Understanding the Importance of Dynamic Landscape Connectivity
Abstract
:1. Introduction
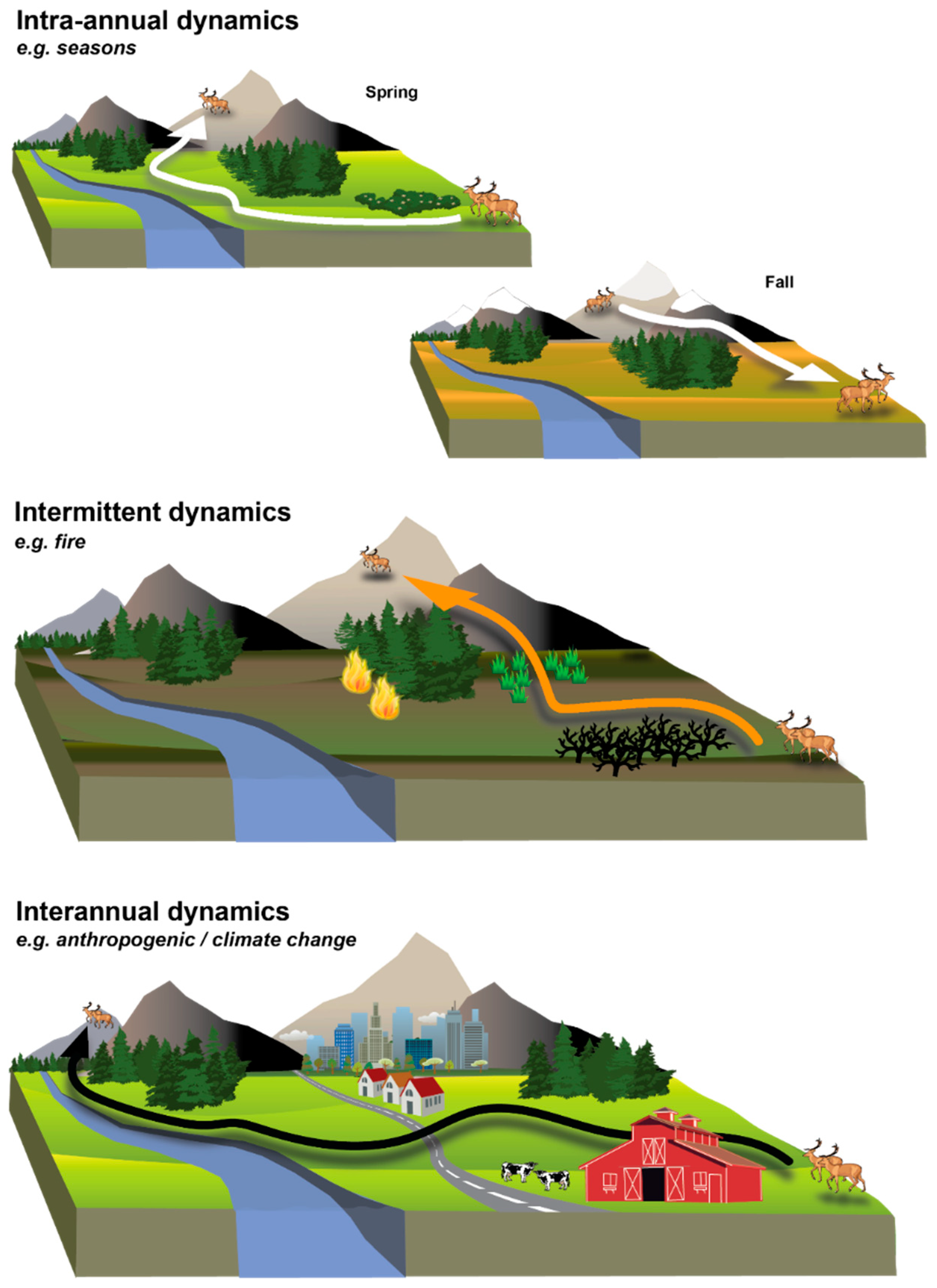
2. Moving from Static to Dynamic Landscapes
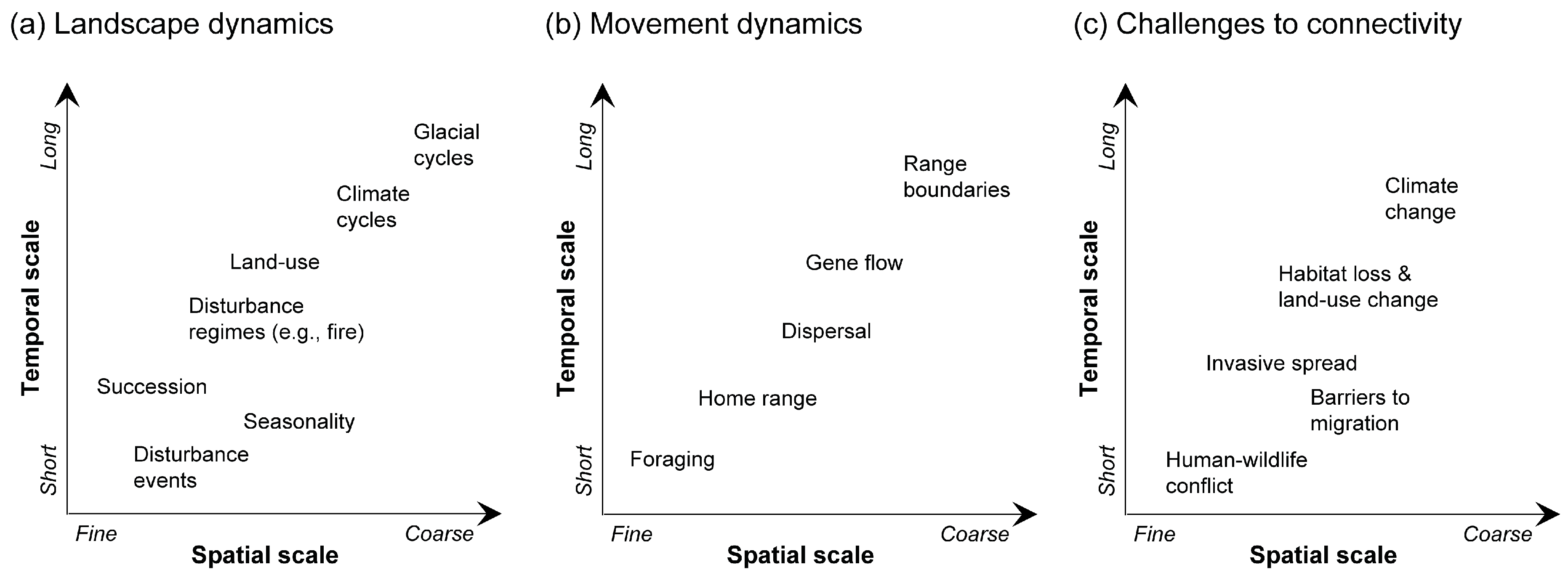
3. Key Features of Dynamic Landscape Connectivity
4. Approaches for Modeling Dynamic Landscape Connectivity
5. Linking Dynamic Landscape Science to Connectivity Action
6. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNEP-WCMC. The World Database on Protected Areas. Available online: https://www.protectedplanet.net/c/protected-planet-report-2016 (accessed on 11 June 2020).
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, C.M.; Oakleaf, J.R.; Theobald, D.M.; Baruch-Mordo, S.; Kiesecker, J. Managing the middle: A shift in conservation priorities based on the global human modification gradient. Glob. Chang. Biol. 2019, 25, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.M.; Levi, T. Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; Palma, A.D.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.R.B.; Di Marco, M.; Rondinini, C.; Boitani, L. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef] [Green Version]
- Damschen, E.I.; Brudvig, L.A.; Burt, M.A.; Fletcher, R.J.; Haddad, N.M.; Levey, D.J.; Orrock, J.L.; Resasco, J.; Tewksbury, J.J. Ongoing accumulation of plant diversity through habitat connectivity in an 18-year experiment. Science 2019, 365, 1478–1480. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [Green Version]
- Gilbert-Norton, L.; Wilson, R.; Stevens, J.R.; Beard, K.H. A Meta-Analytic Review of Corridor Effectiveness. Conserv. Biol. 2010, 24, 660–668. [Google Scholar] [CrossRef]
- Resasco, J. Meta-analysis on a Decade of Testing Corridor Efficacy: What New Have we Learned? Curr. Landsc. Ecol. Rep. 2019, 4, 61–69. [Google Scholar] [CrossRef]
- Fletcher, R.J.; Burrell, N.S.; Reichert, B.E.; Vasudev, D.; Austin, J.D. Divergent Perspectives on Landscape Connectivity Reveal Consistent Effects from Genes to Communities. Curr. Landsc. Ecol. Rep. 2016, 1, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Hilty, J.; Lidicker, W.Z.J.; Merenlender, A.M. Corridor Ecology: The Science and Practice of Linking Landscapes for Biodiversity Conservation; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Brown, J.H.; Kodrick-Brown, A. Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology 1977, 58, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Keeley, A.T.H.; Beier, P.; Creech, T.; Jones, K.; Jongman, R.H.; Stonecipher, G.; Tabor, G.M. Thirty years of connectivity conservation planning: An assessment of factors influencing plan implementation. Environ. Res. Lett. 2019, 14, 103001. [Google Scholar] [CrossRef]
- Levins, R. Extinction. In Some Mathematical Problems in Biology; Gerstenhaber, Ed.; American Mathematical Society: Providence, RI, USA, 1970; pp. 77–107. [Google Scholar]
