The Immobilization of Oxindole Derivatives with Use of Cube Rhombellane Homeomorphs
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
The Binding Affinity and Conformational Diversity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damascelli, B.; Patelli, G.L.; Lanocita, R.; Di Tolla, G.; Frigerio, L.F.; Marchianò, A.; Garbagnati, F.; Spreafico, C.; Tichà, V.; Gladin, C.R.; et al. A Novel Intraarterial Chemotherapy Using Paclitaxel in Albumin Nanoparticles to Treat Advanced Squamous Cell Carcinoma of the Tongue: Preliminary Findings. Am. J. Roentgenol. 2003, 181, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.M.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Cascone, M.G.; Lazzeri, L.; Carmignani, C.; Zhu, Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J. Mater. Sci. Mater. Med. 2002, 13, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, A.K. Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. J. Control. Release 2004, 99, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.M.; Baik, D.J.; Kang, J.S. Toxic characteristics of methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) nanospheres; in vitro and in vivo studies in the normal mice. Biomaterials 2003, 24, 55–63. [Google Scholar] [CrossRef]
- Alyautdin, R.N.; Petrov, V.E.; Langer, K.; Berthold, A.; Kharkevich, D.A.; Kreuter, J. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated poly(butylcyanoacrylate) nanoparticles. Pharm. Res. 1997, 14, 325–328. [Google Scholar] [CrossRef]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; von Briesen, H.; Begley, D.J. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res. 2003, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, A.; Wirth, M.; Gabor, F. WGA-grafted PLGA-nanospheres: Preparation and association with Caco-2 single cells. J. Control. Release 2004, 99, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Da Ros, T. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Springer: Berlin, Germany, 2008; ISBN 9781402068454. [Google Scholar]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Andrievsky, G.; Klochkov, V.; Derevyanchenko, L. Is the C60 Fullerene Molecule Toxic?! Fuller. Nanotub. Carbon Nanostructures 2005, 13, 363–376. [Google Scholar] [CrossRef]
- Szefler, B. Nanotechnology, from quantum mechanical calculations up to drug delivery. Int. J. Nanomed. 2018, 13, 6143–6176. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, R.R.; Prylutska, S.V.; Chumakl, V.V.; Skorokhyd, N.R.; Lehka, L.V.; Evstigneev, M.P.; Prylutskyy, Y.I.; Berger, W.; Heffeter, P.; Scharff, P.; et al. Application of C60 Fullerene-Doxorubicin Complex for Tumor Cell Treatment In Vitro and In Vivo. J. Biomed. Nanotechnol. 2015, 11, 1139–1152. [Google Scholar] [CrossRef]
