Preparation of a Zirconia-Based Ceramic Membrane and Its Application for Drinking Water Treatment
Abstract
1. Introduction
2. Material and Methods
2.1. Characterization of Raw Kaolin Powders
2.2. The Methods of the Membrane Support and Ceramic Membrane Preparation
- Thermal treatment of the clay material at a temperature of 400–600 °C for 30 min for the removal of water contained in kaolin (dehydration) and the combustion of organic matters.
- Grinding of the clay material to obtain small particles.
- Sieving of the small particles to obtain particles smaller than 125 μm.
- Addition of kaolin (75%) and calcium carbonate (CaCO3) (22%) for the appearance of pores with an acceptable number and size in the final support.
- Addition of an organic additive (Methocel) (3%) to improve the elastic properties of the dough and to facilitate the formation process.
- Mixing of the above-mentioned materials with the presence of the solvent (distilled water), by using the mixer until a paste of good elastic properties was obtained. Then, the mixture was placed in a tightly closed plastic bag for 12 h to properly spread the water in the ceramic paste.
- Extrusion of ceramic paste in tubular form.
- Drying of the tubular support with ambient air by placing it on the machine containing the rotating cylinders to dry it uniformly and maintain its shape for 24 h.
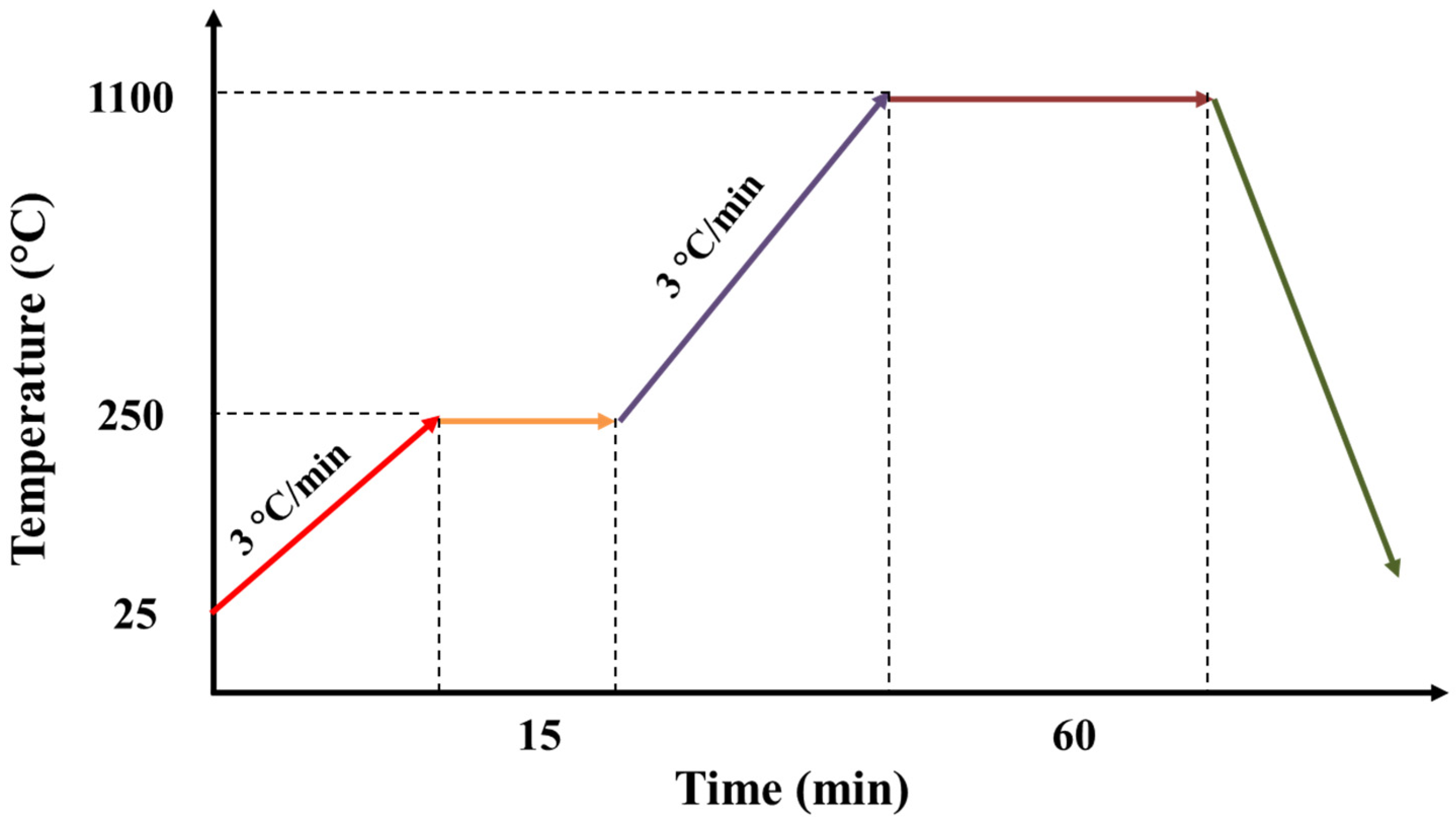
- Sintering the components of the ceramic paste that forms the support at a temperature equal to 1100 °C, which will convert it to anorthite according to a series of reactions during a specific thermal program.