- Levins, R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Am. Entomol. 1969, 15, 237–240. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity Is a Vital Element of Landscape Structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef] [Green Version]
- Wiens, J.A.; Chr, N.; van Horne, B.; Ims, R.A. Ecological Mechanisms and Landscape Ecology. Oikos 1993, 66, 369–380. [Google Scholar] [CrossRef]
- Howell, P.E.; Muths, E.; Hossack, B.R.; Sigafus, B.H.; Chandler, R.B. Increasing connectivity between metapopulation ecology and landscape ecology. Ecology 2018, 99, 1119–1128. [Google Scholar] [CrossRef]
- Hanski, I. Spatially realistic theory of metapopulation ecology. Naturwissenschaften 2001, 88, 372–381. [Google Scholar] [CrossRef]
- Moilanen, A.; Nieminen, M. Simple Connectivity Measures in Spatial Ecology. Ecology 2002, 83, 1131–1145. [Google Scholar] [CrossRef]
- Merriam, G. Connectivity: A fundamental ecological characteristic of landscape pattern. In Methodology in Landscape Ecological Research and Planning, Proceedings of the First Seminar, International Association of Landscape Ecology, Roskilde, Denmark, 15–19 October 1984; Brandt, J., Agger, P., Eds.; Roskilde University Centre: Roskilde, Denmark, 1984; pp. 5–15. [Google Scholar]
- Tischendorf, L.; Fahrig, L. On the usage and measurement of landscape connectivity. Oikos 2000, 90, 7–19. [Google Scholar] [CrossRef] [Green Version]
- With, K. Metapopulation Dynamics: Perspectives from Landscape Ecology. In Ecology, Genetics, and Evolution of Metapopulations; Hanski, I., Gaggiotti, O.E., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2004; pp. 23–44. [Google Scholar]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating landscape resistance to movement: A review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Hanski, I. Habitat Connectivity, Habitat Continuity, and Metapopulations in Dynamic Landscapes. Oikos 1999, 87, 209–219. [Google Scholar] [CrossRef]
- Martensen, A.C.; Saura, S.; Fortin, M.-J. Spatio-temporal connectivity: Assessing the amount of reachable habitat in dynamic landscapes. Methods Ecol. Evol. 2017, 8, 1253–1264. [Google Scholar] [CrossRef]
- Huang, J.-L.; Andrello, M.; Martensen, A.C.; Saura, S.; Liu, D.-F.; He, J.-H.; Fortin, M.-J. Importance of spatio–temporal connectivity to maintain species experiencing range shifts. Ecography 2020, 43, 591–603. [Google Scholar] [CrossRef]
- Bishop-Taylor, R.; Tulbure, M.G.; Broich, M. Evaluating static and dynamic landscape connectivity modelling using a 25-year remote sensing time series. Landsc. Ecol. 2018, 33, 625–640. [Google Scholar] [CrossRef]
- Wilson, K.A.; Carwardine, J.; Possingham, H.P. Setting Conservation Priorities. Ann. N. Y. Acad. Sci. 2009, 1162, 237–264. [Google Scholar] [CrossRef]
- Turner, M.G.; Gardner, R.H.; O’Neill, R.V. Ecological Dynamics at Broad Scales. BioScience 1995, 45, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Fortuna, M.A.; Gómez-Rodríguez, C.; Bascompte, J. Spatial network structure and amphibian persistence in stochastic environments. Proc. R. Soc. B Biol. Sci. 2006, 273, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- O’Farrill, G.; Schampaert, K.G.; Rayfield, B.; Bodin, Ö.; Calmé, S.; Sengupta, R.; Gonzalez, A. The Potential Connectivity of Waterhole Networks and the Effectiveness of a Protected Area under Various Drought Scenarios. PLoS ONE 2014, 9, e95049. [Google Scholar] [CrossRef] [Green Version]
