Extent of Soil Acidity in No-Tillage Systems in the Western Cape Province of South Africa
Abstract
1. Introduction
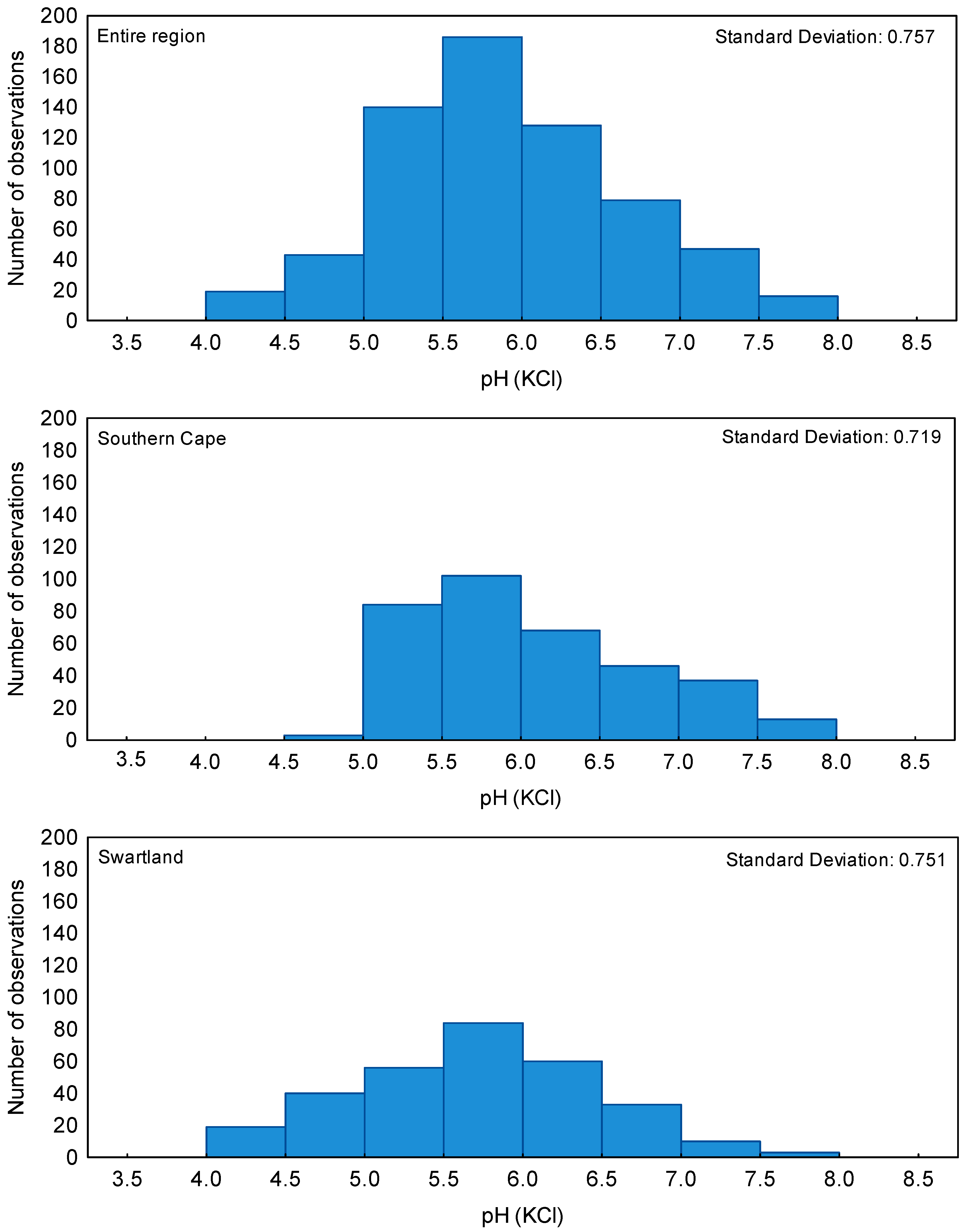
2. Results and Discussion
2.1. Subset Data from Fields with pH(KCl) ≤ 5.0 at Any Depth Increment
2.2. Canola Leaf Nutrient Content
2.3. Recommendations
3. Materials and Methods
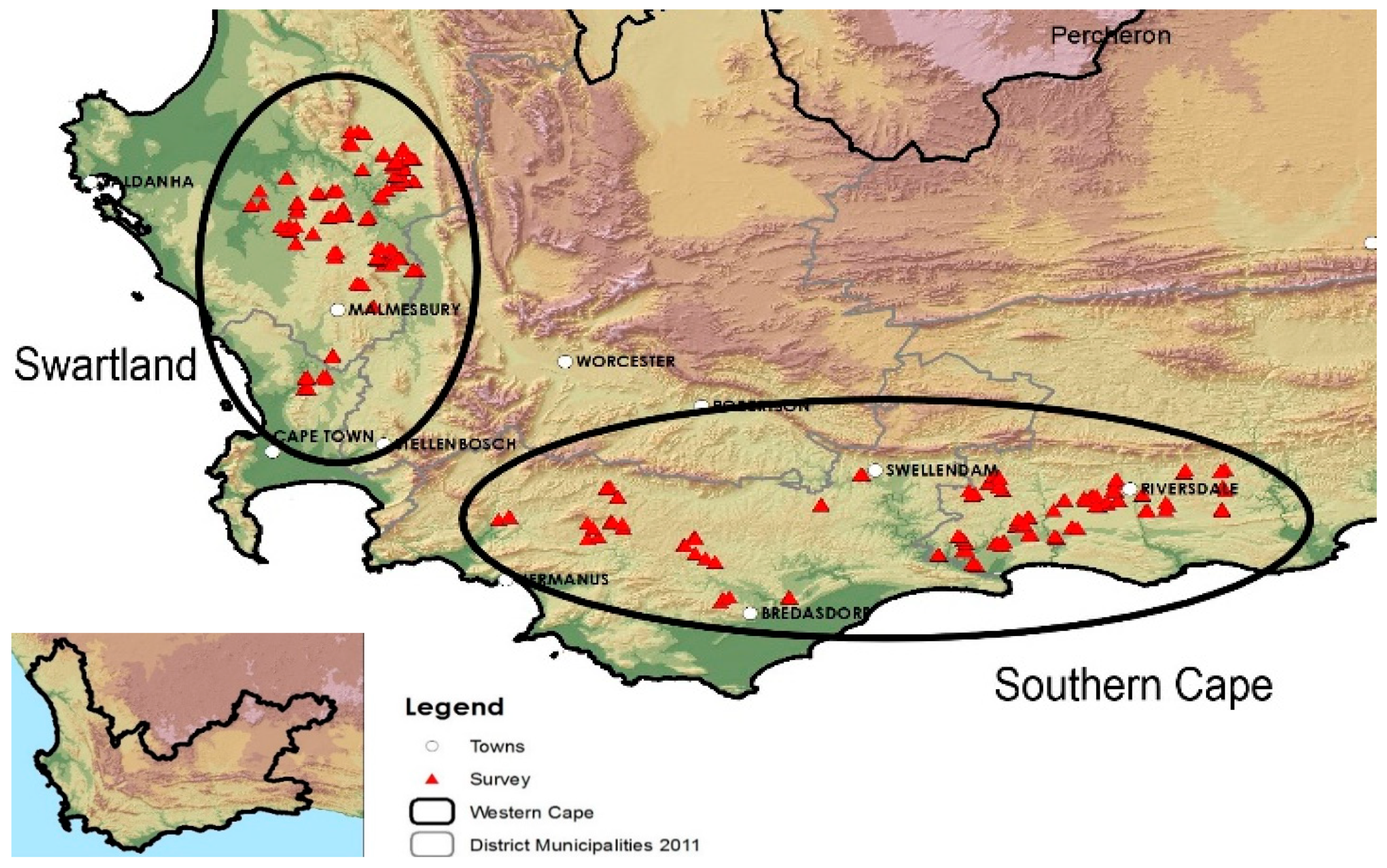
3.1. Description of Climate, Soil Types, and Land Use of the Survey Sites
3.2. Sampling and Analyses
3.3. Data Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, H.J.; Kruger, E.; Knot, J.; Blignaut, J.N. Conservation Agriculture in South Africa: Lessons from case studies. CAB Int. 2017, 214–245. [Google Scholar]
- FAO. Conservation Agriculture Principles; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; p. 1. Available online: http://faostat.fao.org/ (accessed on 18 September 2020).
- Findlater, K.M.; Kandlikar, M.; Satterfield, T. Misunderstanding conservation agriculture: Challenges in promoting, monitoring and evaluating sustainable farming. Environ. Sci. Policy 2019, 100, 47–54. [Google Scholar] [CrossRef]
- Barth, V.P.; Reardon, C.L.; Coffey, T.; Klein, A.M.; McFarland, C.; Huggins, D.R.; Sullivan, T.S. Stratification of soil chemical and microbial properties under no-till after liming. Appl. Soil. Ecol. 2018, 130, 169–177. [Google Scholar] [CrossRef]
- Miller, L. How fast is lime moving and is it treating acidity at depth? South. Farming Syst. 2015, 8, 133–135. [Google Scholar]
- Godsey, C.B.; Pierzynski, G.M.; Mengel, D.B.; Lamond, R.E. Management of soil acidity in no-till production systems through surface application of lime. Agron. J. 2007, 99, 764–772. [Google Scholar] [CrossRef]
- Conyers, M.K.; Heenan, D.P.; McGhie, W.J.; Poile, G.P. Amelioration of acidity with time by limestone under contrasting tillage. Soil Tillage Res. 2003, 72, 85–94. [Google Scholar] [CrossRef]
- USDA. Delayed Winter Rains Reduce South Africas 2015/2016 Wheat Prospects; United States Department of Agriculture: Washington, DC, USA, 2015; p. 1.
- Mogala, M. A Profile of the South African Barley Market Value Chain; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2017; pp. 1–20.
