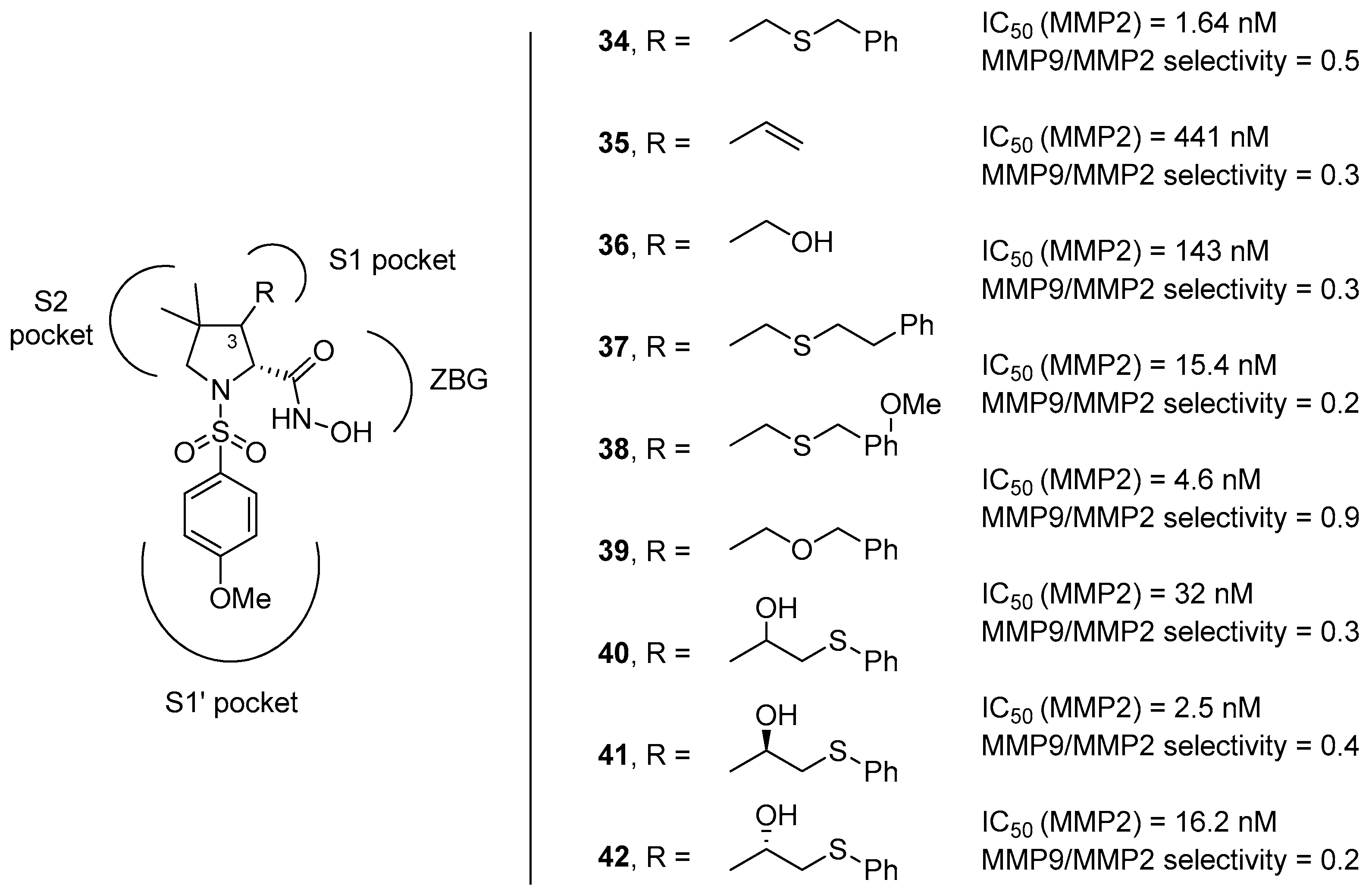Abstract
Natural and nonnatural amino acids represent important building blocks for the development of peptidomimetic scaffolds, especially for targeting proteolytic enzymes and for addressing protein–protein interactions. Among all the different amino acids derivatives, proline is particularly relevant in chemical biology and medicinal chemistry due to its secondary structure’s inducing and stabilizing properties. Also, the pyrrolidine ring is a conformationally constrained template that can direct appendages into specific clefts of the enzyme binding site. Thus, many papers have appeared in the literature focusing on the use of proline and its derivatives as scaffolds for medicinal chemistry applications. In this review paper, an insight into the different biological outcomes of d-proline and l-proline in enzyme inhibitors is presented, especially when associated with matrix metalloprotease and metallo-β-lactamase enzymes.
1. Introduction
More than 500 amino acids are present in nature. Although d-amino acids were long considered to have only a minor function compared to their enantiomers, many peptides that contain d-amino acids have been found in nature correlated to specific biological functions [1]. For example, bacterial cells wall peptidoglycans contain several d-amino acids, such as d-Asp, d-Asn, d-Glu, d-Gln, d-Ala, and d-Ser, that have been shown to give to bacteria the peculiar resistance to proteolytic digestion [2]. For the same reason, d-amino acids have been found in many antibiotics, such as bacitracin penicillin, actinomycin, and valinomycin. In humans, d-amino acids are often involved in pathophysiological processes [3] and can be used as disease markers [4]. Just to give some examples, d-serine is an important neuromodulator recognized by the N-methyl-d-aspartate (NMDA)-type glutamate receptors; its metabolism is altered in many neurodegenerative disorders [5,6]. Also, d-aspartate is implicated in the regulation of adult neurogenesis and in the development of endocrine functions, including hormones synthesis and spermatogenesis [7]. Generally, d-amino acids act antagonistically to l-amino acids by inhibiting the active site of biological targets [8]. This tendency is also demonstrated for synthetic amino acids. Just to give an example, l- and d-morpholine-3-carboxylic acid are able to modulate the conformational preferences [9,10] and the bioactivity of peptidomimetic compounds once inserted in their structure [11]. Specific enzymes, such as the amino acid racemase, the d-amino acid oxidase, and the peptidyl aminoacyl l-d-isomerase are present in our metabolism to convert the stereochemistry of specific amino acids in peptides or proteins [12,13].
Among all the different amino acids derivatives, proline is particularly relevant in chemical biology and medicinal chemistry due to the stabilizing properties and the conformational restriction of the pyrrolidine ring [14]. Proline-rich regions create peculiar secondary structures in proteins, such as β- and γ-turns and loops, which often play determining roles for protein–protein and protein–drug interactions [15]. Also, some proline-rich peptides have been shown to possess unique cell-penetrating properties [16]; others proved to have an antimicrobial activity by inhibiting protein synthesis [17] and to modulate the amyloid formation [18]. Finally, the alteration of hydroxyproline metabolism plays key roles in the many pathophysiological processes [19].
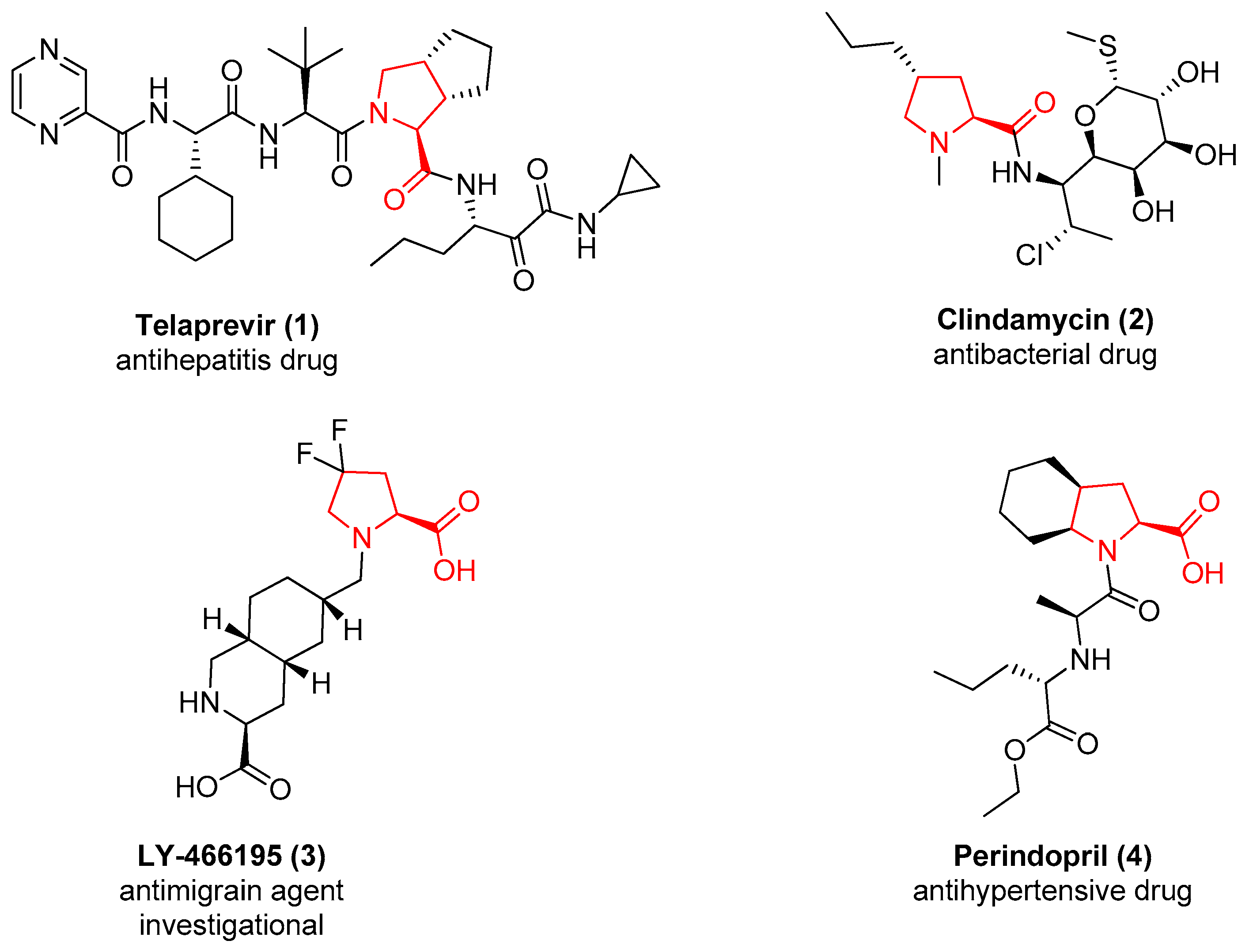
More than 100 drugs contain the l-proline chemotype in their structure. As representative examples, Figure 1 reports the structure of Telaprevir (1), a potent inhibitor of the Hepatitis C virus (HCV) that contains a proline derivative as an interacting motif able to address a binding pocket [20], and the structure of the antibiotic Clindamycin (2), useful for the treatment of a variety of different bacterial infections in which the proline is acting as a core scaffold to direct the substituents in the correct spatial orientation [21]. A substituted proline chemotype is also contained in compound 3, developed by Eli Lilly as a GLUK5 receptor antagonist [22], and in the structure of the antihypertensive drug Perindopril (4), where the proline is fused to a cyclohexane ring to further constrain the flexibility of the molecule [23].