- Bishop-Taylor, R.; Tulbure, M.G.; Broich, M. Surface water network structure, landscape resistance to movement and flooding vital for maintaining ecological connectivity across Australia’s largest river basin. Landsc. Ecol. 2015, 30, 2045–2065. [Google Scholar] [CrossRef]
- Uden, D.R.; Hellman, M.L.; Angeler, D.G.; Allen, C.R. The role of reserves and anthropogenic habitats for functional connectivity and resilience of ephemeral wetlands. Ecol. Appl. 2014, 24, 1569–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, C.K. Spatiotemporal dynamics of prairie wetland networks: Power-law scaling and implications for conservation planning. Ecology 2010, 91, 1924–1930. [Google Scholar] [CrossRef]
- Wimberly, M.C. Species Dynamics in Disturbed Landscapes: When does a Shifting Habitat Mosaic Enhance Connectivity? Landsc. Ecol. 2006, 21, 35–46. [Google Scholar] [CrossRef]
- Mui, A.B.; Caverhill, B.; Johnson, B.; Fortin, M.-J.; He, Y. Using multiple metrics to estimate seasonal landscape connectivity for Blanding’s turtles (Emydoidea blandingii) in a fragmented landscape. Landsc. Ecol. 2017, 32, 531–546. [Google Scholar] [CrossRef]
- Graham, C.H.; VanDerWal, J.; Phillips, S.J.; Moritz, C.; Williams, S.E. Dynamic refugia and species persistence: Tracking spatial shifts in habitat through time. Ecography 2010, 33, 1062–1069. [Google Scholar] [CrossRef]
- Baudry, J.; Burel, F.; Aviron, S.; Martin, M.; Ouin, A.; Pain, G.; Thenail, C. Temporal variability of connectivity in agricultural landscapes: Do farming activities help? Landsc. Ecol. 2003, 18, 303–314. [Google Scholar] [CrossRef]
- Zeigler, S.L.; Fagan, W.F. Transient windows for connectivity in a changing world. Mov. Ecol. 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Saunders, M.I.; Brown, C.J.; Foley, M.M.; Febria, C.M.; Albright, R.; Mehling, M.G.; Kavanaugh, M.T.; Burfeind, D.D. Human impacts on connectivity in marine and freshwater ecosystems assessed using graph theory: A review. Mar. Freshw. Res. 2016, 67, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Kininmonth, S.J.; De’ath, G.; Possingham, H.P. Graph theoretic topology of the Great but small Barrier Reef world. Theor. Ecol. 2010, 3, 75–88. [Google Scholar] [CrossRef]
- Jacobson, B.; Peres-Neto, P.R. Quantifying and disentangling dispersal in metacommunities: How close have we come? How far is there to go? Landsc. Ecol. 2010, 25, 495–507. [Google Scholar] [CrossRef]