- Morgen, M.; Bloom, C.; Beyerinck, R.; Bello, A.; Song, W.; Wilkinson, K.; Steenwyk, R.; Shamblin, S. Polymeric Nanoparticles for Increased Oral Bioavailability and Rapid Absorption Using Celecoxib as a Model of a Low-Solubility, High-Permeability Drug. Pharm. Res. 2012, 29, 427–440. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, L.; Sun, Y. Nanotechnology applied to overcome tumor drug resistance. J. Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef]
- Turov, V.V.; Chehun, V.F.; Barvinchenko, V.N.; Krupskaya, T.V.; Prylutskyy, Y.I.; Scharff, P.; Ritter, U. Low-temperature 1H-NMR spectroscopic study of doxorubicin influence on the hydrated properties of nanosilica modified by DNA. J. Mater. Sci. Mater. Med. 2011, 22, 525–532. [Google Scholar] [CrossRef]
- Schuetze, C.; Ritter, U.; Scharff, P.; Fernekorn, U.; Prylutska, S.; Bychko, A.; Rybalchenko, V.; Prylutskyy, Y. Interaction of N-fluorescein-5-isothiocyanate pyrrolidine-C60 with a bimolecular lipid model membrane. Mater. Sci. Eng. C 2011, 31, 1148–1150. [Google Scholar] [CrossRef]
- Qiao, R.; Roberts, A.P.; Mount, A.S.; Klaine, S.J.; Ke, P.C. Translocation of C60 and Its Derivatives Across a Lipid Bilayer. Nano Lett. 2007, 7, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Bilyy, R.; Overchuk, M.; Bychko, A.; Andreichenko, K.; Stoika, R.; Rybalchenko, V.; Prylutskyy, Y.; Tsierkezos, N.G.; Ritter, U. Water-soluble pristine fullerenes C60 increase the specific conductivity and capacity of lipid model membrane and form the channels in cellular plasma membrane. J. Biomed. Nanotechnol. 2012, 8, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.V.; Grynyuk, I.I.; Grebinyk, S.M.; Matyshevska, O.P.; Prylutskyy, Y.I.; Ritter, U.; Siegmund, C.; Scharff, P. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Materwiss. Werksttech. 2009, 40, 238–241. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The Biological Mechanisms and Physicochemical Characteristics Responsible for Driving Fullerene Toxicity. Toxicol. Sci. 2010, 114, 162–182. [Google Scholar] [CrossRef]
- Evstigneev, M.P.; Buchelnikov, A.S.; Voronin, D.P.; Rubin, Y.V.; Belous, L.F.; Prylutskyy, Y.I.; Ritter, U. Complexation of C60 Fullerene with Aromatic Drugs. Chem Phys Chem 2013, 14, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Czeleń, P. Molecular dynamics study on inhibition mechanism of CDK-2 and GSK-3β by CHEMBL272026 molecule. Struct. Chem. 2016, 27, 1807–1818. [Google Scholar] [CrossRef]
- Czeleń, P. Inhibition mechanism of CDK-2 and GSK-3β by a sulfamoylphenyl derivative of indoline—A molecular dynamics study. J. Mol. Model. 2017, 23, 230. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK Inhibitors: Cell Cycle Regulators and Beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canavese, M.; Santo, L.; Raje, N. Cyclin dependent kinases in cancer. Cancer Biol. Ther. 2012, 13, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Child, E.S.; Hendrychov, T.; McCague, K.; Futreal, A.; Otyepka, M.; Mann, D.J. A cancer-derived mutation in the PSTAIRE helix of cyclin-dependent kinase 2 alters the stability of cyclin binding. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. To cycle or not to cycle: A critical decision in cancer. Nat. Rev. Cancer 2001, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Diudea, M.V.; Lungu, C.N.; Nagy, C.L.; Diudea, M.V.; Lungu, C.N.; Nagy, C.L. Cube-Rhombellane Related Structures: A Drug Perspective. Molecules 2018, 23, 2533. [Google Scholar] [CrossRef] [PubMed]
- Szefler, B.; Czeleń, P.; Diudea, M. V Docking of indolizine derivatives on cube rhombellane functionalized homeomorphs. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 7–18. [Google Scholar] [CrossRef]
- ChEMBL. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 1 March 2016).
- Kim, K.H.; Ko, D.K.; Kim, Y.T.; Kim, N.H.; Paul, J.; Zhang, S.Q.; Murray, C.B.; Acharya, R.; Kim, Y.H.; DeGrado, W.F.; et al. Protein-directed self-assembly of a fullerene crystal. Nat. Commun. 2016, 7, 11429. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Potemkin, V.A.; Grishina, M.A.; Belik, A.V. A Method for Multiconformational Modeling of the Three-Dimensional Shape of a Molecule. J. Struct. Chem. 2002, 43, 1033–1039. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
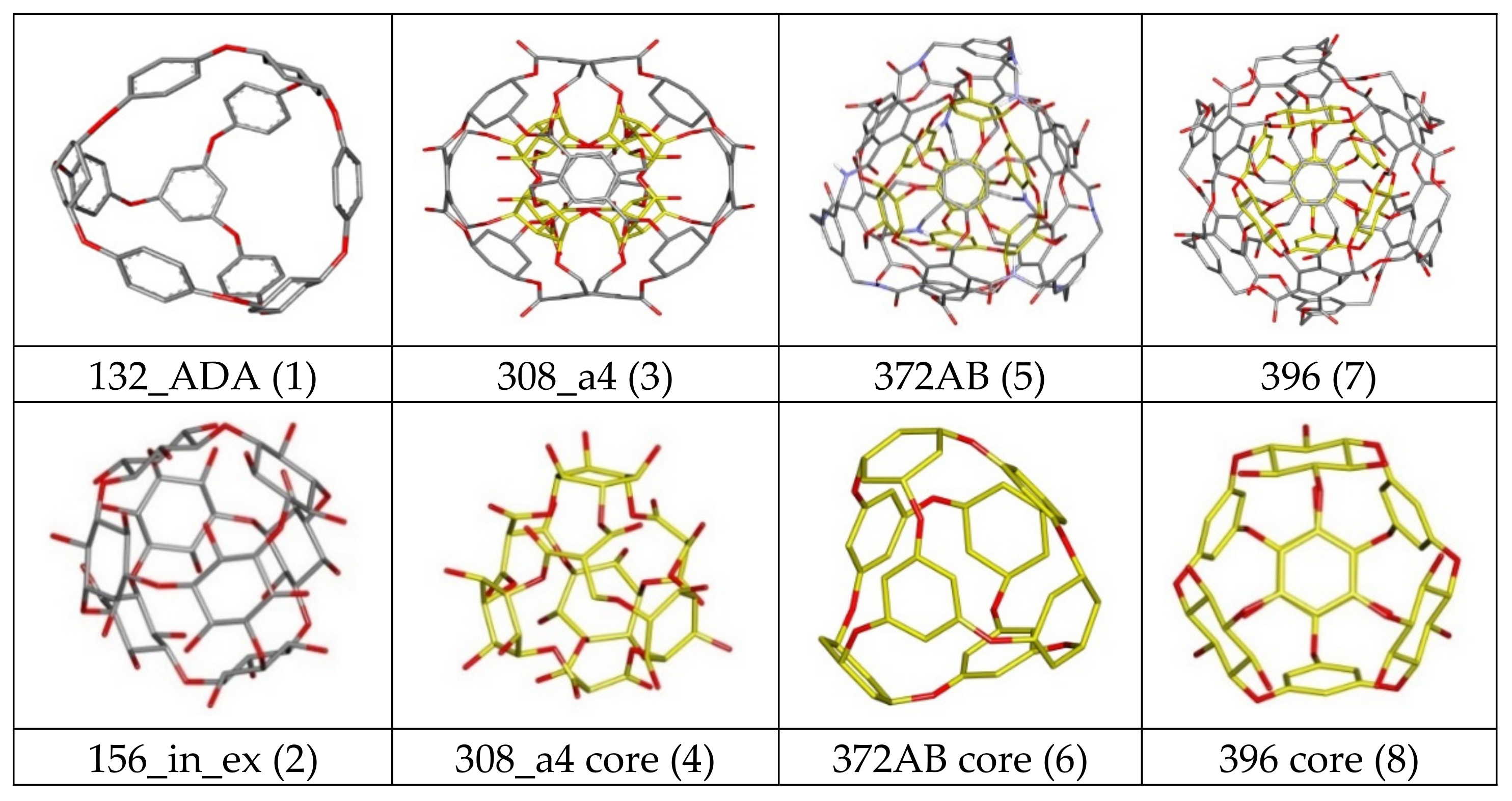
| Nanostructure Name | Structure Type | Chem.Type Core/ex. Shell | Atoms Quantity | ||||
|---|---|---|---|---|---|---|---|
| All | C | H | O | N | |||
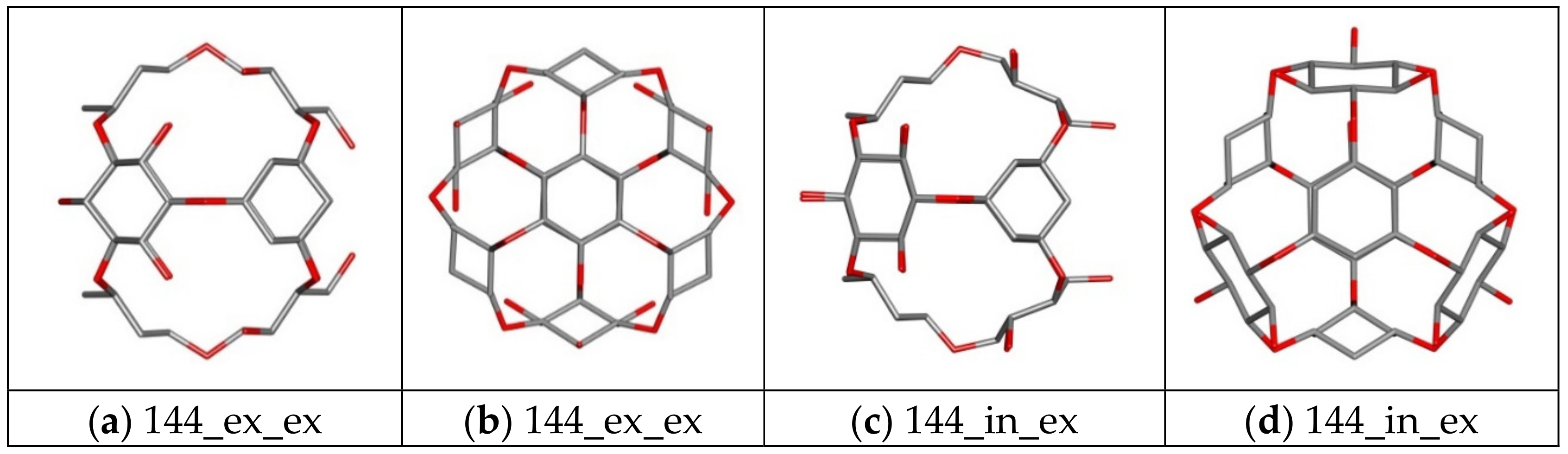
| 144 ex_ex/in_ex | Core | Ether | 144 | 48 | 84 | 24 | 0 |
| 156 ex_ex/in_ex | Core | Ether | 156 | 48 | 84 | 36 | 0 |
