2.1. Transient Expression
A pair of IgG
4 antibodies was previously identified [
12] whereby an Ala to Gly mutation at Kabat position 49 resulted in a four-fold decrease in transient CHO expression. The Gly is the “naturally preferred” amino acid at this position and is present in ~50% of human antibody sequences with Ala and Ser equally contributing to the other 50% [
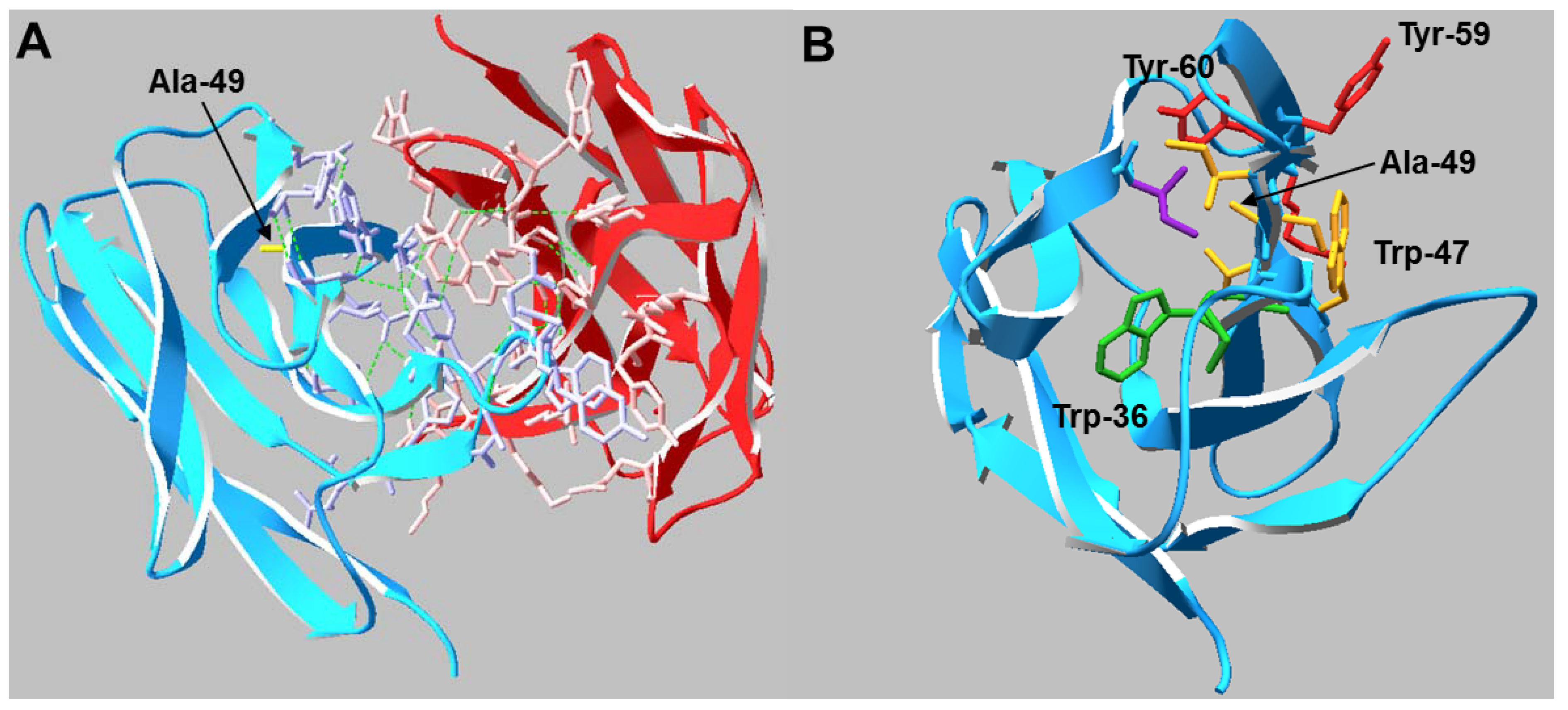
13]. The mutation lies within the heavy chain variable framework, two residues before the CDR2 loop. Analysis of the Fab crystal structure suggests the side chain of any residue in Kabat position 49 may play a critical role in stabilizing the hydrophobic core of the V
H domain as well as maintaining the conformation of adjacent residues which comprise part of the V
H/V
L interface (
Figure 1). To determine if mild hypothermia alters the innate differential expression of this antibody pair, CHO-S cells were transiently transfected with Ala or Gly double gene vectors (DGV, described in
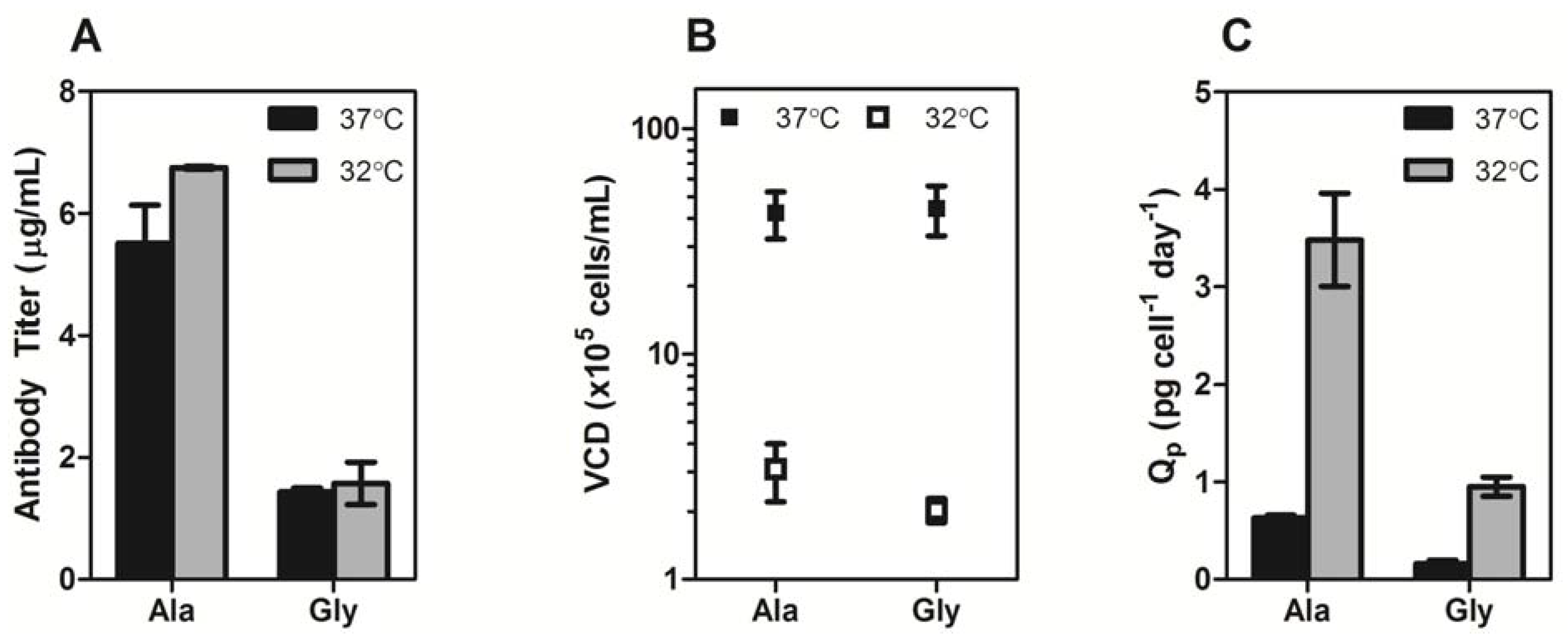
Section 3.2) and immediately incubated at 37 °C or 32 °C for five days. The Ala variant exhibited four-fold higher monoclonal antibody (mAb) expression than the Gly variant at 37 °C and 32 °C (
Figure 2a). Culturing the cells at 32 °C gave a slight improvement in mAb titer for both constructs (~1.2-fold), however the total viable cell densities for the cultures at 37 °C were at least 10-fold higher than the cultures at 32 °C (
Figure 2b), indicating that the average specific productivity was higher for the low temperature cultures (
Figure 2c).