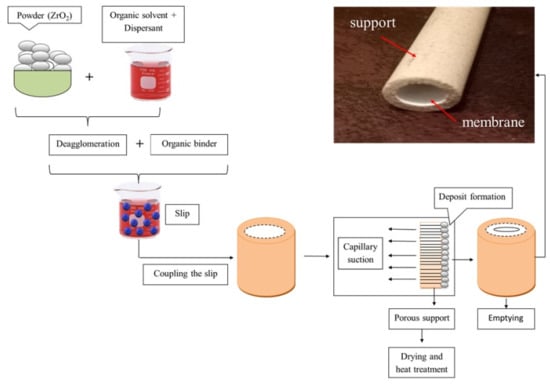
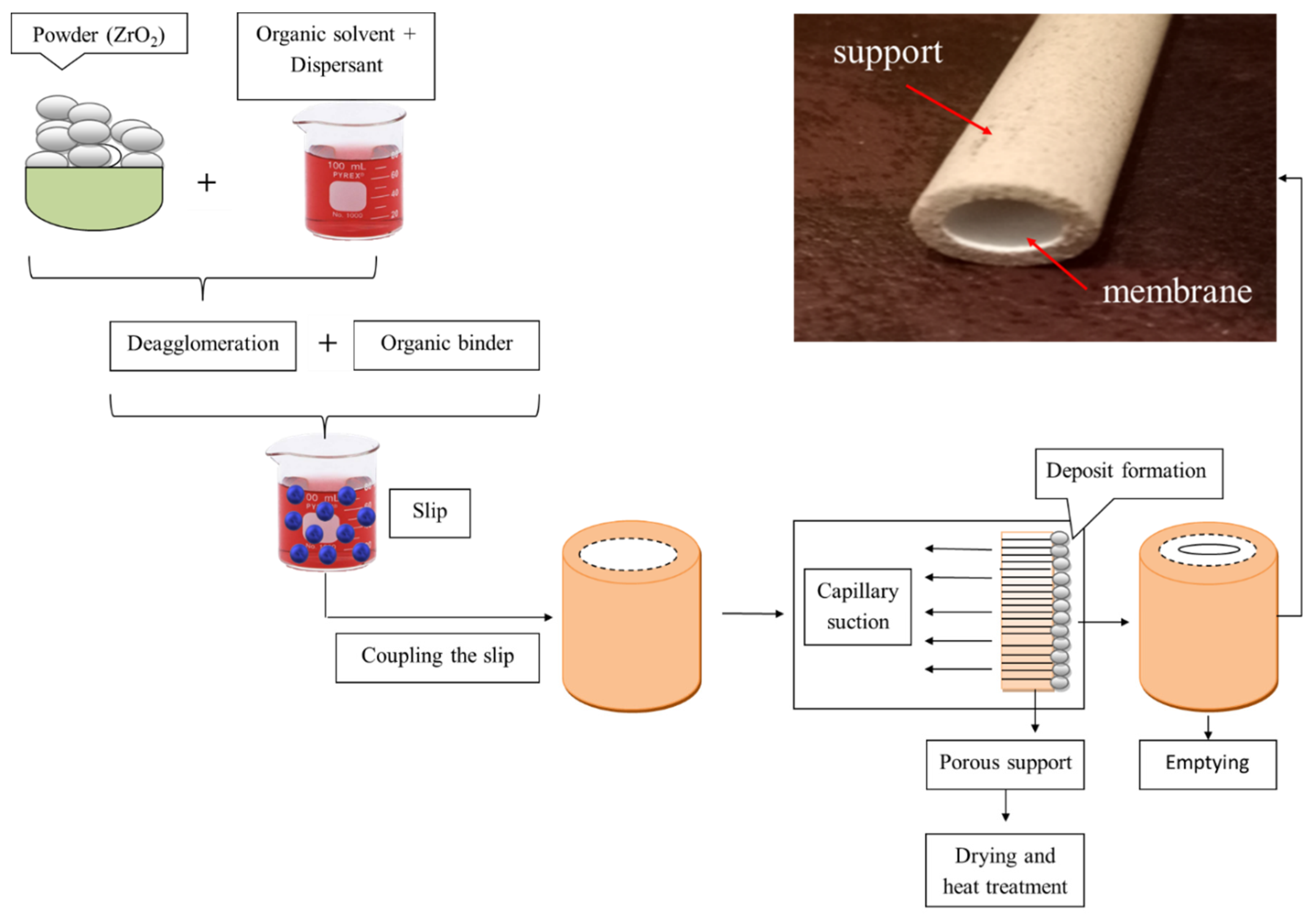
2.3. The Method of the Slip Casting Membrane Preparation
- Take 70% of the distilled water and add in 4% by weight of ZrO2 powder to mix the mixture until a good homogeneous mixture was obtained.
- Place the mixture in an ultrasonic bath for 10 min to dispel the granules and dissolve the sediments.
- Then, add 26% polyvinyl alcohol (PVA) and mix for 12 h to obtain the suspension solution.
- The solution is poured into the support for 10 min and dried for 5 min.
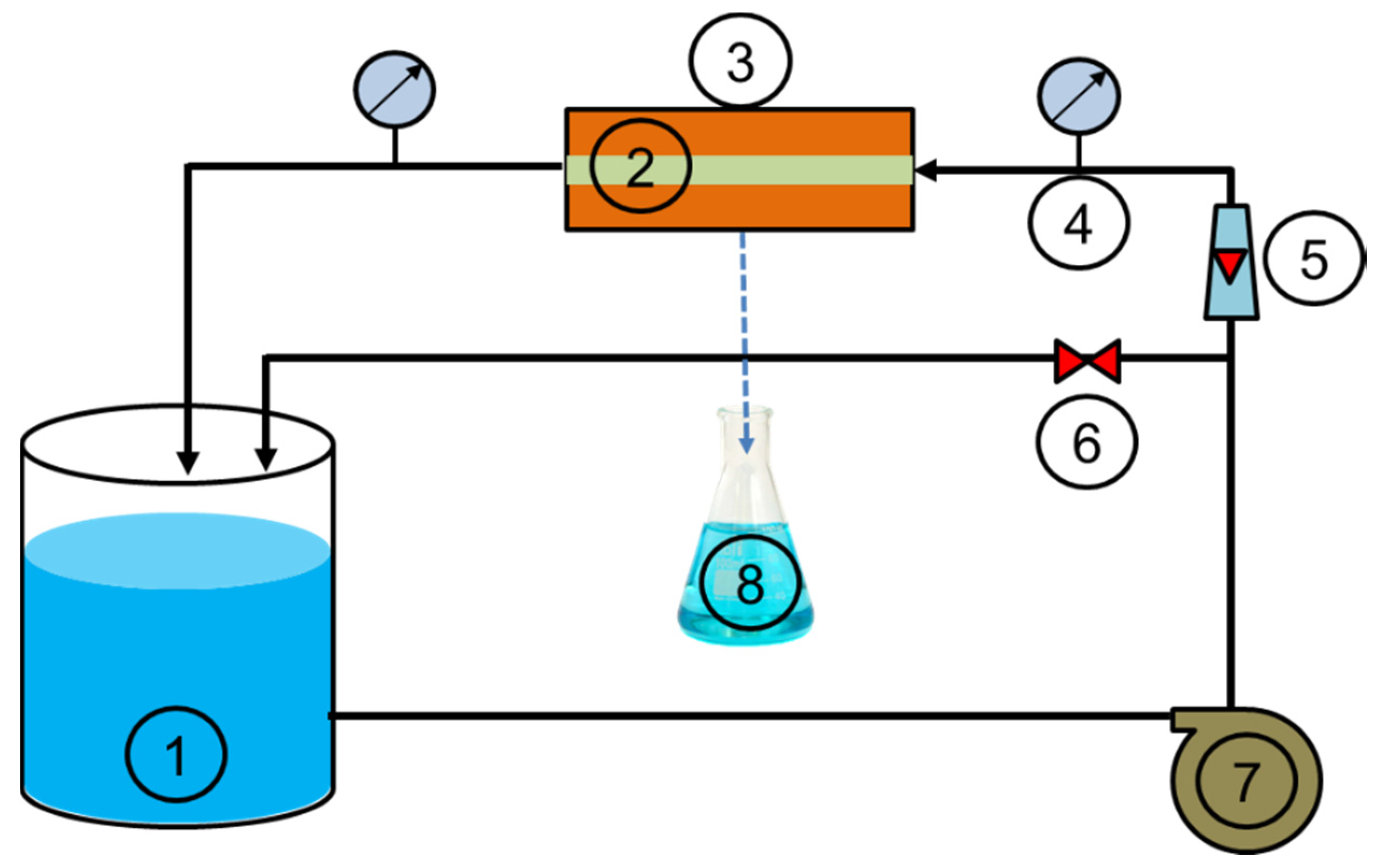
2.4. Experimental Setup of Filtration
2.5. Determination Method of Total Coliform Bacteria
3. Results
3.1. Characterization of the Support and Zirconia-Based Ceramic Membrane
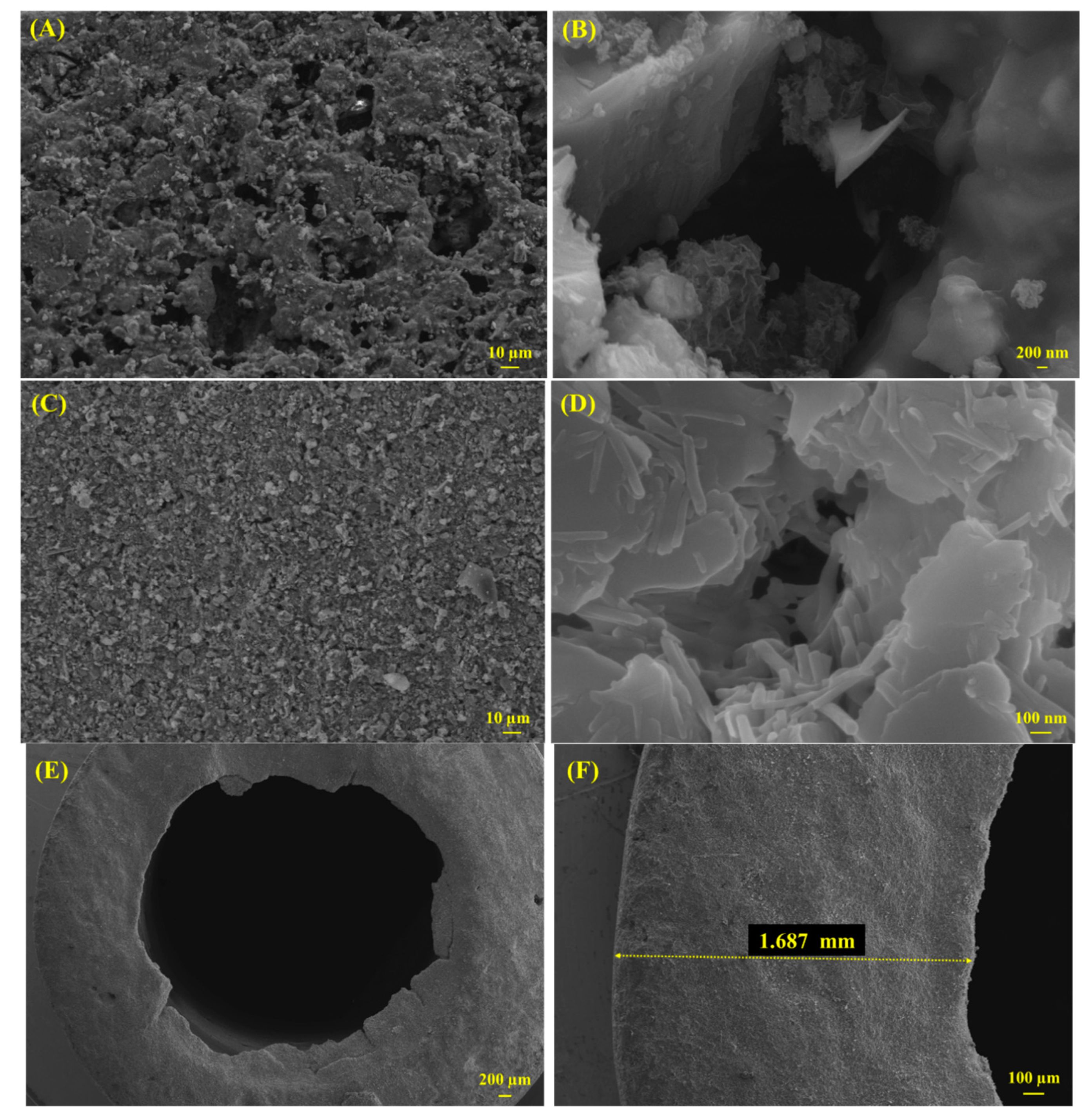
3.1.1. Scanning Electron Microscopy (SEM)
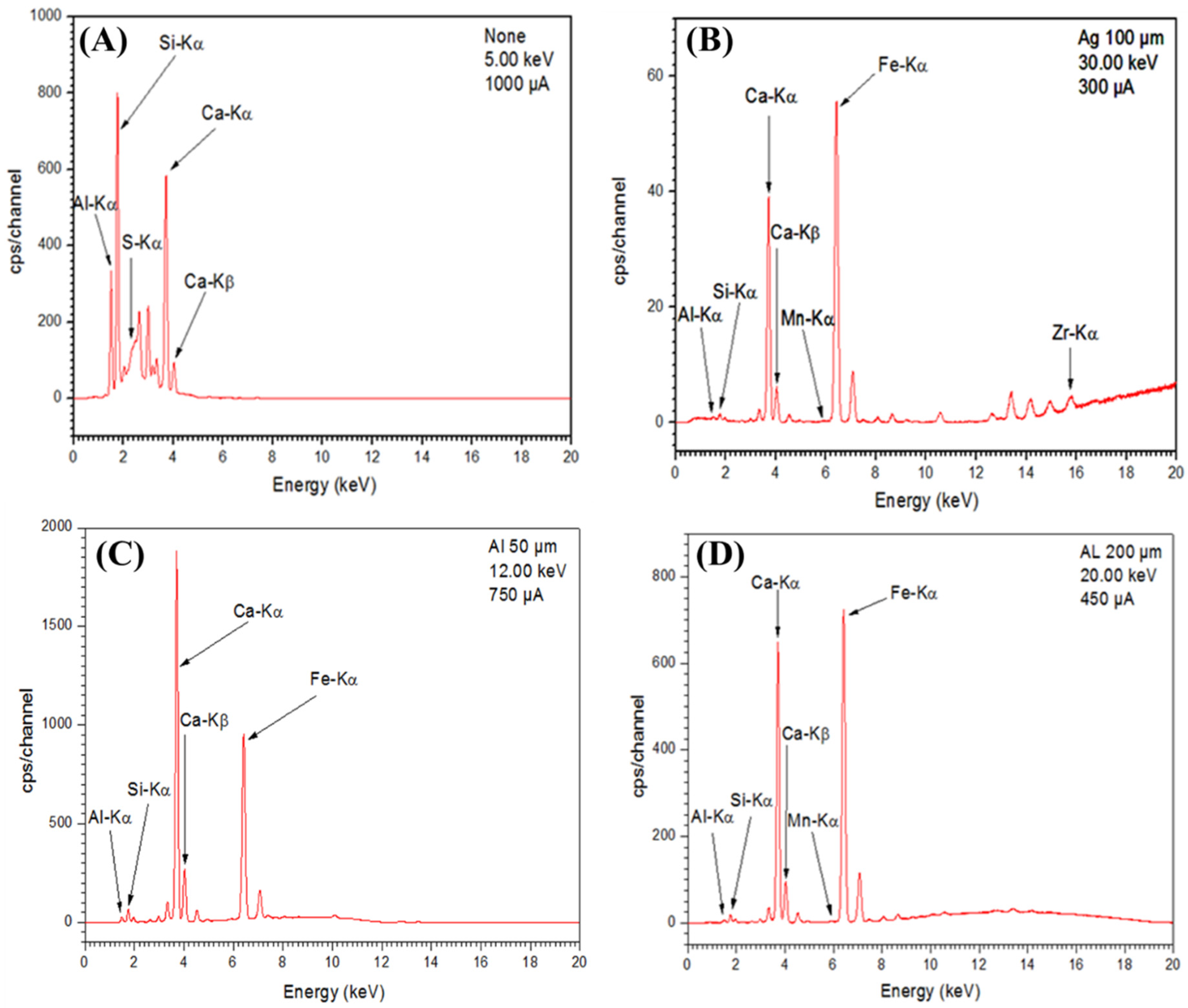
3.1.2. X-ray Fluorescence (XRF)
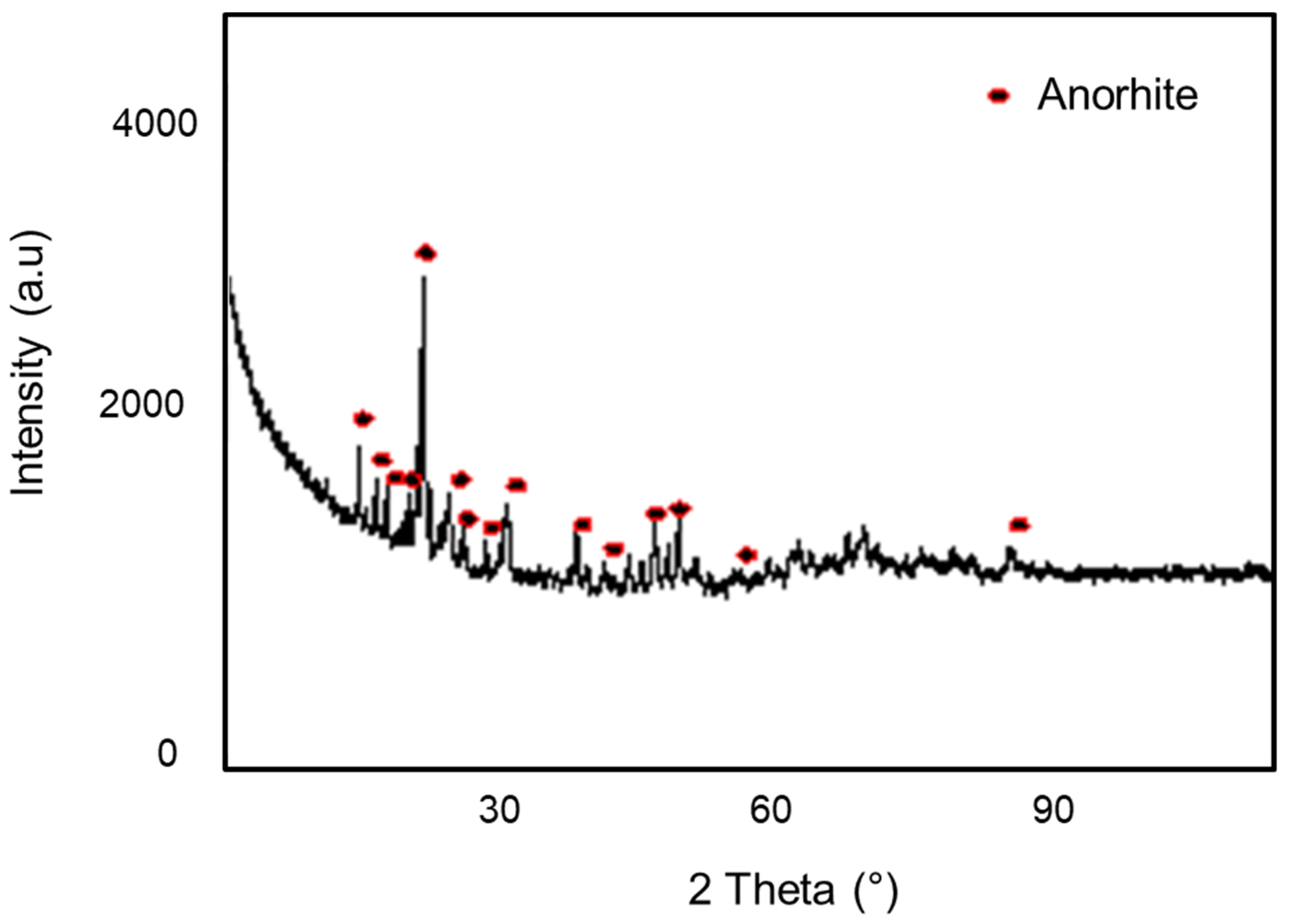
3.1.3. X-ray Diffraction (XRD)
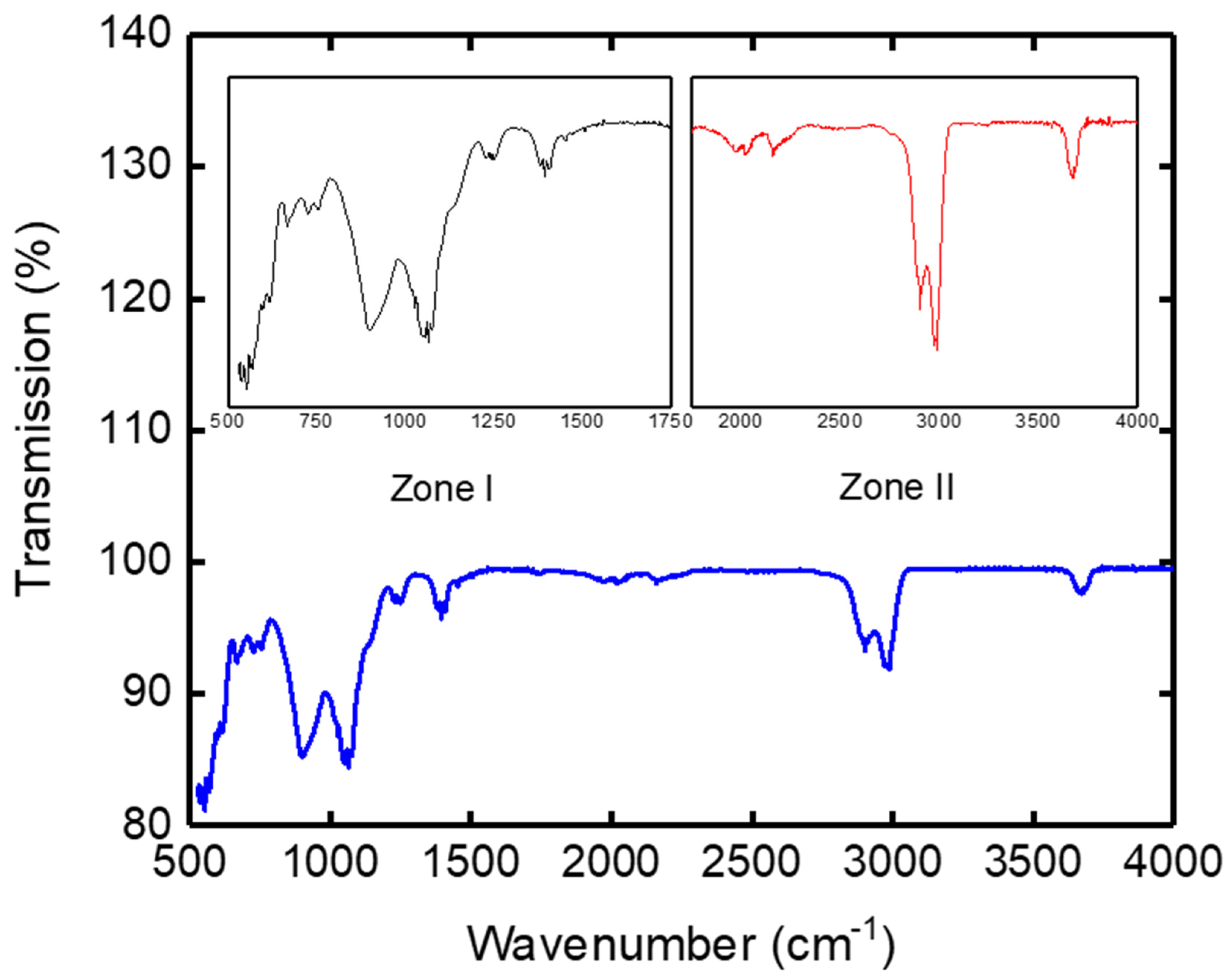
3.1.4. Fourier-Transform Infrared Spectroscopy (FTIR)
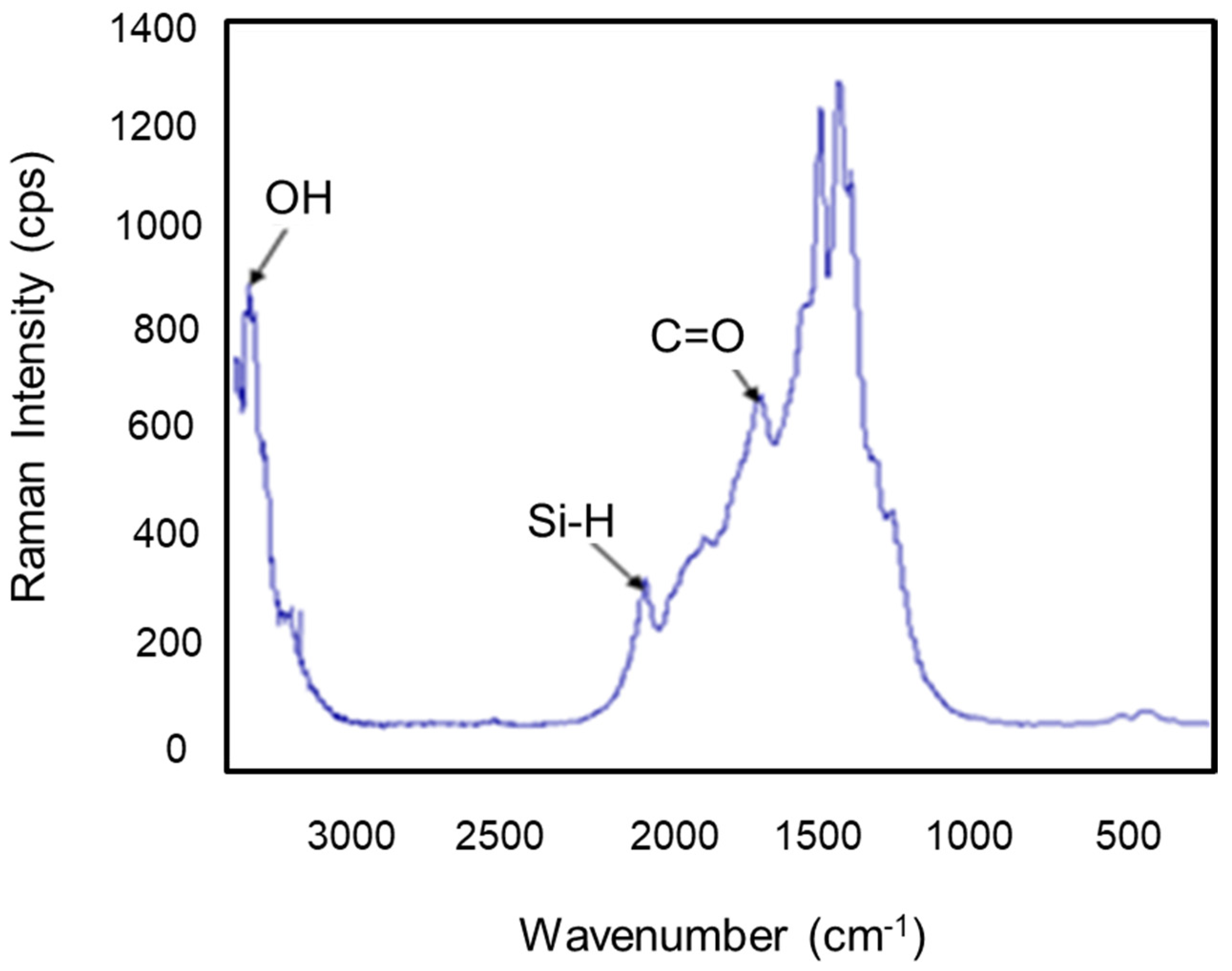
3.1.5. Raman Spectroscopy
3.2. Filtration Experiments
3.2.1. Permeate Flux Variation versus TMP and Time
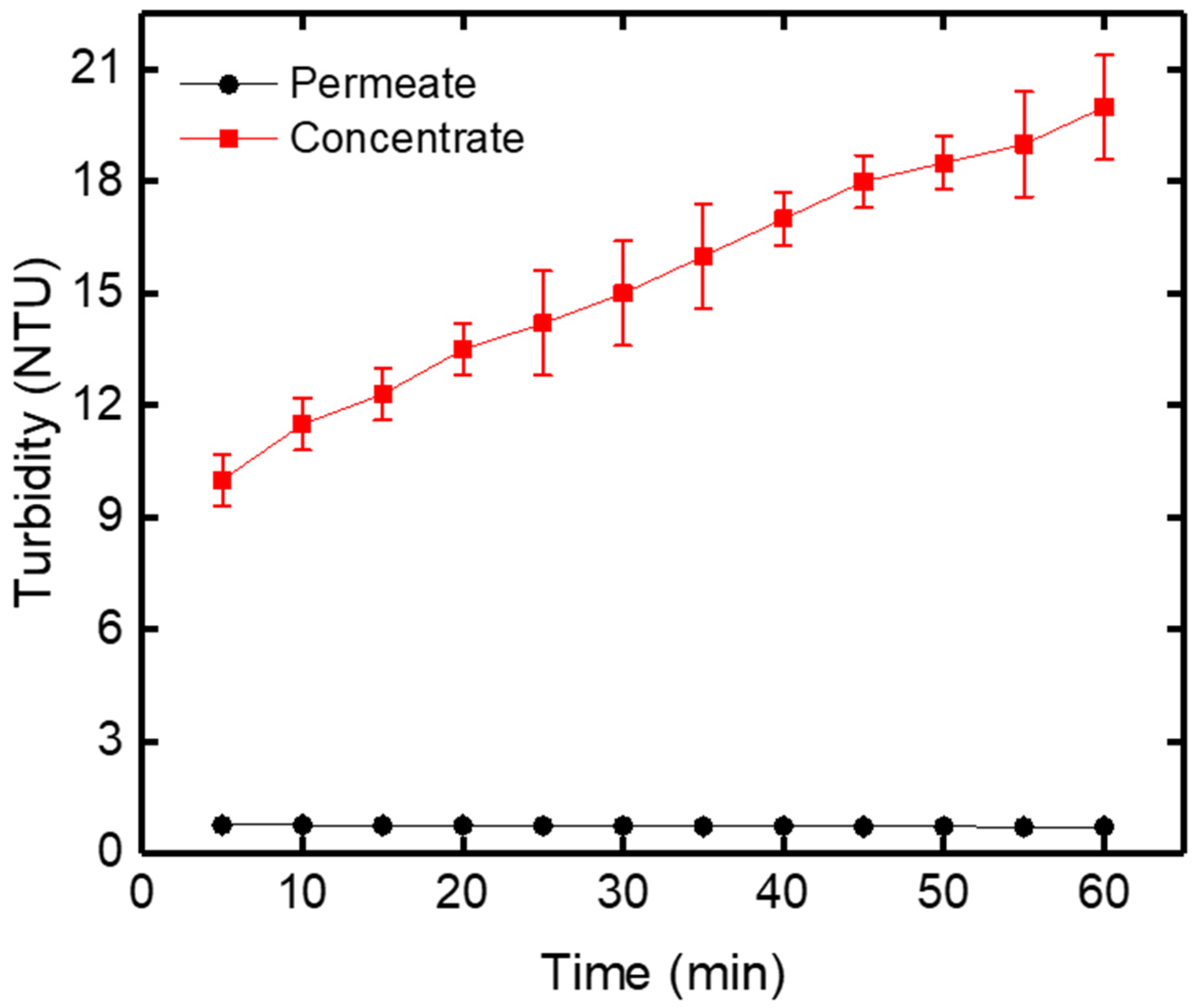
3.2.2. Turbidity Variation Versus Time
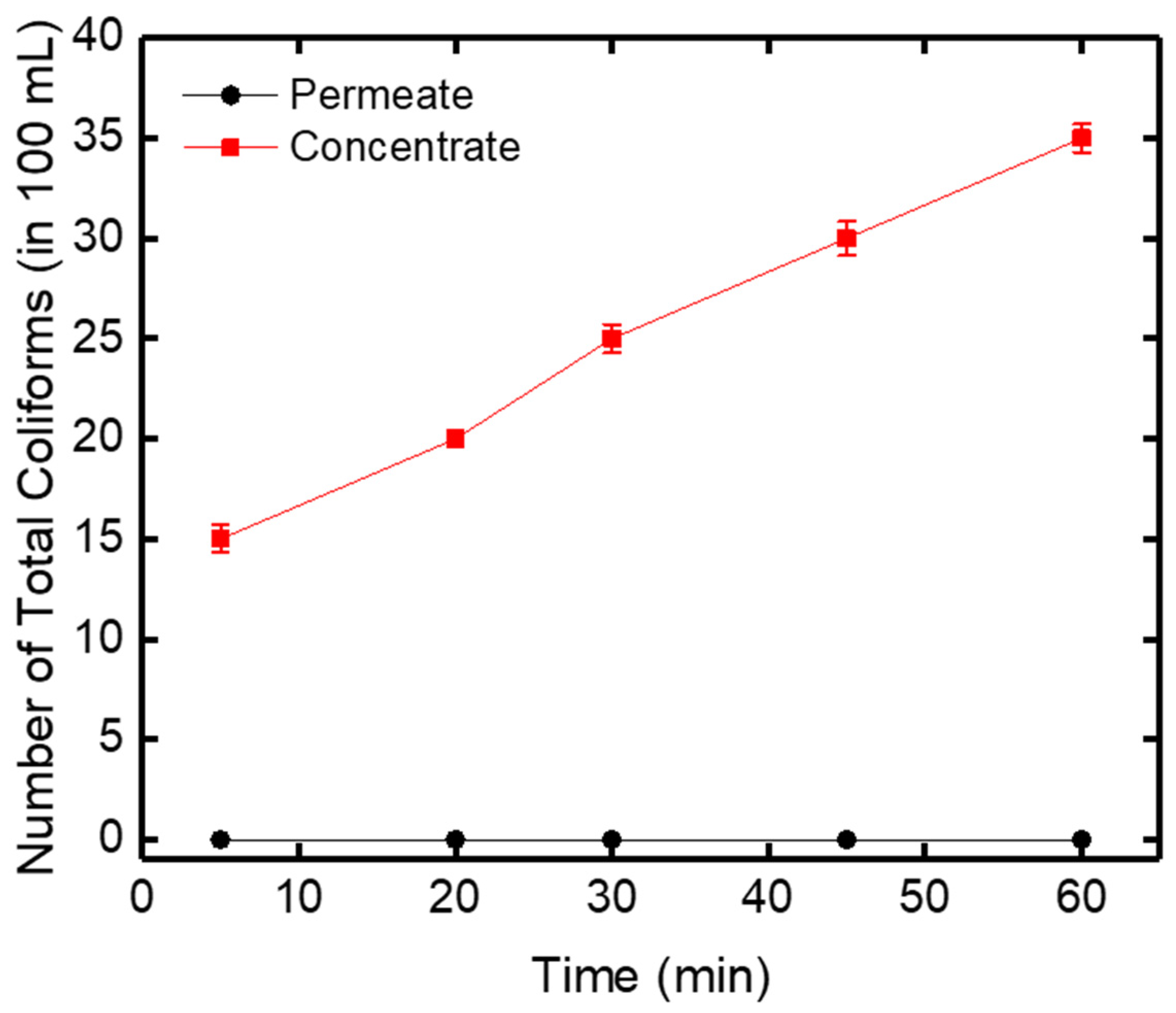
3.2.3. Total Coliform Bacteria Variation versus Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.-H.; Chung, K.-C.; Shin, M.-C.; Dong, J.-I.; Lee, H.-S.; Auh, K.H. Preparation of ceramic membrane and application to the crossflow microfiltration of soluble waste oil. Mater. Lett. 2002, 52, 266–271. [Google Scholar] [CrossRef]
