Docking of Cisplatin on Fullerene Derivatives and Some Cube Rhombellane Functionalized Homeomorphs
Abstract
1. Introduction
2. Methods
2.1. Docking Procedure
2.2. Results and Discussion
3. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Dou, Q.P. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B.; Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 317–325. [Google Scholar] [CrossRef]
- Fraval, H.N.; Rawlings, C.J.; Roberts, J.J. Increased sensitivity of UV-repair-deficient human cells to DNA bound platinum products which unlike thymine dimers are not recognized by an endonuclease extracted from micrococcus luteus. Mutat. Res. 1978, 51, 121–132. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Yeap, B.; Vogl, S.E.; Kaplan, B.H.; Comis, R.L.; Falkson, G.; Davis, T.E.; Fazzini, E.; Cheuvart, B.; Horton, J. Hexamethylmelamine and low or moderate dose cisplatin with or without pyridoxine for treatment of advanced ovarian carcinoma: A study of the eastern cooperative oncology group. Cancer Invest. 1992, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wiltshaw, E.; Kroner, T. Phase II study of cis-dichlorodiammineplatinum[II] (NSC-119875] in advanced adenocarcinoma of the ovary. Cancer Treat. Rep. 1976, 60, 55–60. [Google Scholar] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Szefler, B.; Czeleń, P.; Szczepanik, A.; Cysewski, P. Does affinity of cisplatin to B-Vitamins impair the therapeutic effect in the case of patient with lung cancer consuming carrot or beet juice? Anticancer Agents Med Chem. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- ISI Web of Science. 2010.
- Gomes, J.A.N.F.; Mallion, R.B. Aromaticity and ring currents. Chem. Rev. 2001, 101, 1349–1383. [Google Scholar] [CrossRef]
- Cyrański, M.K.; Krygowski, T.M.; Katritzky, A.R.; Schleyer, P.v.R. To what extent can aromaticity be defined uniquely? J. Org. Chem. 2002, 67, 1333–1338. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Crominboeuf, C.; Puchta, R.; Schleyer, R.v.P. nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef]
- Diudea, M.V.; Lungu, C.N.; Nagy, C.L. Cube-rhombellane related structures: A drug perspective. Molecules 2018, 23, 2533. [Google Scholar] [CrossRef] [PubMed]
