Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function
Abstract
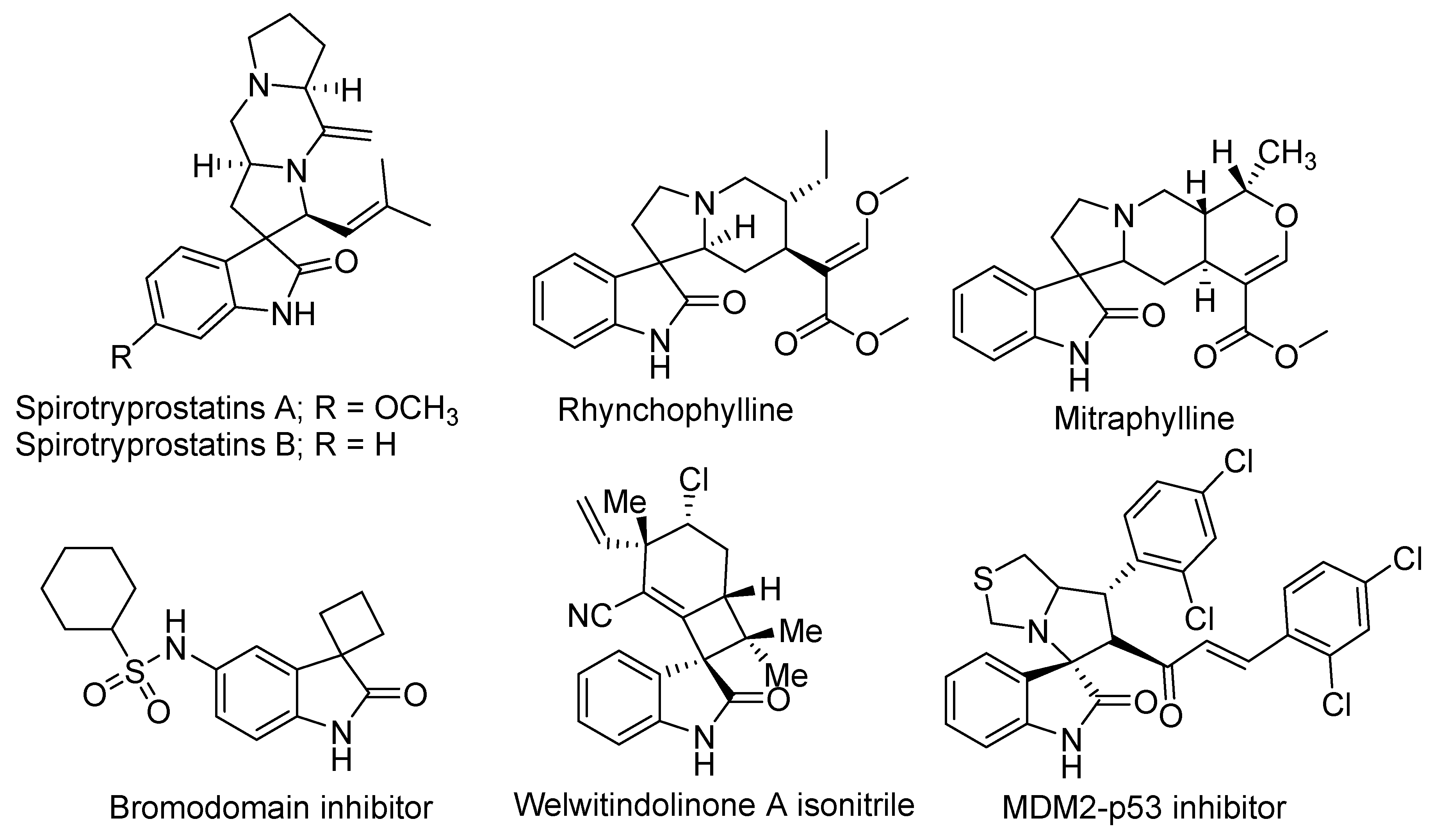
1. Introduction
2. Materials and Methods
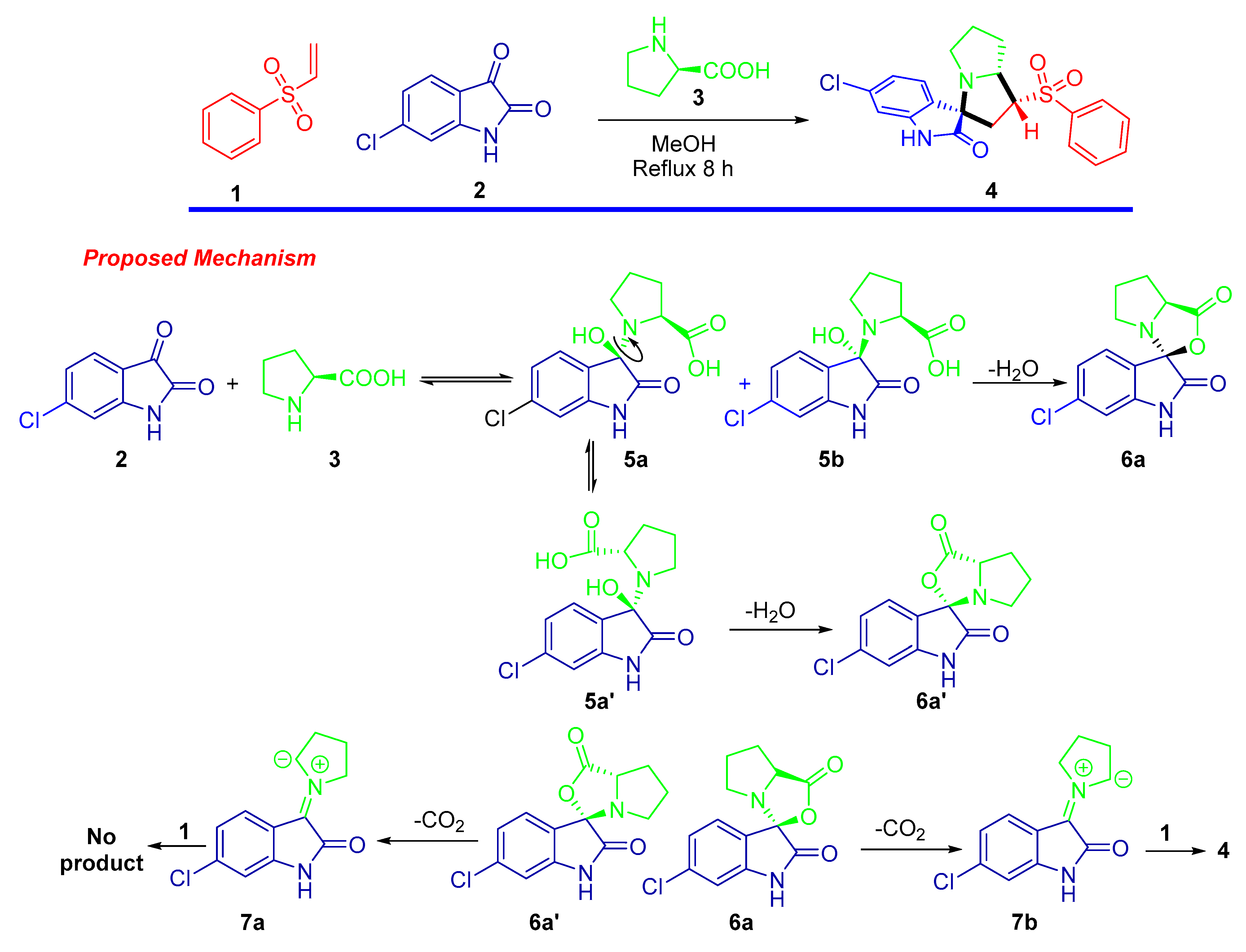
2.1. Synthesis of the Spirooxindole-Based Phenylsulphone ((1′R,3R,7a′R)-6-Chloro-1′-(phenylsulfonyl)-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one 4)
2.2. X-ray Structure Determinations
2.3. Computational Methods
3. Results
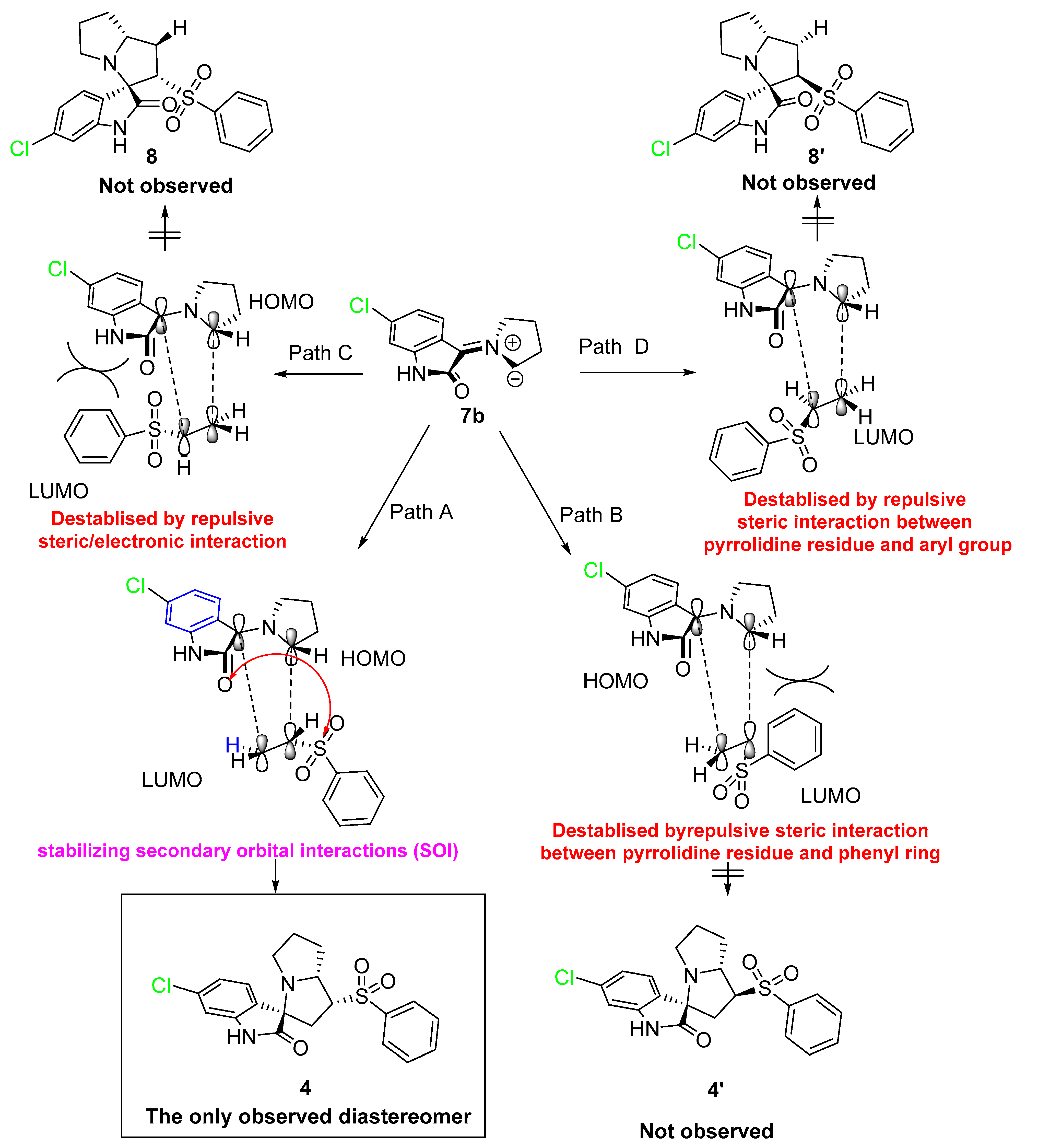
3.1. Synthesis of the Spirooxindole-Based Phenylsulphone 4
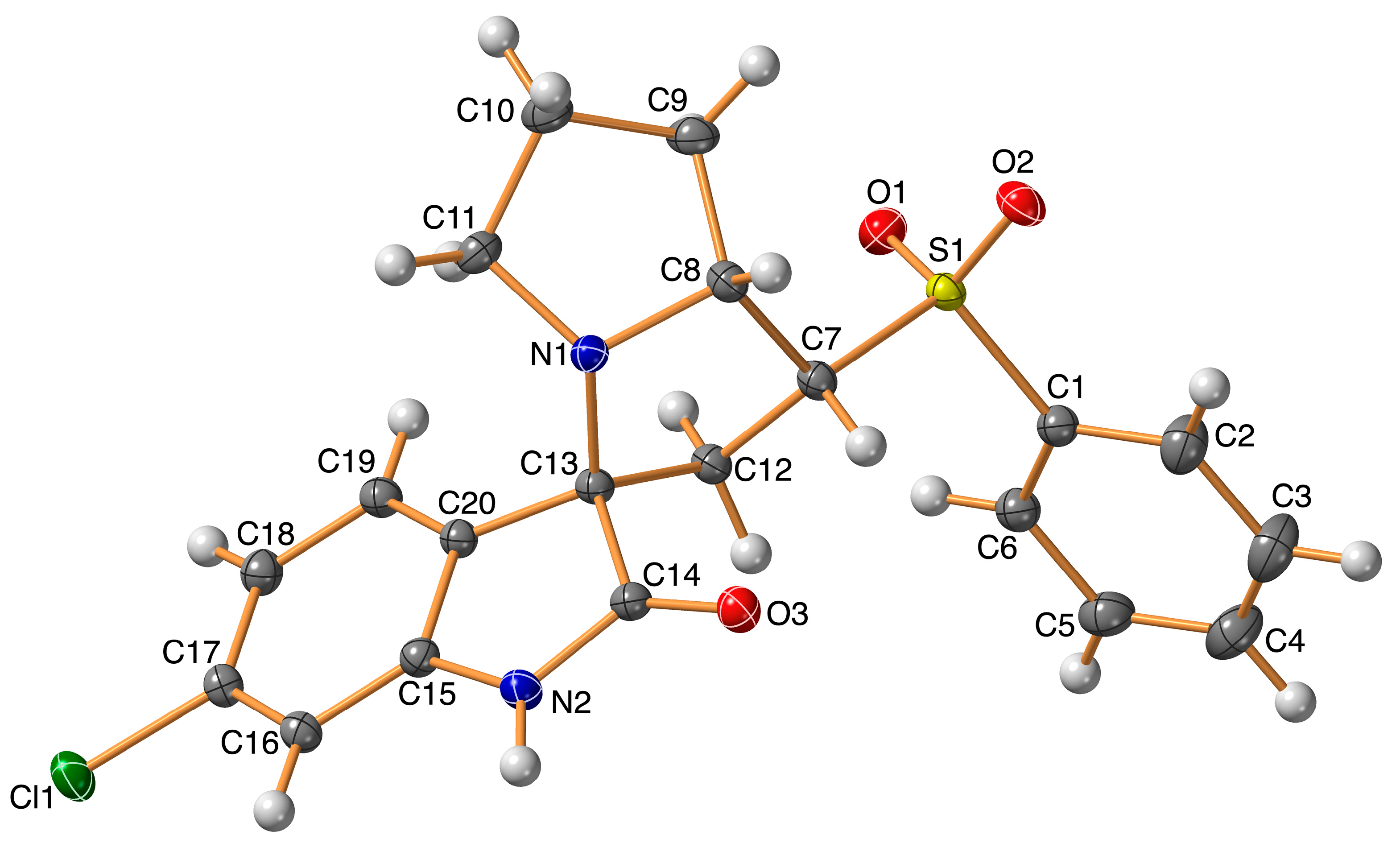
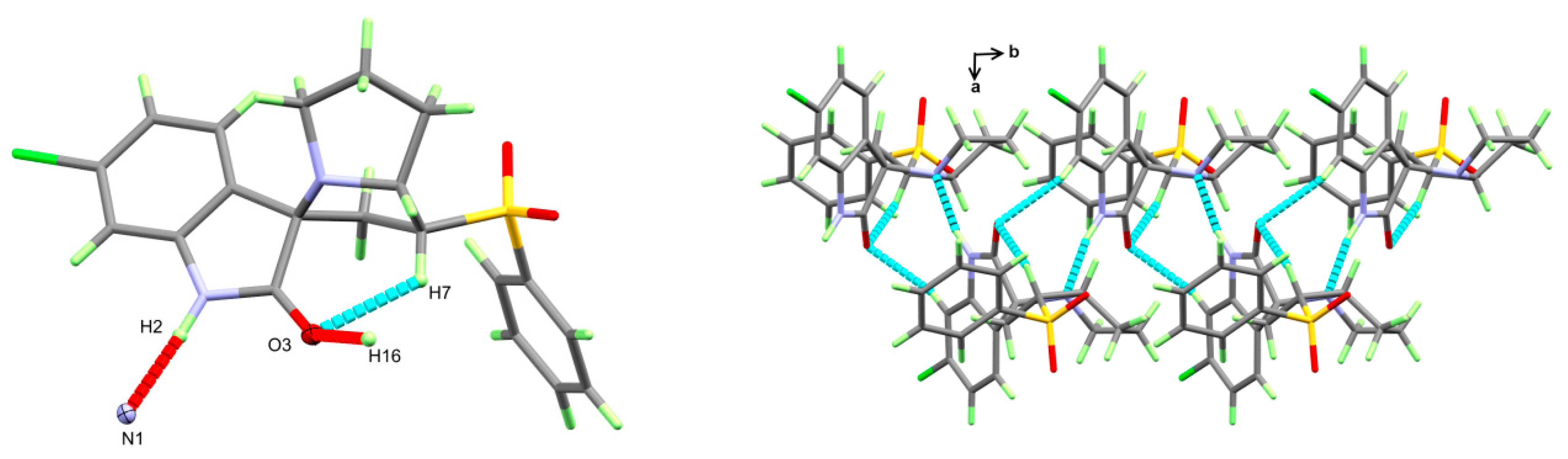
3.2. X-ray Structure of 4
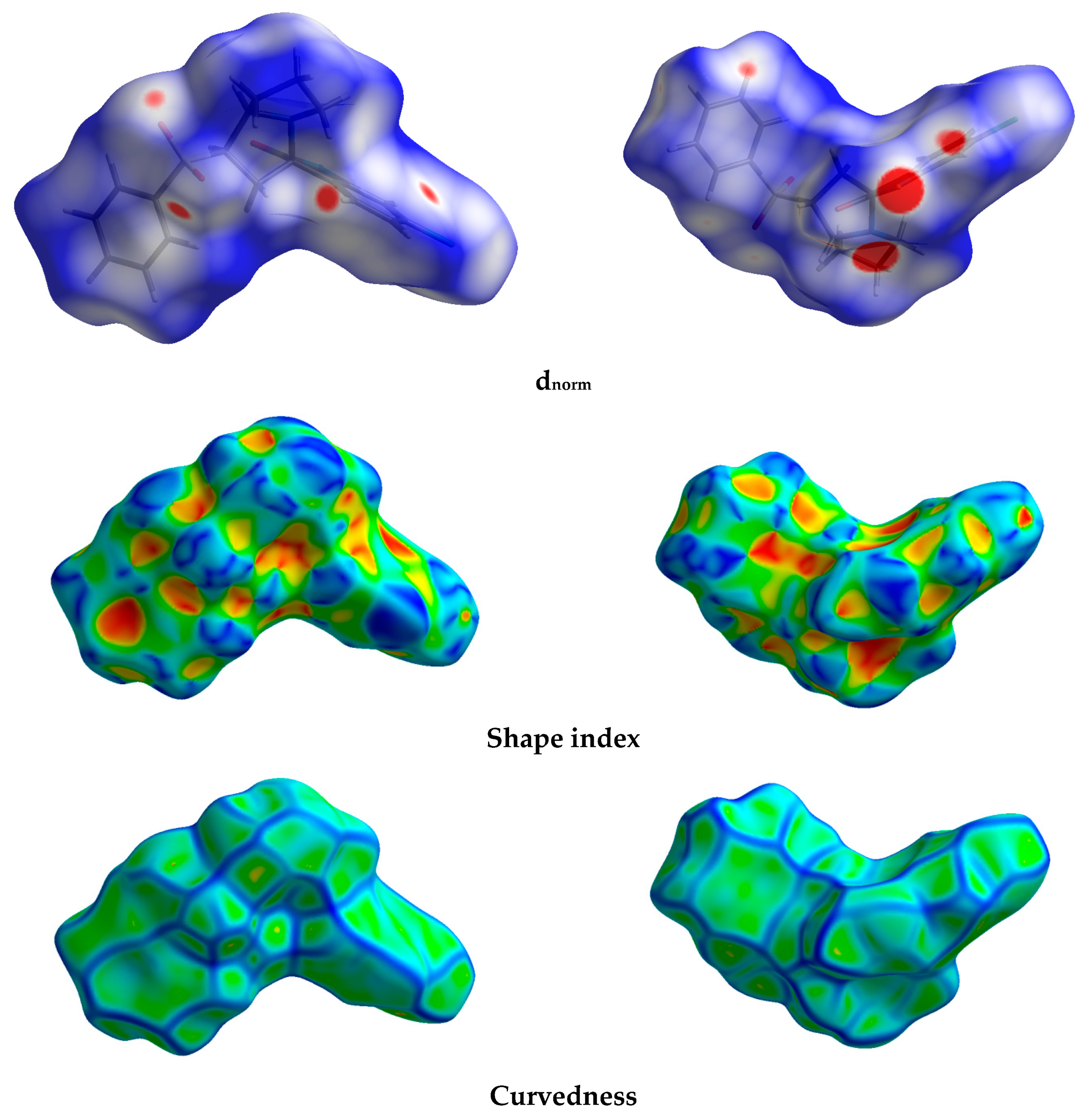
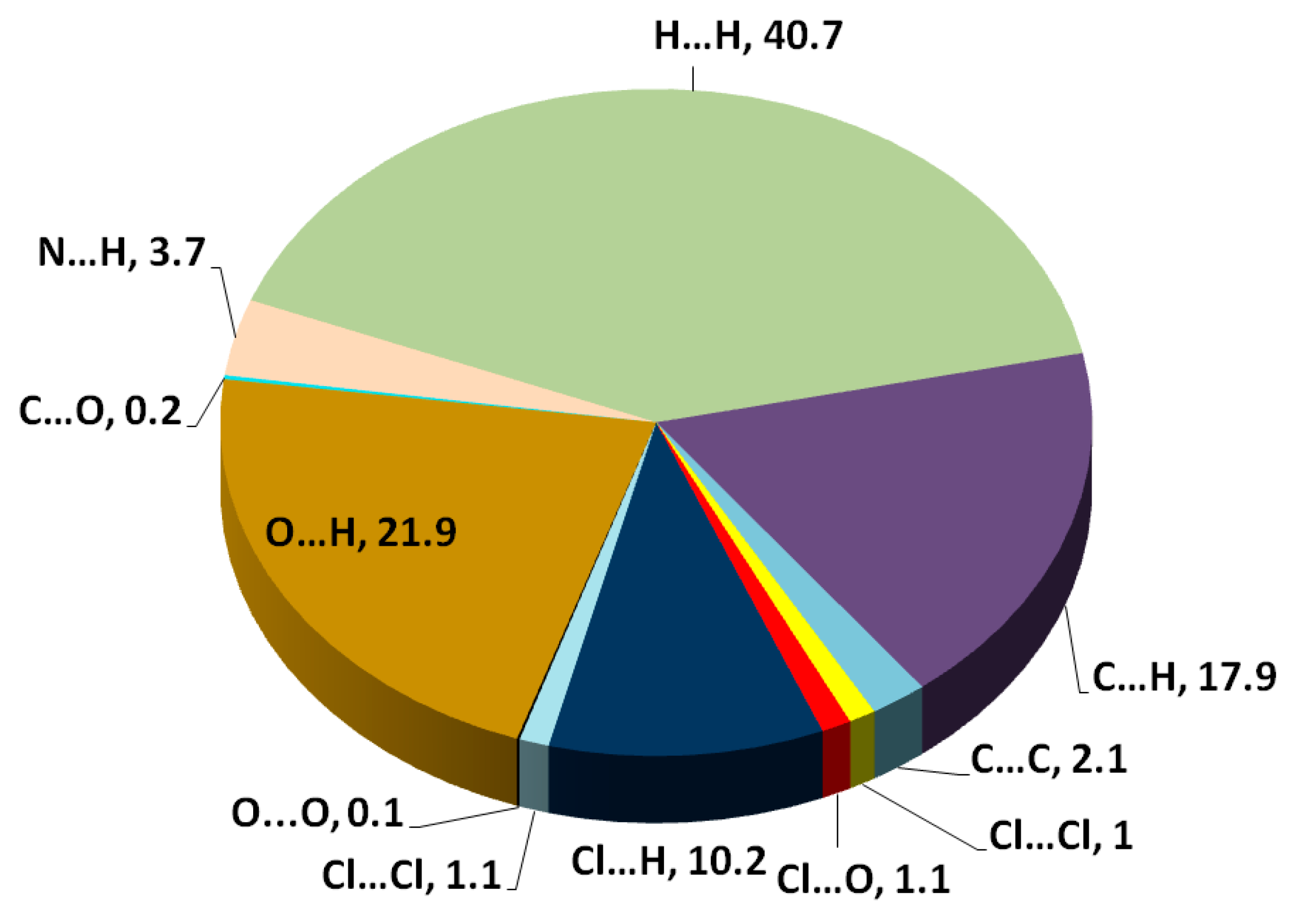
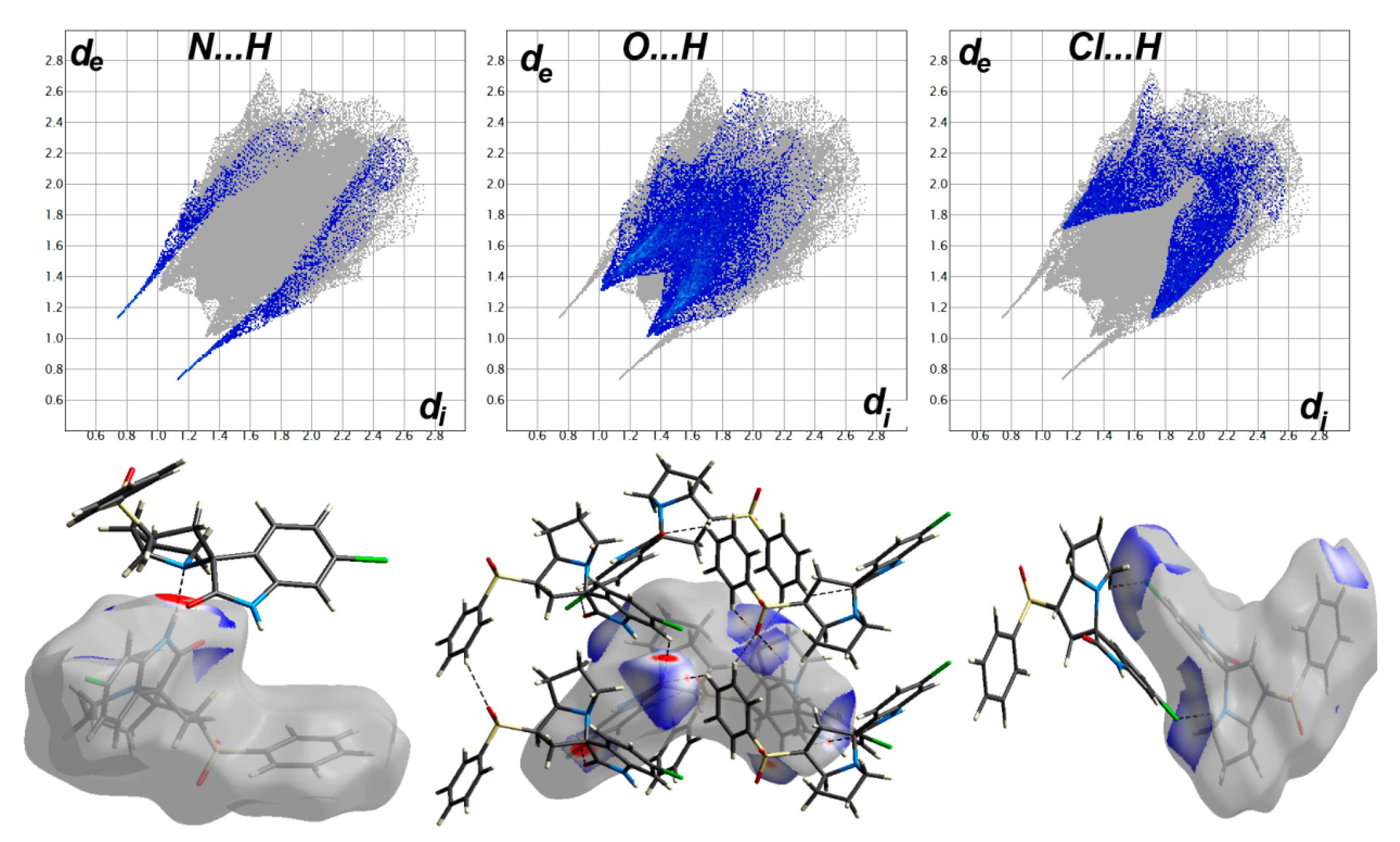
3.3. Hirshfeld Analysis of Molecular Packing
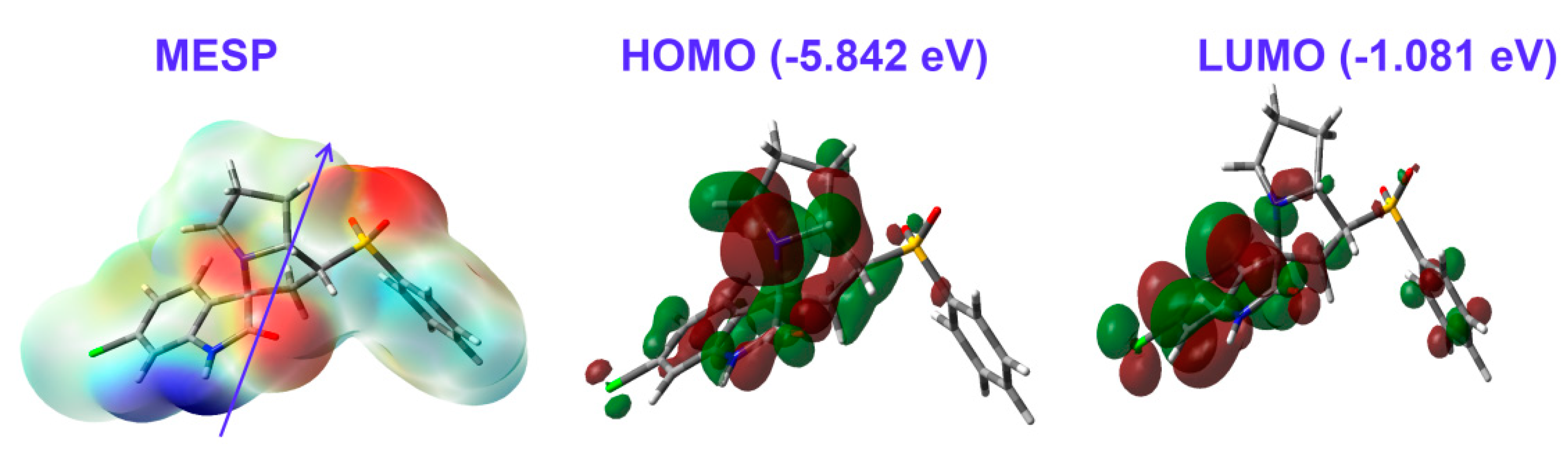
3.4. DFT Studies
3.5. NBO Analysis
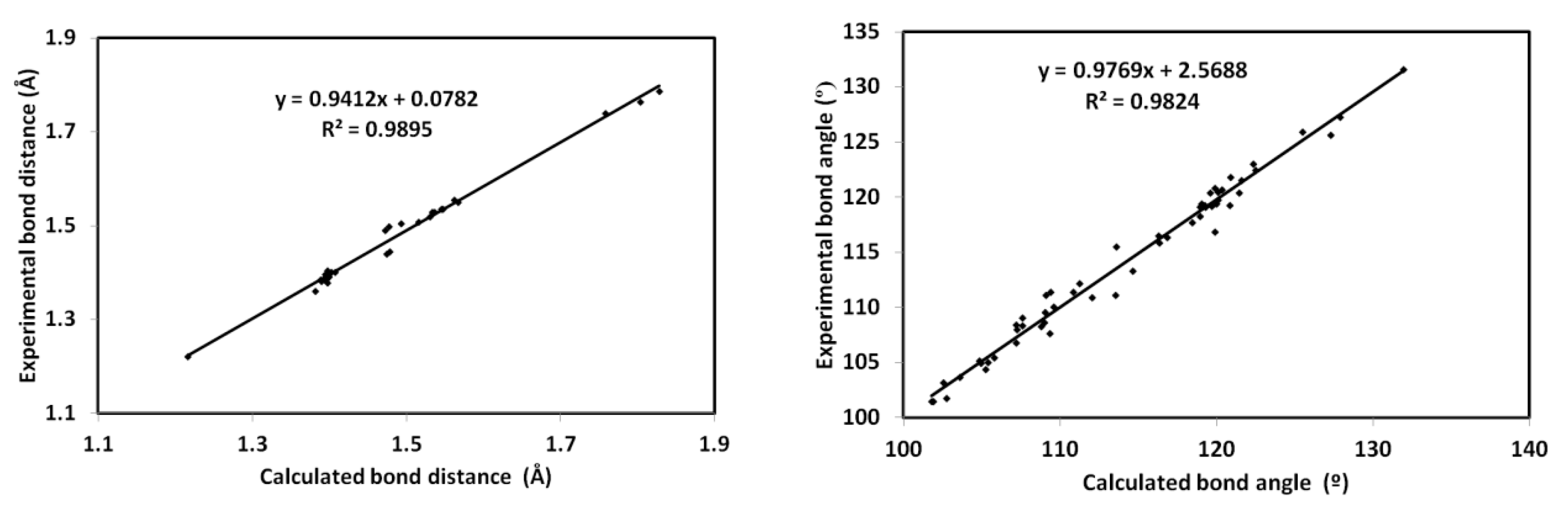
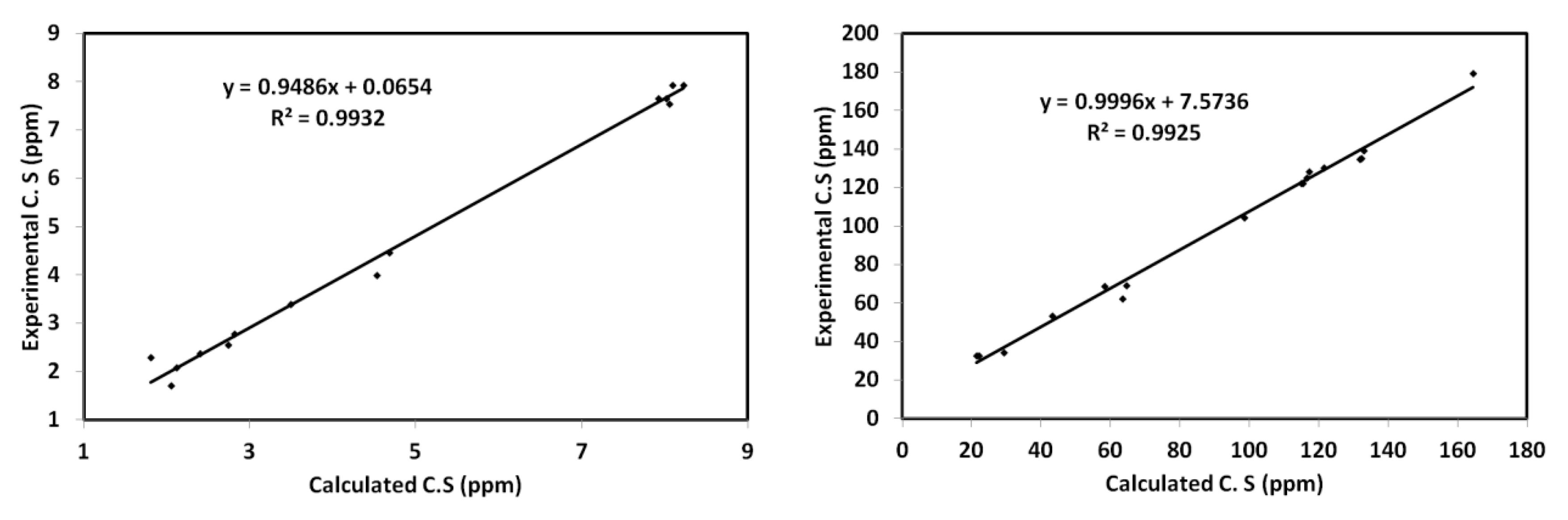
3.6. NMR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojima, I. Catalytic Asymmetric Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Zhou, L.M.; Qu, R.Y.; Yang, G.F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Murakami, Y.; Matsumoto, K.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Rhynchophylline and isorhynchophylline inhibit NMDA receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 2002, 455, 27–34. [Google Scholar] [CrossRef]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.H.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of Mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef]