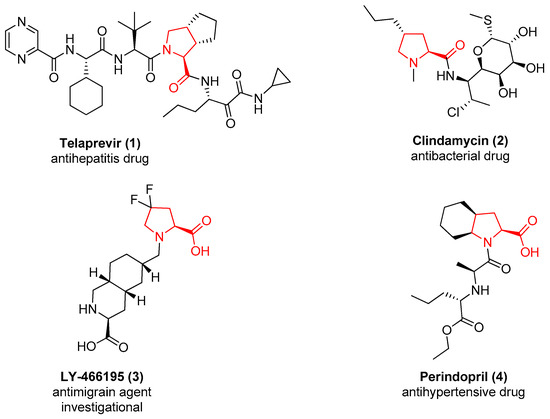
Figure 1.
The approved or investigational drugs containing the l-proline chemotype.
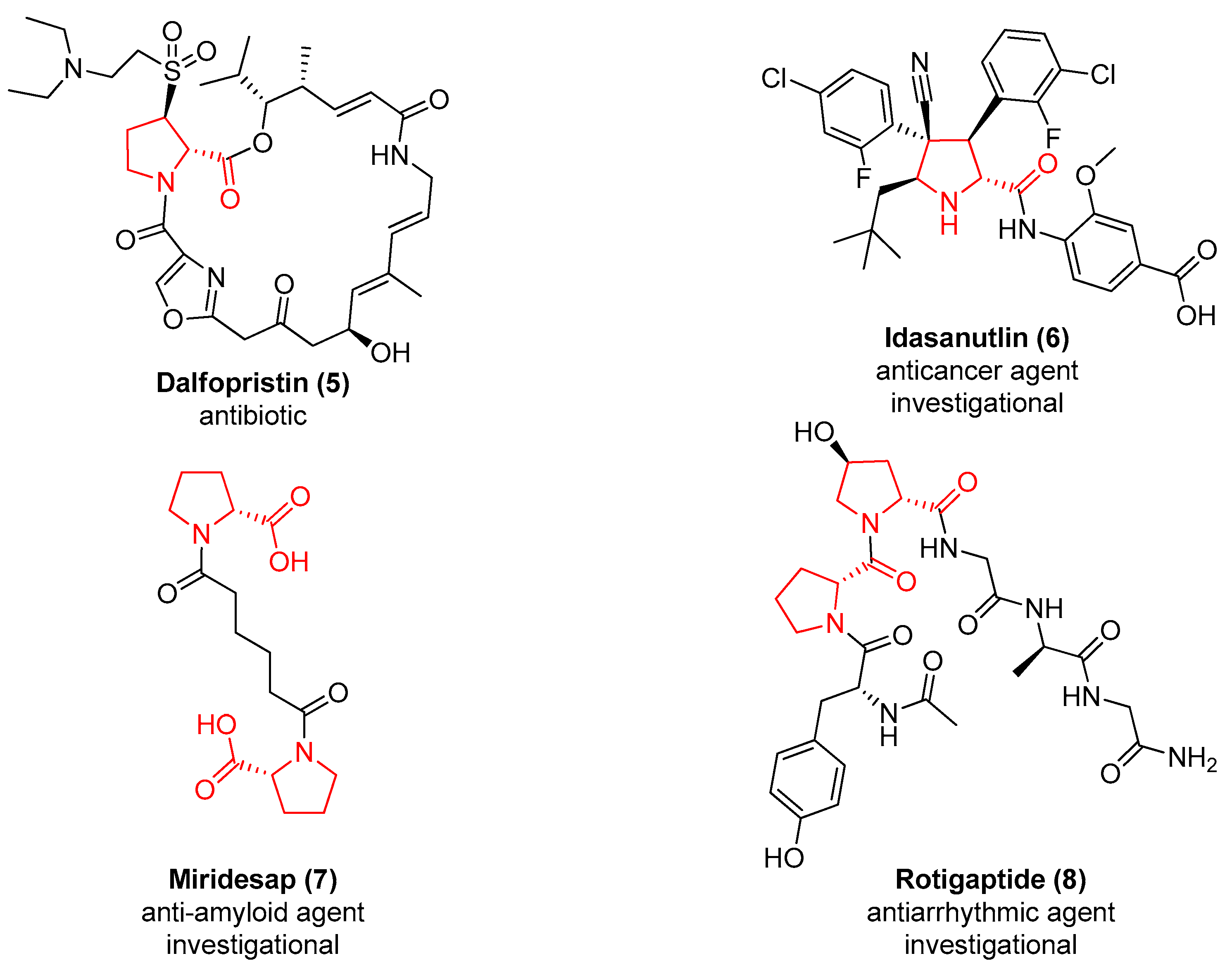
Also, 21 entries of the drug bank database [24] contain the d-proline chemotype in their structure. In Figure 2 are represented the structures of the antibiotic Dalfopristin (5), which is used in combination with quinupristin to treat staphylococci infections [25], and of the antitumoral agent MDM2 inhibitor Idasanutlin (6) [26]. Two d-proline are present in Miridesap (7), a therapeutic agent able to enhance the degradation of amyloid P component [27], and in the structure of the antiarrhythmic Rotigaptide (8) [28].
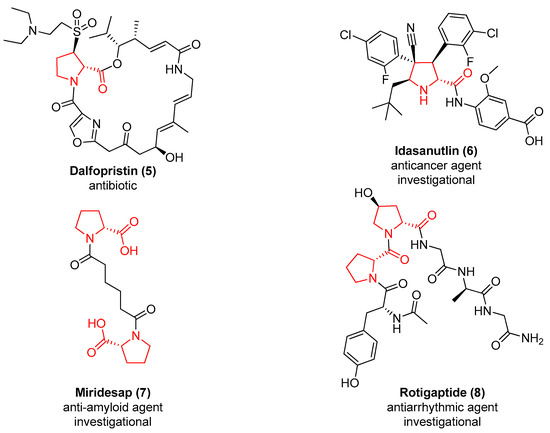
Figure 2.
The approved or investigational drugs containing the d-proline chemotype.
d-proline plays key roles also in the pathophysiological processes of other organisms. Just to give some examples, adipokinetic neurohormones of cicadas differ only for the presence of l- or d-proline in their sequence [29]. Also, one of the mechanisms of the development of Chagas disease, caused by the infection of the protozoan parasite Trypanosoma cruzi [30], seems to be correlated to the overexpression of proline racemase. During the infection, T. cruzi secretes this enzyme, thus leading to the formation of several d-proline-rich peptides that showed a resistance against host proteolytic mechanisms [31]. Thus, proline racemase is a potential drug target against this parasite, and the development of its inhibitors can be used in the treatment of Chaga disease [32].
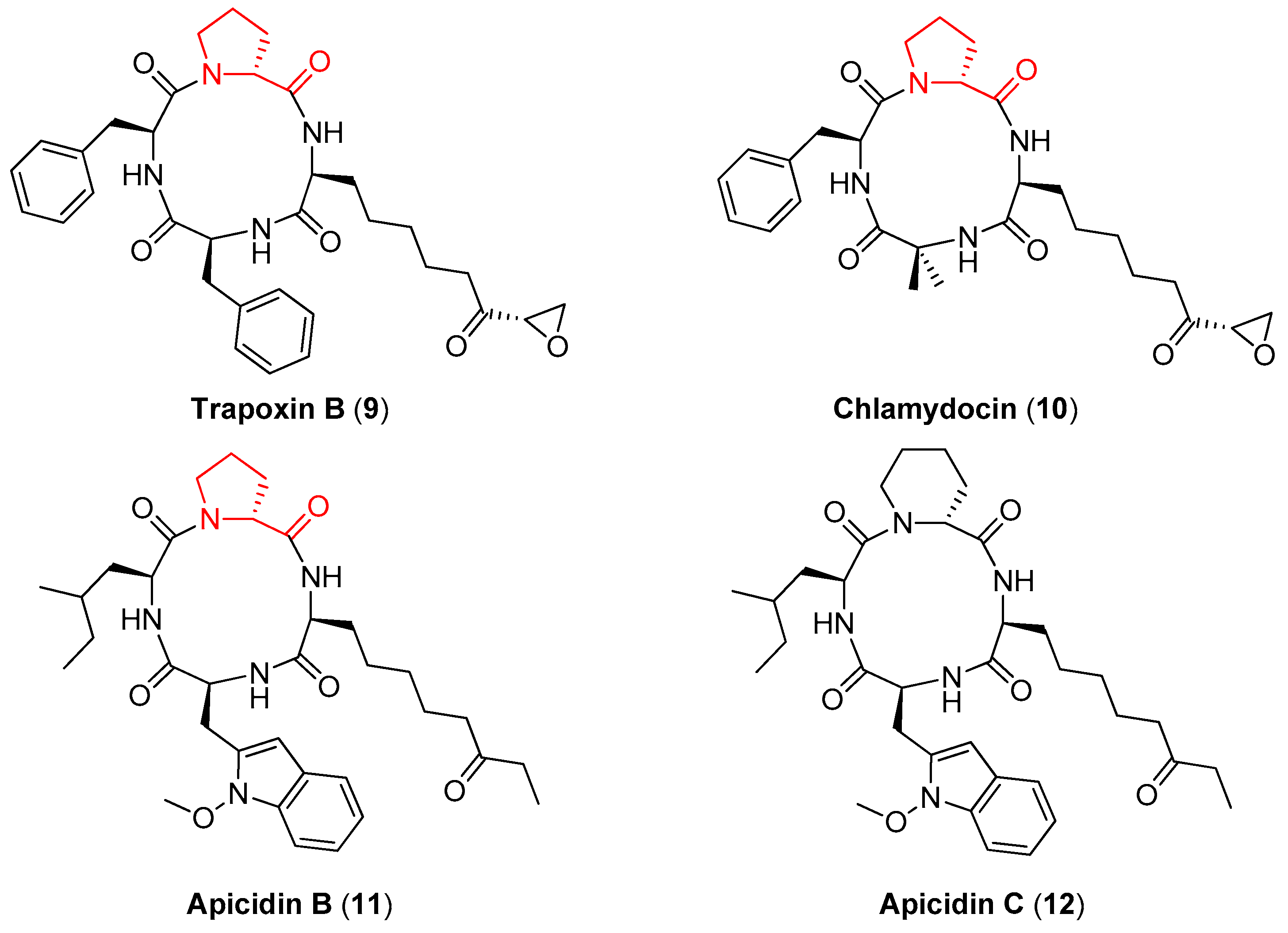
d-proline is also contained in Trapoxin B (9), Chlamydocin (10), and Apicidin B (11) (Figure 3), naturally occurring tetrapeptides that show a potent antiprotozoal activity by inhibiting histone deacetylase (HDAC), an enzyme that plays a key role in inducing or reversing gene transcription [33,34]. The importance of the d-proline ring in these compounds for the antiprotozoal activity has been clarified by Singh and coworkers [35]. In particular, while Apicidin B showed a 5-fold selectivity between the HDAC of the parasite and the HDAC of the liver of the chicken host, its synthetic analogue Apicidin C (12), where the (R)-proline ring is substituted with a (R)-piperidine ring, did not show any selectivity.