Figure 1.
(A) Crystal structure of the VH/VL interactions in the Ala Fab domain. Residues that participate in the HC (blue) /LC (red) interface are shown in wire representation and the Ala-49 side chain is labeled (yellow). Hydrogen bonds are shown as green dashed lines. (B) Top view of the VH domain. Residues that may be conformationally influenced by Ala-49 (<5 Å distance) are shown in wire representation. Side chains sharing the same color reside on the same β-strand.
Figure 1.
(A) Crystal structure of the VH/VL interactions in the Ala Fab domain. Residues that participate in the HC (blue) /LC (red) interface are shown in wire representation and the Ala-49 side chain is labeled (yellow). Hydrogen bonds are shown as green dashed lines. (B) Top view of the VH domain. Residues that may be conformationally influenced by Ala-49 (<5 Å distance) are shown in wire representation. Side chains sharing the same color reside on the same β-strand.
Figure 2.
(A) Transient expression titers of Ala and Gly antibodies after a 5-day incubation at 37 °C (black bars) or 32 °C (gray bars). (B) The viable cell density (VCD) of the Ala and Gly cultures at 37 °C (■) and 32 °C (□). (C) Specific productivities of Ala and Gly antibodies at 37 °C (black bars) or 32 °C (gray bars). The error bars represent ± 1 standard deviation from the mean of experimental replicates (n = 2).
Figure 2.
(A) Transient expression titers of Ala and Gly antibodies after a 5-day incubation at 37 °C (black bars) or 32 °C (gray bars). (B) The viable cell density (VCD) of the Ala and Gly cultures at 37 °C (■) and 32 °C (□). (C) Specific productivities of Ala and Gly antibodies at 37 °C (black bars) or 32 °C (gray bars). The error bars represent ± 1 standard deviation from the mean of experimental replicates (n = 2).
Exposing CHO cultures to sub-physiological temperatures is known to arrest cells primarily in the G0/G1 phase and can correlate with high levels of biosynthetic activity [
5,
10,
11,
14]. The growth arrest of the 32 °C cultures over the entirety of the five day incubation is in agreement with this observation. A study by Wurm and coworkers found that performing a temperature shift from 37 °C to 31 °C at 0–4 h post-transfection resulted in the highest transient expression levels of an IgG in CHO cells [
14]. They could not attribute the increase in expression to a specific mechanism but suggested DNA uptake into the nucleus, DNA/mRNA stability, transcription, translation, and/or protein modification and transport may be responsible. Here, we observed that an immediate shift to 32 °C resulted in high apparent specific productivity, but only a marginal increase in volumetric productivity. Because the Amaxa Nucelofector method allows the transgenic DNA to directly enter the nucleus, the cells are capable of expression immediately and cell division is not required. This could suggest that the inhibition of cell division at 32 °C prevents dilution of intracellular recombinant DNA; in the 37 °C cultures the same amount of DNA is spread throughout a larger number of cells resulting in constant volumetric productivity, but decreased apparent specific productivity.
2.2. Stable Expression
To assess if specific productivity of stable systems is improved under mild hypothermia, multiple clonal cell lines stably expressing identical constructs to those used in the transient study were established as previously described [
12]. Cell lines were subcultured in the presence of 50 μM MSX and inoculated into shake flasks at 3 × 10
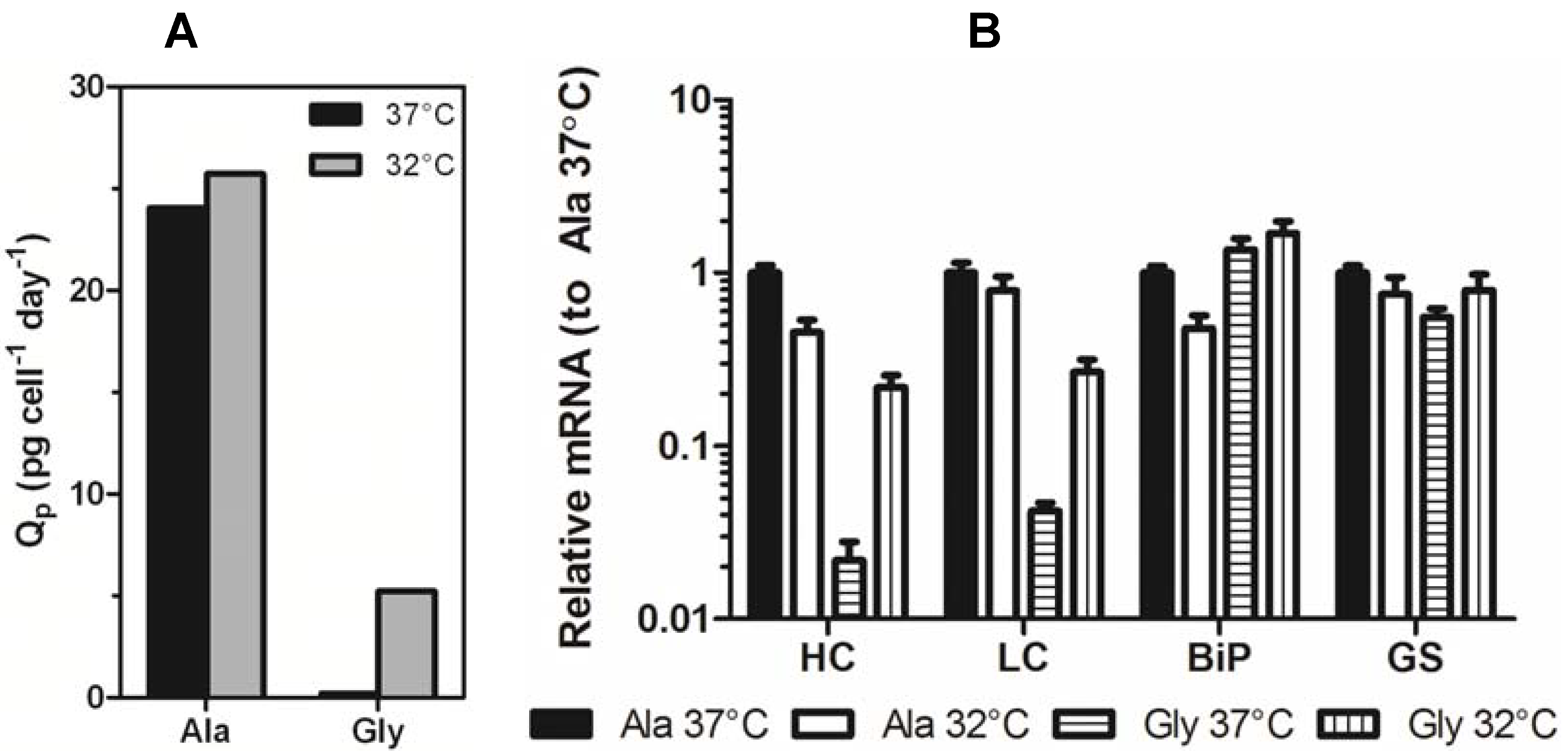
5 cells/mL followed by immediate incubation at either 37 °C or 32 °C. Samples were removed periodically to measure mAb titer and cell number. The initial experiments were performed on high expressing Ala (Ala-138, high expressing variant) and Gly (Gly-26, low expressing variant) clones. These cell lines exhibited a 100-fold difference in expression when cultured at 37 °C. Culturing the clones at 32 °C reduced the difference in expression between the Ala and Gly cell lines to 20-fold due to a 2-fold decrease in Ala-138 titer and a 10-fold increase in Gly-26 titer. When the decrease in growth rate was taken into account, the Ala specific productivity remained largely unchanged, whereas the Gly specific productivity increased 25-fold (
Figure 3a).