- Stratmann, K.; Moore, R.E.; Patterson, G.M.L.; Bonjouklian, R.; Deeter, J.B.; Shaffer, S.; Smitka, T.A.; Smith, C.D. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to fischerindoles and hapalinodoles. J. Am. Chem. Soc. 1994, 116, 9935–9942. [Google Scholar] [CrossRef]
- Santos, M.M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 52, 9735–9757. [Google Scholar] [CrossRef]
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers. 2016, 20, 299–344. [Google Scholar] [CrossRef]
- Arun, Y.; Bhaskar, G.; Balachandran, C.; Ignacimuthu, S.; Perumal, P. Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against a549 human lung adenocarcinoma cancer cell line. Bioorg. Med. Chem. Lett. 2013, 23, 1839–1845. [Google Scholar] [CrossRef]
- Girgis, A.S. Regioselective synthesis of dispiro [1H-indene-2,3′-pyrrolidine-2′,3″-[3H]indole]-1,2″(1″h)-diones of potential anti-tumor properties. Eur. J. Med. Chem. 2009, 44, 91–100. [Google Scholar] [CrossRef]
- Islam, M.S.; Park, S.; Song, C.; Kadi, A.A.; Kwon, Y.; Rahman, A.M. Fluorescein hydrazones: A series of novel non-intercalative topoisomerase IIα catalytic inhibitors induce G1 arrest and apoptosis in breast and colon cancer cells. Eur. J. Med. Chem. 2017, 125, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ismail, R.; Choon, T.S.; Yoon, Y.K.; Wei, A.C.; Pandian, S.; Kumar, R.S.; Osman, H.; Manogaran, E. Substituted spiro [2.3′] oxindolespiro [3.2″]-5, 6-dimethoxy-indane-1″-one-pyrrolidine analogue as inhibitors of acetylcholinesterase. Bioorg. Med. Chem. Lett. 2010, 20, 7064–7066. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Sahu, V.; Upadhyay, S.; Singh, J. Synthesis of bioactive spiro-2-[3′-(2′-phenyl)-3h-indolyl]-1-aryl-3-phenylaziridines and SAR studies on their antimicrobial behavior. Med. Chem. Res. 2009, 18, 383–395. [Google Scholar] [CrossRef]
- Uchida, R.; Imasato, R.; Shiomi, K.; Tomoda, H.; Ōmura, S. Yaequinolones j1 and j2, novel insecticidal antibiotics from penicillium sp. Fki-2140. Org. Lett. 2005, 7, 5701–5704. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2–p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Biol. Sci. 2020, 27, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H. Spiroindolone analogues as potential hypoglycemic with dual inhibitory activity on α-amylase and α-glucosidase. Molecules 2019, 24, 2342. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ghawas, H.M.; El-Senduny, F.F.; Al-Majid, A.M.; Elshaier, Y.A.; Badria, F.A.; Barakat, A. Synthesis of new thiazolo-pyrrolidine-(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorg. Chem. 2019, 82, 423–430. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Al Majid, A.M.; Ghawas, H.M.; El-Senduny, F.F.; Badria, F.A.; M.Elshaier, Y.A.M. Ghabbour, Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335. [Google Scholar] [CrossRef]
- Lotfy, G.; El Sayed, H.; Said, M.M.; Aziz, Y.M.A.; Al-Dhfyan, A.; Al-Majid, A.M.; Barakat, A. Regio- and stereoselective synthesis of novel spiro-oxindole via 1,3-dipolar cycloaddition reaction. Anti-cancer and molecular docking studies. J. Photochem. Photobiol. B 2018, 180, 98–108. [Google Scholar] [CrossRef]