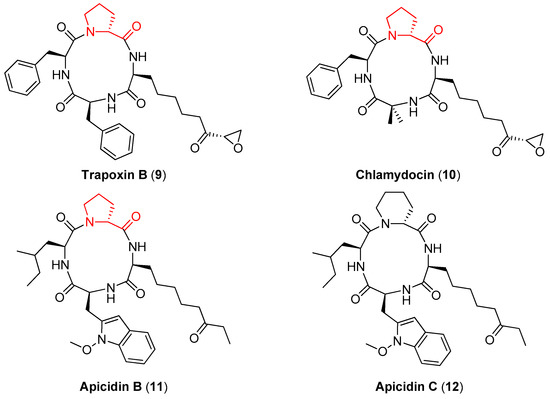
Figure 3.
The naturally occurring cyclic tetrapeptides containing d-proline (9–11) and the structurally related Apicidin C (12).
d- and l-proline have long attracted the interest of the medicinal chemistry community, and many papers are present in the literature focusing on the generation of proline derivatives as molecular scaffolds for the synthesis of peptidomimetics and enzyme inhibitors [36,37], also in a combinatorial chemistry [38] and Diversity-Oriented Synthesis perspective [39]. In this review paper, an insight into the different biological outcomes of d-proline and l-proline in enzyme inhibitors is presented, considering as case studies the matrix metalloprotease (MMP) and metallo-β-lactamase (MBL) enzymes.
2. d-Proline Derivatives as Metallo-β-Lactamase (MBL) Inhibitors
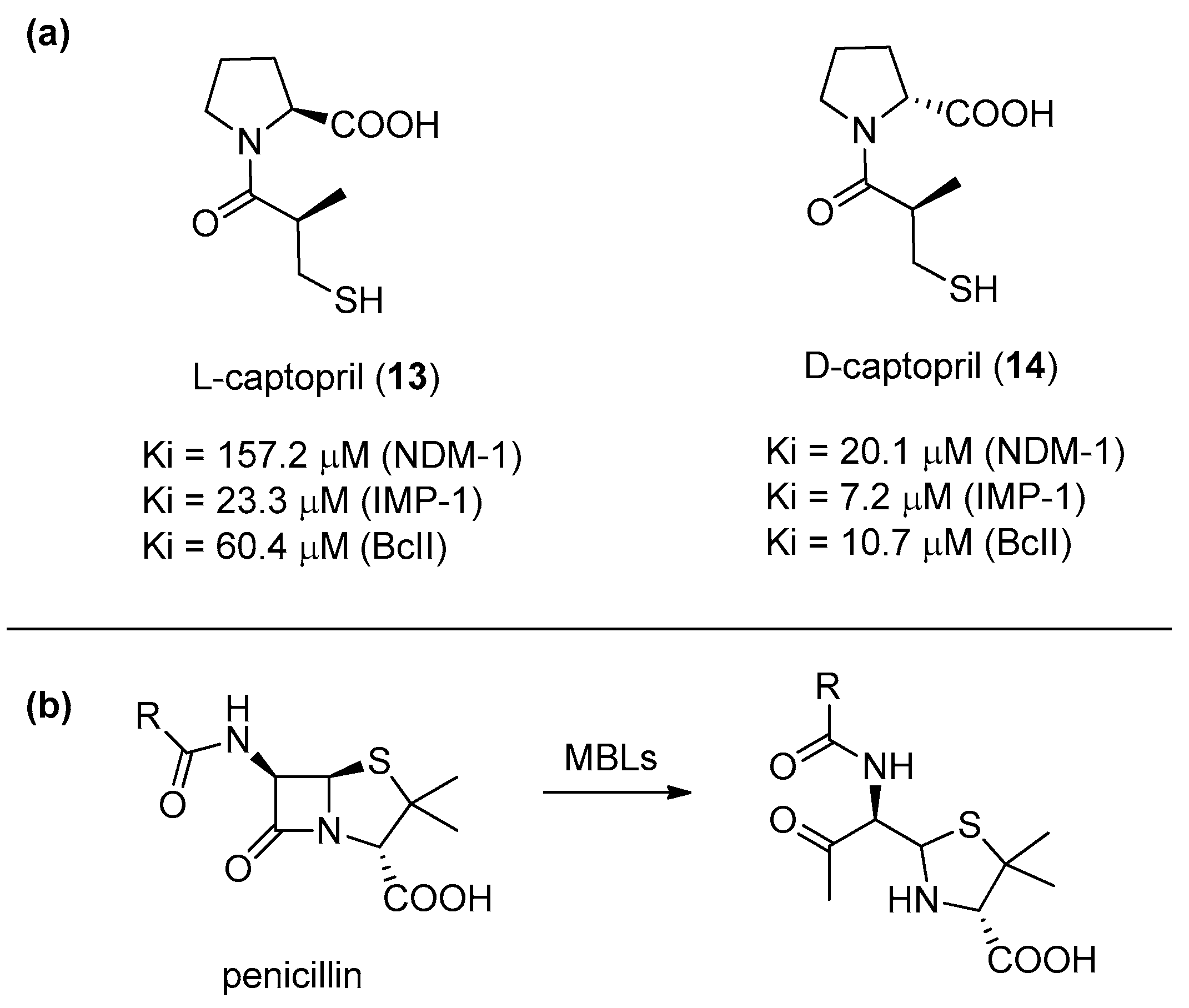
Antibiotic-resistant bacteria are becoming a serious threat of our century. Although many serine-dependent metallo-β-lactamases (SBL) are already currently used in combination with antibiotics, no clinically useful metallo-β-lactamase (MBL) inhibitors have been reported yet [40]. This is mainly due to the need of achieving a selective inhibition against MBL enzymes, while avoiding the inhibition of other structurally similar enzymes. Depending on the number of zinc ions in the catalytic sites, MBLs can be categorized into three subclasses (B1, B2, and B3). l-Captopril (13) (Figure 4a), developed in the 1970s as a human angiotensin-converting enzyme (ACE) inhibitor, is able also to block several MBLs. In particular, its diastereoisomer d-captopril (14) has proven to be more potent against MBLs, especially against those belonging to the B1 subclass (such as NDM-1, IMP-1, and BcII) because its conformation is more similar to the one adopted by the antibiotic hydrolysis product (Figure 4b). Also, considering that d-captopril is not particularly active against the ACE enzyme, this compound provides an interesting starting point for the development of selective therapeutic agents [41] because the B1 subclass is the most diffuse and clinically relevant [42,43].
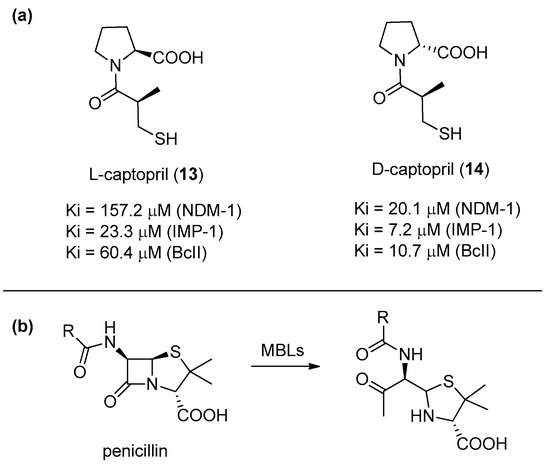
Figure 4.
(a) l- and d-captopril and their inhibition activities towards different metallo-β-lactamase (MBLs) of the B1 subclass: (b) The hydrolyzed penicillin product shows a higher structural similarity to d-captopril as compared to l-captopril.
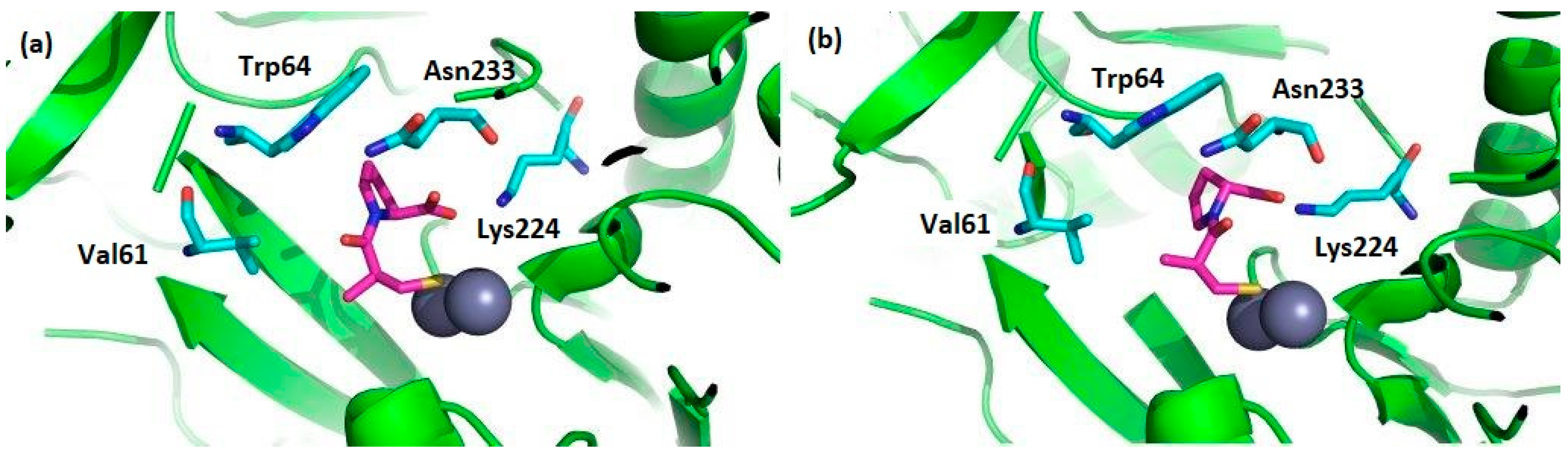
In particular, numerous cases of antibiotic resistance have been associated to the activity of imipenemase (IMP-1) [44], one of the most potent MBL from B1 subclass. The binding mode of the two captopril stereoisomers to IMP-1 has been determined by crystallography (Figure 5) [45]. In both cases, the two Zn2+ ions in the active site are located at the end of the two β-sheets; one is chelated by three histidines, while the other one is coordinated by an aspartic acid, a histidine, and a cysteine [46,47]. In the complex with l-captopril, a decreased number of hydrogen bonds and weaker electrostatic interactions are found, which reflects its smaller inhibition.
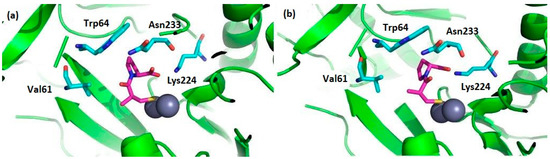
Figure 5.
(a) The selected amino acids of the IMP-1 catalytic site interacting with l-captopril (PDB code: 4C1F) and (b) with d-captopril (PDB code: 4C1G). Zinc ions are shown as black spheres, amino acid residues are shown in light blue, and captopril molecules are shown in fuchsia.