Samples from mid-exponential phase Ala-138 and Gly-26 clones cultured at 37 °C and 32 °C were analyzed using qRT-PCR to determine if decreased culture incubation temperature correlated with an increase in transgene mRNA. Decreasing culture temperature reduced the Ala HC and LC mRNA levels, whereas it resulted in a 10-fold increase in Gly HC and LC mRNA (
Figure 3b). GAPDH was used as an endogenous control. Similar, though less significant, changes were seen in immunoglobulin binding protein (BiP) and glutamine synthetase (GS). The decrease in Ala mRNA and increase in Gly mRNA contradicts previous studies attributing increased mRNA half-life and transcription as the primary mechanisms responsible for improvements in recombinant gene expression at reduced temperature. If mRNA-related mechanisms were the sole contributors to increased expression at reduced temperatures, then a universal increase in message for both constructs should be observed.
Figure 3.
(A) Specific productivity (Qp) of Ala-138 and Gly-26 mAb-producing clones in the mid-exponential growth phase at 37 °C, days 3–6 (black bars) and 32 °C, days 6–9 (gray bars). (B) Expression of HC, LC, BiP, and GS mRNA in Ala-138 and Gly-26 clones on day 4 at 37 °C or 32 °C incubation temperature. The mRNA levels for each gene were calibrated to Ala-138 at 37 °C. The error bars represent ±1 standard deviation of technical replicates (n = 3). 37 °C cultures were compared with 32 °C using a paired t-test (GraphPad Prism 5.03) and found to be statistically different (p = 0.0229 for Ala cultures and p = 0.0038 for Gly cultures).
Figure 3.
(A) Specific productivity (Qp) of Ala-138 and Gly-26 mAb-producing clones in the mid-exponential growth phase at 37 °C, days 3–6 (black bars) and 32 °C, days 6–9 (gray bars). (B) Expression of HC, LC, BiP, and GS mRNA in Ala-138 and Gly-26 clones on day 4 at 37 °C or 32 °C incubation temperature. The mRNA levels for each gene were calibrated to Ala-138 at 37 °C. The error bars represent ±1 standard deviation of technical replicates (n = 3). 37 °C cultures were compared with 32 °C using a paired t-test (GraphPad Prism 5.03) and found to be statistically different (p = 0.0229 for Ala cultures and p = 0.0038 for Gly cultures).
Clonal variation in recombinant protein expression is typically attributed to integration events that affect the ability of the DNA to be readily transcribed and therefore, change the level of transgene mRNA available for translation. A study by Yoon
et al. [
15] showed that the degree of Q
p enhancement under reduced temperature conditions varied between clones and that the enhancement decreased with increasing gene amplification. To ensure the differential effect of temperature on the expression of the mAb variants was not due to clonal variation, several clones (previously described by Mason
et al. [
12]) exhibiting different growth and expression profiles were analyzed in parallel. All clones exhibited the same differential effect of temperature on expression that was previously observed; all Ala clones exhibited compromised mAb expression when cultured at lower temperatures, whereas the Gly clones benefited (
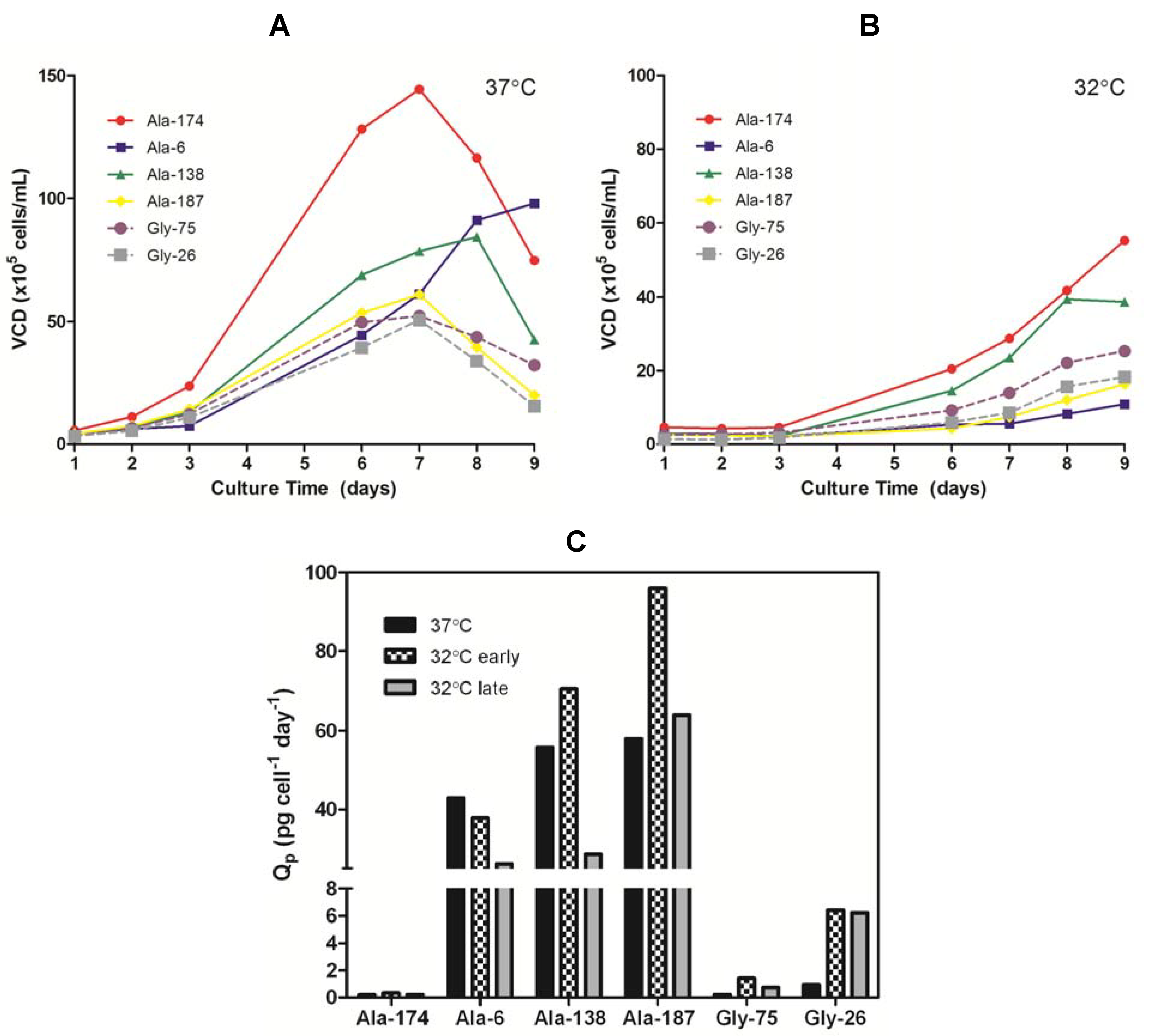
Table 1). Comparison of the growth curves for all clones at 37 °C
versus 32 °C (
Figure 4a,b) showed the cells cultured at the lower temperature had an extended lag phase up to three days, followed by a dynamic exponential growth phase as the cells adapted to the lower temperature, and finally a plateau in the stationary phase around day nine. The change in growth rate during the exponential phase, presumably due to the cells adapting to the lower temperature, resulted in a shift in Q