- Oh, H.K.; Takizawa, S.; Ohgaki, S.; Katayama, H.; Oguma, K.; Yu, M.J. Removal of organics and viruses using hybrid ceramic MF system without draining PAC. Desalination 2007, 202, 191–198. [Google Scholar] [CrossRef]
- Emani, S.; Uppaluri, R.; Purkait, M.K. Preparation and characterization of low cost ceramic membranes for mosambi juice clarification. Desalination 2013, 317, 32–40. [Google Scholar] [CrossRef]
- Bouazizi, A.; Saja, S.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Younssi, S.A. Elaboration and characterization of a new flat ceramic MF membrane made from natural Moroccan bentonite. Application to treatment of industrial wastewater. Appl. Clay Sci. 2016, 132–133, 33–40. [Google Scholar] [CrossRef]
- Ghouil, B.; Harabi, A.; Bouzerara, F.; Boudaira, B.; Guechi, A.; Demir, M.M.; Figoli, A. Development and characterization of tubular composite ceramic membranes using natural alumino-silicates for microfiltration applications. Mater. Charact. 2015, 103, 18–27. [Google Scholar] [CrossRef]
- Jana, S.; Purkait, M.K.; Mohanty, K. Preparation and Characterizations of Ceramic Microfiltration Membrane: Effect of Inorganic Precursors on Membrane Morphology. Sep. Sci. Technol. 2010, 46, 33–45. [Google Scholar] [CrossRef]
- Verweij, H. Inorganic membranes. Curr. Opin. Chem. Eng. 2012, 1, 156–162. [Google Scholar] [CrossRef]
- Ismail, A.F.; Chandra Khulbe, K.; Matsuura, T. Gas. Separation Membranes; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-01094-6. [Google Scholar]