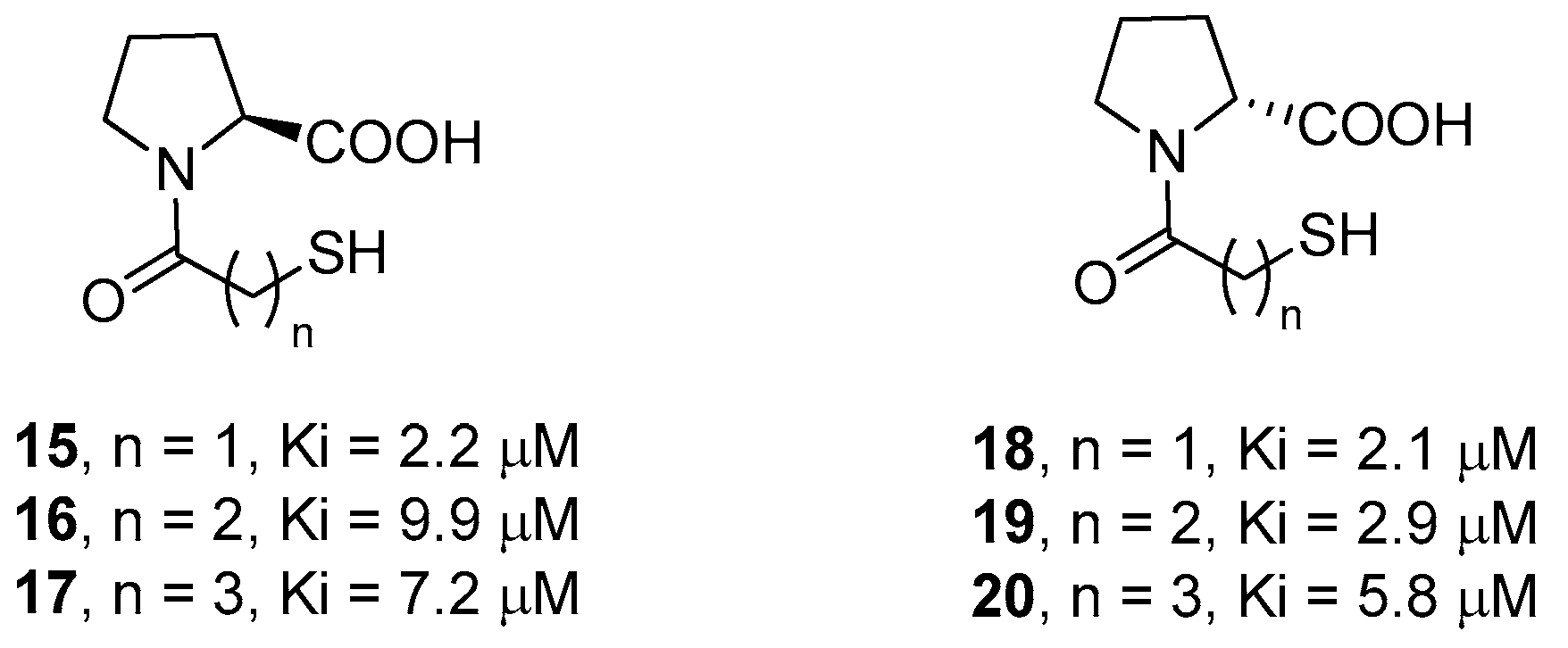
Novel broad-spectrum inhibitors have been designed by using l-proline and d-proline as molecular scaffolds to direct the interacting groups in the correct spatial orientations. In particular, in order to determine the optimal distance between the thiol and the carboxylate functional groups, McGeary and coworkers synthesized compounds 15–20 (Figure 6), containing a different number of carbon atoms in the amide appendage [48]. The biological data showed that compounds 15 and 18, characterized by the shortest distance between these groups, are the most active, although only slightly more than the parent d- and l-captopril. Also, d-proline derivatives 18–20 were, in all cases, more potent than the corresponding l-proline derivatives, demonstrating the higher affinity of this conformation in coordinating both metal ions through the thiolate and the carboxylate functional groups.
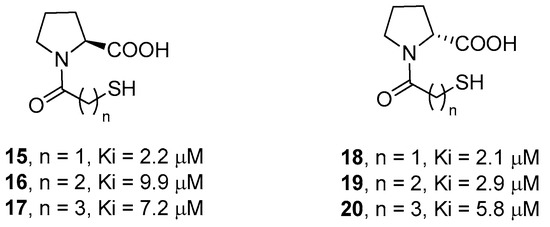
Figure 6.
l- and d-proline derivatives 15–20 and their inhibition activity against IMP-1.
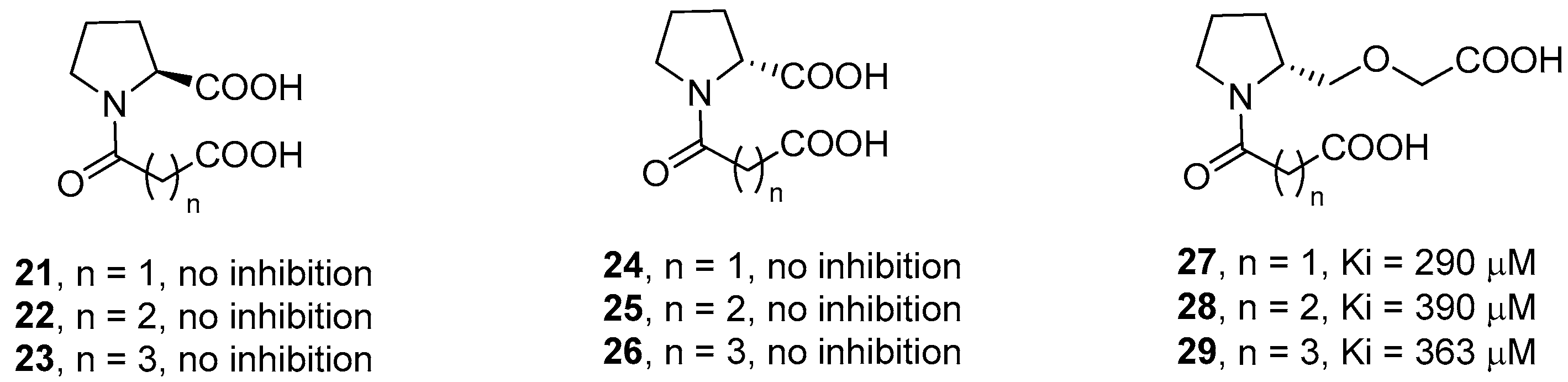
The authors also synthesized l-proline and d-proline derivatives with two carboxylic acids (compounds 21–26, Figure 7). However, these compounds, including the succinic and glutaric anhydride derivatives, did not show any inhibition activity against IMP-1, thus revealing the importance of the thiol group for the binding affinity. The relevance of the carboxylate group linked to the proline ring was also demonstrated by derivatives 27–29, where the carboxylic group is spaced out by an ether group, that showed a significantly lower biological activity as compared to compounds 18–20.
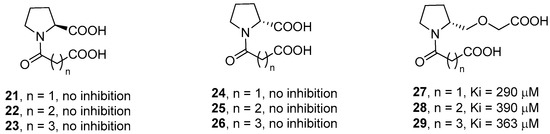
Figure 7.
l- and d-proline derivatives 21–29 and their inhibition activity against IMP-1.
3. d-Proline Derivatives as Matrix Metalloproteases (MMP) Inhibitors
Matrix metalloproteases (MMP) are a class of Zn2+-dependent proteases of the metzincin superfamily that are key players in the modeling of the extracellular matrix (ECM) [49]. The 23 members of the human MMP family are classified into six categories [50]. Many of them are involved in cancer development [51], as well as in inflammatory and cardiovascular diseases [52,53]. Unfortunately, the use of MMP inhibitors (MMPi) in the clinics has been stalled due to the development of side effects related to the nonselective inhibition of several metalloproteases [54]. Among the different types of MMPs, gelatinase A (MMP-2) and gelatinase B (MMP-9) have been shown to play a key role in the angiogenic switch, which is related to tumor cell invasiveness. However, MMP-2 is considered a better candidate target as compared than MMP-9, of which the inhibition can be even detrimental in advanced stages of cancer [55].
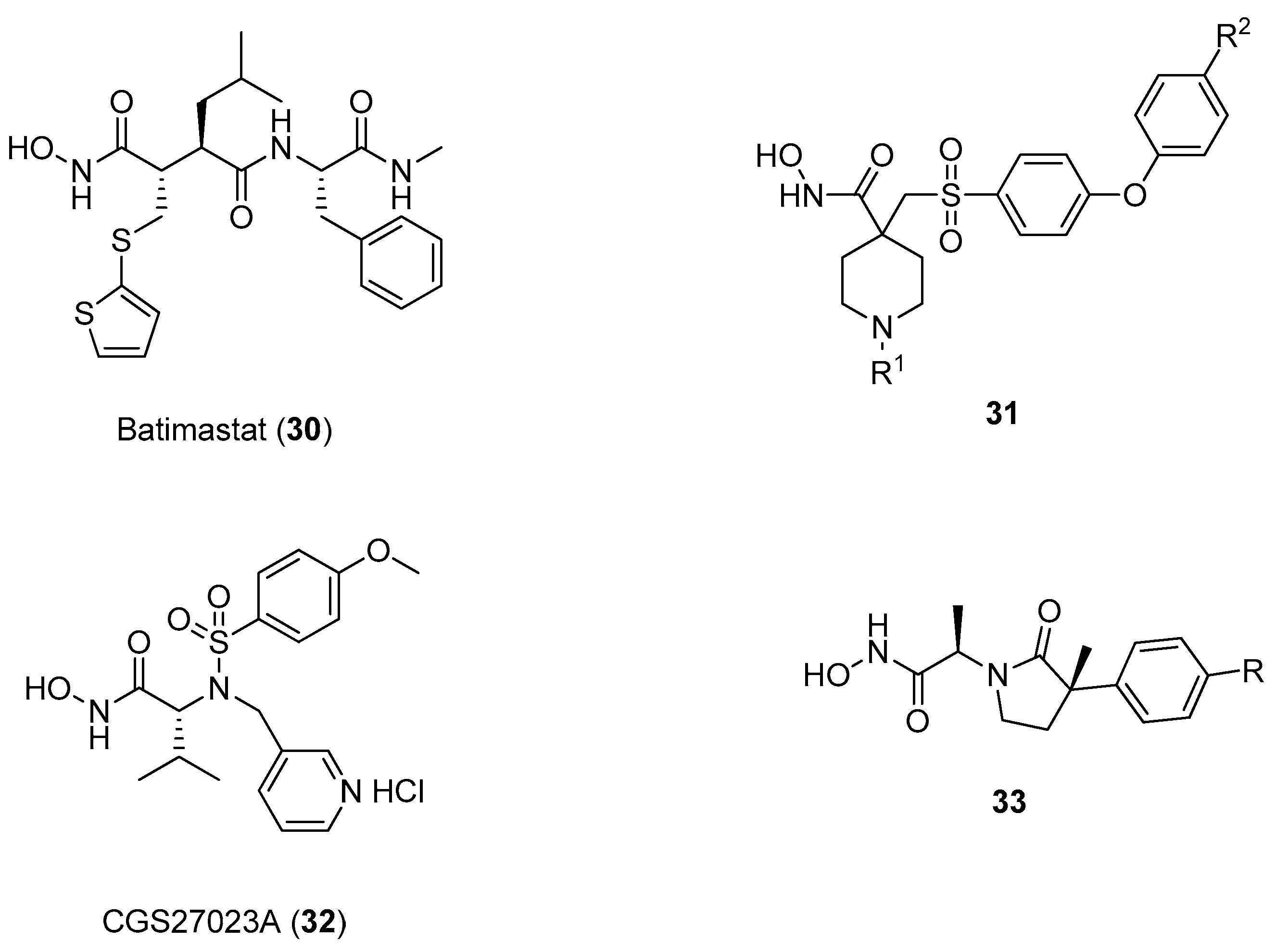
Several types of MMP-2 inhibitors have been developed during the last decades, most of them containing the hydroxamate functional group as an effective zinc binding element and at least one hydrophobic side chain effectively interacting with S1’ and S2’ pockets [56]. Early stage hydroxamates, such as batimastat (30), belong to the class of succinylate aminoacid hydroxamates, one of the most effective MMP binders, that unfortunately did not show any selectivity and failed the clinical trials [57]. Another class of effective MMPi are represented by α-sulfonyl-piperidine and α-sulfonyl-pyran hydroxamic acids, such as compound 31 in which the SO2 moiety is involved in several strong hydrogen bonds with amino acid residues from the active site cleft and in directing the hydrophobic substituent into the deep S1’ cleft [58]. In this field, sulfonylated amino acid hydroxamates with general formula HONH-CO-CHR1-NR2-SO2R3, such as compound 32 (CGS27023A) developed by Novartis, have proven to be very effective, although toxicities related to arthralgia and myalgia were observed in clinical trials [59]. Other hydroxamate inhibitors containing a cyclic scaffold have been also reported, such as the ϒ-lactam hydroxamate 33 as in Figure 8, but these compounds were found to have only a sub-micromolar affinity for MMP-2.