| 308 a4/b4 | C-rbl | Ether/Ester | 308 | 100 | 124 | 84 | 0 |
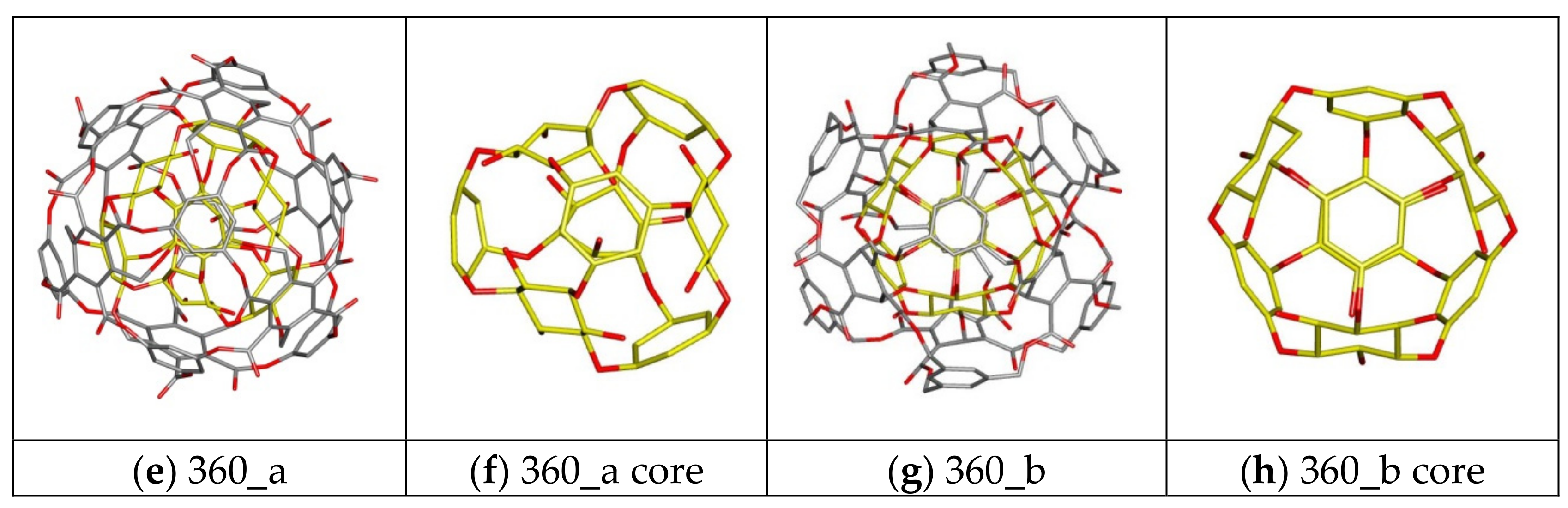
| 360 a/b | C-rbl | Ether/Ester | 360 | 168 | 108 | 84 | 0 |
| 372AB | C-rbl | Ether/Amide; Ester | 372 | 180 | 120 | 60 | 12 |
| 396 | C-rbl | Ether/Ester | 396 | 192 | 132 | 72 | 0 |
| 420 | C-rbl | Ether/Amide | 420 | 192 | 156 | 48 | 24 |
| 444 | C-rbl | Ether/Amide | 444 | 192 | 180 | 48 | 24 |
| 456 | C-rbl | Ether/Amide | 456 | 192 | 180 | 60 | 24 |
| ADA_132 | ADA/rbl | Ether | 132 | 60 | 60 | 12 | 0 |
| Nanostructure Name | ΔG [kcal/mol] | Binding Constant [Kmax] | Difference of Kmax Relative to C60 [%] | |||
|---|---|---|---|---|---|---|
| MAX | MIN | AVERAGE | SD | |||
| 144_ex_ex | −3.50 | −3.20 | −3.30 | 0.12 | 367.73 | −97.11 |
| 144_in_ex | −3.90 | −3.70 | −3.81 | 0.09 | 722.33 | −94.33 |
| 156_ex_ex | −4.10 | −3.70 | −3.88 | 0.11 | 1012.37 | −92.05 |
| 156_in_ex | −4.20 | −3.80 | −3.92 | 0.14 | 1198.51 | −90.59 |
| 308a4 | −6.00 | −5.60 | −5.76 | 0.13 | 25,006.81 | 96.43 |
| 308b4 | −5.90 | −5.50 | −5.74 | 0.14 | 21,123.08 | 65.92 |
| 360a | −6.20 | −5.90 | −6.02 | 0.11 | 35,047.76 | 175.30 |
| 360b | −5.50 | −5.10 | −5.26 | 0.11 | 10,753.59 | −15.53 |
| 372AB | −6.10 | −5.50 | −5.67 | 0.21 | 29,604.61 | 132.54 |
| 396 | −5.60 | −5.20 | −5.37 | 0.13 | 12,730.76 | 0.00 |
| 420 | −5.70 | −5.20 | −5.42 | 0.15 | 15,071.46 | 18.39 |
| 444 | −5.30 | −5.00 | −5.12 | 0.10 | 7672.76 | −39.73 |