- Barakat, A.; Soliman, S.M.; Al-majid, A.M.; Ali, M.; Islam, M.S.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Regioselective synthesis of novel spiro-oxindole constructed with pyrrolidine/thioxothiazolidin-4-one derivatives: X-ray crystal structures, Hirshfeld surface analysis, DFT, docking and antimicrobial studies. J. Mol. Struct. 2018, 1152, 101–114. [Google Scholar] [CrossRef]
- Lotfy, G.; Said, M.M.; El Sayed, H.; El Sayed, H.; Al-Dhfyan, A.; Aziz, Y.M.A.; Barakat, A. Synthesis of new spirooxindole-pyrrolothiazoles derivatives: Anti-cancer activity and molecular docking. Bioorg. Med. Chem. 2017, 25, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Al Majid, A.M.; Ghawas, H.M.; El-Senduny, F.F.; Badria, F.A.; M.Elshaier, Y.A.M.; Ghabbour, H.A. Substituted Spirooxindoles. U.S. Patent Application No. 9822128B1, 21 November 2017. [Google Scholar]
- Rios, R. Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 2012, 41, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, R. Recent advances in asymmetric organocatalytic construction of 3, 3′-spirocyclic oxindoles. Adv. Synth. Catal. 2013, 355, 1023–1052. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Yao, J.; Cao, Y.; Kai, M.; He, N.; Zhang, X.; Wang, Y.; Wang, R. Core Scaffold-Inspired Concise Synthesis of Chiral Spirooxindole-Pyranopyrimidines with Broad-Spectrum Anticancer Potency. Adv. Synth. Catal. 2012, 354, 917–925. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17 (2017) University of Western Australia. Available online: http://hirshfeldsurface.net (accessed on 12 June 2017).