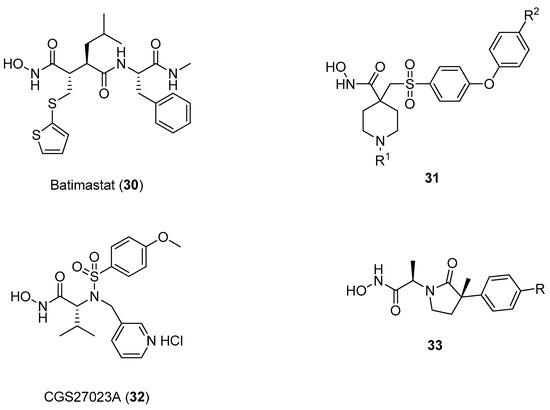
Figure 8.
Different MMPi developed in the last twenty years.
In this context, proline has been taken into account as a molecular scaffold by several research groups, starting from the observation that collagen, one of the main components of the ECM, is composed by the repetition of the XaaYaaGly sequence, in which Xaa is often l-proline and Yaa is often trans-(2S,4R)-4-hydroxyproline [60,61]. Hannessian and coworkers developed a class of d-proline derivatives (compounds 34–42 as in Figure 9) possessing different appendages on position 3 of the pyrrolidine ring that can address the S1 pocket by an additional hydrogen bond donor [62]. However, these compounds did not show a satisfactory selectivity, as the p-methoxyphenyl moiety addressing the S1’ pocket did not prove to be sufficient in discriminating between the two gelatinases.
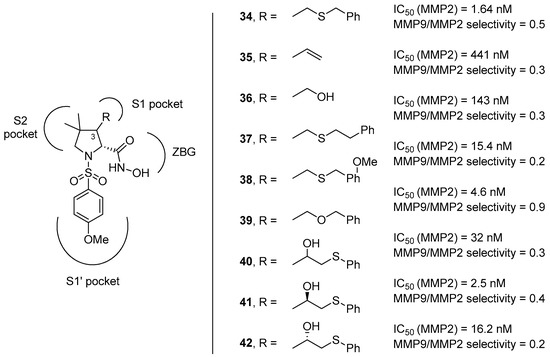
Figure 9.
d-proline derivatives 35–42 developed by Hannessian and coworkers, their binding activity for MMP-2, and their selectivity between the two gelatinases (MMP-9/MMP-2).
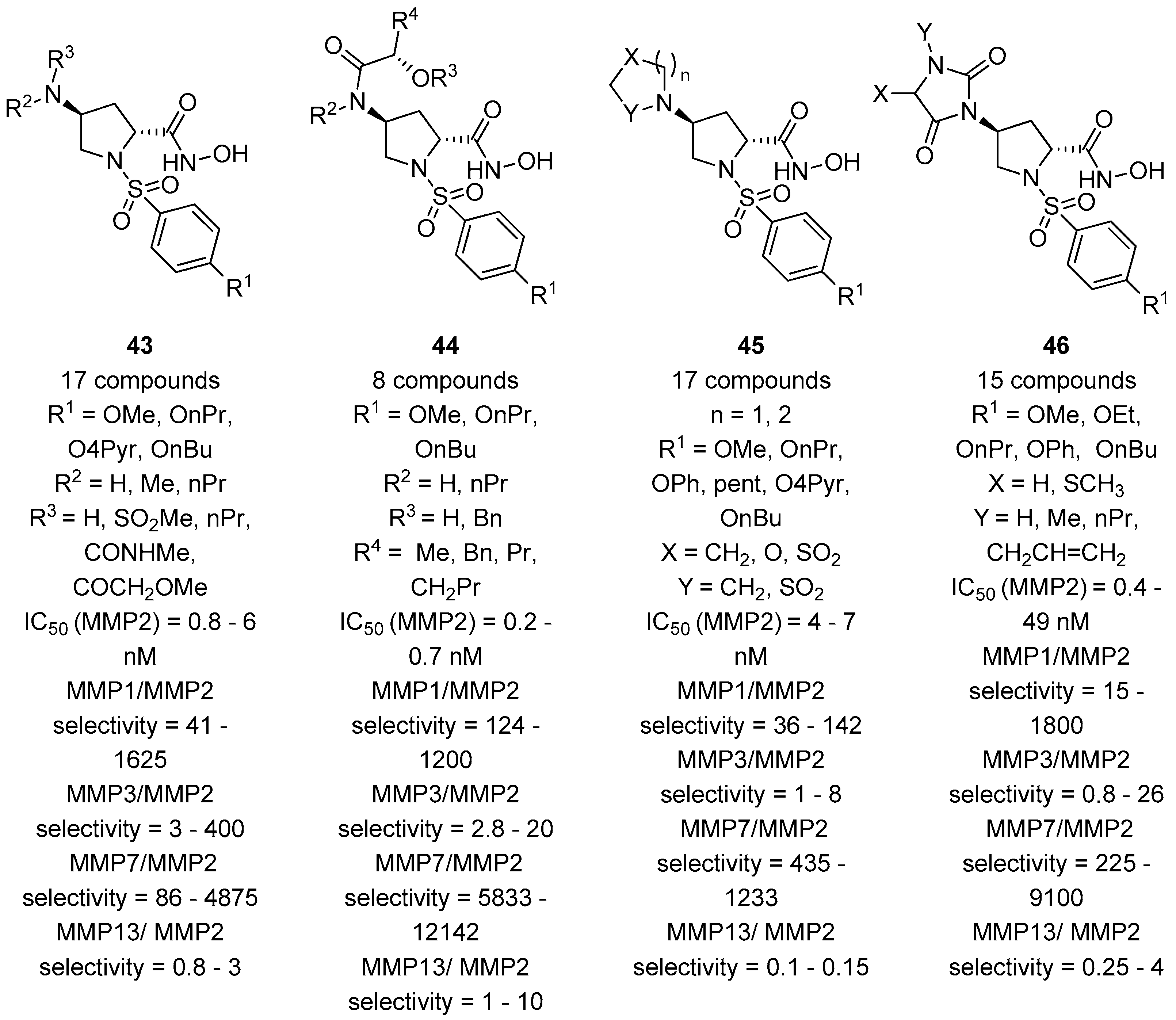
In a complementary approach, the group of Taiwo and coworkers [63] reasoned to address the selectivity for the MMP-2 by introducing on position 4 of the pyrrolidine ring a panel of different functionalities, including amides, sulfonamides, and ureas. Also, different sulfonyl groups at the nitrogen atom of the proline core were attached in order to better study the influence of the aromatic group in addressing the S1’ pocket. Fifty-six compounds (with general formula 43–46, Figure 10) were developed by this group starting from cis-4-hydroxy-d-proline and tested in vitro for the inhibition against collagenase-1 (MMP-1), gelatinase-A (MMP-2), stromelysin (MMP-3), matrilysin (MMP-7), and collagenase-3 (MMP-13). This class of compounds inhibits effectively MMP-2 and -13, with a good selectivity towards MMP-7. The inhibition of MMP-1 and MMP-3 proved to be more variable, depending on the functional group on position 4. However, while longer aliphatic substituents in the sulfonamide appendage resulted in an improvement of the selectivity for the MMP-2 enzyme, the insertion of different groups on position 4 did not show any remarkable effects.
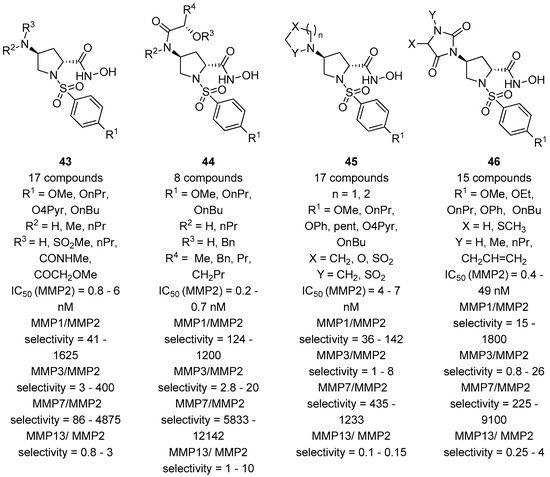
Figure 10.
The general formula of d-proline derivatives 43–46 developed by Taiwo and coworkers, their binding activity for MMP-2, and their selectivity between other MMPs (MMP-1, MMP-3, MMP-7, and MMP-13).