- Weir, M. Fabrication, characterization and preliminary testing of all-inorganic ultrafiltration membranes composed entirely of a naturally occurring sepiolite clay mineral. J. Membr. Sci. 2001, 182, 41–50. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Ghouil, B.; Medjemem, N.; Boudaira, B.; Condom, S. Elaboration and Properties of Zirconia Microfiltration Membranes. Procedia Eng. 2012, 33, 278–284. [Google Scholar] [CrossRef]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Ceramic-based microbial fuel cells (MFCs): A review. Int. J. Hydrogen Energy 2017, 42, 1672–1690. [Google Scholar] [CrossRef]
- Xing, W.-H. Ceramic Membranes. In Membrane-Based Separations in Metallurgy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 357–370. ISBN 9780128034279. [Google Scholar]
- Zhang, S.; Wang, R.; Zhang, S.; Li, G.; Zhang, Y. Treatment of wastewater containing oil using phosphorylated silica nanotubes (PSNTs)/polyvinylidene fluoride (PVDF) composite membrane. Desalination 2014, 332, 109–116. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials (Basel) 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Chakrabarty, B.; Barkakati, P. Preparation and characterization of novel ceramic membranes for micro-filtration applications. Ceram. Int. 2016, 42, 14326–14333. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Raja, M.; Angulakshmi, N.; Nahm, K.S.; Stephan, A.M. A flexible zirconium oxide based-ceramic membrane as a separator for lithium-ion batteries. RSC Adv. 2016, 6, 92020–92027. [Google Scholar] [CrossRef]