- De Kock, J. Development Plan for Canola 2018; Protein Research Foundation: Rivonia, South Africa, 2018; p. 1. [Google Scholar]
- Tang, C.; Rengel, Z.; Diatloff, E.; Gazey, C. Responses of wheat and barley to liming on a sandy soil with subsoil acidity. Field Crops Res. 2003, 80, 235–244. [Google Scholar] [CrossRef]
- Angus, J.; Swan, T.; Kirkegaard, J.; Beven, A.; Duff, C.; Conyers, M. Canola and the Acid Throttle. Grains Res. Dev. Corp. Updates Pap. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2008/06/canola-and-the-acid-throttle (accessed on 25 September 2020).
- Foy, C.D.; Atkinson, D. Soil Chemical Factors Limiting Plant Root Growth. Adv. Soil Sci. 1991, 19, 471. [Google Scholar]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Naidu, R. Functional Relationships of Soil Acidification, Liming, and Greenhouse Gas Flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar]
- Kochian, K.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Krstic, D.; Djalovic, I.; Nikezic, D.; Bjelic, D. Aluminium in Acid Soils: Chemistry, Toxicity and Impact on Maize Plants. Intech 2012, 13, 231–242. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Plant nutrient efficiency: Towards the second paradigm. Braz. Soc. Soil Sci. 1999, 31, 183–204. [Google Scholar]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.D.; Abbott, L.K. The effect of soil acidity on microbial activity in soils. Soil Acidity Plant Growth 1989, 4, 139–165. [Google Scholar]
- Kunito, T.; Isomura, I.; Sumi, H.; Park, H.D.; Toda, H.; Otsuka, S.; Nagaoka, K.; Saeki, K.; Senoo, K. Aluminum and acidity suppress microbial activity and biomass in acidic forest soils. Soil Biol. Biochem. 2016, 97, 23–30. [Google Scholar] [CrossRef]
- Grieve, I.C. Effects of parent material on the chemical composition of soil drainage waters. Geoderma 1999, 90, 49–64. [Google Scholar] [CrossRef]
- Fooladmand, H.R. Estimating cation exchange capacity using soil textural data and soil organic matter content: A case study for the south of Iran. Arch. Agron. Soil Sci. 2008, 54, 381–386. [Google Scholar] [CrossRef]
- Nathan, M.V. Soils, Plant Nutrition and Nutrient Management. Univ. Mo. Ext. Available online: https://extension2.missouri.edu/mg4 (accessed on 25 September 2020).
- Jacobsen, O.H.; Moldrup, P.; Larsen, C.; Konnerup, L.; Petersen, L.W. Particle transport in macropores of undisturbed soil columns. J. Hydrol. 1997, 196, 185–203. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- FERTASA. Fertilization Handbook; Fertilizer Association of Southern Africa: Pretoria, South Africa, 2016; p. 224. [Google Scholar]
- Gazey, C.; Davies, S. Soil Acidity: A Guide for WA Farmers and Consultants; Bulletin; Department of Agriculture and Food Western Australia: Perth, Australia, 2009; Volume 4784, pp. 1–52.
- Miller, L. Acidity–Soil Sampling and Lime Incorporation under Review. Grain Res. Dev. Corp. Updates Pap. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2020/02/acidity-soil-sampling-and-lime-incorporation-under-review (accessed on 25 September 2020).
- Van Lierop, W. Conversion of organic soil pH values measured in water, 0.01 M CaCl2 or 1 N KCl. Can. J. Soil Sci. 1981, 61, 577–579. [Google Scholar] [CrossRef]
- Whitten, M.G.; Wong, M.T.F.; Rate, A.W. Amelioration of subsurface acidity in the south-west of Western Australia: Downward movement and mass balance of surface-incorporated lime after 2–15 years. Aust. J. Soil Res. 2000, 38, 711–728. [Google Scholar] [CrossRef]
- Dang, Y.P.; Moodt, P.W.; Bell, M.J.; Seymour, N.P.; Dalal, R.C.; Freebairn, D.M.; Walker, S.R. Strategic-tillage in no-till farming systems in Australia’s northern grains-growing region: II. Implications for agronomy, soil and environment. Soil Tillage Res. 2015, 152, 115–123. [Google Scholar] [CrossRef]
- Foy, C.D. Tolerance of barley cultivars to an acid, aluminum-toxic subsoil related to mineral element concentrations in their shoots. J. Plant Nutr. 1996, 19, 1361–1380. [Google Scholar] [CrossRef]
- DAFF. Production Guideline for Canola. In Production Guidelines; Department of Agriculture, Forestry & Fisheries: Pretoria, South Africa, 2016; Volume 5, pp. 1–25. [Google Scholar]
- DAFF. Production Guidelines for Wheat; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2016; pp. 1–27.
- Norton, R. Focus on Calcium: Its role in crop production. GRDC Updates Pap. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2013/02/focus-on-calcium-its-role-in-crop-production (accessed on 25 September 2020).
- Botta, C. Understanding Your Soil Test Step by Step; Yea River Catchment Land-care Group: Yea, Australia, 2015; pp. 1–36. [Google Scholar]
- Magdoff, F. Building soils for better crops: Organic matter management. Soil Sci. 1993, 156, 371. [Google Scholar] [CrossRef]
- Parnes, R. Soil fertility: Chapter 14-Calcium and soil pH. Northeast. Org. Farming Assoc. Available online: https://www.nofa.org/soil/html/calcium.php (accessed on 25 September 2020).