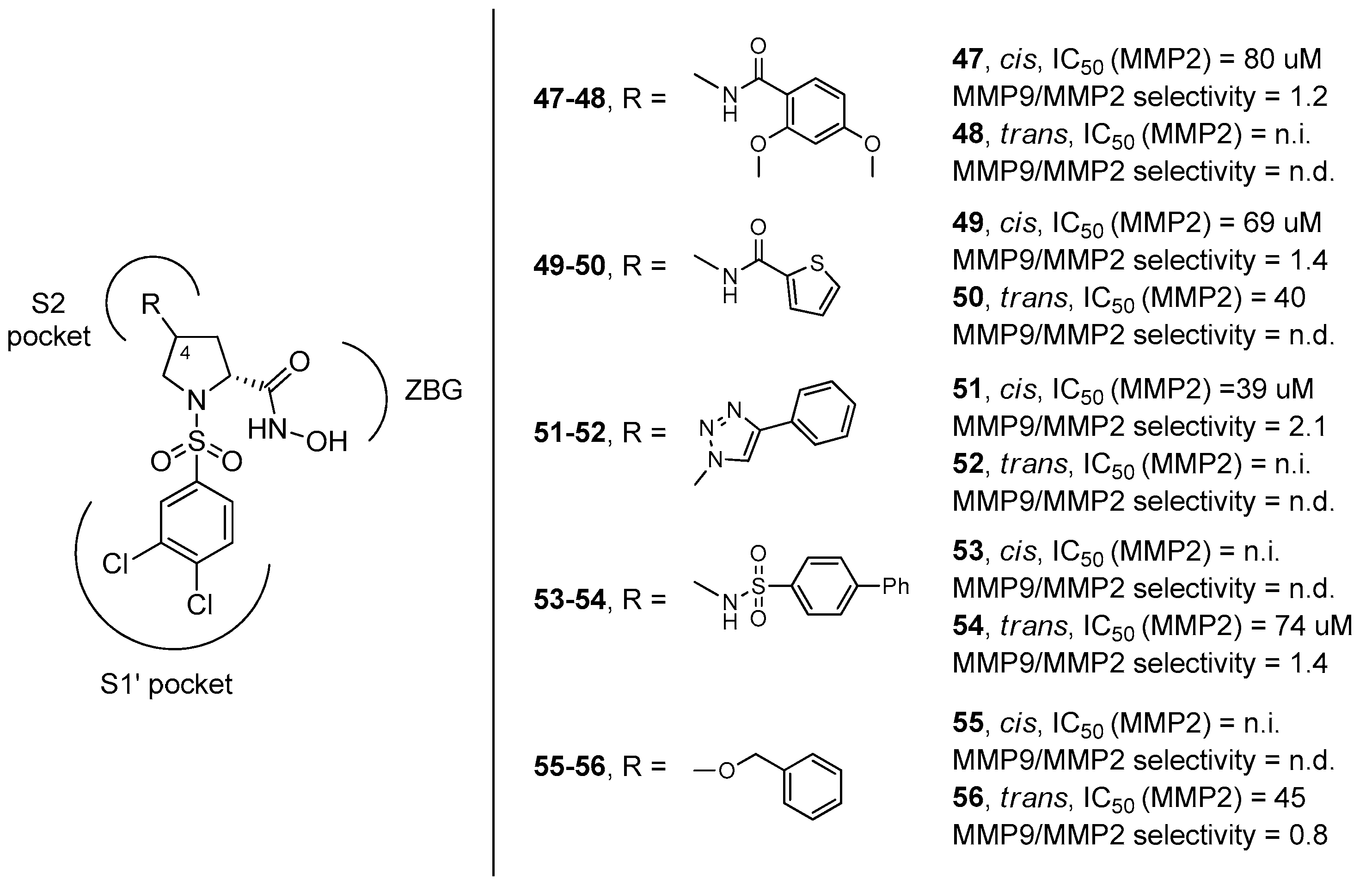
The fact that functional groups at position 4 did not improve significantly the selectivity for MMP-2 versus MMP-9 was also assessed by our research group when we evaluated the inhibition profile of d-proline derivatives 47–57 (Figure 11) [64]. These compounds showed a general preference for the inhibition towards MMP-9 because the substituent and the stereochemistry at C-4, addressing the S2 pocket, were not sufficiently tuned to balance the strong interaction of the 3,4-dichlorophenyl moiety with the S1’ pocket of MMP-9. This work also revealed that the R configuration at the C-4 stereocenter proved to give a better inhibition, as also confirmed in the paper by Xu and coworkers [65], where an array of α-sulfonyl γ-(glycinyl-amino)proline peptidomimetic derivatives with a cis-configuration at the proline scaffold showed a good selectivity for the MMP-2 enzyme over APN/CD13 (aminopeptidases) and HDACs.
Figure 11.
d-proline derivatives 47–56 developed by Trabocchi and coworkers, their binding activity for MMP-2, and their selectivity between the two gelatinases (MMP-9/MMP-2).
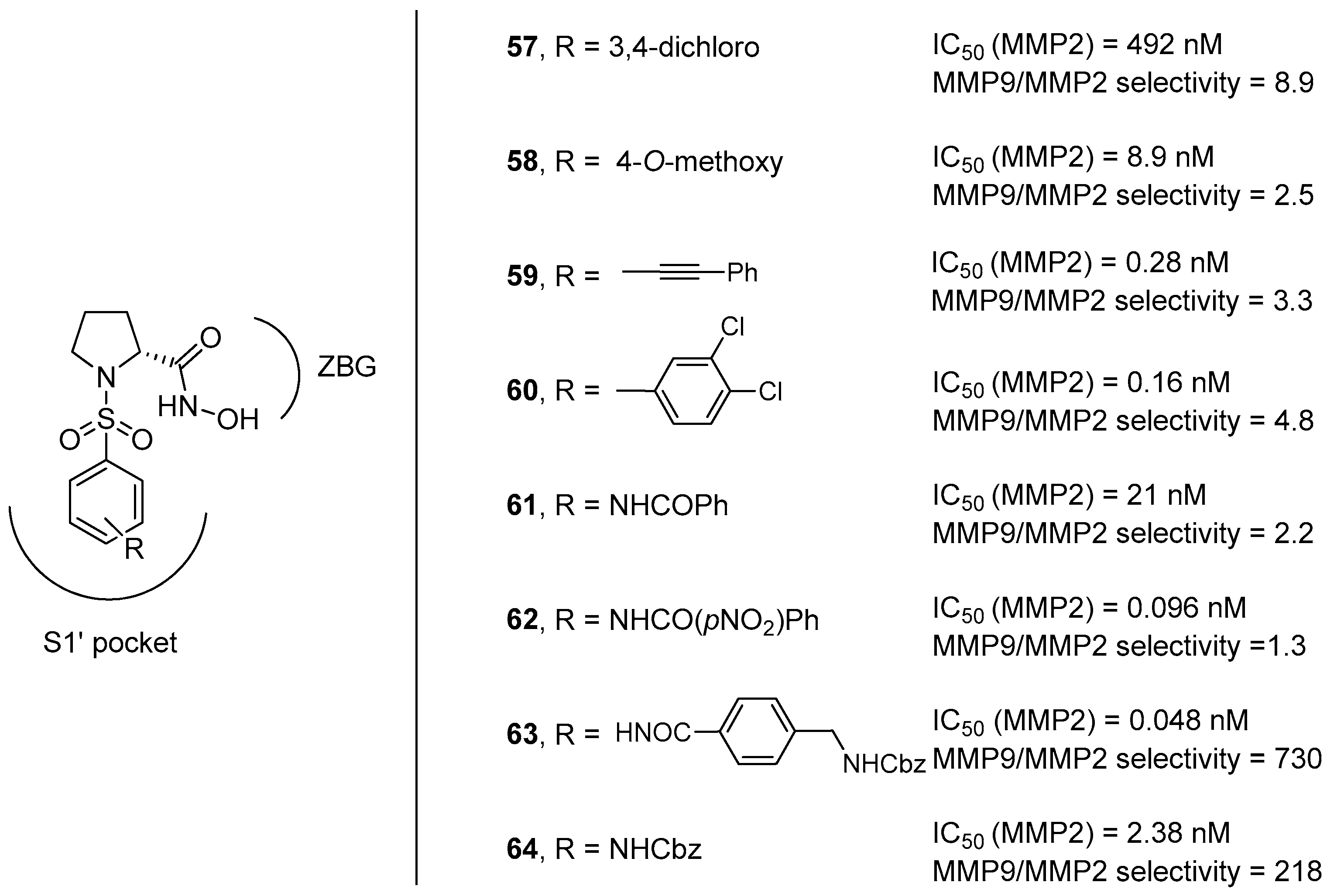
For this reason, our research group tried to advance towards this direction by exploring the interaction of different N-arylsulfonyl moieties at the nitrogen atom within the S1’ subsite, considering that the S1’ pocket of MMP-9 showed a higher degree of hydrophobic nature with respect to that of MMP-2 [66]. Eight different d-proline derivatives were developed, possessing appendages with 1 to 3 rings (compounds 59–64 in Figure 12) and compared to the model compounds 57 and 58, synthesized as the unsubsituted reference compounds corresponding to those previously reported in the literature. The results of the in vitro screening against MMP-2 and MMP-9 purified enzymes, as well as of the cell-based assays, showed that compounds 63–64 with long hydrophobic chains showed a remarkable selectivity thanks to the existence of a long hydrophobic channel at the bottom of the S1’ subsite in the MMP-2 catalytic cleft.
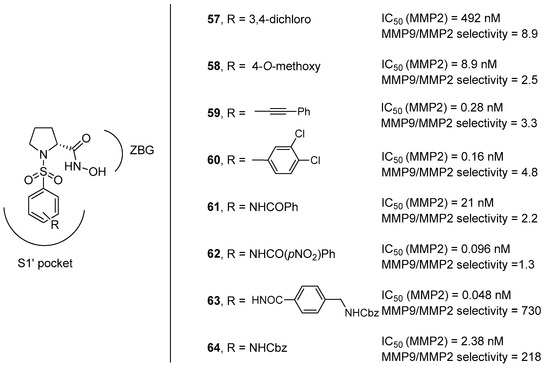
Figure 12.
d-proline derivatives 57–64 developed by Trabocchi and coworkers, their binding activity for MMP-2, and their selectivity between the two gelatinases (MMP-9/MMP-2).