- Wang, P. A pilot study of the treatment of waste rolling emulsion using zirconia microfiltration membranes. J. Membr. Sci. 2000, 173, 159–166. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, Q.; Wang, Y.; Wang, J.; Meng, G. Separation of stable oil–water emulsion by the hydrophilic nano-sized ZrO2 modified Al2O3 microfiltration membrane. Sep. Purif. Technol. 2010, 75, 243–248. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Kroll, S.; Treccani, L.; Rezwan, K.; Grathwohl, G. Development and characterisation of functionalised ceramic microtubes for bacteria filtration. J. Membr. Sci. 2010, 365, 447–455. [Google Scholar] [CrossRef]
- Krajewski, S.R.; Kujawski, W.; Dijoux, F.; Picard, C.; Larbot, A. Grafting of ZrO2 powder and ZrO2 membrane by fluoroalkylsilanes. Colloids Surf. A Physicochem. Eng. Asp. 2004, 243, 43–47. [Google Scholar] [CrossRef]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Fabrication of zirconia composite membrane by in-situ hydrothermal technique and its application in separation of methyl orange. Ecotoxicol. Environ. Saf. 2015, 121, 73–79. [Google Scholar] [CrossRef]
- Erdem, İ.; Çiftçioğlu, M.; Harsa, Ş. Separation of whey components by using ceramic composite membranes. Desalination 2006, 189, 87–91. [Google Scholar] [CrossRef]
- Dey, S.; Bhattacharya, P.; Bandyopadhyay, S.; Roy, S.N.; Majumdar, S.; Sahoo, G.C. Single step preparation of zirconia ultrafiltration membrane over clay-alumina based multichannel ceramic support for wastewater treatment. J. Membr. Sci. Res. 2018, 4, 28–33. [Google Scholar]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and applications for low-cost ceramic membranes. Membranes (Basel) 2019, 9, 105. [Google Scholar] [CrossRef]