- Diudea, M.V.; Pîrvan-Moldovan, A.; Pop, R.; Medeleanu, M. Medeleanu, Energy of graphs and remote graphs, in hypercubes, rhombellanes and fullerenes. MATCH Commun. Math. Comput. Chem. 2018, 80, 835–852. [Google Scholar]
- Pauling, L.; Wheland, G.W. The nature of the chemical bond. V. The quantum mechanical calculation of the resonance energy of benzene and naphthalene and the hydrocarbon free radicals. J. Chem. Phys. 1933, 1, 362–374. [Google Scholar] [CrossRef]
- Daudel, R.; Lefebre, R.; Moser, C. Quantum Chemistry; Interscience: New York, NY, USA, 1959. [Google Scholar]
- Diudea, M.V. Rhombellanic diamond. Fullerenes, Nanotubes and Carbon. Nanomaterials 2018. [Google Scholar] [CrossRef]
- Pop, R.; Medeleanu, M.; Diudea, M.V.; Szefler, B.; Cioslowski, J. Fullerenes patched by flowers. Cent. Eur. J. Chem. 2013, 11, 527–534. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Randić, M. Aromaticity of Polycyclic Conjugated Hydrocarbons. Chem. Rev. 2003, 103, 3449–3605. [Google Scholar]