- Schlecht, E.; Buerkert, A.; Tielkes, E.; Bationo, A. A critical analysis of challenges and opportunities for soil fertility restoration in Sudano-Sahelian West Africa. Nutr. Cycl. Agroecosystems 2006, 76, 109–136. [Google Scholar] [CrossRef]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results. In What Do all the Numbers Mean; CSIRO Publishing: Victoria, Australia, 2007. [Google Scholar]
- White, P.J.; Holland, J.E. Calcium in Plant Physiology and Its Availability from the Soil; IFC: Colchester, UK, 2018; p. 2. [Google Scholar]
- Espinoza, L.; Slaton, N.A.; Mozaffari, M. Understanding the Numbers on Your Soil Test Report; Cooperative Extension Service; University of Arkansas, US Department of Agriculture, and County Government’s Cooperating: Washington, DC, USA, 2006; pp. 1–4.
- Hirzel, J.; Matus, I. Effect of soil depth and increasing fertilization rate on yield and its components of two durum wheat varieties. Chil. J. Agric. Res. 2013, 73, 55–59. [Google Scholar] [CrossRef]
- Busscher, W.J.; Frederick, J.R.; Bauer, P.J. Effect of penetration resistance and timing of rain on grain yield of narrow-row corn in a coastal plain loamy sand. Soil Tillage Res. 2001, 63, 15–24. [Google Scholar] [CrossRef]
- McDonald, C.K. Effect of soil properties on variation in growth, grain yield and nutrient concentration of wheat and barley. Aust. J. Exp. Agric. 2006, 46, 93–105. [Google Scholar] [CrossRef]
- Christopher, J.T.; Manschadi, A.M.; Hammer, G.L.; Borrell, A.K. Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Aust. J. Agric. Res. 2008, 59, 354–364. [Google Scholar] [CrossRef]
- Whitmore, A.P.; Whalley, W.R. Physical effects of soil drying on roots and crop growth. J. Exp. Bot. 2009, 60, 2845–2857. [Google Scholar] [CrossRef]
- Richards, R. Genetic opportunities to improve cereal root systems for dryland agriculture. Plant Prod. Sci. 2008, 11, 12–16. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. Effect of deep and shallow root systems on the dynamics of soil inorganic nitrogen during 3-year crop rotations. Plant Soil 2006, 288, 233–248. [Google Scholar] [CrossRef]
- Ismail, L.; Blevins, R.L.; Frye, W.W. Long-term no-tillage effects on soil properties and continuous corn yields. Soil Sci. Soc. Am. J. 1994, 54, 193–198. [Google Scholar] [CrossRef]
- Rahman, M.H.; Okubo, A.; Sugiyama, S.; Mayland, H.F. Physical, chemical and microbiological properties of an Andisol as related to land use and tillage practice. Soil Tillage Res. 2008, 101, 10–19. [Google Scholar] [CrossRef]