- Zenikheri, F.; Harabi, A.; Boudaira, B.; Bouzerara, F.; Guechi, A.; Barama, S.-E.; Foughali, L.; Karboua, N. Elaboration of porous gehlenite and anorthite based ceramics using low price raw materials. Cerâmica 2016, 62, 242–248. [Google Scholar] [CrossRef]
- Kolli, M.; Hamidouche, M.; Fantozzi, G.; Chevalier, J. Elaboration and characterization of a refractory based on Algerian kaolin. Ceram. Int. 2007, 33, 1435–1443. [Google Scholar] [CrossRef]
- Ouali, A.; Sahnoune, F.; Belhouchet, H.; Heraiz, M. Effect of CaO addition on the sintering behaviour of anorthite formed from kaolin and CaO. Acta Phys. Pol. A 2017, 131, 159–161. [Google Scholar] [CrossRef]
- Mukasa-Tebandeke, I.Z.; Ssebuwufu, P.J.M.; Nyanzi, S.A.; Schumann, A.; Nyakairu, G.W.A.; Ntale, M.; Lugolobi, F. The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Adv. Mater. Phys. Chem. 2015, 5, 67–86. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Characterization of Colombian Clay and Its Potential Use as Adsorbent. Sci. World J. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D. Production of γ-Al2O3 from Kaolin. Open J. Phys. Chem. 2011, 1, 23–27. [Google Scholar] [CrossRef]
- Johnston, C.T.; Elzea Kogel, J.; Bish, D.L.; Kogure, T.; Murray, H.H. Low-temperature Ftir Study of Kaolin-Group Minerals. Clays Clay Miner. 2008, 56, 470–485. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G.; Borah, R.R.; Borthakur, R. Raman and FTIR Spectroscopic Evaluation of Clay Minerals and Estimation of Metal Contaminations in Natural Deposition of Surface Sediments from Brahmaputra River. Int. J. Geosci. 2016, 7, 873–883. [Google Scholar] [CrossRef]