- Diudea, M.V.; Nagy, C.L. Periodic Nanostructures; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Szefler, B. Nanotechnology, from quantum mechanical calculations up to drug delivery. Int. J. Nanomed. 2018, 13, 6143–6176. [Google Scholar] [CrossRef] [PubMed]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov// (accessed on 11 June 2019).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Shoichet, B.K.; Kuntz, I.D.; Bodian, D.L. Molecular docking using shape descriptors. J. Comput. Chem. 2004, 13, 380–397. [Google Scholar] [CrossRef]
- Dhananjayan, K.; Kalathil, K.; Sumathy, A.; Sivanandy, P. A computational study on binding affinity of bio-flavonoids on the crystal structure of 3-hydroxy-3-methyl-glutaryl-CoA reductase—An insilico molecular docking approach. Der Pharma Chemica. 2014, 6, 378–387. [Google Scholar]
- Abagyan, R.; Totrov, M. High-throughput docking for lead generation. Current Opin. Chem. Biol. 2001, 5, 375. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
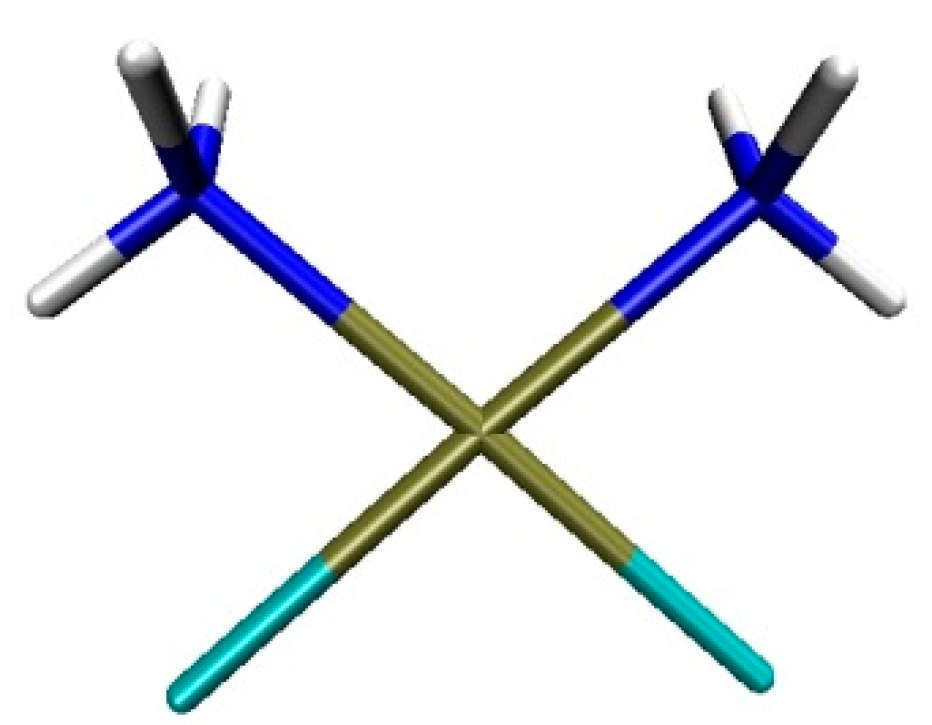
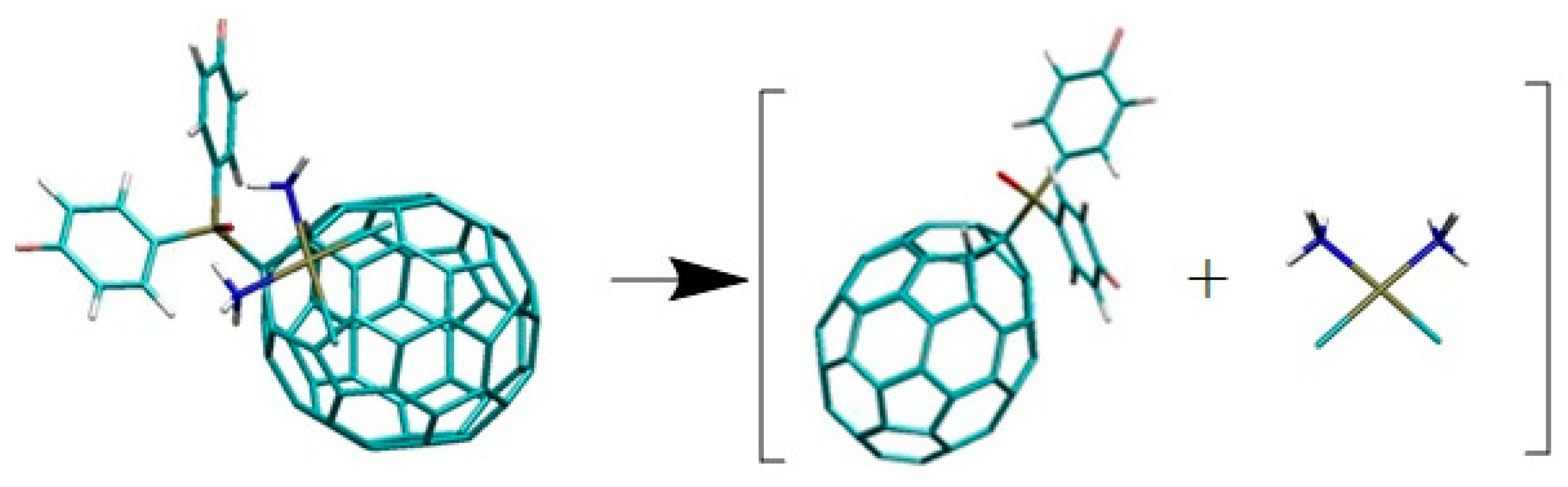

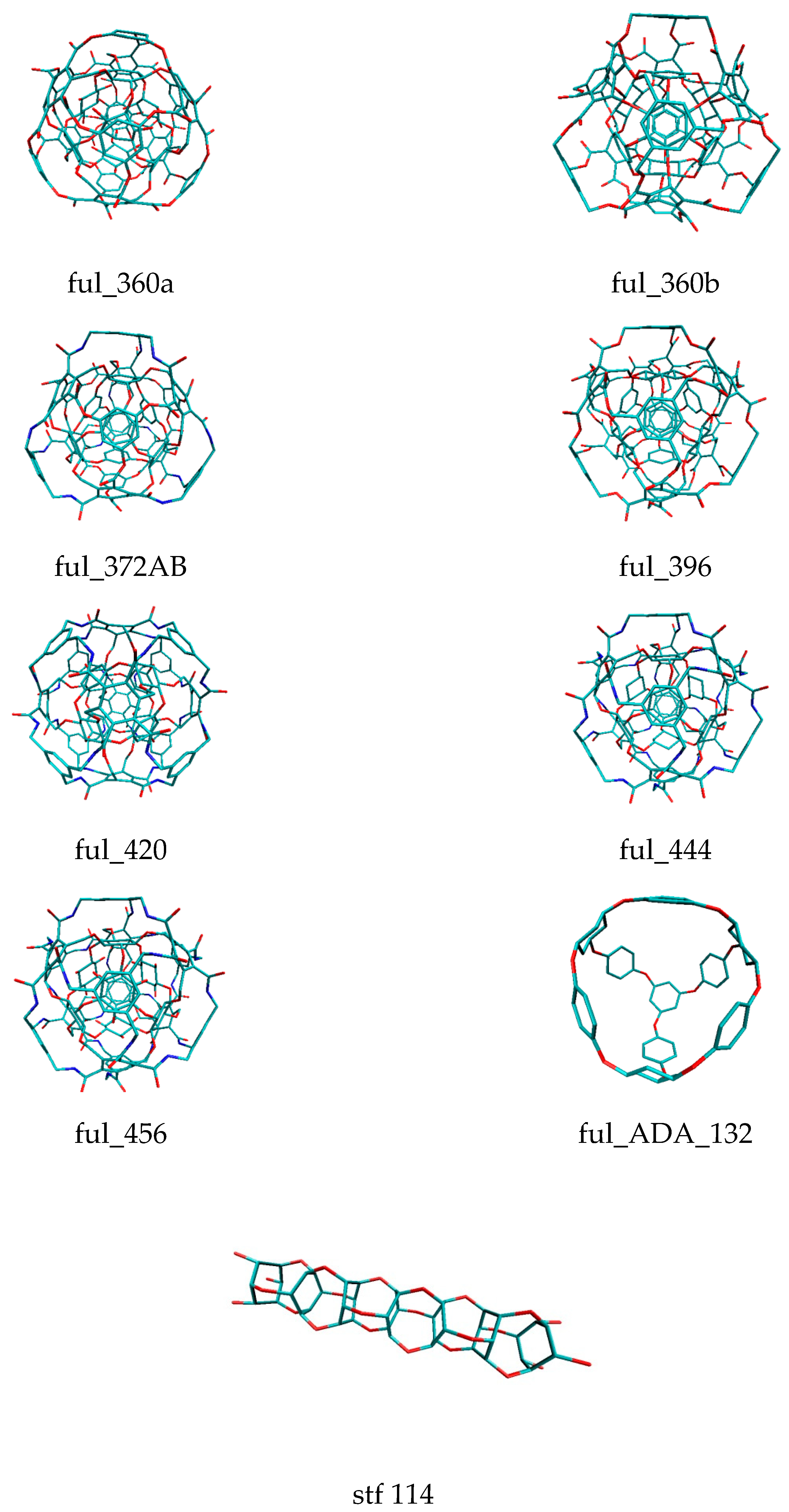
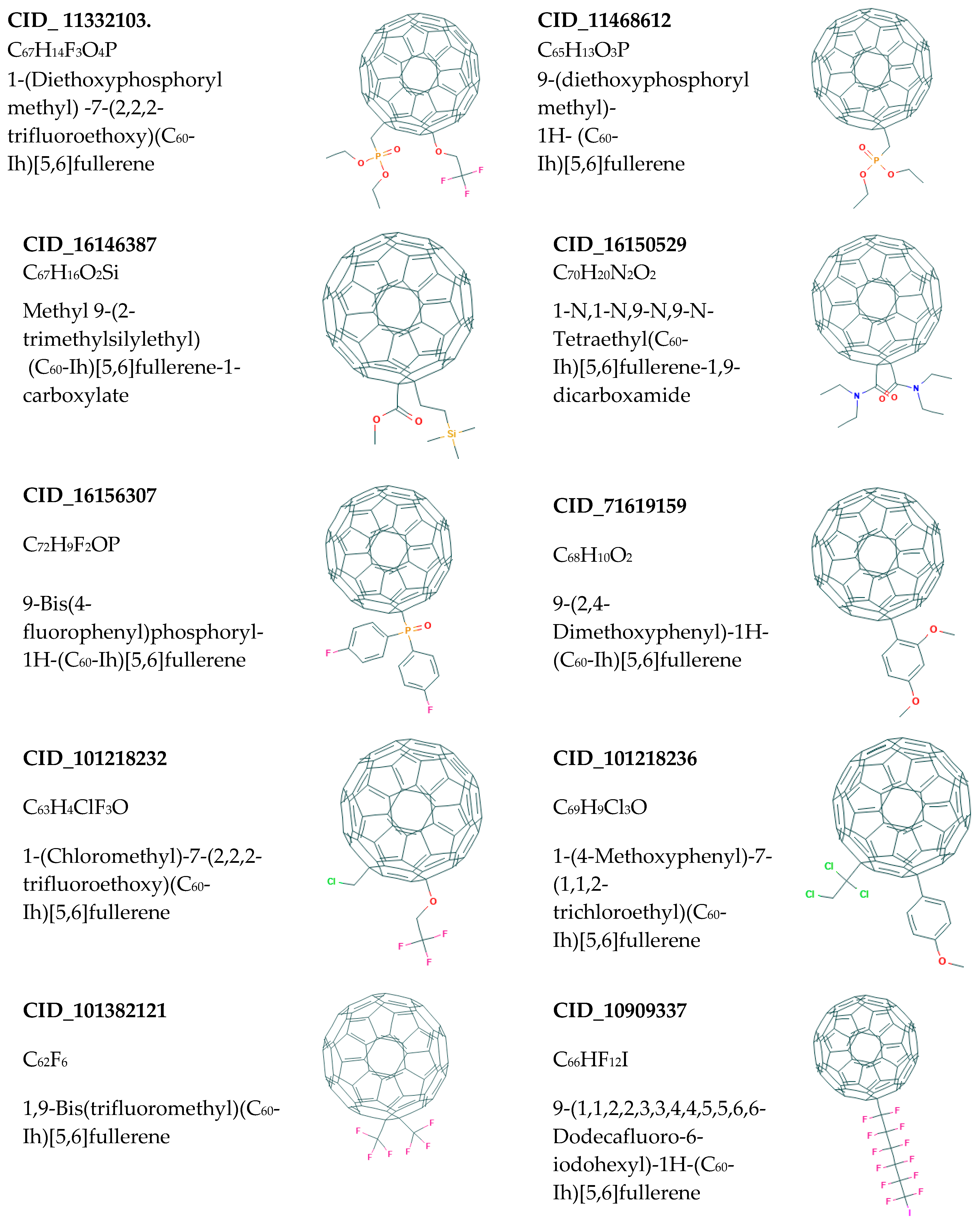

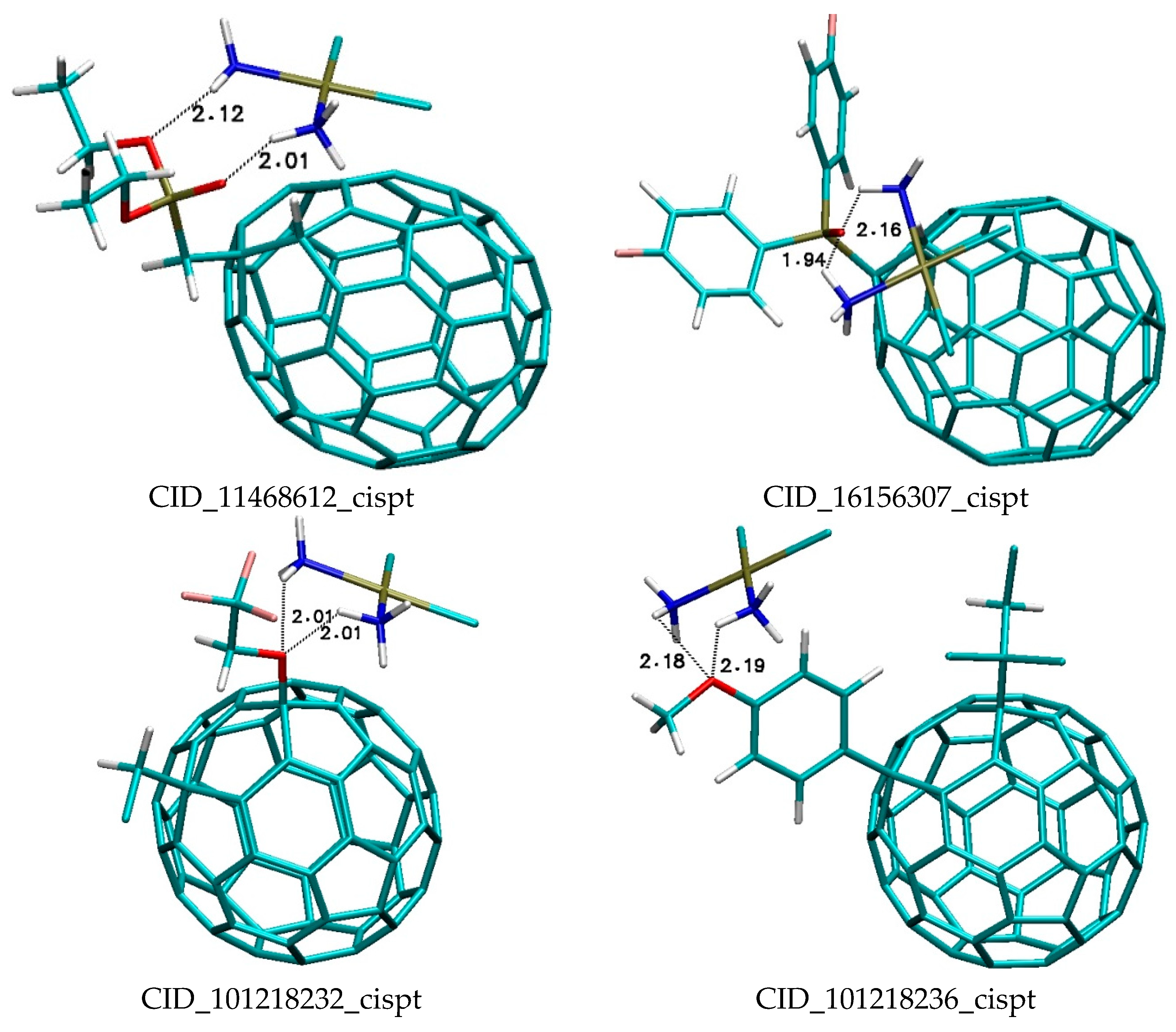
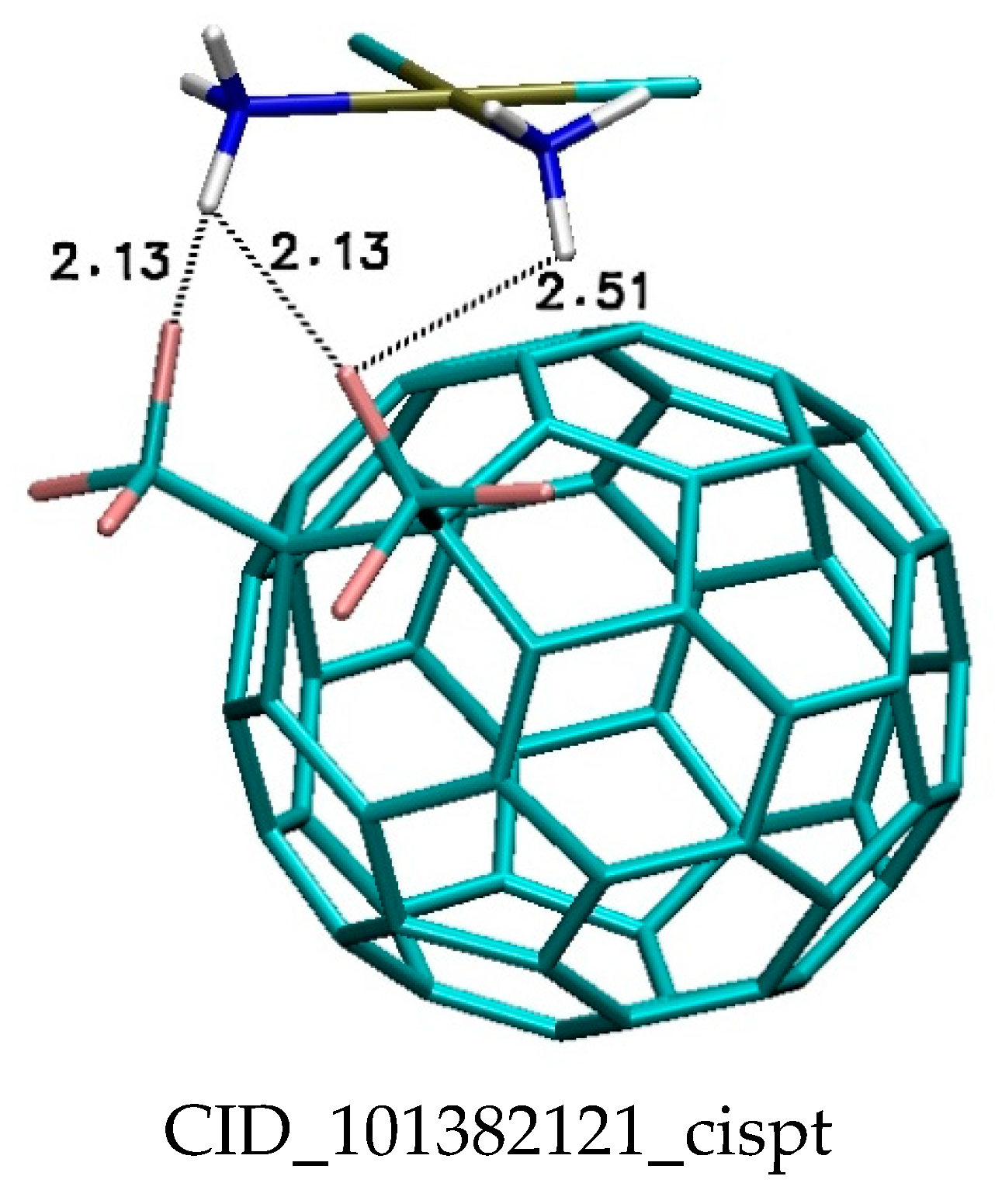