- Canola Council of Canada. Identifying Fertilizer Requirements. 2017. Available online: https://www.canolacouncil.org/canola-encyclopedia/fertilizer-management/identifying-fertilizer-requirements/ (accessed on 18 September 2020).
- Campbell, C. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; North Carolina Department of Agriculture and Consumer Services Agronomic Division: Raleigh, NC, USA, 2000.
- Kettler, T.A.; Lyon, D.J.; Doran, J.W.; Powers, W.L.; Stroup, W.W. Soil quality assesment after weed-control tillage in a no-till wheat fallow cropping system. Soil Sci. Soc. Am. J. 2000, 64, 339–346. [Google Scholar] [CrossRef]
- Quincke, J.A.; Wortmann, C.S.; Mamo, M.; Franti, T.; Drijber, R.A. Occasional tillage of no-till systems: Carbon dioxide flux and changes in total and labile soil organic carbon. Agron. J. 2007, 99, 1158–1168. [Google Scholar] [CrossRef]
- Quincke, J.A.; Wortmann, C.S.; Mamo, M.; Franti, T.; Drijber, R.A.; García, J.P. One-time tillage of no-till systems: Soil physical properties, phosphorus runoff, and crop yield. Agron. J. 2007, 99, 1104–1110. [Google Scholar] [CrossRef]
- Baan, C.D.; Grevers, M.C.J.; Schoenau, J.J. Effects of a single cycle of tillage on long-term no-till prairie soils. Can. J. Soil Sci. 2009, 89, 521–530. [Google Scholar] [CrossRef]
- Crawford, M.H.; Rincon-Florez, V.; Balzer, A.; Dang, Y.P.; Carvalhais, L.C.; Liu, H.; Schenk, P.M. Changes in the soil quality attributes of continuous no-till farming systems following a strategic tillage. Soil Res. 2015, 53, 263–273. [Google Scholar] [CrossRef]
- Leygonie, I.R. The Effect of Once-Off Tillage on Selected Soil Physical and Chemical Properties and Resultant Crop Response on Shale Derived Soil under No-Tillage in the Swartland Sub-Region of the Western Cape; University of Stellenbosch: Stellenbosch, South Africa, 2016; pp. 1–117. [Google Scholar]
- Liu, H.; Crawford, M.; Carvalhais, L.C.; Dang, Y.P.; Dennis, P.G.; Schenk, P.M. Strategic tillage on a Grey Vertosol after fifteen years of no-till management had no short-term impact on soil properties and agronomic productivity. Geoderma 2016, 267, 146–155. [Google Scholar] [CrossRef]
- Van Zyl, J.G. Response of Wheat (Triticum Aestivum L.), Canola (Brassica Napus) and Medic (Medicago) To a Once-Off Mouldboard and Deep Tine Tillage in the Swartland Sub-Region of the Western Cape; University of Stellenbosch: Stellenbosch, South Africa, 2017; pp. 1–101. [Google Scholar]
- Dang, Y.P.; Balzer, A.; Crawford, M.; Rincon-Florez, V.; Liu, H.; Melland, A.R.; Schenk, P. Strategic tillage in conservation agricultural systems of north-eastern australia: Why, where, when and how? Environ. Sci. Pollut. Res. 2018, 25, 1000–1015. [Google Scholar] [CrossRef] [PubMed]
