (transparent circle in
Figure 1) and dead cells (blue circle in
Figure 1), denoted as
A and
, respectively, in the mathematical model developed by us. Dead cells (
) are formed because of apoptosis and/or necrosis in culture after the death of living A cells. The death rate of living
A cells depends on several conditions in the culture environment: the concentration of essential nutrients in the cells, the supply and uptake of oxygen, and the disposal of waste products such as ammonia and various acidic products [
29,
30,
31]. The total number of living and dead cells cannot exceed a certain capacity (
K) in the closed space of the culture. The dynamics of living and dead cells of type A20 in a closed environment is described using differential equations as follows:
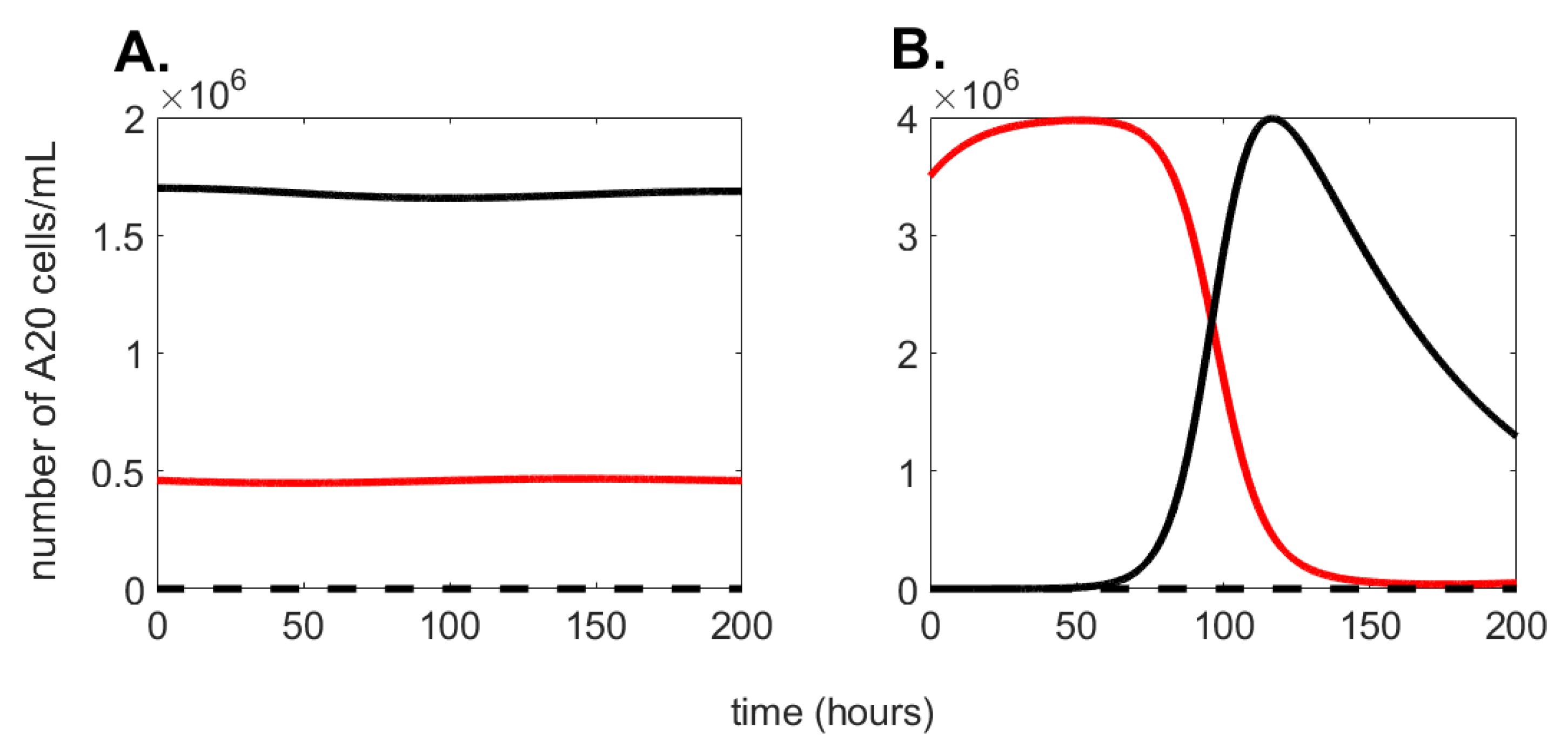
describes the dynamics of living A20 cells. It comprises two terms: one positive, corresponding to the logistic cancer growth characterized by the coefficient, r, which is limited by the maximal tumor cell number, K; one negative term, corresponding to living cells becoming dead at a rate of .
describes the dynamic of dead A20 cells. It is comprised of two terms: the positive term is the death of A20 cells with a rate coefficient of due to apoptosis or necrosis and depends on living A20 cells competing for survival (oxygen consumption and nutrition) in an enclosed space; the negative term corresponds to dissolution of dead cells at a rate of d.