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Yarnton, Oxfordshire, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09, Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R., II; Keith, T.; Millam, J. (Eds.) GaussView, Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New Model for Calculation of Solvation Free Energies: Correction of Self-Consistent Reaction Field Continuum Dielectric Theory for Short-Range Hydrogen-Bonding Effects. J. Phys. Chem. 1996, 100, 11775–11788. [Google Scholar] [CrossRef]
- Tannor, D.J.; Marten, B.; Murphy, R.; Friesner, R.A.; Sitkoff, D.; Nicholls, A.; Ringnalda, M.; Goddard, W.A.; Honig, B. Accurate first principles calculation of molecular charge distributions and solvation energies from ab initio quantum mechanics and continuum dielectric theory. J. Am. Chem. Soc. 1994, 116, 11875–11882. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A Comparison of Models for Calculating Nuclear Magnetic Resonance Shielding Tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Al-Qubati, M.; Ghabbour, H.A.; Soliman, S.M.; Al-Majid, A.M.; Barakat, A.; Sultan, M.A. Synthesis of N-(Anthracen-9-ylmethyl)-N-methyl-2-(phenylsulfonyl)ethanamine via Microwave Green Synthesis Method: X-ray Characterization, DFT and Hirshfeld Analysis. Crystals 2020, 10, 643. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, E. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Hubert Joe, I.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Pinzaru, S.C. Theoretical and vibrational spectral investigation of sodium salt of acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar]