p with time. The Q
p in the early exponential growth phase (days 3–6) of all 32 °C cultures was comparable or higher than the 37 °C cultures. Once the growth rate began to accelerate (days 6–9), the Q
p for the Ala cultures decreased to a value at or below the 37 °C Q
p whereas the Gly clones retained an elevated Q
p (
Figure 4c). The maximum achievable antibody titers (<10% viable cells remaining in culture) obtained from the Ala clones were always lower when cultured at 32 °C than at 37 °C (
Table 1). There was some clonal variation in the impact of the reduced temperature on the Ala clones, but this appeared to be linked with growth rate. The clone that exhibited the slowest growth at 37 °C (Ala-6, 3.3-fold decrease) was the most affected by the reduction in temperature whereas the fastest growing clone (Ala-174, 1.1-fold decrease) showed little change in expression. Both Gly clones showed a five-fold improvement in titer when cultured at the lower temperature. Therefore, the universal negative impact on Ala expression and positive impact on Gly expression indicates that the effect of reduced temperature on productivity was predominantly a function of the protein being expressed with minor effects from clonal variation.
Table 1.
Effect of culture temperature on antibody expression.
Table 1.
Effect of culture temperature on antibody expression.
| Construct | Max. Titer at 37 °C (µg/mL ) | Max. Titer at 32 °C (µg/mL ) | Titer 32 °C/37 °C |
|---|
| Ala-174 | 7.42 | 6.72 | 0.91 |
| Ala-6 | 814 | 247 | 0.30 |
| Ala-138 | 1124 | 547 | 0.49 |
| Ala-187 | 848 | 389 | 0.46 |
| Gly-75 | 3.31 | 14.9 | 4.50 |
| Gly-26 | 12.2 | 63.3 | 5.19 |
2.3. Product Stability
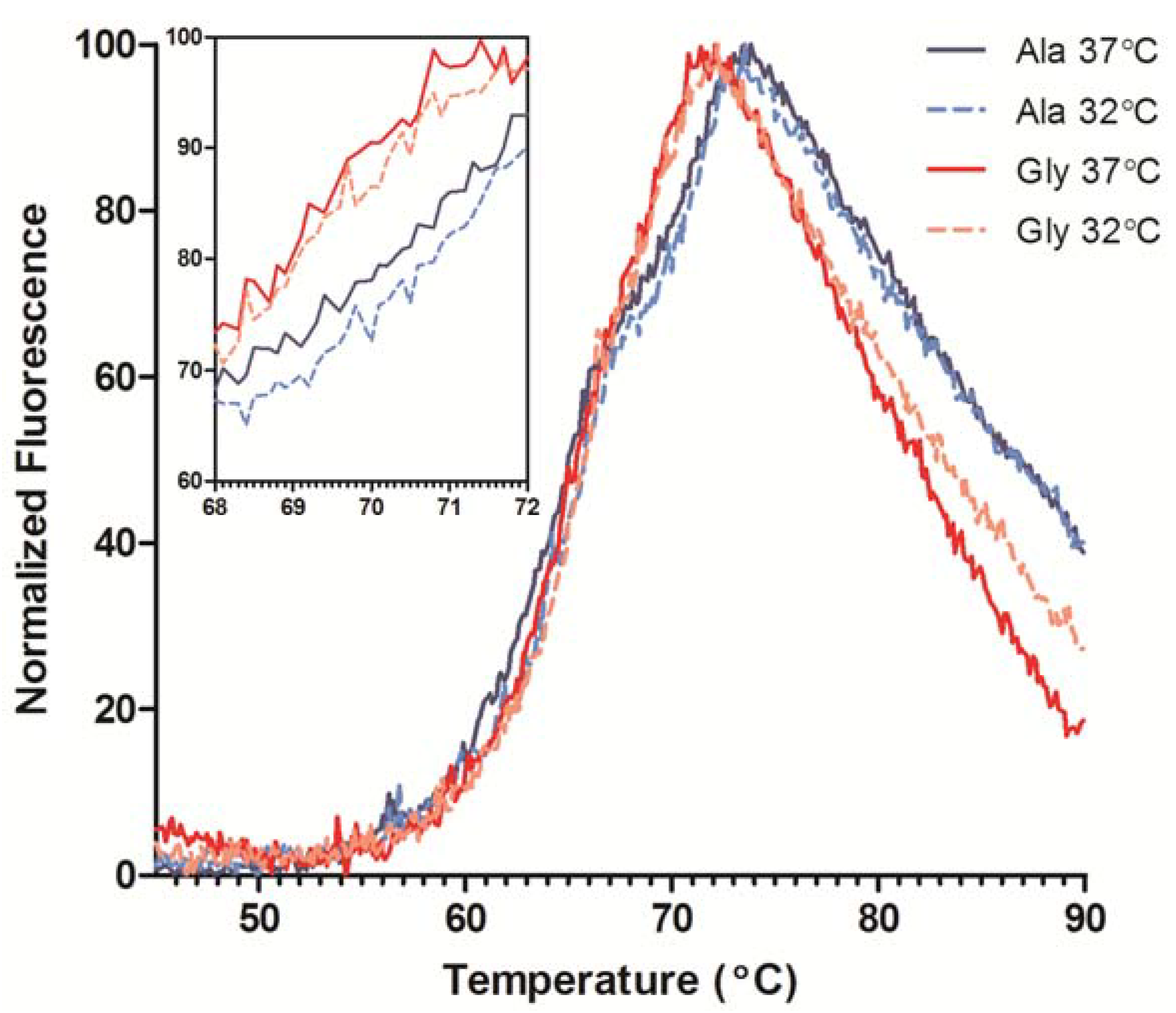
The stability of all samples was assessed using differential scanning fluorimetry (DSF). Instead of measuring the heat flux as in traditional calorimetric methods, DSF measures the fluorescence of an environmentally sensitive dye (SYPRO Orange) that binds to hydrophobic areas of protein [
16]. As the protein unfolds, the increase in exposed hydrophobic residues coincides with an increase in dye binding, and therefore, fluorescence signal. The melting temperature (T
m) is the temperature at which 50% of the protein is unfolded and can be estimated by finding the inflection point of each unfolding transition. For IgG
4 antibodies, 65 °C is the expected melting temperature (T
m) for a glycosylated Fc region and only one peak is observed due to cooperative unfolding of the C
H2 and C
H3 domains [
17]. The humanized Fab domain can exhibit a wide range of T
m’s typically between 60–80 °C [
18]. The DSF traces indicated two transitions, one for the unfolding of the C
H2 domain around 65 °C and the more intense Fab unfolding ~70 °C (
Figure 5). The Ala and Gly variants exhibited similar C
H2 melting profiles, but the Fab T
m’s differed by ~1.5 °C. DSF was also performed on transiently-expressed His-tagged Fabs of the Ala and Gly variants to ensure the Fab T
m is independent of the Fc T
m. The Gly Fab variant exhibited a T
m ~2 °C lower than the Ala Fab T
m, which is consistent with the full-length results (data not shown). Therefore, the reduction in mAb stability could be attributed to the Ala to Gly mutation and not from any Fc domain influences. A comparison of antibody product obtained from cultures performed at 37 °C and 32 °C resulted in a slight increase in stability (~0.5 °C) of the antibodies from the low temperature cultures (
Figure 5, inset); antibodies from both 32 °C cultures exhibited similar C
H2 traces.