We extended the model by adding a new equation that represents the dynamics of chemotherapeutic drugs. Based on previous studies [
23] and our experiments in vitro, we formulated an ODE model to mathematically explain the interaction between CLL cells and chemotherapeutic drugs:
describes the first-order pharmacokinetics of a drug [
32]. The drug was given only once at the beginning of the experiment, i.e.,
is a constant value and depends on the dose of the drug.
is the deactivation rate calculated by formula
, where
is the in vitro elimination half-life, about 1.5 h for Chl, 2 h for Mel, and 1–3 h (biphasic) for Cyt (
www.drugbank.ca accessed on 17 September 2021).
The terms 1 and 2 of the Equations (3) and (4) represent the log-kill hypothesis [
33], with a Michaelis–Menten drug saturation response [
34],
;
is the death rate resulting from the action of the drug on the cancer cells. The parameter
changes depending on the drug dose and on the particular drug. We chose the parameter
to be ten-times more than
, assuming that there are 10–100 drug molecules attacking each cancer cell. Ultimately, the parameter
does not play a significant role in the model, since there are many more drug molecules than cancer cells, and it only reflects the amount of available drug molecules. The parameter
a represents the drug concentration that produces 50% of the maximum activity of the drug in each cell population [
27].
We performed a mathematical analysis of our model by identifying fixed points and their stability. It was found that the system is characterized by three fixed points, one of which is stable asymptotically (
Appendix A,
Table A4).
3.4. Estimation of the Parameters of the Model
In this section, we evaluate the model parameters (
Table 1), together with the detailed methods and the literature sources for their evaluation:
(cells/mL)—the initial number of A20 cells;
(cells/mL)—the initial number of dead A20 cells (cell cultures commonly consist of at least 5% of dead cells);
dose (M) (number of drug molecules/mL)—the dose concentration of Chl, Mel, or Cyt (this number may vary depending on the drug, but not significantly since all these drugs are related to the same type of small molecules).
The number of drug molecules was calculated using the formula:
where:
m = the mass of drug in kg;
= Avogadro number = (constant);
M = the molar mass of drug (Chl 304.212 g/mol; Mel 305.2 g/mol; Cyt 243.217 g/mol).
For example, for 50 µM of Chl, i.e., 50 µM = 15.15 µL = 0.00001515 kg, it would be:
The cell doubling time was calculated using an exponential growth rate:
or
where:
= the number of cells at time t;
= the number of cells at Time 0;
r = growth rate;
t = time (usually in hours).