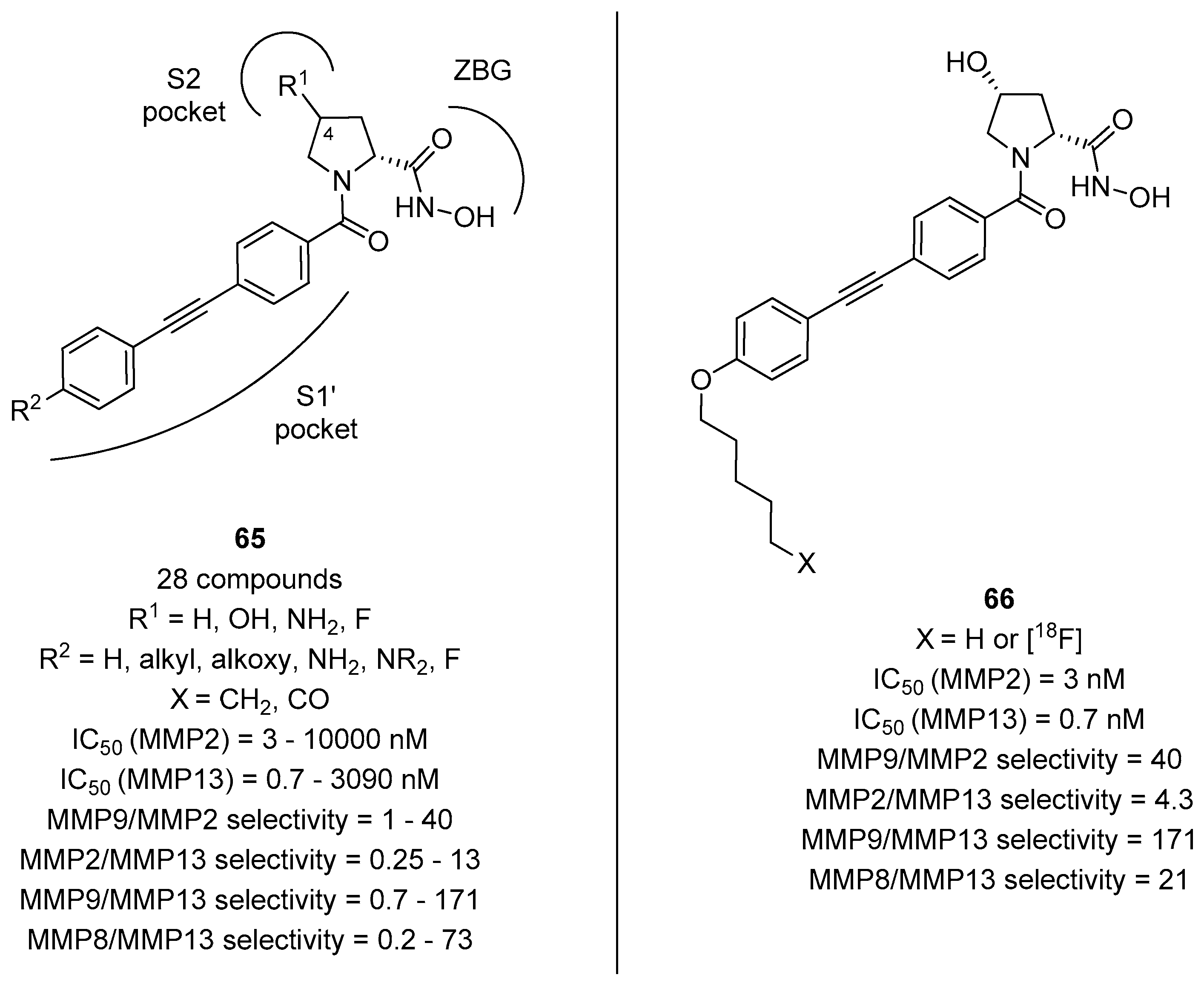
The importance of the long hydrophobic chain addressing the S1’ pocket was also found to be important in achieving selectivity for other MMPs, as evinced by the work of Holl and coworkers [67]. In fact, d- and l-proline derivatives with general formula 65 and a long lipophilic tail (Figure 13) were found to be highly selective also for collagenase 3 (MMP-13), an important target not only for anticancer drug development but also in Alzheimer’s disease [68]. Even in this case, it was evinced that the cis relative stereochemistry was found to be extremely important for the selectivity and the binding activity, as compounds derived from (2R,4R)-4-hydroxyproline were more potent than analogues with a (2S,4R)-4-hydroxyproline or a (2S)-proline scaffold. The best compound was found to be 66 possessing an n-pentyl moiety, as this compound showed a picomolar activity for MMP-13 and a great selectivity towards other MMPs as well as towards other Zn2+-dependent metalloproteases, such as ADAMs and meprins. Also, the [18F]-radiolabeled analogue was synthesized by this group and subjected to in vivo studies, revealing that this ligand possesses an excellent sereum stability and a relative rapid clearance in mice, demonstrating the valence of this ligand as a PET imaging tracer able to quantify the MMP-13 activity in a variety of in vivo pathological model systems.
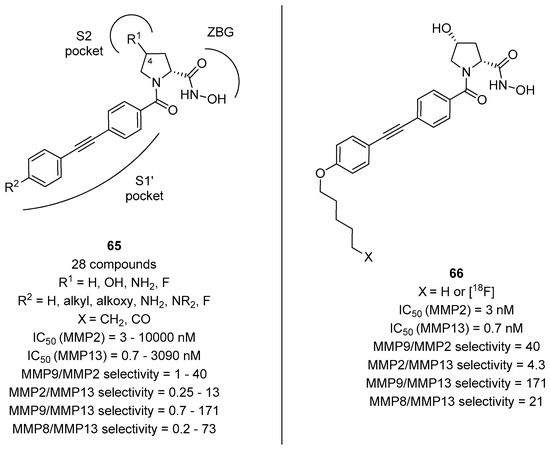
Figure 13.
d-proline derivatives with general formula 65 developed by Holl and coworkers, their binding activity for MMP-2 and MMP-13, and their selectivity towards MMP-9 and MMP-8: The structure of the most potent and selective compound 66 and of its radiolabeled analogue.
4. Conclusions
Proline is one of the most relevant amino acids for chemical biology and medicinal chemistry applications due to the stabilizing properties and the conformational restriction of the pyrrolidine ring. Although for many years, researchers believed that d-amino acids have only a minor function as compared to their l-enantiomers, d-proline was shown to play a crucial role in many pathophysiological events. The d-Proline chemotype is contained in more than 20 approved or investigational drugs present in the drug bank database, as well as in the naturally occurring HDAC inhibitors of the Trapoxin family. d-Proline-rich peptides were shown to provide bacteria and parasites the peculiar resistance against host proteolytic mechanism. Also, the d-proline chemotype were shown to possess the right conformation to accommodate itself in the catalytic clefts of different metalloenzymes. Accordingly, d-proline derivatives have proven to efficiently inhibit the IMP-1 enzyme, belonging to the B1 subclass of metallo-β-lactamases (MBLs), one of the main targets responsible for antibiotic resistance, demonstrating the higher affinity of this conformation, as compared to the one adopted by l-proline derivatives, in coordinating both Zn2+ ions through the thiolate and the carboxylate functional groups. Also, d-proline derivatives, possessing different appendages on position 3/4 of the pyrrolidine ring and different N-arylsulfonyl moieties, have been designed to selectively inhibit some matrix metalloprotease (MMP) enzymes, including gelatinase A (MMP-2), a validated molecular target in cancer drug discovery. Even in this case, the relative cis-configuration of the two hydrophobic groups linked to the pyrrolidine scaffold was found to be extremely important for selectivity and binding activity, as compounds derived from (2R,4R)-4-hydroxy-d-proline were more potent than analogues with a (2S,4R)-4-hydroxy-d-proline or a l-proline scaffold. One of the main limitations of using d-proline for the development of enzyme inhibitors is related to the cis/trans isomerization process. As for l-proline, the small energy difference between the two conformations results in a high probability of finding both the cis and trans isomers in solution even at room temperature. This isomerization plays a key role in affecting the geometry and, consequently, the biological output of the peptides or of the peptidomimetic compounds. However, this limitation can be controlled intentionally by replacing d-proline with suitable analogues in which the rotational flexibility is reduced by bulky substituents, heteroatoms, or different sizes of the ring. Thus, the potential of d-proline as a conformationally restricted scaffold for the development of enzyme inhibitors has drawn considerable interest in the organic and medicinal chemistry community, and there is still much room left for the generation of novel small molecules for a drug discovery purpose around this scaffold.
Author Contributions
E.L. and A.T. conceived and wrote the paper.
Funding
This research was funded by MIUR (PRIN2015, cod. 20157WW5EH), Fondazione CR Firenze (cod. 2017.0721), CNR roadmap europea ESFRI: CISPIM, and the University of Florence.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Genchi, G. An overview on D-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Piquet, S.; Maisons, A.; Furlan, S.; Courtin, P.; Chapot-Chartier, M.P.; Kulakauskas, S. Identification of an essential gene responsible for d-Asp incorporation in the Lactococcus lactis peptidoglycan crossbridge. Mol. Microbiol. 2006, 62, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Hamase, K.; Morikawa, A.; Zaitsu, K. D-amino acids in mammals and their diagnostic value. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2002, 781, 73–91. [Google Scholar] [CrossRef]
- Hamase, K. Sensitive two-dimensional determination of small amounts of D-amino acids in mammals and the study on their functions. Chem. Pharm. Bull. 2007, 55, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.A.; Berger, R.; Klomp, L.W.; de Koning, T.J. D-amino acids in the central nervous system in health and disease. Mol. Genet. Metab. 2005, 85, 168–180. [Google Scholar] [CrossRef]
- Bauer, D.; Hamacher, K.; Bröer, S.; Pauleit, D.; Palm, C.; Zilles, K.; Coenen, H.; Langen, K.J. Preferred stereoselective brain uptake of d-serine-a modulator of glutamatergic neurotransmission. Nucl. Med. Biol. 2005, 32, 793–797. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, A. D-aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Nutritional and medicinal aspects of D-amino acids. Amino Acids 2012, 42, 1553–1582. [Google Scholar] [CrossRef]
- Sladojevich, F.; Guarna, A.; Trabocchi, A. Evaluation of stereochemically dense morpholine-based scaffolds as proline surrogates in β-turn peptides. Org. Biomol. Chem. 2010, 7, 916–924. [Google Scholar] [CrossRef]
- Sladojevich, F.; Trabocchi, A.; Guarna, A. Configurationally-driven folding of model tetrapeptides containing l- or d-morpholine-3-carboxylic acids as β-turn nucleators. Chirality 2009, 21, 584–594. [Google Scholar]
- Cini, N.; Trabocchi, A.; Menchi, G.; Bottoncetti, A.; Raspanti, S.; Pupi, A.; Guarna, A. Morpholine-based RGD-cyclopentapeptides as αvβ3/αvβ5 integrin ligands: Role of configuration towards receptor binding affinity. Biorg. Med. Chem. 2009, 17, 1542–1549. [Google Scholar] [CrossRef]
- Yoshimura, T.; Esak, N. Amino acid racemases: Functions and mechanisms. J. Biosci. Bioeng. 2003, 96, 103–109. [Google Scholar] [CrossRef]
- Ollivaux, C.; Soyez, D.; Toullec, J.Y. Biogenesis of d-amino acid containing peptides/proteins: Where, when and how? J. Pept. Sci. 2014, 20, 595–612. [Google Scholar] [CrossRef]
- Mauger, A.B. Naturally Occurring Proline Analogues. J. Nat. Prod. 1996, 59, 1205–1211. [Google Scholar] [CrossRef]
- Newberry, R.W.; Raines, R.T. The n → π * interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [Google Scholar] [CrossRef]
- Sanchez-Navarro, M.; Teixido, M.; Giralt, E. Jumping hurdles: Peptides able to overcome biological barriers. Acc. Chem. Res. 2017, 50, 1847–1854. [Google Scholar] [CrossRef]
- Graf, M.; Mardirossian, M.; Nguyen, F.; Seefeldt, A.C.; Guichard, G.; Scocchi, M.; Innis, C.A.; Wilson, D.N. Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep. 2017, 34, 702–711. [Google Scholar] [CrossRef]
- Kraus, A. Proline and lysine residues provide modulatory switches in amyloid formation: Insights from prion protein. Prion 2016, 10, 57–62. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Khare, P.; Nagar, H.K.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases. Curr. Protein. Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef]
- Dienstag, J.L. Antiviral Drugs against Hepatitis Viruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2015; Volume 1, pp. 563–575. [Google Scholar]
- Smieja, M. Current indications for the use of clindamycin: A critical review. Can. J. Infect. Dis. 1998, 9, 22–28. [Google Scholar] [CrossRef]
- Weiss, B.; Alt, A.; Ogden, A.M.; Gates, M.; Dieckman, D.K.; Clemens-Smith, A.; Ho, K.H.; Jarvie, K.; Rizkalla, G.; Wright, R.A.; et al. Pharmacological characterization of the competitive GLUK5 receptor antagonist decahydroisoquinoline LY466195 in vitro and in vivo. J. Pharm. Exp. 2006, 318, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Butlin, M.; Avolio, A.P. Persistent effect of early, brief angiotensin-converting enzyme inhibition on segmental pressure dependency of aortic stiffness in spontaneously hypertensive rats. J. Hypertens. 2012, 30, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Drug Bank. Available online: https://www.drugbank.ca (accessed on 20 February 2019).