- Conyers, M.; van der Rijt, V.; Oates, A.; Poile, G.; Kirkegaard, J.; Kirkby, C. The strategic use of minimum tillage within conservation agriculture in southern New South Wales, Australia. Soil Tillage Res. 2019, 193, 17–26. [Google Scholar] [CrossRef]
- Caires, E.F.; Barth, G.; Garbuio, F.J. Lime application in the establishment of a no-till system for grain crop production in Southern Brazil. Soil Tillage Res. 2006, 89, 3–12. [Google Scholar] [CrossRef]
- Caires, E.F.; Joris, H.A.W.; Churka, S. Long-term effects of lime and gypsum additions on no-till corn and soybean yield and soil chemical properties in southern Brazil. Soil Use Manag. 2011, 27, 45–53. [Google Scholar] [CrossRef]
- Tiritan, C.S.; Büll, L.T.; Crusciol, C.A.C.; Carmeis Filho, A.C.A.; Fernandes, D.M.; Nascente, A.S. Tillage system and lime application in a tropical region: Soil chemical fertility and corn yield in succession to degraded pastures. Soil Tillage Res. 2016, 155, 437–447. [Google Scholar] [CrossRef]
- Western Cape Department of Agriculture. CapeFarmMapper, ver. 2.3.1.1. Spatial Information & Mapping Services. 2020. Available online: https://gis.elsenburg.com/apps/cfm/ (accessed on 18 September 2020).
- Non-Affiliated Soil Analysis Work Committee. Handbook of Standard Soil Testing Methods for Advisory Purposes; Soil Science Society of South Africa: Pretoria, South Africa, 1990. [Google Scholar]
- United States Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils. In Agriculture Handbook no. 60; Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Kenward, M.G.; Roger, J.H. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput. Stats Data Anal. 2009, 53, 2583–2595. [Google Scholar] [CrossRef]
- TIBCO Software. Statistica (Data Analysis Software System), Version 13.5.0.17; TIBCO Software: Palo Alto, CA, USA, 2020. [Google Scholar]

| n | Mean | Median | Minimum | Maximum | SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Southern Cape | Swart-Land | Depth (cm) | Southern Cape | Swart-Land | Southern Cape | Swartland | Southern Cape | Swart-Land | Southern Cape | Swart-Land | Southern Cape | Swart-Land | |
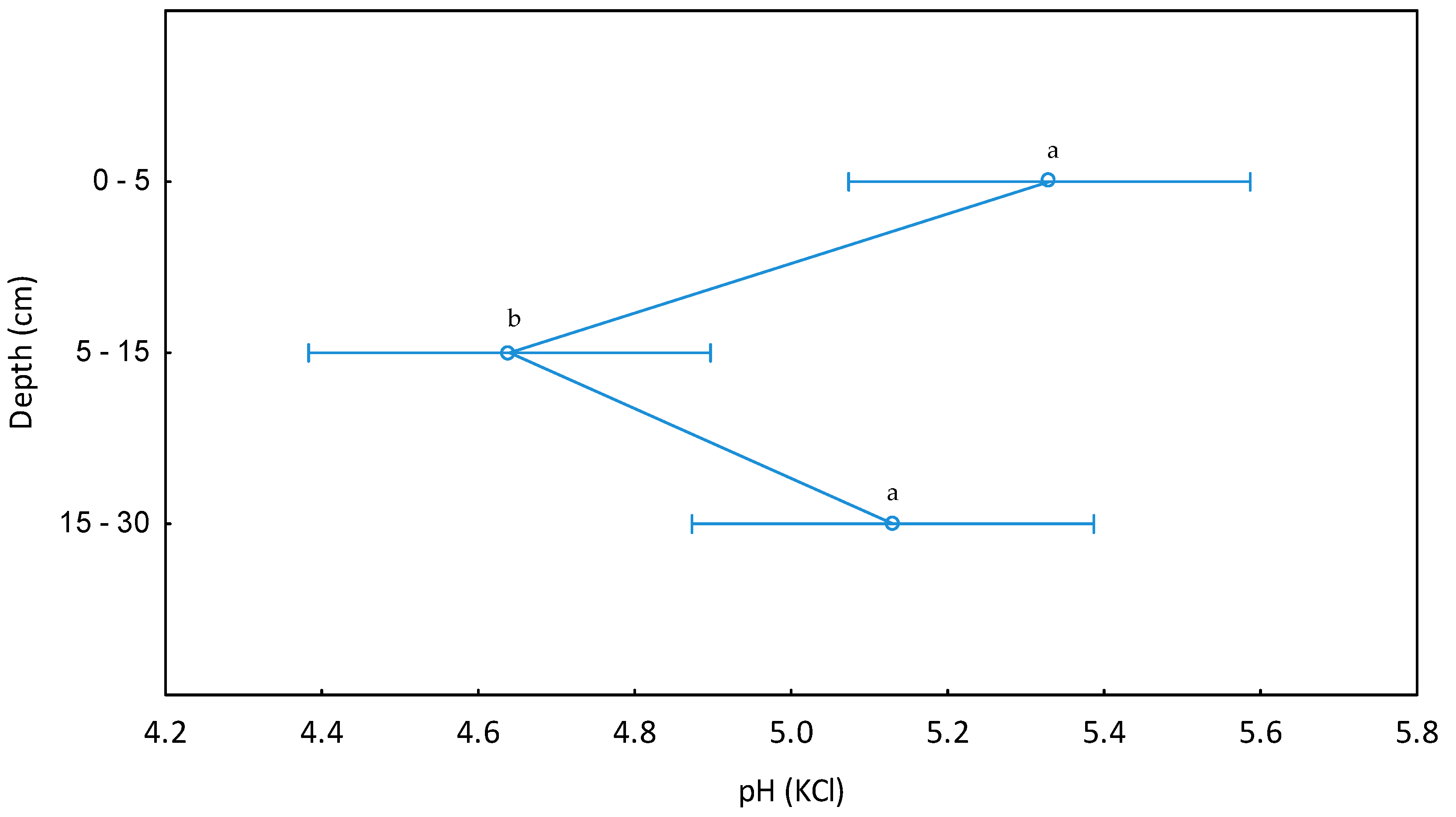
| pH (KCl) | 118 | 99 | 0–5 | 6.2 | 6.0 | 6.2 | 6.1 | 4.8 | 4.5 | 7.5 | 7.4 | 0.64 | 0.69 |
| 115 | 106 | 5–15 | 6.0 | 5.5 | 5.9 | 5.6 | 5.0 | 4.2 | 7.7 | 7.6 | 0.70 | 0.73 | |
| 115 | 100 | 15–30 | 6.2 | 5.8 | 5.8 | 5.7 | 5.1 | 4.1 | 7.9 | 7.9 | 0.87 | 0.76 | |
| Ca (mg kg −1) | 118 | 99 | 0–5 | 2514 | 1728 | 1761 | 1390 | 430 | 344 | 12,898 | 10,134 | 2193 | 1473 |
| 115 | 106 | 5–15 | 1857 | 985 | 1127 | 763 | 366 | 116 | 9634 | 8562 | 1818 | 1007 | |