- Sebastian, S.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef]

| 4 | |
|---|---|
| empirical formula | C20H19ClN2O3S |
| fw | 402.88 |
| temp (K) | 120(2) |
| λ(Å) | 0.71073 |
| cryst syst | Monoclinic |
| space group | I2/a |
| a (Å) | 22.2636(6) |
| b (Å) | 7.70240(10) |
| c (Å) | 24.3057(6) |
| β (deg) | 116.521(3) |
| V (Å3) | 3729.42(17) Å3 |
| Z | 8 |
| ρcalc (Mg/m3) | 1.435 Mg/m3 |
| μ(Mo Kα) (mm–1) | 0.341 mm–1 |
| No. reflns. | 10411 |
| Unique reflns. | 5073 |
| GOOF (F2) | 1.037 |
| Rint | 0.0147 |
| R1a (I ≥ 2σ) | 0.0333 |
| wR2 b (I ≥ 2σ) | 0.0831 |
| CCDC | 2020273 |
| Cl(1)–C(17) | 1.7395(13) |
| S(1)–O(2) | 1.4402(10) |
| S(1)–O(1) | 1.4450(10) |
| S(1)–C(1) | 1.7652(13) |
| S(1)–C(7) | 1.7873(12) |
| O(3)–C(14) | 1.2208(15) |
| N(1)–C(11) | 1.4912(16) |
| N(1)–C(13) | 1.4986(15) |
| N(1)–C(8) | 1.5055(15) |
| N(2)–C(14) | 1.3606(16) |
| N(2)–C(15) | 1.4052(16) |
| N(2)–H(2) | 0.913(18) |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| N2–H2...N1#1 | 0.913(2) | 1.963(2) | 2.862(1) | 168.0(2) |
| C7–H7...O3 | 1.00 | 2.37 | 3.001(2) | 120.5 |
| C16–H16...O3#1 | 0.95 | 2.41 | 3.106(2) | 129.0 |
| Atom | Charge | Atom | Charge | Atom | Charge |
|---|---|---|---|---|---|
| Cl1 | 0.0020 | H 17 | 0.2481 | H 32 | 0.2020 |
| S2 | 2.2162 | C 18 | −0.2195 | C 33 | −0.4776 |
| O3 | −0.9644 | H 19 | 0.2682 | H 34 | 0.2623 |
| O4 | −0.9524 | C 20 | −0.5068 | H 35 | 0.2630 |
| O5 | −0.5983 | H 21 | 0.2960 | C 36 | 0.0524 |
| N6 | −0.5097 | C 22 | −0.0639 | C 37 | 0.7268 |
| N7 | −0.6325 | H 23 | 0.2621 | C 38 | 0.1914 |