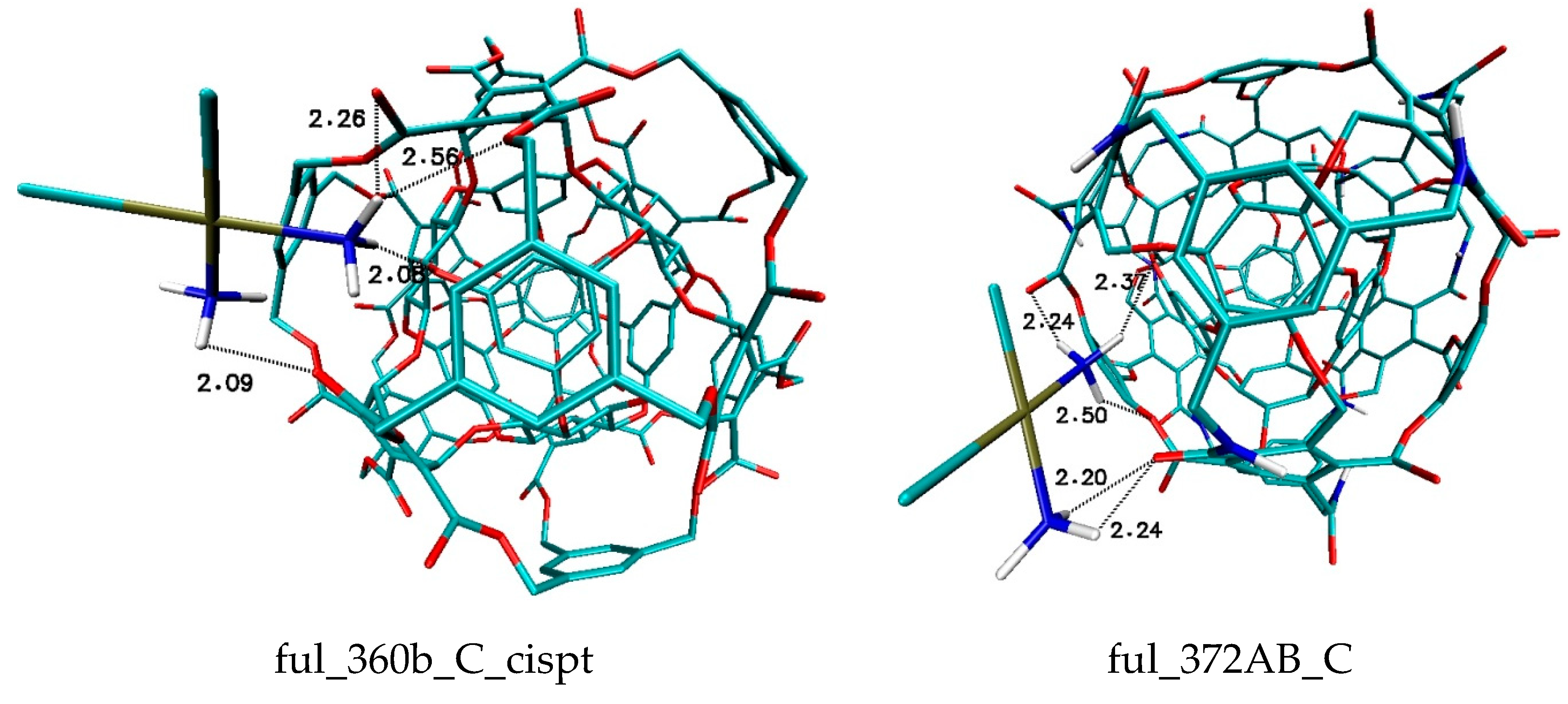
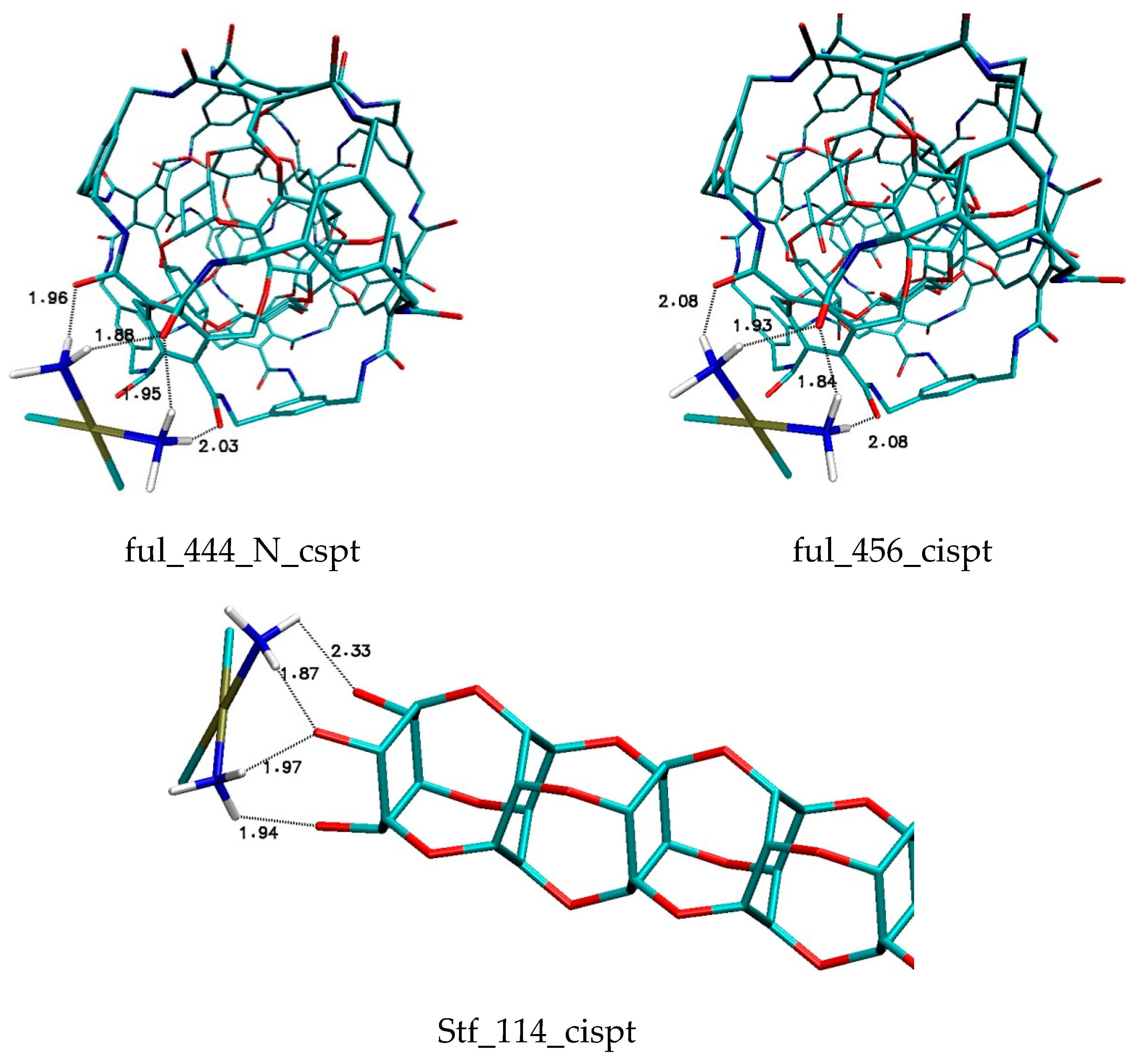
| Binding Energy (kcal/mol) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 360b | −2.66 | −2.6 | −2.61 | −2.49 | −2.45 | −2.42 | −2.33 | −2.44 | −2.25 | −2.42 |
| 372 | −2.51 | −2.38 | −2.36 | −2.31 | −2.25 | −2.25 | −2.3 | −2.2 | −2.19 | −2.17 |
| 396 | −2.34 | −2.29 | −2.28 | −2.19 | −2.23 | −2.23 | −2.22 | −2.22 | −2.16 | −2.13 |
| 420 | −2.17 | −2.16 | −2.16 | −2.16 | −2.16 | −2.16 | −2.16 | −2.09 | −2.09 | −2.09 |
| 444 | −2.72 | −2.71 | −2.72 | −2.69 | −2.67 | −2.67 | −2.65 | −2.63 | −2.63 | −2.63 |
| 456 | −2.62 | −2.62 | −2.56 | −2.56 | −2.56 | −2.55 | −2.52 | −2.45 | −2.43 | −2.41 |
| ADA132 | −2.3 | −2.23 | −2.27 | −2.26 | −2.23 | −2.05 | −2.16 | −2.16 | −2.04 | −1.98 |
| 308a4 | −2.16 | −2.11 | −1.97 | −2.16 | −2.14 | −2.14 | −2.14 | −2.04 | −1.95 | −1.84 |
| 308b4 | −2.09 | −1.9 | −2.08 | −2.01 | −1.98 | −2.05 | −1.94 | −1.89 | −1.84 | −1.8 |
| 360a | −2.35 | −2.23 | −2.23 | −2.17 | −2.11 | −2.22 | −2.19 | −2.17 | −2.09 | −2.02 |
| stf114 | −1.9 | −1.88 | −1.87 | −1.85 | −1.78 | −1.83 | −1.76 | −1.74 | −1.82 | −1.67 |