- Cowen, R.K.; Sponaugle, S. Larval Dispersal and Marine Population Connectivity. Annu. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lett, C.; Ayata, S.-D.; Huret, M.; Irisson, J.-O. Biophysical modelling to investigate the effects of climate change on marine population dispersal and connectivity. Prog. Oceanogr. 2010, 87, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Soria, G.; Torre-Cosio, J.; Munguia-Vega, A.; Marinone, S.G.; Lavín, M.F.; Cinti, A.; Moreno-Báez, M. Dynamic connectivity patterns from an insular marine protected area in the Gulf of California. J. Mar. Syst. 2014, 129, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Halpern, B.S. The Impact of Marine Reserves: Do Reserves Work and Does Reserve Size Matter? Ecol. Appl. 2003, 13, 117–137. [Google Scholar] [CrossRef]
- Beger, M.; Grantham, H.S.; Pressey, R.L.; Wilson, K.A.; Peterson, E.L.; Dorfman, D.; Mumby, P.J.; Lourival, R.; Brumbaugh, D.R.; Possingham, H.P. Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biol. Conserv. 2010, 143, 565–575. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Collins, S.D.; Heintzman, L.J.; Starr, S.M.; van Gestel, N. The challenge of assaying landscape connectivity in a changing world: A 27-year case study in the southern Great Plains (USA) playa network. Ecol. Indic. 2018, 91, 607–616. [Google Scholar] [CrossRef]
- Tulbure, M.G.; Kininmonth, S.; Broich, M. Spatiotemporal dynamics of surface water networks across a global biodiversity hotspot—implications for conservation. Environ. Res. Lett. 2014, 9, 114012. [Google Scholar] [CrossRef]
- Bishop-Taylor, R.; Tulbure, M.G.; Broich, M. Impact of hydroclimatic variability on regional-scale landscape connectivity across a dynamic dryland region. Ecol. Indic. 2018, 94, 142–150. [Google Scholar] [CrossRef]
- Ruiz, L.; Parikh, N.; Heintzman, L.J.; Collins, S.D.; Starr, S.M.; Wright, C.K.; Henebry, G.M.; van Gestel, N.; McIntyre, N.E. Dynamic connectivity of temporary wetlands in the southern Great Plains. Landsc. Ecol. 2014, 29, 507–516. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Wright, C.K.; Swain, S.; Hayhoe, K.; Liu, G.; Schwartz, F.W.; Henebry, G.M. Climate forcing of wetland landscape connectivity in the Great Plains. Front. Ecol. Environ. 2014, 12, 59–64. [Google Scholar] [CrossRef]
- Gurarie, E.; Ovaskainen, O. Characteristic Spatial and Temporal Scales Unify Models of Animal Movement. Am. Nat. 2011, 178, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelnik, Y.R.; Arnoldi, J.-F.; Loreau, M. The Impact of Spatial and Temporal Dimensions of Disturbances on Ecosystem Stability. Front. Ecol. Evol. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Newman, E.A.; Kennedy, M.C.; Falk, D.A.; McKenzie, D. Scaling and Complexity in Landscape Ecology. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Leibowitz, S.G.; Vining, K.C. Temporal connectivity in a prairie pothole complex. Wetlands 2003, 23, 13–25. [Google Scholar] [CrossRef]