- Allington, D.R.; Rivey, M.P. Quinupristin/dalfopristin: A therapeutic review. Clin. Ther. 2001, 23, 24–44. [Google Scholar] [CrossRef]
- Seipel, K.; Marques, M.A.T.; Sidler, C.; Mueller, B.U.; Pabst, T. The Cellular p53 Inhibitor MDM2 and the Growth Factor Receptor FLT3 as Biomarkers for Treatment Responses to the MDM2-Inhibitor Idasanutlin and the MEK1 Inhibitor Cobimetinib in Acute Myeloid Leukemia. Cancers 2018, 10, 170. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Tennent, G.A.; Hutchinson, W.L.; Gallimore, J.R.; Lachmann, H.J.; Goodman, H.J.B.; Offer, M.; Millar, D.J.; Petrie, A.; Hawkins, P.N.; et al. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br. J. Haematol. 2010, 148, 760–767. [Google Scholar] [CrossRef]
- Pedersen, C.M.; Venkatasubramanian, S.; Vase, H.; Hyldebrandt, J.A.; Contractor, H.; Schmidt, M.R.; Bøtker, H.E.; Cruden, N.L.; Newby, D.E.; Kharbanda, R.K.; et al. Rotigaptide protects the myocardium and arterial vasculature from ischaemia reperfusion injury. Br. J. Clin. Pharmacol. 2016, 81, 1037–1045. [Google Scholar] [CrossRef]
- König, S.; Marco, H.; Gäde, G. The hypertrehalosemic neuropeptides of cicadas are structural isomers—evidence by ion mobility mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- Reina-San-Martin, B.; Degrave, W.; Rougeot, C.; Cosson, A.; Chamond, N.; Cordeiro-Da-Silva, A.; Arala-Chaves, M.; Coutinho, A.; Minoprio, P. A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat. Med. 2000, 6, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Coatnoan, N.; Berneman, A.; Chamond, N.; Minoprio, P. Proline racemases: Insights into Trypanosoma cruzi peptides containing D-proline. Mem. Inst. Oswaldo Cruz 2009, 104, 295–300. [Google Scholar] [CrossRef]
- Chamond, N.; Goytia, M.; Coatnoan, N.; Barale, J.C.; Cosson, A.; Degrave, W.M.; Minoprio, P. Trypanosoma cruzi proline racemases are involved in parasite differentiation and infectivity. Mol. Microbiol. 2005, 58, 46–60. [Google Scholar] [CrossRef]
- Islam, M.S.; Bhuiyan, M.P.I.; Islam, M.N.; Nsiama, T.K.; Oishi, N.; Kato, T.; Nishino, N.; Ito, A.; Yoshida, M. Evaluation of functional groups on amino acids in cyclic tetrapeptides in histone deacetylase inhibition. Amino Acids 2012, 42, 2103–2110. [Google Scholar] [CrossRef]
- Yoshida, M.; Matsuayama, A.; Komatsu, Y.; Nishino, N. From discovery to the coming generation of histone deacetylase inhibitors. Curr. Med. Chem. 2003, 10, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Zink, D.L.; Liesch, J.M.; Dombrowski, A.W.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goet, M.A. Structure, Histone Deacetylase, and Antiprotozoal Activities of Apicidins B and C, Congeners of Apicidin with Proline and Valine Substitutions. Org. Lett. 2001, 3, 2815–2818. [Google Scholar] [CrossRef] [PubMed]
- Trabocchi, A.; Guarna, A. Cyclic α-Amino Acids as Proline Mimetics. In Peptidomimetics in Organic and Medicinal Chemistry: The Art of Transforming Peptides in Drugs, 1st ed.; Trabocchi, A., Guarna, A., Eds.; John Wiley and Sons, Ltd: Chichester, UK, 2014; pp. 165–190. [Google Scholar]
- Luppi, G.; Lanci, D.; Trigari, V.; Garavelli, M.; Garelli, A.; Tomasini, A. Development and conformational analysis of a pseudoproline-containing turn mimic. J. Org. Chem. 2003, 68, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Kern, D.; Schutkowski, M.; Drakenberg, T. cis–trans-Amide isomerism of the 3,4-dehydroproline residue, the ‘unpuckered’ proline. J. Am. Chem. Soc. 1997, 119, 8403–8408. [Google Scholar] [CrossRef]
- Beausoleil, E.; L’Archevêque, B.; Bélec, L.; Atfani, M.; Lubell, W.D. 5-tert-Butylproline. J. Org. Chem. 1996, 61, 9447–9454. [Google Scholar] [CrossRef]
- Cornaglia, G.; Giamarellou, H.; Rossolini, G.M. Metallo-β-lactamases: A last frontier for -lactams? Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar] [CrossRef]
- Heinz, U.; Bauer, R.; Wommer, S.; Meyer-Klaucke, W.; Papamichaels, C.; Bateson, J.; Adolph, H.W. Coordination Geometries of Metal Ions in d- or l-Captopril-inhibited Metallo-β-lactamases. J. Biol. Chem. 2003, 278, 20659–20666. [Google Scholar] [CrossRef]
- Karsisiotis, A.I.; Damblon, C.F.; Roberts, G.C.K. A Variety of Roles for versatile zinc in Metallo-β-lactamases. Metallomics 2014, 6, 1181–1197. [Google Scholar] [CrossRef]
- Brem, J.; van Berkel, S.S.; Zollman, D.; Lee, S.Y.; Gileadi, O.; McHugh, P.J.; Walsh, T.R.; McDonough, M.A.; Schofield, C.J. Structural Basis of Metallo-β-Lactamase Inhibition by Captopril Stereoisomers. Antimicrob. Agents Chemother. 2015, 60, 142–150. [Google Scholar] [CrossRef]
- Faridoon; Hussein, W.M.; Vella, P.; Nazar, U.I.; Ollis, D.L.; Schenk, G.; McGeary, R.P. 3-Mercapto-1,2,4-triazoles and N-acylated Thiosemicarbazides as Metallo-β-lactamase Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 380–386. [Google Scholar] [CrossRef]
- Page, M.I.; Badarau, A. The Mechanism of Catalysis by Metallo-β-Lactamases. Bioinorg. Chem. Appl. 2008, 1–14. [Google Scholar] [CrossRef]
- Bebrone, C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharm. 2007, 74, 1686–1701. [Google Scholar] [CrossRef]
- Goto, M.; Takahashi, T.; Yamashita, F.; Koreeda, A.; Mori, H.; Ohta, M.; Arakawa, Y. Inhibition of the Metallo-β-lactamase Produced from Serratia marcescens by Thiol Compounds. Biol. Pharm. Bull. 1997, 20, 1136–1140. [Google Scholar] [CrossRef]
- Yusof, Y.; Tan, D.T.C.; Arjomandi, O.K.; Schenk, G.; McGeary, R.P. Captopril Analogues as Metallo-β-lactamase Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1589–1593. [Google Scholar] [CrossRef]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Vihinen, P.; Ala-aho, R.; Kahari, V.M. Matrix metalloproteinases as therapeutic targets in cancer. Curr. Cancer Drug Targets 2005, 5, 203–220. [Google Scholar] [CrossRef]
- Fingleton, B. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin. Tar. 2003, 7, 385–390. [Google Scholar] [CrossRef]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix Metalloproteinase Inhibitors and Cancer—Trials and Tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Li, N.G.; Shi, Z.H.; Tang, Y.P.; Duan, J.A. Selective matrix metalloproteinase inhibitors for cancer. Curr. Med. Chem. 2009, 16, 3805–3827. [Google Scholar] [CrossRef]
- Beattie, G.J.; Young, H.A.; Smyth, J.F. Phase I study of intra-peritoneal metalloproteinase inhibitor BB-94 in patients with malignant ascites. Ann. Oncol. 1994, 5, 72a. [Google Scholar]
- Rothenberg, M.L.; Nelson, A.R.; Hande, K.R. New Drugs on the Horizon: Matrix Metalloproteinase Inhibitors. Oncologist 1998, 3, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Levitt, N.C.; Eskens, F.A.; O’Byrne, K.J.; Propper, D.J.; Denis, L.J.; Owen, S.J.; Choi, L.; Foekens, J.A.; Wilner, S.; Wood, J.M.; et al. A phase one pharmacokinetic study of CGS27023A, a matrix metalloproteinase inhibitor. Proc. Am. Soc. Clin. Oncol. 1998, 17, 213a. [Google Scholar]
- Jenkins, C.L.; McCloskey, A.I.; Guzei, I.A.; Eberhardt, E.S.; Raines, R.T. O-acylation of hydroxyproline residues: Effect on peptide-bond isomerization and collagen stability. Biopolymers 2005, 80, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, L.; Berisio, R.; Mazzarella, L.; Zagari, A. Structural bases of collagen stabilization induced by proline hydroxylation. Biopolymers 2001, 58, 459–464. [Google Scholar] [CrossRef]
- Hanessian, S.; MacKay, D.B.; Moitessier, N. Design and Synthesis of Matrix Metalloproteinase Inhibitors Guided by Molecular Modeling. Picking the S1 Pocket Using Conformationally Constrained Inhibitors. J. Med. Chem. 2001, 44, 3074–3082. [Google Scholar] [CrossRef]
- Natchus, M.G.; Bookland, R.G.; De, B.; Almstead, N.G.; Pikul, S.; Janusz, M.J.; Heitmeyer, S.A.; Hookfin, E.B.; Hsieh, L.C.; Dowty, M.E.; et al. Development of new hydroxamate matrix metalloproteinase inhibitors derived from functionalized 4-aminoprolines. J. Med. Chem. 2000, 43, 4948–4963. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Calugi, C.; Ruzzolini, J.; Menchi, G.; Calorini, L.; Guarna, A.; Trabocchi, A. A study of a D-proline peptidomimetic inhibitor of melanoma and endothelial cell invasion through activity towards MMP-2 and MMP-9. Med. Chem. Comm. 2015, 6, 277–282. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Jiang, Y.; Feng, J.; Li, X.; Zhang, Y.; Xu, W. Design, synthesis and preliminary evaluation of α-sulfonyl γ-(glycinyl-amino)proline peptidomimetics as matrix metalloproteinase inhibitors. Bioorg. Med. Chem. 2014, 22, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Innocenti, R.; Di Francescantonio, T.; Menchi, G.; Bianchini, F.; Contini, A.; Trabocchi, A. Identification of highly potent and selective MMP2 inhibitors addressing the S1’ subsite with d-proline-based compounds. Bioorg. Med. Chem. 2019, 27, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, D.V.; Wagner, S.; Riemann, B.; Hermann, S.; Schmidt, F.; Becker-Pauly, C.; Rose-John, S.; Schäfers, M.; Holl, R. Novel Potent Proline-Based Metalloproteinase Inhibitors: Design, (Radio)Synthesis, and First in Vivo Evaluation as Radiotracers for Positron Emission Tomography. J. Med. Chem. 2016, 59, 9541–9559. [Google Scholar] [CrossRef]
- Paumier, J.M.; Thinakaran, G. Matrix metalloproteinase 13, a new target for therapy in Alzheimer’s disease. Genes Dis. 2019, 16, 1–2. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).