| CID_11332103 | −2.47 | −2.47 | −2.41 | −2.41 | −2.4 | −2.4 | −2.4 | −2.37 | −2.35 | −2.19 |
| CID_11468612 | −2.54 | −2.53 | −2.51 | −2.51 | −2.5 | −2.49 | −2.48 | −2.47 | −2.47 | −2.46 |
| CID_16146387 | −2.1 | −2.09 | −2.08 | −2.08 | −2.06 | −2.01 | −2 | −2.06 | −2.05 | −2.04 |
| CID_16150529 | −2.14 | −2.1 | −2.09 | −2.06 | −2.04 | −2.02 | −1.98 | −1.98 | −1.97 | −1.97 |
| CID_16156307 | −3.44 | −3.41 | −3.39 | −3.37 | −3.37 | −3.37 | −3.36 | −3.36 | −3.35 | −3.33 |
| CID_71619159 | −1.74 | −1.67 | −1.73 | −1.72 | −1.57 | −1.57 | −1.56 | −1.53 | −1.49 | −1.48 |
| CID_101218232 | −2.83 | −2.82 | −2.77 | −2.76 | −2.71 | −2.63 | −2.48 | −2.46 | −2.45 | −2.4 |
| CID_101218236 | −3.13 | −3.09 | −3.07 | −3.06 | −3.05 | −2.98 | −2.93 | −2.92 | −2.91 | −2.79 |
| CID_101382121 | −0.82 | −0.8 | −0.78 | −0.78 | −0.75 | −0.8 | −0.8 | −0.77 | −0.74 | −0.73 |
| CID_10909337_C | −0.97 | −0.96 | −0.96 | −0.96 | −0.96 | −0.95 | −0.94 | −0.94 | −0.94 | −0.93 |
| Name of Nanostructure | Maximum Binding Energy | Minimum Binding Energy | Average | SD | Binding Constant [Kmax] |