| 115 | 100 | 15–30 | 1818 | 658 | 889 | 545 | 326 | 106 | 11,644 | 3798 | 2339 | 550 | |
| Mg (mg kg −1) | 118 | 99 | 0–5 | 241 | 255 | 206 | 218 | 46 | 53 | 679 | 888 | 116 | 150 |
| 115 | 106 | 5–15 | 201 | 141 | 173 | 119 | 41 | 18 | 481 | 408 | 99 | 81 | |
| 115 | 100 | 15–30 | 247 | 148 | 190 | 124 | 60 | 17 | 1463 | 720 | 182 | 107 | |
| Exchangeable acidity (cmolc kg−1) | 118 | 99 | 0–5 | 0.13 | 0.18 | 0 | 0 | 0 | 0 | 1.30 | 1.39 | 0.33 | 0.40 |
| 115 | 106 | 5–15 | 0.19 | 0.39 | 0 | 0 | 0 | 0 | 1.22 | 1.54 | 0.35 | 0.46 | |
| 115 | 100 | 15–30 | 0.12 | 0.24 | 0 | 0 | 0 | 0 | 1.00 | 1.12 | 0.28 | 0.36 | |
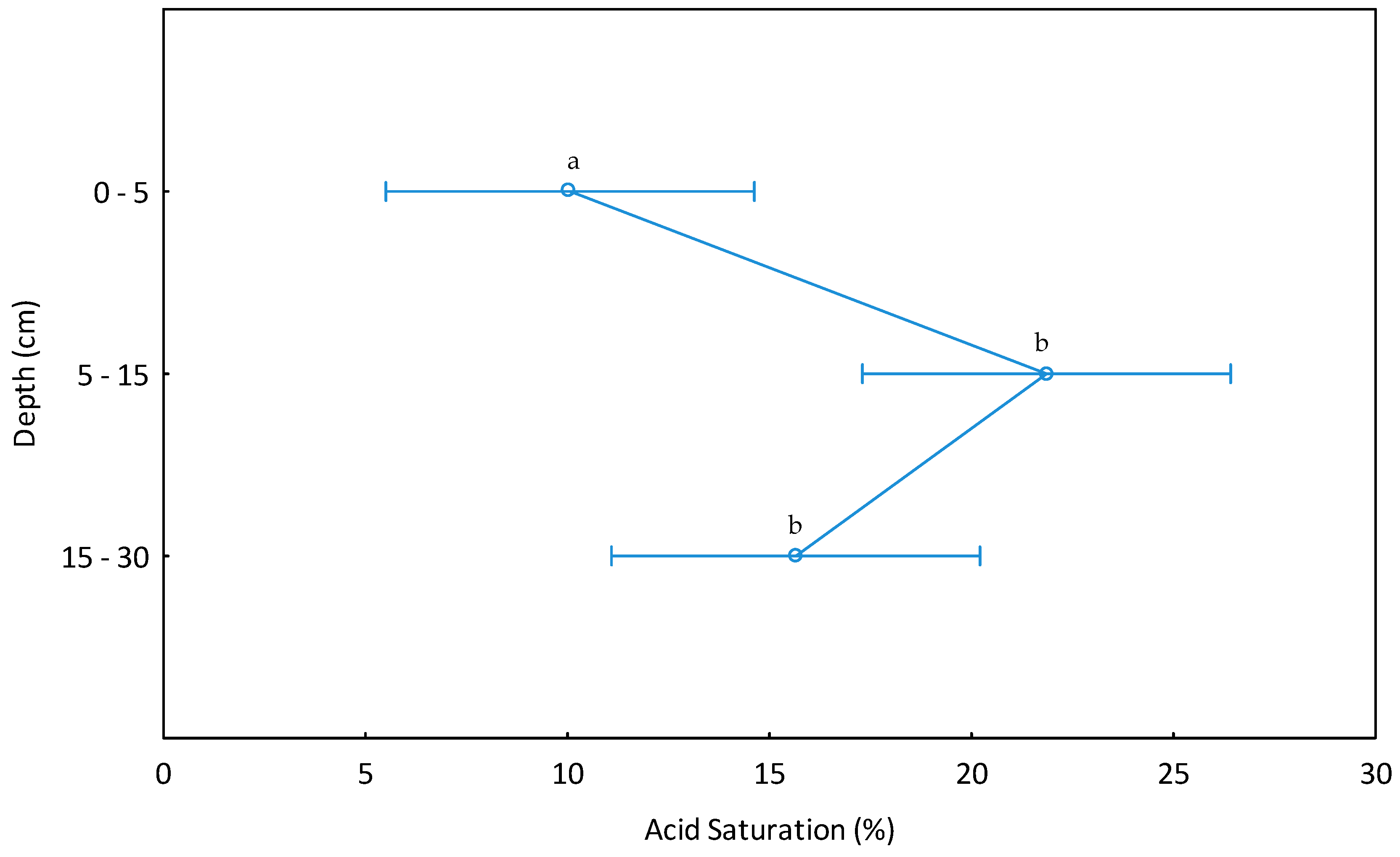
| Acid saturation (%) | 118 | 99 | 0–5 | 1.49 | 2.64 | 0 | 0 | 0 | 0 | 15.53 | 23.00 | 3.78 | 5.88 |
| 115 | 106 | 5–15 | 2.72 | 8.17 | 0 | 0 | 0 | 0 | 17.44 | 44.16 | 5.07 | 10.38 | |
| 115 | 100 | 15–30 | 2.00 | 6.51 | 0 | 0 | 0 | 0 | 16.63 | 44.65 | 4.56 | 10.71 | |
| Soil Chemical Properties | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| pH(KCl) | 0.465 | 0.073 | 0.747 | 0.060 |
| Electrical resistance (Ohm) | −0.021 | −0.628 | −0.183 | −0.498 |
| Electrical conductivity (mS m−1) | −0.036 | 0.787 | 0.072 | 0.433 |
| Exchangeable acidity (cmolc kg−1) | −0.110 | −0.057 | −0.941 | −0.056 |
| Ca (mg kg−1) | 0.919 | −0.070 | 0.165 | 0.225 |
| Mg (mg kg−1) | 0.690 | 0.543 | 0.172 | −0.101 |
| Na (mg kg−1) | 0.052 | 0.829 | 0.0531 | −0.160 |
| K (mg kg−1) | 0.194 | 0.072 | 0.154 | 0.766 |
| P (mg kg−1) | 0.131 | 0.009 | 0.009 | 0.719 |
| Effective cation exchange capacity (cmolc kg−1) | 0.944 | 0.042 | 0.151 | 0.212 |
| Acid saturation (%) | −0.126 | −0.172 | −0.916 | −0.146 |
| Eigenvalue | 5.302 | 2.072 | 1.632 | 1.426 |
| Total variance (%) | 40.8 | 15.9 | 12.6 | 10.7 |
| Cumulative variance (%) | 40.8 | 56.7 | 69.3 | 80.2 |
| Factor | Factor 1 | Factor 2 | Factor 3 | Factor 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | Ca, Mg, ECEC | Electrical Resistance, Conductivity, Na | pH(KCl), Exchangeable Acidity, Acid Saturation | K, P | ||||
| F | P | F | P | F | P | F | P | |
| Depth | 14.25 | <0.001 | 16.21 | <0.001 | 12.06 | <0.001 | 306.59 | <0.001 |
| Region | 18.85 | <0.001 | 0.01 | 0.938 | 12.62 | 0.001 | 8.24 | 0.004 |
| Rainfall | 3.32 | 0.001 | 0.39 | 0.924 | 6.33 | <0.001 | 3.43 | 0.001 |
| Texture | 2.37 | 0.070 | 12.84 | <0.001 | 1.71 | 0.166 | 0.57 | 0.636 |
| Years since liming | 0.92 | 0.500 | 0.73 | 0.667 | 0.98 | 0.451 | 1.69 | 0.103 |
| Texture | Percentage of Samples | ||
|---|---|---|---|
| All Samples | Southern Cape | Swartland | |
| Sandy loam | 89.13 | 92.55 | 85.20 |
| Sand | 9.04 | 7.45 | 10.86 |