| H8 | 0.4439 | C 24 | −0.4907 | C 39 | −0.2913 |
| C9 | −0.3220 | H 25 | 0.2645 | H 40 | 0.2604 |
| C 10 | −0.2168 | H 26 | 0.2526 | C 41 | −0.0222 |
| H 11 | 0.2671 | C 27 | −0.4692 | C 42 | −0.2735 |
| C 12 | −0.2300 | H 28 | 0.2403 | H 43 | 0.2605 |
| H 13 | 0.2492 | H 29 | 0.2501 | C 44 | −0.1958 |
| C 14 | −0.2167 | C 30 | −0.2589 | H 45 | 0.2517 |
| H 15 | 0.2464 | H 31 | 0.2428 | C 46 | −0.0759 |
| C 16 | −0.2318 |
| Donor NBO | Acceptor NBO | E(2) | Donor NBO | Acceptor NBO | E(2) |
|---|---|---|---|---|---|
| σ→σ* | π→π* | ||||
| BD (1) C9–C10 | BD*(1) C9–C18 | 4.29 | BD (2) C9–C18 | BD*(2) C10–C12 | 20.61 |
| BD (1) C9–C18 | BD*(1) C9–C10 | 4.29 | BD (2) C9–C18 | BD*(2) C14–C16 | 17.09 |
| BD (1) C36–C37 | BD*(1) C44–C46 | 4.29 | BD (2) C10–C12 | BD*(2) C9–C18 | 19.55 |
| BD (1) C38–C39 | BD*(1)Cl1–C41 | 4.31 | BD (2) C10–C12 | BD*(2) C14–C16 | 21.02 |
| BD (1) C38–C39 | BD*(1) C38–C46 | 4.68 | BD (2) C14–C16 | BD*(2) C9–C18 | 23.62 |
| BD (1) C38–C46 | BD*(1) C38–C39 | 4.15 | BD (2) C14–C16 | BD*(2) C10–C12 | 18.94 |
| BD (1) C39–C41 | BD*(1) N7–C38 | 5.62 | BD (2) C38–C39 | BD*(2) C41–C42 | 22.22 |
| BD (1) C42–C44 | BD*(1) C36–C46 | 5.11 | BD (2) C38–C39 | BD*(2) C44–C46 | 16.53 |
| BD (1) C42–C44 | BD*(1)Cl1–C41 | 4.71 | BD (2) C41–C42 | BD*(2) C38–C39 | 15.95 |
| BD (1) C44–C46 | BD*(1) C38–C46 | 4.13 | BD (2) C41–C42 | BD*(2) C44–C46 | 19.37 |
| BD (2) C44–C46 | BD*(1) N6–C36 | 4.38 | BD (2) C44–C46 | BD*(2) C38–C39 | 22.64 |
| BD (2) C44–C46 | BD*(2) C41–C42 | 18.29 | |||
| n→σ* | n→π* | ||||