|---|---|---|---|---|---|
| 360b | −2.66 | −2.25 | −2.47 | 0.12 | 89.1 |
| 372 | −2.51 | −2.17 | −2.29 | 0.10 | 69.2 |
| 396 | −2.34 | −2.13 | −2.23 | 0.06 | 51.9 |
| 420 | −2.17 | −2.09 | −2.14 | 0.03 | 39.0 |
| 444 | −2.72 | −2.63 | −2.67 | 0.03 | 98.6 |
| 456 | −2.62 | −2.41 | −2.53 | 0.07 | 83.3 |
| ADA132 | −2.30 | −1.98 | −2.17 | 0.10 | 48.5 |
| 308a4 | −2.16 | −1.84 | −2.07 | 0.11 | 38.3 |
| 308b4 | −2.09 | −1.80 | −1.96 | 0.10 | 34.0 |
| 360a | −2.35 | −2.02 | −2.18 | 0.09 | 52.8 |
| stf114 | −1.90 | −1.67 | −1.81 | 0.07 | 24.7 |
| Name of Nanostructure | Maximum Binding Energy | Minimum Binding Energy | Average | SD | Binding Constant [Kmax] | Type |
|---|---|---|---|---|---|---|
| CID_11332103 | −2.47 | −2.19 | −2.39 | 0.07 | 64.6 | C67H14F3O4P |
| CID_11468612 | −2.54 | −2.46 | −2.50 | 0.03 | 72.8 | C65H13O3P |
| CID_16146387 | −2.10 | −2.00 | −2.06 | 0.03 | 34.6 | C67H16O2Si |
| CID_16150529 | −2.14 | −1.97 | −2.04 | 0.06 | 37.0 | C70H20N2O2 |
| CID_16156307 | −3.44 | −3.33 | −3.38 | 0.03 | 332.3 | C72H9F2OP |
| CID_71619159 | −1.74 | −1.48 | −1.61 | 0.09 | 18.9 | C68H10O2 |