The tumor doubling time is calculated as:
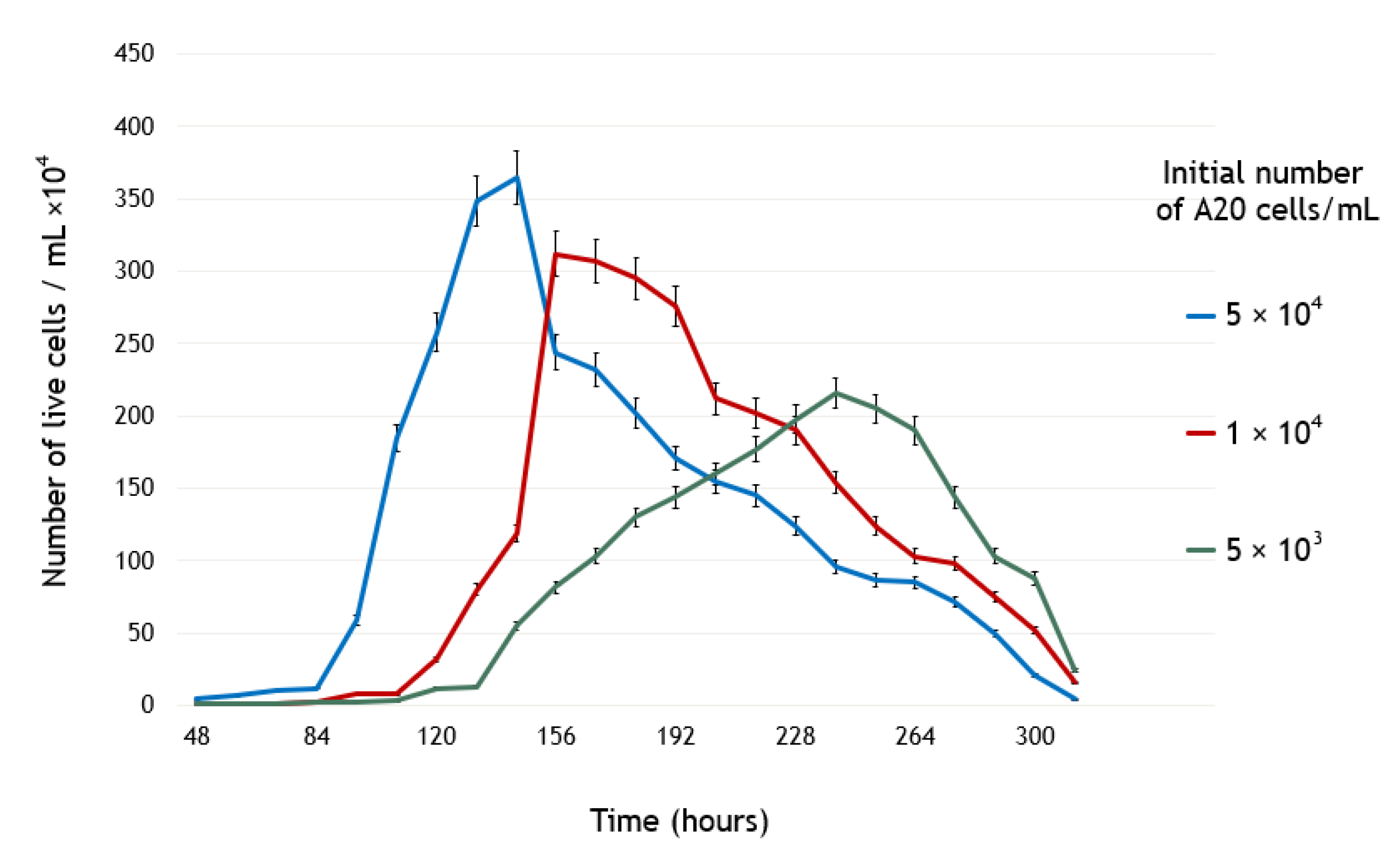
Thus, according to
Figure 2, when
by the 84th hour,
by the 132th hour, and
, the growth rate of A20 cells would be:
and the cell doubling time would be:
Thus, according to
Figure 2 the number of A20 cells double in less than 12 h.
The parameters used in this study are summarized in
Table 1.
3.5. Validation of the Cancer Cell Growth Dynamics Model
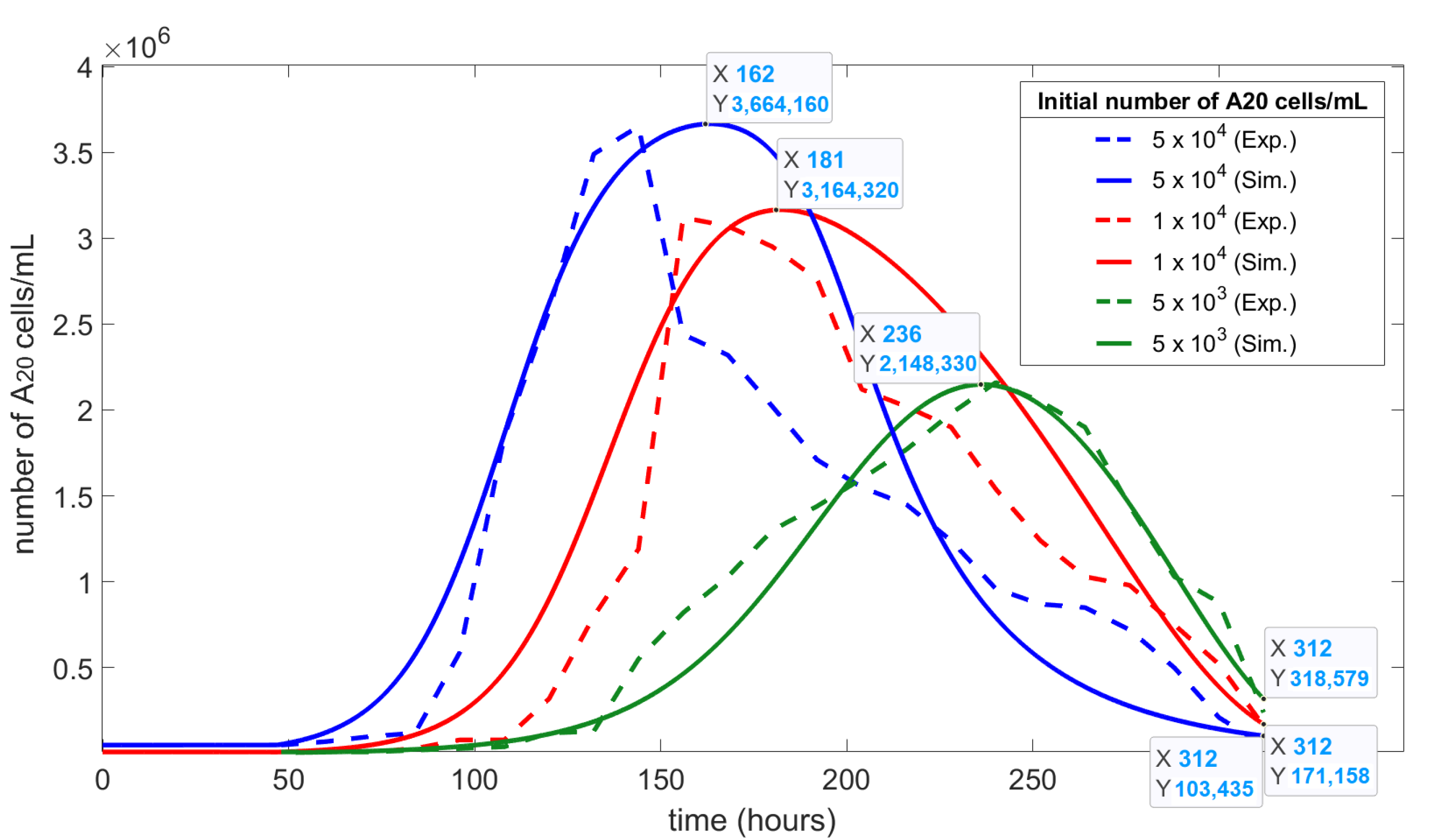
Figure 4 compares the numerical simulations and the in vitro experimental growth and natural death curves for A20 cells at different starting cell concentrations.
To assess the closeness of the fit between the simulation and experimental curves, the root-mean-squared errors (RMSEs) were calculated using the formula:
where
is the numerical simulation data (predicted value),
is the experimental data (observed value), and
n is the number of observations (see
Appendix A,
Table A5).
The RMSEs for the initial cell concentrations are: cells/mL = 0.016; cells/mL = 0.013; cells/mL = 0.008. The data showed a strong correlation between the simulation and experimental results for both the time and cell concentration at both the maximum and final data points. The correlation held for all three starting concentrations (all RMSEs were < 0.1), but was most symmetric for the lowest starting concentration ( cells/mL).