Figure 4.
(A) Viable cell density (VCD) profiles for Ala and Gly clones grown at 37 °C in serum-free batch culture. (B) VCD profiles for Ala and Gly clones grown at 32 °C in serum-free batch culture. (C) Specific productivity (Qp) of Ala and Gly clones in mid-exponential growth phase at 37 °C (days 3–6) and 32 °C (early phase: days 3–6; late phase: days 7–8).
Figure 4.
(A) Viable cell density (VCD) profiles for Ala and Gly clones grown at 37 °C in serum-free batch culture. (B) VCD profiles for Ala and Gly clones grown at 32 °C in serum-free batch culture. (C) Specific productivity (Qp) of Ala and Gly clones in mid-exponential growth phase at 37 °C (days 3–6) and 32 °C (early phase: days 3–6; late phase: days 7–8).
Figure 5.
Differential scanning fluorimetry (DSF) profiles of purified Ala-138 and Gly-26 mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). The unfolding transitions for the CH2, CH3, and Fab domains are labeled. Each trace is the mean of four technical replicates. The inset graph shows detail over the Fab Tm range.
Figure 5.
Differential scanning fluorimetry (DSF) profiles of purified Ala-138 and Gly-26 mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). The unfolding transitions for the CH2, CH3, and Fab domains are labeled. Each trace is the mean of four technical replicates. The inset graph shows detail over the Fab Tm range.
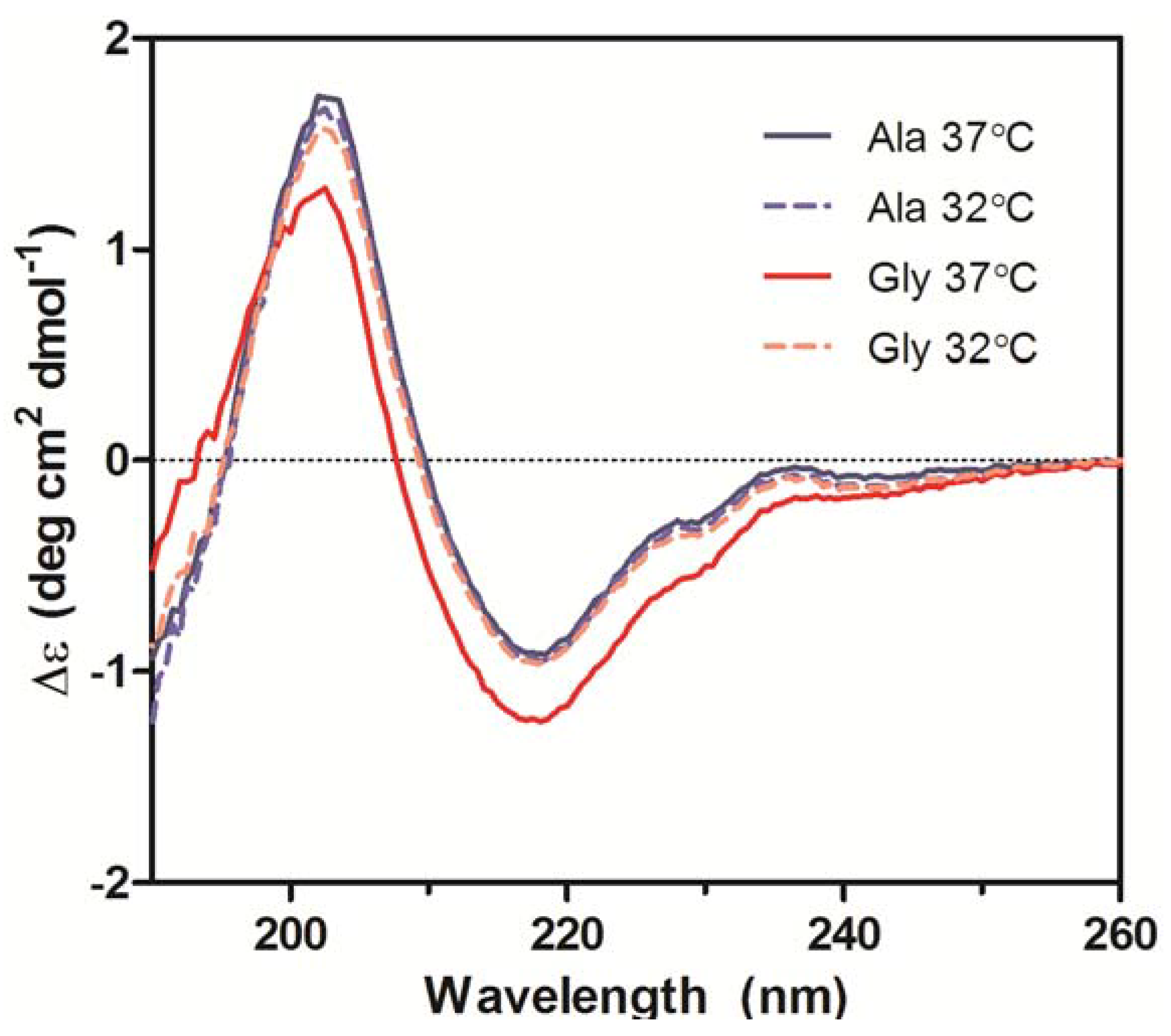
2.4. Circular Dichroism
Secondary structures of the purified Ala-138 and Gly-26 antibodies obtained from cultures at 37 °C and 32 °C were compared using far-UV CD. All spectra exhibited profiles characteristic of high β-sheet content proteins, which consist of a trough at ~217 nm and a peak at ~200 nm (
Figure 6) [
19,
20,
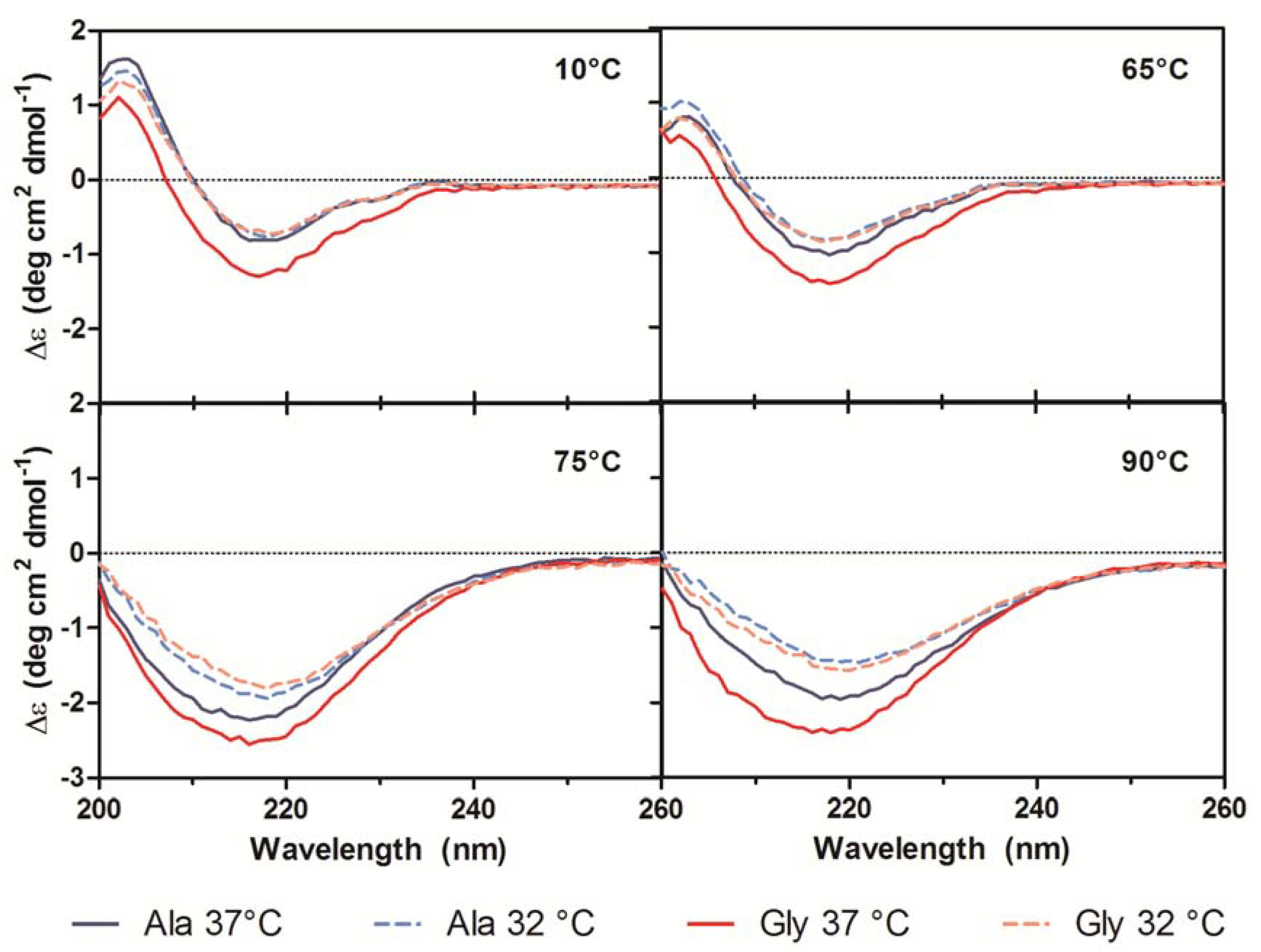
21]. A small shoulder at 230 nm was also present in all spectra and was attributed to contributions from aromatic side chain asymmetry. The CD traces for the Ala mAb cultured at both temperatures (37 °C and 32 °C) and the Gly mAb cultured at 32 °C were in good agreement with one another and exhibited similar behavior upon thermal denaturation. The spectrum of the Gly antibody purified from 37 °C culture deviated slightly from the other spectra. Monitoring the change in far-UV spectra as temperature was increased from 10 °C to 90 °C indicated little to no change in secondary structure until 65 °C (