| CID_101218232 | −2.83 | −2.40 | −2.63 | 0.16 | 118.7 | C63H4ClF3O |
| CID_101218236 | −3.13 | −2.79 | −2.99 | 0.10 | 196.9 | C69H9Cl3O |
| CID_101382121 | −0.82 | −0.73 | −0.78 | 0.03 | 4.0 | C62F6 |
| CID_10909337 | −0.97 | −0.93 | −0.95 | 0.01 | 5.1 | C66HF12I |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szefler, B.; Czeleń, P. Docking of Cisplatin on Fullerene Derivatives and Some Cube Rhombellane Functionalized Homeomorphs. Symmetry 2019, 11, 874. https://doi.org/10.3390/sym11070874
Szefler B, Czeleń P. Docking of Cisplatin on Fullerene Derivatives and Some Cube Rhombellane Functionalized Homeomorphs. Symmetry. 2019; 11(7):874. https://doi.org/10.3390/sym11070874
Chicago/Turabian StyleSzefler, Beata, and Przemysław Czeleń. 2019. "Docking of Cisplatin on Fullerene Derivatives and Some Cube Rhombellane Functionalized Homeomorphs" Symmetry 11, no. 7: 874. https://doi.org/10.3390/sym11070874
APA StyleSzefler, B., & Czeleń, P. (2019). Docking of Cisplatin on Fullerene Derivatives and Some Cube Rhombellane Functionalized Homeomorphs. Symmetry, 11(7), 874. https://doi.org/10.3390/sym11070874