- Bowne, D.R.; Bowers, M.A.; Hines, J.E. Connectivity in an Agricultural Landscape as Reflected by Interpond Movements of a Freshwater Turtle. Conserv. Biol. 2006, 20, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z.; Ptacnik, R.; Vad, C.F.; Chase, J.M. Habitat loss over six decades accelerates regional and local biodiversity loss via changing landscape connectance. Ecol. Lett. 2019, 22, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, P.J.; Harvey, P.H. The Natal and Breeding Dispersal of Birds. Annu. Rev. Ecol. Syst. 1982, 13, 1–21. [Google Scholar] [CrossRef]
- Reichert, B.E.; Fletcher, R.J.; Cattau, C.E.; Kitchens, W.M. Consistent scaling of population structure across landscapes despite intraspecific variation in movement and connectivity. J. Anim. Ecol. 2016, 85, 1563–1573. [Google Scholar] [CrossRef] [Green Version]
- Lowe, W.H.; Allendorf, F.W. What can genetics tell us about population connectivity? Mol. Ecol. 2010, 19, 3038–3051. [Google Scholar] [CrossRef]
- Anderson, C.D.; Epperson, B.K.; Fortin, M.-J.; Holderegger, R.; James, P.M.A.; Rosenberg, M.S.; Scribner, K.T.; Spear, S. Considering spatial and temporal scale in landscape-genetic studies of gene flow. Mol. Ecol. 2010, 19, 3565–3575. [Google Scholar] [CrossRef]
- Talluto, M.V.; Boulangeat, I.; Vissault, S.; Thuiller, W.; Gravel, D. Extinction debt and colonization credit delay range shifts of eastern North American trees. Nat. Ecol. Evol. 2017, 1, 0182. [Google Scholar] [CrossRef]
- Perry, G.L.W.; Lee, F. How does temporal variation in habitat connectivity influence metapopulation dynamics? Oikos 2019, 128, 1277–1286. [Google Scholar] [CrossRef]
- Taylor, C.M. Effects of Natal Dispersal and Density-Dependence on Connectivity Patterns and Population Dynamics in a Migratory Network. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Kahilainen, A.; van Nouhuys, S.; Schulz, T.; Saastamoinen, M. Metapopulation dynamics in a changing climate: Increasing spatial synchrony in weather conditions drives metapopulation synchrony of a butterfly inhabiting a fragmented landscape. Glob. Chang. Biol. 2018, 24, 4316–4329. [Google Scholar] [CrossRef] [Green Version]
- Wiens, J.A. Spatial Scaling in Ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Jarvis, L.E.; Hartup, M.; Petrovan, S.O. Road mitigation using tunnels and fences promotes site connectivity and population expansion for a protected amphibian. Eur. J. Wildl. Res. 2019, 65, 27. [Google Scholar] [CrossRef] [Green Version]
- Cormont, A.; Malinowska, A.H.; Kostenko, O.; Radchuk, V.; Hemerik, L.; WallisDeVries, M.F.; Verboom, J. Effect of local weather on butterfly flight behaviour, movement, and colonization: Significance for dispersal under climate change. Biodivers. Conserv. 2011, 20, 483–503. [Google Scholar] [CrossRef] [Green Version]
- Zeller, K.A.; Wattles, D.W.; Conlee, L.; DeStefano, S. Black bears alter movements in response to anthropogenic features with time of day and season. Mov. Ecol. 2019, 7, 19. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of Fear: Spatial Patterns of Risk Perception and Response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Aikens, E.O.; Kauffman, M.J.; Merkle, J.A.; Dwinnell, S.P.H.; Fralick, G.L.; Monteith, K.L. The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecol. Lett. 2017, 20, 741–750. [Google Scholar] [CrossRef]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Krosby, M.; Tewksbury, J.; Haddad, N.M.; Hoekstra, J. Ecological Connectivity for a Changing Climate. Conserv. Biol. 2010, 24, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Keeley, A.T.H.; Ackerly, D.D.; Cameron, D.R.; Heller, N.E.; Huber, P.R.; Schloss, C.A.; Thorne, J.H.; Merenlender, A.M. New concepts, models, and assessments of climate-wise connectivity. Environ. Res. Lett. 2018, 13, 073002. [Google Scholar] [CrossRef]
- Lawler, J.J.; Ruesch, A.S.; Olden, J.D.; McRae, B.H. Projected climate-driven faunal movement routes. Ecol. Lett. 2013, 16, 1014–1022. [Google Scholar] [CrossRef]
- Fleishman, E.; Thomson, J.R.; Kalies, E.L.; Dickson, B.G.; Dobkin, D.S.; Leu, M. Projecting current and future location, quality, and connectivity of habitat for breeding birds in the Great Basin. Ecosphere 2014, 5, 82. [Google Scholar] [CrossRef]
- Littlefield, C.E.; McRae, B.H.; Michalak, J.L.; Lawler, J.J.; Carroll, C. Connecting today’s climates to future climate analogs to facilitate movement of species under climate change. Conserv. Biol. 2017, 31, 1397–1408. [Google Scholar] [CrossRef]
- Veloz, S.; Williams, J.W.; Lorenz, D.; Notaro, M.; Vavrus, S.; Vimont, D.J. Identifying climatic analogs for Wisconsin under 21st-century climate-change scenarios. Clim. Chang. 2012, 112, 1037–1058. [Google Scholar] [CrossRef]
- Nuñez, T.A.; Lawler, J.J.; Mcrae, B.H.; Pierce, D.J.; Krosby, M.B.; Kavanagh, D.M.; Singleton, P.H.; Tewksbury, J.J. Connectivity Planning to Address Climate Change. Conserv. Biol. 2013, 27, 407–416. [Google Scholar] [CrossRef]
- Carroll, C.; Parks, S.A.; Dobrowski, S.Z.; Roberts, D.R. Climatic, topographic, and anthropogenic factors determine connectivity between current and future climate analogs in North America. Glob. Chang. Biol. 2018, 24, 5318–5331. [Google Scholar] [CrossRef] [Green Version]
- Epps, C.W.; Palsbøll, P.J.; Wehausen, J.D.; Roderick, G.K.; Mccullough, D.R. Elevation and connectivity define genetic refugia for mountain sheep as climate warms. Mol. Ecol. 2006, 15, 4295–4302. [Google Scholar] [CrossRef]
- Morelli, T.L.; Maher, S.P.; Lim, M.C.W.; Kastely, C.; Eastman, L.M.; Flint, L.E.; Flint, A.L.; Beissinger, S.R.; Moritz, C. Climate change refugia and habitat connectivity promote species persistence. Clim. Chang. Responses 2017, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Brost, B.M.; Beier, P. Use of land facets to design linkages for climate change. Ecol. Appl. 2012, 22, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Krosby, M.; Breckheimer, I.; John Pierce, D.; Singleton, P.H.; Hall, S.A.; Halupka, K.C.; Gaines, W.L.; Long, R.A.; McRae, B.H.; Cosentino, B.L.; et al. Focal species and landscape “naturalness” corridor models offer complementary approaches for connectivity conservation planning. Landsc. Ecol. 2015, 30, 2121–2132. [Google Scholar] [CrossRef]