Figure 7). Between 65 °C and 75 °C, the trough at 217 nm became increasingly negative, widened, and exhibited a slight blueshift to 216 nm; the peak at 203 nm gradually disappeared. As the temperature was increased beyond 75 °C the trough amplitude decreased and the minima shifted to ~219 nm. Heating the 37 °C Gly sample resulted in similar behavior to all other samples up to 75 °C; no further changes in the far-UV spectra occurred above this temperature. Around 75 °C all spectra exhibited an increase in absorbance, coinciding with the formation of aggregates. The concomitant change in absorbance with temperature suggests that significant aggregation occurred in the 37 °C Gly antibody, but the differences in far-UV CD traces suggest that it may be a different type of aggregation than the other three samples. Further study is needed to characterize the aggregates formed upon thermal denaturation and determine if reduced culture temperature results in differential aggregation behavior.
Figure 6.
Far-UV CD spectra of purified Ala-138 and Gly-26 mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). Each trace is the average of three acquisitions and has had the phosphate buffer (20 mM, pH 8) signal subtracted. All measurements were performed at 10 °C.
Figure 6.
Far-UV CD spectra of purified Ala-138 and Gly-26 mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). Each trace is the average of three acquisitions and has had the phosphate buffer (20 mM, pH 8) signal subtracted. All measurements were performed at 10 °C.
Figure 7.
Far-UV CD traces at 10 °C, 65 °C, 75 °C, and 90 °C for purified Ala-138 (blue) and Gly-26 (red) mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). A heating rate of 1 °C/min was used.
Figure 7.
Far-UV CD traces at 10 °C, 65 °C, 75 °C, and 90 °C for purified Ala-138 (blue) and Gly-26 (red) mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). A heating rate of 1 °C/min was used.
The spectra obtained from the far-UV CD measurements were analyzed using the CDSSTR, CONTIN/LL, and SELCON3 programs contained in the CDPro analytical package. These algorithms attempt to fit the sample spectrum to an appropriate set of reference spectra compiled from proteins with well-characterized and diverse secondary structures [
22]. All three algorithms gave similar fits of the spectra, which is required for a reliable analysis. The results of the analysis using the largest reference set available (56 proteins) indicated that all four samples contained ~45% β-sheet structures, 20% turns, 2.5% α-helical structures, and the remaining 32.5% did not fall under any of these classifications. The deviation of the 37 °C-cultured Gly sample is attributed to an increase in the amount of distorted α-helix compared to the other samples. The output from the CDPro analysis was compared to secondary structure assignment of the Ala variant Fab and an IgG
4 Fc (Protein Data Bank entry: 1ADQ_A) crystal structure using the STRIDE algorithm (based on the DSSP algorithm) [
23]. The CD analysis slightly underestimated the helical content, but was otherwise in agreement with the crystal structure data (
Table 2).