| LP (2) O3 | BD*(1) S2–C9 | 13.08 | LP (1) N7 | BD*(2) O5–C37 | 55.29 |
| LP (2) O3 | BD*(1) S2–C20 | 15.29 | LP (1) N7 | BD*(2) C38–C39 | 39.34 |
| LP (3) O3 | BD*(1) S2–O4 | 22.48 | LP (3)Cl1 | BD*(2) C41–C42 | 12.43 |
| LP (3) O3 | BD*(1) S2–C9 | 6.45 | |||
| LP (3) O3 | BD*(1) S2–C20 | 4.24 | |||
| LP (2) O4 | BD*(1) S2–C9 | 13.03 | |||
| LP (2) O4 | BD*(1) S2–C20 | 15.61 | |||
| LP (3) O4 | BD*(1) S2–O3 | 23.29 | |||
| LP (3) O4 | BD*(1) S2–C9 | 6.98 | |||
| LP (2) O5 | BD*(1) N7–C37 | 27.9 | |||
| LP (2) O5 | BD*(1) C36–C37 | 22.37 | |||
| LP (1) N6 | BD*(1) C33–C36 | 4.96 | |||
| LP (1) N6 | BD*(1) C36–C37 | 3.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Majid, A.M.; Soliman, S.M.; Haukka, M.; Ali, M.; Islam, M.S.; Shaik, M.R.; Barakat, A. Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function. Symmetry 2020, 12, 1337. https://doi.org/10.3390/sym12081337
Al-Majid AM, Soliman SM, Haukka M, Ali M, Islam MS, Shaik MR, Barakat A. Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function. Symmetry. 2020; 12(8):1337. https://doi.org/10.3390/sym12081337
Chicago/Turabian StyleAl-Majid, Abdullah Mohammed, Saied M. Soliman, Matti Haukka, M. Ali, Mohammad Shahidul Islam, Mohammed Rafi Shaik, and Assem Barakat. 2020. "Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function" Symmetry 12, no. 8: 1337. https://doi.org/10.3390/sym12081337
APA StyleAl-Majid, A. M., Soliman, S. M., Haukka, M., Ali, M., Islam, M. S., Shaik, M. R., & Barakat, A. (2020). Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function. Symmetry, 12(8), 1337. https://doi.org/10.3390/sym12081337