- Theobald, D.M.; Crooks, K.R.; Norman, J.B. Assessing effects of land use on landscape connectivity: Loss and fragmentation of western U.S. forests. Ecol. Appl. 2011, 21, 2445–2458. [Google Scholar] [CrossRef]
- Acevedo, M.A.; Sefair, J.A.; Smith, J.C.; Reichert, B.; Fletcher, R.J. Conservation under uncertainty: Optimal network protection strategies for worst-case disturbance events. J. Appl. Ecol. 2015, 52, 1588–1597. [Google Scholar] [CrossRef]
- Jennings, M.K.; Lewison, R.L.; Vickers, T.W.; Boyce, W.M. Puma response to the effects of fire and urbanization. J. Wildl. Manag. 2016, 80, 221–234. [Google Scholar] [CrossRef]
- Pressey, R.L.; Cabeza, M.; Watts, M.E.; Cowling, R.M.; Wilson, K.A. Conservation planning in a changing world. Trends Ecol. Evol. 2007, 22, 583–592. [Google Scholar] [CrossRef]
- Van Teeffelen, A.J.A.; Vos, C.C.; Opdam, P. Species in a dynamic world: Consequences of habitat network dynamics on conservation planning. Biol. Conserv. 2012, 153, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Pinto, N.; Keitt, T.H. Beyond the least-cost path: Evaluating corridor redundancy using a graph-theoretic approach. Landsc. Ecol. 2009, 24, 253–266. [Google Scholar] [CrossRef]
- Peterson, G.D.; Cumming, G.S.; Carpenter, S.R. Scenario Planning: A Tool for Conservation in an Uncertain World. Conserv. Biol. 2003, 17, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.L.; Lawler, J.J.; Roberts, D.R.; Carroll, C. Distribution and protection of climatic refugia in North America. Conserv. Biol. 2018, 32, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.G.; Comer, P.J.; Beier, P.; Lawler, J.J.; Schloss, C.A.; Buttrick, S.; Albano, C.M.; Faith, D.P. Case studies of conservation plans that incorporate geodiversity. Conserv. Biol. 2015, 29, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.K.; Zeller, K.A.; Lewison, R.L. Supporting Adaptive Connectivity in Dynamic Landscapes. Land 2020, 9, 295. [Google Scholar] [CrossRef]
- Gregory, R.; Ohlson, D.; Arvai, J. Deconstructing Adaptive Management: Criteria for Applications to Environmental Management. Ecol. Appl. 2006, 16, 2411–2425. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeller, K.A.; Lewison, R.; Fletcher, R.J.; Tulbure, M.G.; Jennings, M.K. Understanding the Importance of Dynamic Landscape Connectivity. Land 2020, 9, 303. https://doi.org/10.3390/land9090303
Zeller KA, Lewison R, Fletcher RJ, Tulbure MG, Jennings MK. Understanding the Importance of Dynamic Landscape Connectivity. Land. 2020; 9(9):303. https://doi.org/10.3390/land9090303
Chicago/Turabian StyleZeller, Katherine A., Rebecca Lewison, Robert J. Fletcher, Mirela G. Tulbure, and Megan K. Jennings. 2020. "Understanding the Importance of Dynamic Landscape Connectivity" Land 9, no. 9: 303. https://doi.org/10.3390/land9090303
APA StyleZeller, K. A., Lewison, R., Fletcher, R. J., Tulbure, M. G., & Jennings, M. K. (2020). Understanding the Importance of Dynamic Landscape Connectivity. Land, 9(9), 303. https://doi.org/10.3390/land9090303