Table 2.
Secondary structure estimation from CD data using the CDPro software package. Abbreviations: αR, regular α-helix; αD, distorted α-helix; αT, total α-helix; βR, regular β-sheet; βD, distorted β-sheet; βT, total β-sheet; T, turns; U, unordered protein; RMSD, root mean square deviation; NRMSD, normalized root mean square deviation.
Table 2.
Secondary structure estimation from CD data using the CDPro software package. Abbreviations: αR, regular α-helix; αD, distorted α-helix; αT, total α-helix; βR, regular β-sheet; βD, distorted β-sheet; βT, total β-sheet; T, turns; U, unordered protein; RMSD, root mean square deviation; NRMSD, normalized root mean square deviation.
| Algorithm | Sample | αR | αD | αT | βR | βD | βT | T | U | RMSD | NRMSD |
|---|
| | Ala Fab X-Ray | - | - | 0.060 | - | - | 0.485 | 0.239 | 0.216 | - | - |
| | Ala Fab + Fc X-Ray | - | - | 0.071 | - | - | 0.490 | 0.232 | 0.207 | - | - |
| CONTIN/LL | Ala-138 37 °C | 0.000 | 0.024 | 0.024 | 0.298 | 0.149 | 0.447 | 0.198 | 0.331 | 0.066 | 0.083 |
| Ala-138 32 °C | 0.000 | 0.027 | 0.027 | 0.290 | 0.148 | 0.438 | 0.200 | 0.335 | 0.055 | 0.070 |
| Gly-26 37 °C | 0.001 | 0.031 | 0.032 | 0.292 | 0.142 | 0.434 | 0.207 | 0.327 | 0.058 | 0.074 |
| Gly-26 32 °C | 0.000 | 0.025 | 0.025 | 0.298 | 0.148 | 0.446 | 0.201 | 0.328 | 0.058 | 0.076 |
| CDSSTR | Ala-138 37 °C | 0.000 | 0.001 | 0.001 | 0.300 | 0.150 | 0.450 | 0.231 | 0.308 | 0.099 | 0.126 |
| Ala-138 32 °C | 0.000 | 0.006 | 0.006 | 0.306 | 0.143 | 0.449 | 0.238 | 0.300 | 0.129 | 0.165 |
| Gly-26 37 °C | 0.000 | 0.016 | 0.016 | 0.297 | 0.140 | 0.437 | 0.236 | 0.308 | 0.085 | 0.108 |
| Gly-26 32 °C | 0.000 | 0.005 | 0.005 | 0.303 | 0.149 | 0.452 | 0.234 | 0.299 | 0.092 | 0.121 |
| SELCON3 | Ala-138 37 °C | 0.000 | 0.000 | 0.000 | 0.246 | 0.154 | 0.400 | 0.237 | 0.354 | 0.224 | 0.284 |
| Ala-138 32 °C | 0.000 | 0.000 | 0.000 | 0.240 | 0.152 | 0.392 | 0.244 | 0.365 | 0.215 | 0.274 |
| Gly-26 37 °C | 0.000 | 0.019 | 0.019 | 0.253 | 0.146 | 0.399 | 0.232 | 0.353 | 0.172 | 0.220 |
| Gly-26 32 °C | 0.000 | 0.004 | 0.004 | 0.243 | 0.151 | 0.394 | 0.235 | 0.343 | 0.237 | 0.314 |
To estimate the secondary structure content of the thermally denatured samples, the far-UV spectra acquired at 25 °C, 50 °C, 65 °C, 70 °C, 75 °C, 80 °C, and 90 °C were also analyzed by the CDPro package. The fits were only used to determine trends in the data because the temperature ramping was performed using a reduced acquisition range of 200–260 nm. Truncating the data below 200 nm decreases the reliability of the fit, especially for proteins with high β-sheet structure [
19]. The signal from helical structures is much stronger than signal from β-sheets and therefore can result in difficulty estimating relative proportions of structure [
21]. As temperature increased, the observed trends included a decrease in β-sheet and an increase in the fractions of α-helices, turns, and unordered protein. The analysis also suggested that the samples cultured at 32 °C retain more β-sheet structure and adopt less α-helix structure upon heating than their 37 °C counterparts. Again, the 37 °C-cultured Gly variant adopted a higher proportion of α-helix than all the other samples. The CONTIN/LL analysis was repeated on all samples at 10 °C and 75 °C to obtain more detail about the temperature-induced helix formation. A different set of reference proteins was used in the analysis which included the 3
10-helix and the poly-L-proline II (PP2) helix motifs [
24]. This analysis showed an increase in α-helix, 3
10-helix, and turn content with a subsequent decrease in fractions of β-sheet, PP2, and unordered protein (
Table 3). The same trends were observed between samples, with the 37 °C cultures adopting more helical structure and the 32 °C cultures retaining more β-sheet upon thermal denaturation.
Table 3.
Secondary structure estimation from CD data of native and denatured antibodies.
Table 3.
Secondary structure estimation from CD data of native and denatured antibodies.
| Temperature | Sample | α-helix | 310-helix | Β-sheet | Turn | PP2 | Unordered | RMSD | NRMSD |
|---|
| 10°C | Ala-138 37 °C | 0.001 | 0.013 | 0.351 | 0.106 | 0.105 | 0.425 | 0.078 | 0.129 |
| Ala-138 32 °C | 0.002 | 0.013 | 0.344 | 0.108 | 0.107 | 0.426 | 0.057 | 0.105 |
| Gly-26 37 °C | 0.018 | 0.023 | 0.336 | 0.114 | 0.095 | 0.414 | 0.081 | 0.125 |
| Gly-26 32 °C | 0.002 | 0.013 | 0.338 | 0.110 | 0.109 | 0.428 | 0.061 | 0.123 |
| 75°C | Ala-138 37 °C | 0.114 | 0.045 | 0.243 | 0.132 | 0.079 | 0.386 | 0.025 | 0.020 |
| Ala-138 32 °C | 0.089 | 0.038 | 0.260 | 0.138 | 0.080 | 0.395 | 0.029 | 0.027 |
| Gly-26 37 °C | 0.141 | 0.049 | 0.226 | 0.135 | 0.073 | 0.374 | 0.031 | 0.021 |
| Gly-26 32 °C | 0.080 | 0.038 | 0.267 | 0.139 | 0.081 | 0.396 | 0.036 | 0.036 |
The increase in α-helix content of IgGs upon thermal denaturation is supported by previous experimental work. One study attributed the increase in α-helix content to hydrogen bonding between aggregate interfaces [
25]. Other proteins with high β-sheet content, such as TNF-α, have shown an increased propensity for α-helix formation upon heating [
26]. It was thought that the loss of tertiary structure removes critical long-range interactions, and new short-range interactions result in adoption of intermediate structures containing more α-helix structure. Here, both protein sequence and culture incubation temperature appear to affect the adopted secondary structure upon thermal denaturation. Using a reduced culture temperature of 32 °C appeared to allow the antibodies to adopt a β-sheet structure that was more resistant to heat-induced α-helix formation. The same resistance of the 32 °C cultures to α-helix formation was also observed in isothermal denaturation at 65 °C (data not shown). In the case of the Gly variant, reduced culture temperature had a more dramatic effect and seemed to prevent the mutation-induced formation of α-helix. The reduced culture temperature only had a modest effect on thermal stability of the molecules, but may have more important consequences for aggregation propensity. Further work is needed to elucidate the exact changes to secondary structure imposed by reduced temperature culture.