- Chikhi, M.; Meniai, A.-H.; Balaska, F.; Bencheikh-Lehocine, M. Modeling of the Ultrafiltration of a Dextran T500 Solution in a Tubular Membrane Module. Chem. Eng. Technol. 2008, 31, 501–506. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, C.; Del Borghi, A.; Colombino, M.; Conio, O. Water treatment for drinking purpose: Ceramic microfiltration application. Desalination 2001, 141, 75–79. [Google Scholar] [CrossRef]

| Oxide | Weight (%) |
|---|---|
| SiO2 | 55.080 |
| Al2O3 | 29.041 |
| Fe2O3 | 2.813 |
| K2O | 1.422 |
| Na2O | 0.320 |
| MgO | 0.172 |
| CaO | 0.010 |
| P2O5 | 0.082 |
| TiO2 | 0.066 |
| MnO | 0.014 |
| Loss on ignition | 10.980 |
| Properties | Support Material |
|---|---|
| Outside diameter | 9 mm |
| Inside diameter | 4.6 mm |
| Thickness | 2.2 mm |
| Length | 190 mm |
| Operating pH range | 1–14 |
| Washing pH range | 1–14 |
| Properties | Ceramic Membrane |
|---|---|
| Total area | 4.48 × 10−3 m2 |
| Average pore diameter | 0.2 µm |
| Operating pH range | 1–14 |
| Washing pH range | 1–14 |
| Wave Number υ in (cm−1) of the Clay Support | Wave Number υ in (cm−1) Observed in Literature | Kaolin Band Assignment |
|---|---|---|
| 3670 | 3695 | ν O-H interlayer |
| 3670 | ν O-H grain surface | |
| 1070 | 1096 | νSi-O |
| 1010–1033 | νSi-O-Si | |
| 897 | 875 | Al-OH-Fe3+ |
| 937 | δAl-OH-Al intern with Feuillet | |
| 912–915 | δAl-OH-Al external with layer | |
| 780 | 800–778 | Si-O of Quartz |
| 760 | 757–700 | Al-OH |
| 540 | 540 | Al-O |
| Physico-Chemical Parameters | Units | Raw Water | Permeate | Concentrate |
|---|---|---|---|---|
| pH | - | 8.35 | 8.17 | 8.39 |
| Conductivity | µS/cm | 1120 | 1100 | 1133 |
| Dissolved Salt Rate (DSR) | mg/L | 617 | 610 | 631 |