3.6. Validation of the Cancer Cell Drug Cytotoxicity Dynamics Model
By inserting the parameters from
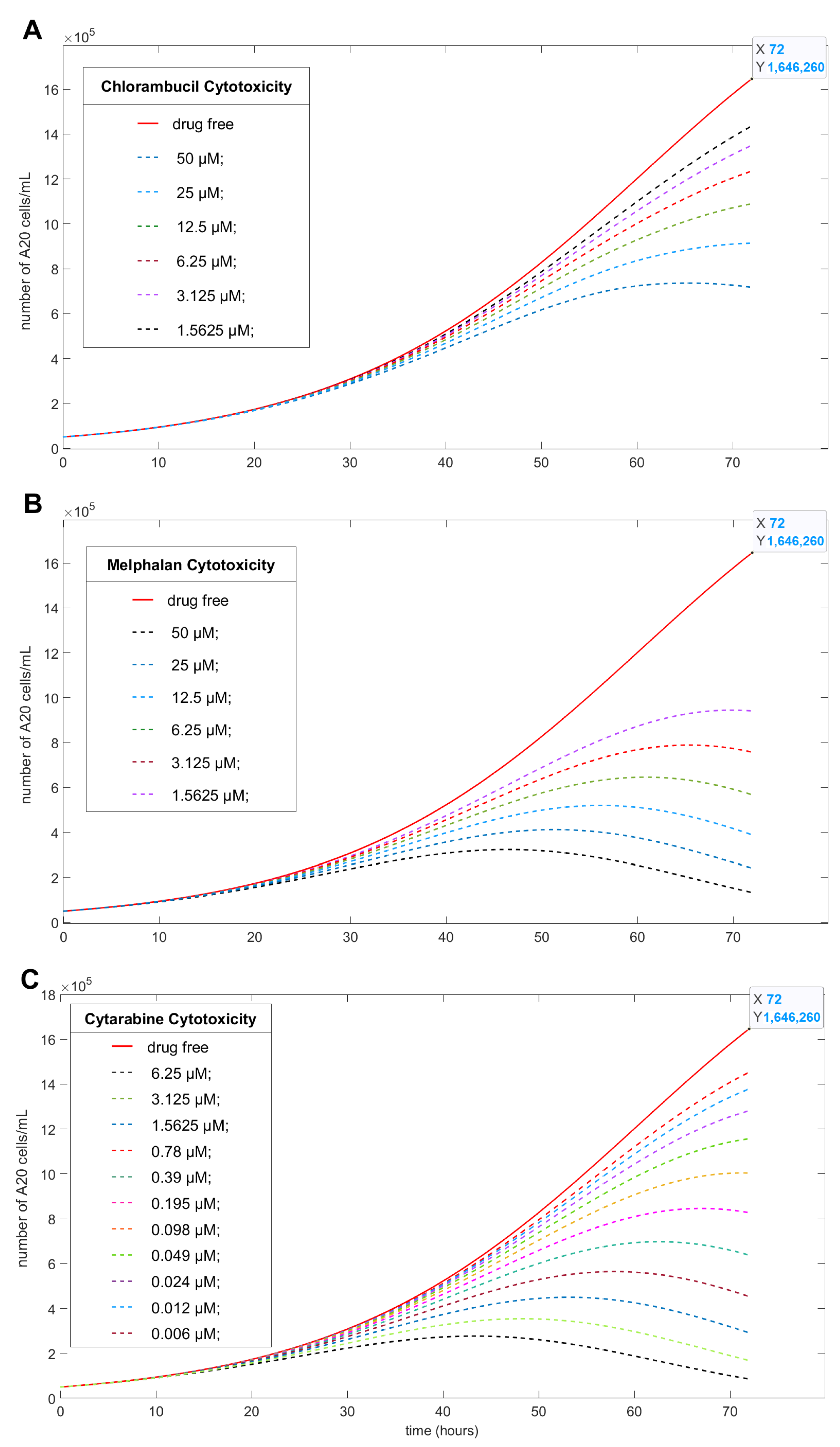
Table 1 into Equations (2) and (3), we could numerically simulate the effect of Chl, Mel, or Cyt on A20 cells, as depicted in
Figure 5.
After 72 h, the concentration of A20 growth without drug increased to
A(72) = 1,646,260. The additional 50 µM of Chl (A) reduced the growth to
A(72) = 717,369 cells/mL, which equals 56.4% growth inhibition; 50 µM of Mel induced
growth inhibition (B), and 6.25 µM of Cyt induced
growth inhibition (C). The complete set of calculated data for all drug concentrations is presented in
Appendix A (
Table A1,
Table A2 and
Table A3).
We then compared the degree of A20 growth inhibition from the numerical simulation of the model (Equations (3)–(5)) (
Figure 5) with the output obtained by the in vitro experiments (
Figure 3). The results are shown in
Figure 6.
Figure 6 demonstrates that our simulation coincided with the experimental data for the high doses of each drug. For the intermediate doses, there appeared to be a slight deviation between the two sets of data. This deviation was artificial since we tried to maintain consistency in the decrease of the parameter
in accordance with the decrease in the drug dose. This resulted in a discrepancy of 30% for each drug. However, it is important to note that maintaining this consistency is not necessary, and it is possible to juxtapose the experimental and simulation results with absolute accuracy by choosing the appropriate parameter
.
The RMSE for each drug was calculated (see
Appendix A,
Table A6). The data showed a strong correlation between the simulation and experimental results for cell growth inhibition by various drug concentrations. The correlation held for all three drugs (all RMSEs were < 0.1), but was most apparent for Cyt (RMSE = 0.018).