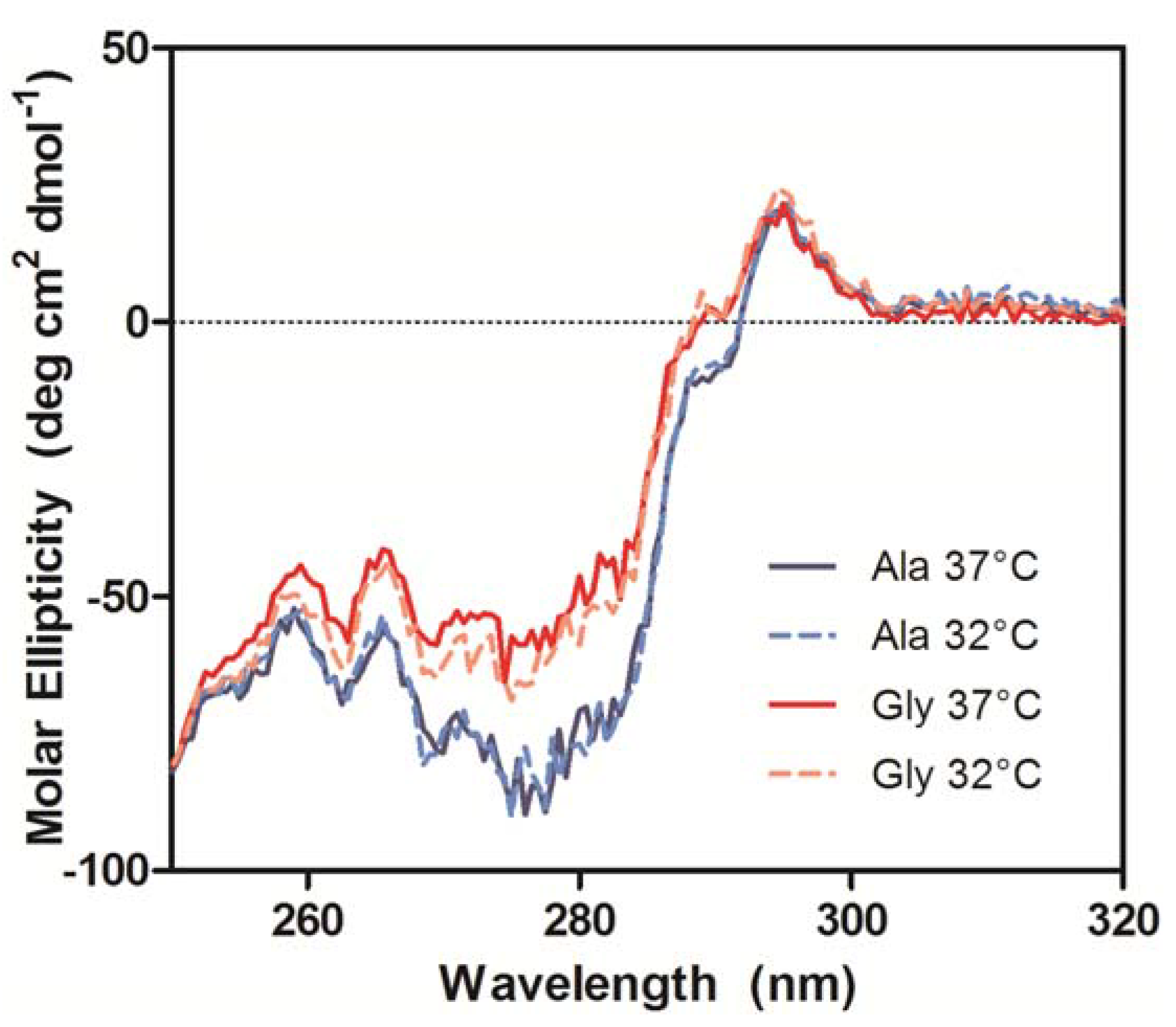
The near-UV CD spectra, which were used to compare the tertiary conformation of the samples, indicated that the Ala mAbs were in the same conformation, but the Gly mAbs not only differed from the Ala samples, but also from each other (
Figure 8). The most significant deviations in the spectra occurred between 270–295 nm, which is mainly comprised of Tyr (270–290 nm) and Trp (280–300 nm) signals [
20]. Analysis of the antibody crystal structure indicated the majority of these residues are either buried in the hydrophobic core of domains or present at the HC/LC interface. The amino acid occupying Kabat position 49 potentially interacts with or impacts the conformation of two Trp and two Tyr residues. The side chain of Ala interacts with the buried Trp-36 and Tyr-60 residues whereas the Trp-47 and Tyr-59 reside on the surface (
Figure 1). Trp-47 is a highly conserved residue that directly interacts with the V
L interface [
27]. Replacing Ala with Gly may eliminate several interactions within the hydrophobic core of the V
H domain that could result in the observed alternate conformation.
Figure 8.
Near-UV CD spectra of purified Ala-138 and Gly-26 mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). Each trace is the average of three acquisitions and has had the phosphate buffer (20 mM, pH 8) signal subtracted.
Figure 8.
Near-UV CD spectra of purified Ala-138 and Gly-26 mAb cultured at 37 °C (solid lines) and 32 °C (dashed lines). Each trace is the average of three acquisitions and has had the phosphate buffer (20 mM, pH 8) signal subtracted.
The combined biophysical data indicates that the Ala to Gly mutation at Kabat position 49 resulted in a change in the V
H conformation, which coincided with decreased expression in CHO cells. Introducing a Gly residue to a β sheet is known to be intrinsically destabilizing [
28]; stability can be reinstated by cross-strand pairing with an aromatic residue in a process known as aromatic rescue [
29]. The aromatic partner can adopt a rare conformation to “shield” the Gly, which is impossible with any other residues due to the steric restrictions imposed by all other amino acid side chains [
30,
31]. If aromatic shielding occurs in the Gly antibody, then the side chain of its aromatic neighbors could lose their native interactions in the V
H hydrophobic core or the HC/LC interface. The increased proportion of α-helix observed in the 37 °C-cultured Gly construct could be the result of an intermediate conformation that remains upon assembly with the LC. If the HC/LC variable region interface is disturbed, the interface may be more solvent-accessible, causing local burying of hydrophobic residues. This may account for the differences in near UV CD spectra. Decreasing the culture temperature results in the Gly variant adopting the same secondary structure content as the Ala variant, potentially through mechanisms such as aromatic shielding or increased contact time between the HC and LC, but does not fully rescue the tertiary conformation. This result is expected, as the changes required to stabilize the secondary structure would compromise interactions that dictate the tertiary conformation.