| Turbidity | NTU | 8.10 | 0.69 | 21.10 |
| Total hardness | mg/L | 400 | 380 | 410 |
| Phosphate (PO43−) | mg/L | 0.07 | 0.00 | 0.16 |
| Ammonium (NH4+) | mg/L | 0.03 | 0.02 | 0.06 |
| Nitrite (NO2−) | mg/L | 0.0 | 0.0 | 0.0 |
| Nitrate (NO3−) | mg/L | 7.00 | 6.18 | 7.40 |
| Ferrous iron (Fe2+) | mg/L | 0.17 | 0.03 | 0.33 |
| Manganese (Mn2+) | mg/L | 0.1 | 0.0 | 0.7 |
| Aluminum (Al3+) | mg/L | 0.0 | 0.0 | 0.0 |
| Zinc (Zn2+) | mg/L | 0.43 | 0.30 | 0.60 |
| Chloride (Cl−) | mg/L | 177.27 | 173.72 | 180.81 |
| Calcium (Ca+2) | mg/L | 84.17 | 80.16 | 92.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussemghoune, M.; Chikhi, M.; Balaska, F.; Ozay, Y.; Dizge, N.; Kebabi, B. Preparation of a Zirconia-Based Ceramic Membrane and Its Application for Drinking Water Treatment. Symmetry 2020, 12, 933. https://doi.org/10.3390/sym12060933
Boussemghoune M, Chikhi M, Balaska F, Ozay Y, Dizge N, Kebabi B. Preparation of a Zirconia-Based Ceramic Membrane and Its Application for Drinking Water Treatment. Symmetry. 2020; 12(6):933. https://doi.org/10.3390/sym12060933
Chicago/Turabian StyleBoussemghoune, Mohamed, Mustapha Chikhi, Fouzia Balaska, Yasin Ozay, Nadir Dizge, and Brahim Kebabi. 2020. "Preparation of a Zirconia-Based Ceramic Membrane and Its Application for Drinking Water Treatment" Symmetry 12, no. 6: 933. https://doi.org/10.3390/sym12060933
APA StyleBoussemghoune, M., Chikhi, M., Balaska, F., Ozay, Y., Dizge, N., & Kebabi, B. (2020). Preparation of a Zirconia-Based Ceramic Membrane and Its Application for Drinking Water Treatment. Symmetry, 12(6), 933. https://doi.org/10.3390/sym12060933