| Sandy clay loam | 1.68 | 0 | 3.62 |
| Clay | 0.15 | 0 | 0.32 |
| Ca (mg kg−1) | Mg (mg kg−1) | |
|---|---|---|
| Very low | <400 | <36 |
| Low | 400–1000 | 36–120 |
| Moderate | 1000–2000 | 120–360 |
| High | 2000–4000 | 360–960 |
| Very high | >4000 | >960 |
| Depth (cm) | S. Cape (%) | Swartland (%) |
|---|---|---|
| 0–5 | 0.00 | 11.11 |
| 5–15 | 1.74 | 29.25 |
| 15–30 | 0.87 | 17.00 |
| Total | 0.86 | 19.30 |
| F Value | p Value | |
|---|---|---|
| pH (KCl) | 7.76 | <0.001 |
| Ca (mg kg−1) | 13.58 | <0.001 |
| Mg (mg kg−1) | 6.88 | <0.001 |
| Exchangeable acidity (cmolc kg−1) | 3.59 | 0.040 |
| Acid saturation (%) | 6.13 | 0.040 |
| Soil Depth (cm) | Exchangeable Ca (mg kg−1) | Exchangeable Mg (mg kg−1) | Exchangeable Acidity (cmol kg−1) |
|---|---|---|---|
| 0–5 | 1039 a | 188 a | 0.68 ab |
| 5–15 | 535 b | 103 b | 0.95 a |
| 15–30 | 417 b | 149 b | 0.56 b |
| N (%) | P (%) | S (%) | Ca (%) | Mg (%) | K (%) | Fe (mg kg−1) | Cu (mg kg−1) | Mn (mg kg−1) | Zn (mg kg−1) | B (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Mean | 5.46 (0.62) | 0.53 (0.11) | 0.83 (0.10) | 4.28 (1.24) | 0.86 (0.38) | 4.10 (2.10) | 250.99 (185.28) | 6.76 (5.55) | 74.02 (27.94) | 33.45 (6.58) | 30.28 (12.71) |
| Canadian threshold [49] | 2.40 | 0.24 | 0.24 | 0.49 | 0.19 | 1.40 | 19.00 | 2.60 | 14.00 | 14.00 | 29.00 |
| USA threshold [50] | 3.60 | 0.37 | 0.47 | 1.60 | 0.10 | 2.15 | 82.00 | 4.00 | 20.00 | 28.00 | 20.00 |
| RSA threshold [23] | 3.50 | 0.3–0.6 | 0.50 | 1.4–3.0 | 0.2–0.6 | 2.20 | 50–300 | 3–5 | 30–200 | 20.00 | 20–50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebenberg, A.; van der Nest, J.R.; Hardie, A.G.; Labuschagne, J.; Swanepoel, P.A. Extent of Soil Acidity in No-Tillage Systems in the Western Cape Province of South Africa. Land 2020, 9, 361. https://doi.org/10.3390/land9100361
Liebenberg A, van der Nest JR, Hardie AG, Labuschagne J, Swanepoel PA. Extent of Soil Acidity in No-Tillage Systems in the Western Cape Province of South Africa. Land. 2020; 9(10):361. https://doi.org/10.3390/land9100361
Chicago/Turabian StyleLiebenberg, Adriaan, John Richard (Ruan) van der Nest, Ailsa G. Hardie, Johan Labuschagne, and Pieter Andreas Swanepoel. 2020. "Extent of Soil Acidity in No-Tillage Systems in the Western Cape Province of South Africa" Land 9, no. 10: 361. https://doi.org/10.3390/land9100361
APA StyleLiebenberg, A., van der Nest, J. R., Hardie, A. G., Labuschagne, J., & Swanepoel, P. A. (2020). Extent of Soil Acidity in No-Tillage Systems in the Western Cape Province of South Africa. Land, 9(10), 361. https://doi.org/10.3390/land9100361



_Van_der_Nest.jpg)