| 456 | −5.50 | −5.00 | −5.26 | 0.17 | 10,753.59 | −15.53 |
| ADA_132 | −5.80 | −5.50 | −5.58 | 0.10 | 17,842.53 | 40.15 |
| C60 | −5.60 | −5.40 | −5.50 | 0.07 | 12,730.76 | — |
| Nanostructure Name | ΔG [kcal/mol] | Binding Constant [Kmax] | Difference of Kmax Relative to C60 [%] | |||
|---|---|---|---|---|---|---|
| MAX | MIN | AVERAGE | SD | |||
| 144_ex_ex | −3.60 | −3.50 | −3.57 | 0.05 | 435.35 | −94.33 |
| 144_in_ex | −4.00 | −3.70 | −3.80 | 0.10 | 855.14 | −88.85 |
| 156_ex_ex | −3.90 | −3.70 | −3.79 | 0.06 | 722.33 | −90.59 |
| 156_in_ex | −4.20 | −3.90 | −4.03 | 0.09 | 1198.51 | −84.38 |
| 308a4 | −5.80 | −5.50 | −5.59 | 0.11 | 17,842.53 | 132.54 |
| 308b4 | −5.80 | −5.60 | −5.72 | 0.08 | 17,842.53 | 132.54 |
| 360a | −6.30 | −5.80 | −6.08 | 0.15 | 41,491.70 | 440.77 |
| 360b | −5.10 | −4.80 | −4.92 | 0.13 | 5474.56 | −28.65 |
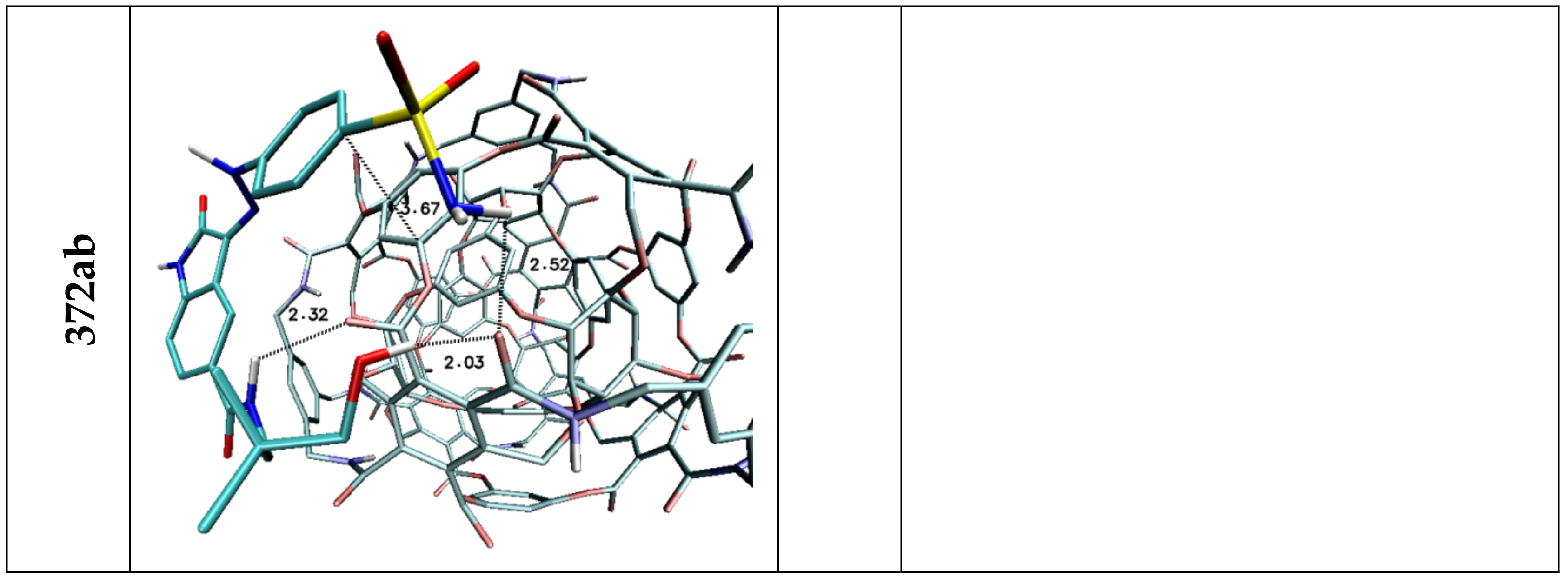
| 372AB | −5.80 | −5.40 | −5.58 | 0.13 | 17,842.53 | 132.54 |
| 396 | −5.80 | −5.50 | −5.68 | 0.11 | 17,842.53 | 132.54 |
| 420 | −5.60 | −5.40 | −5.49 | 0.08 | 12,730.76 | 65.92 |
| 444 | −5.00 | −4.70 | −4.83 | 0.11 | 4624.33 | −39.73 |
| 456 | −5.00 | −4.80 | −4.91 | 0.08 | 4624.33 | −39.73 |
| ADA_132 | −5.40 | −5.10 | −5.19 | 0.11 | 9083.48 | 18.39 |
| C60 | −5.30 | −5.20 | −5.21 | 0.03 | 7672.76 | — |
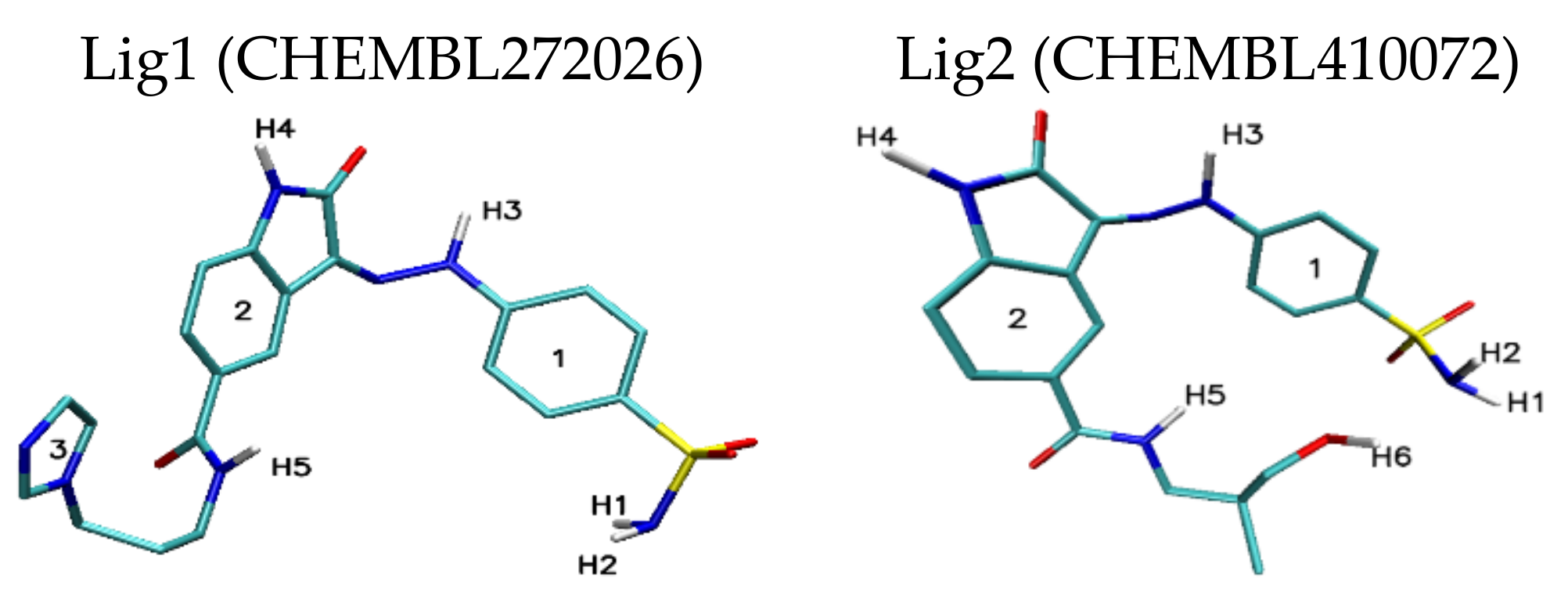
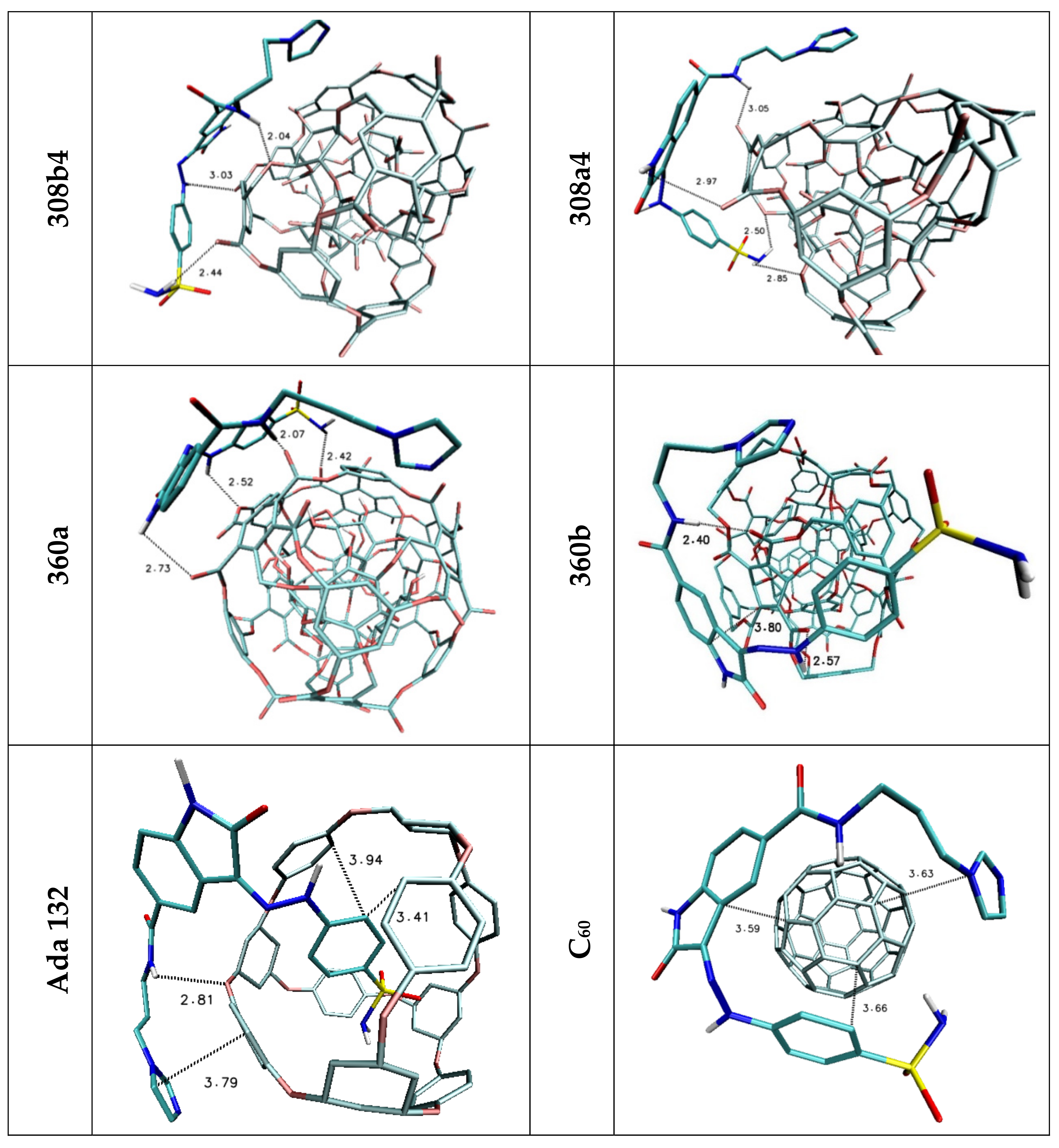
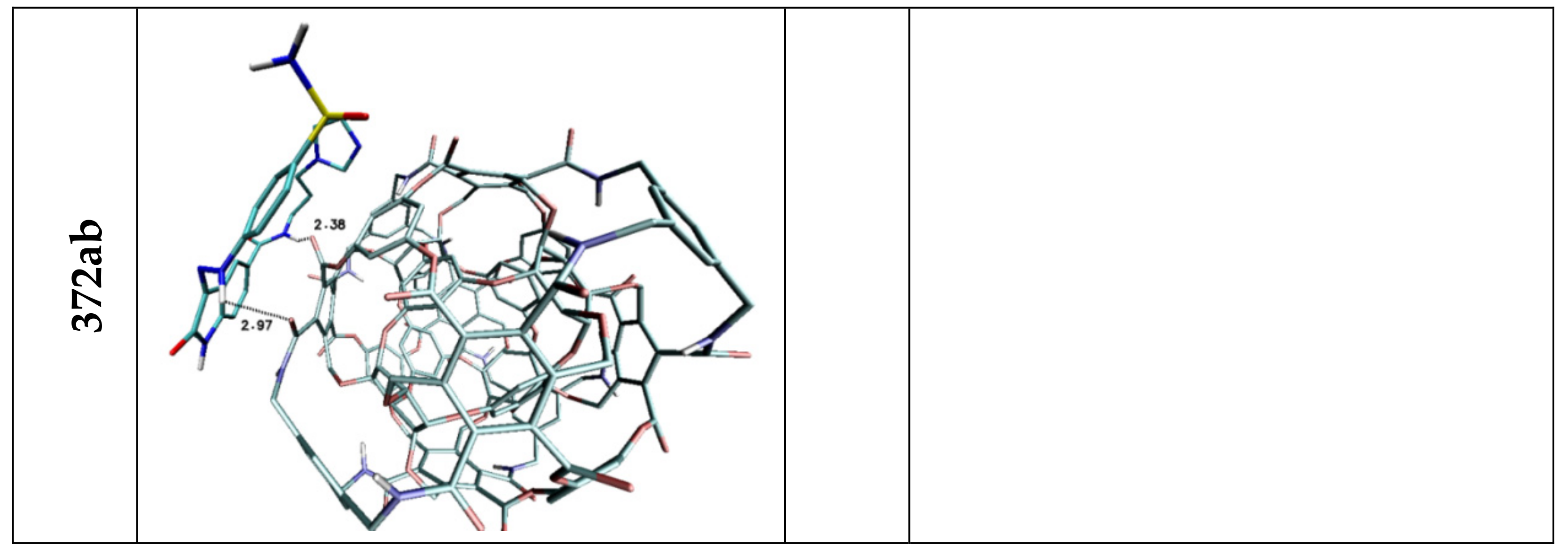
| Lig1 | ||||||
| 308b4 | 308a4 | 360a | 372AB | ADA 132 | C60 | |
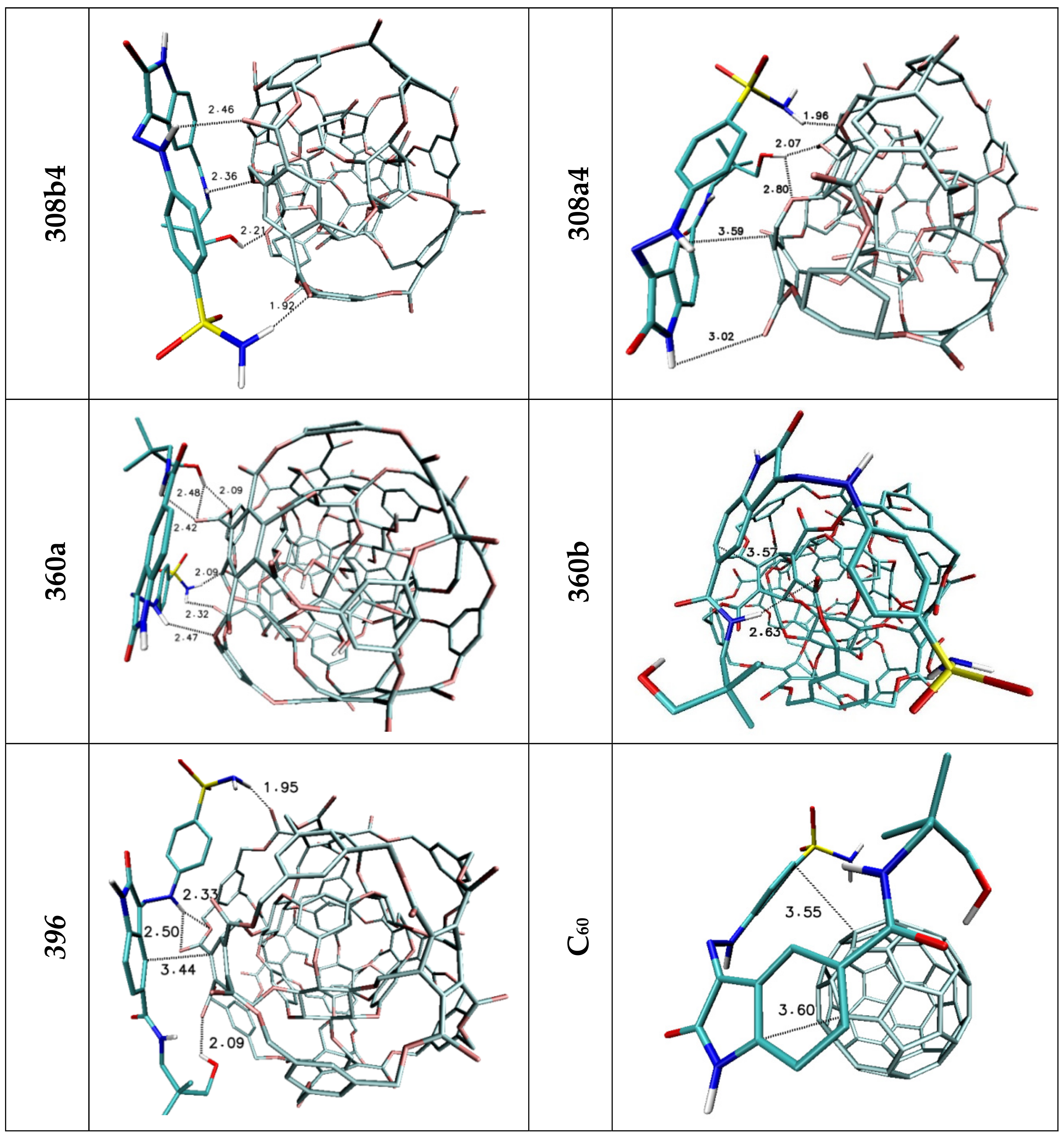
| H1/H2 | 2.44 | 2.50/2.85 | 2.42 | — | — | — |
| H3 | 3.03 | — | 2.52 | 2.97 | — | — |
| H4 | — | 2.97 | 2.73 | — | — | — |
| H5 | 2.04 | 3.05 | 2.07 | 2.38 | 2.81 | — |
| Stacking 1/2/3 | 3.74/—/3.96 | —/3.74/4.34 | —/3.68/— | 3.55/3.95/— | 3.94; 3.41/—/3.79 | 3.66/3.59/3.63 |
| Lig2 | ||||||
| 308b4 | 308a4 | 360a | 372AB | 396 | C60 | |
| H1/H2 | 1.92 | 1.96 | 2.09/2.32 | 2.52 | 1.95 | — |
| H3 | 2.46 | — | 2.47 | — | 2.33/2.50 | — |
| H4 | — | 3.02 | — | — | — | — |
| H5 | 2.36 | — | 2.42 | 2.32 | 3.15 | — |
| H6 | 2.21 | 2.07/2.80 | 2.09/2.48 | 2.03 | 2.09 | — |
| Stacking 1/2 | 3.59/— | —/3.59 | —/3.66 | 3.67/— | —/3.44 | 3.55/3.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czeleń, P.; Szefler, B. The Immobilization of Oxindole Derivatives with Use of Cube Rhombellane Homeomorphs. Symmetry 2019, 11, 900. https://doi.org/10.3390/sym11070900
Czeleń P, Szefler B. The Immobilization of Oxindole Derivatives with Use of Cube Rhombellane Homeomorphs. Symmetry. 2019; 11(7):900. https://doi.org/10.3390/sym11070900
Chicago/Turabian StyleCzeleń, Przemysław, and Beata Szefler. 2019. "The Immobilization of Oxindole Derivatives with Use of Cube Rhombellane Homeomorphs" Symmetry 11, no. 7: 900. https://doi.org/10.3390/sym11070900
APA StyleCzeleń, P., & Szefler, B. (2019). The Immobilization of Oxindole Derivatives with Use of Cube Rhombellane Homeomorphs. Symmetry, 11(7), 900. https://doi.org/10.3390/sym11070900





