Monitoring Forest Restoration in Berenty Reserve, Southern Madagascar
Abstract
1. Introduction
- (1)
- What are the growth patterns, height, canopy widths and basal stem diameter of the planted species?
- (2)
- How does growth vary between species, and what might this indicate concerning site-specific conditions or management history?
- (3)
- What were the mortality rates, and what species were recruited from the forest (2017–2018)?
- (4)
- What insights are provided by longer-term monitoring that could be missed in short-term studies?
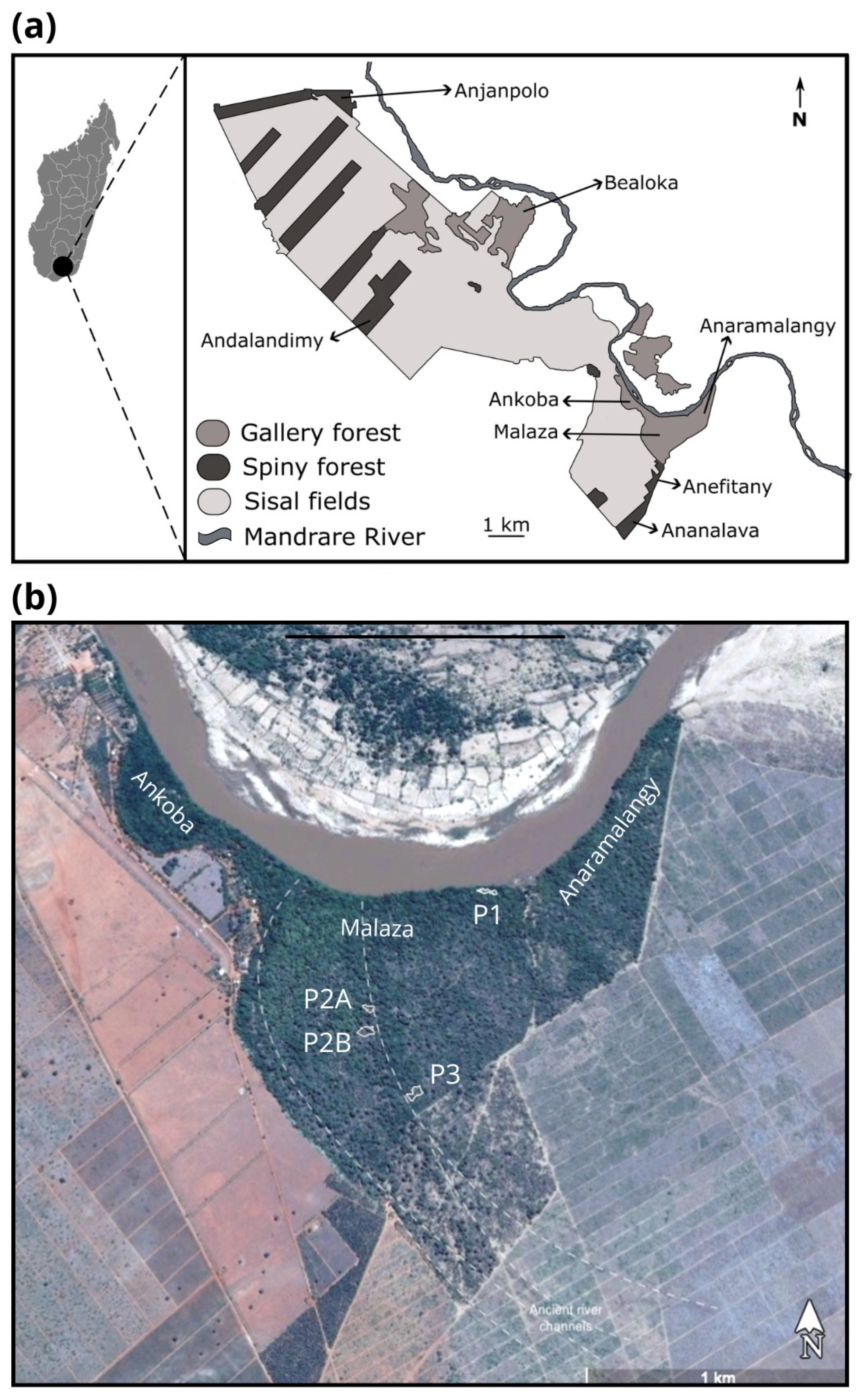
2. Methods
2.1. Experimental Design and Planting Protocol
2.2. Data Handling and Analysis
3. Results
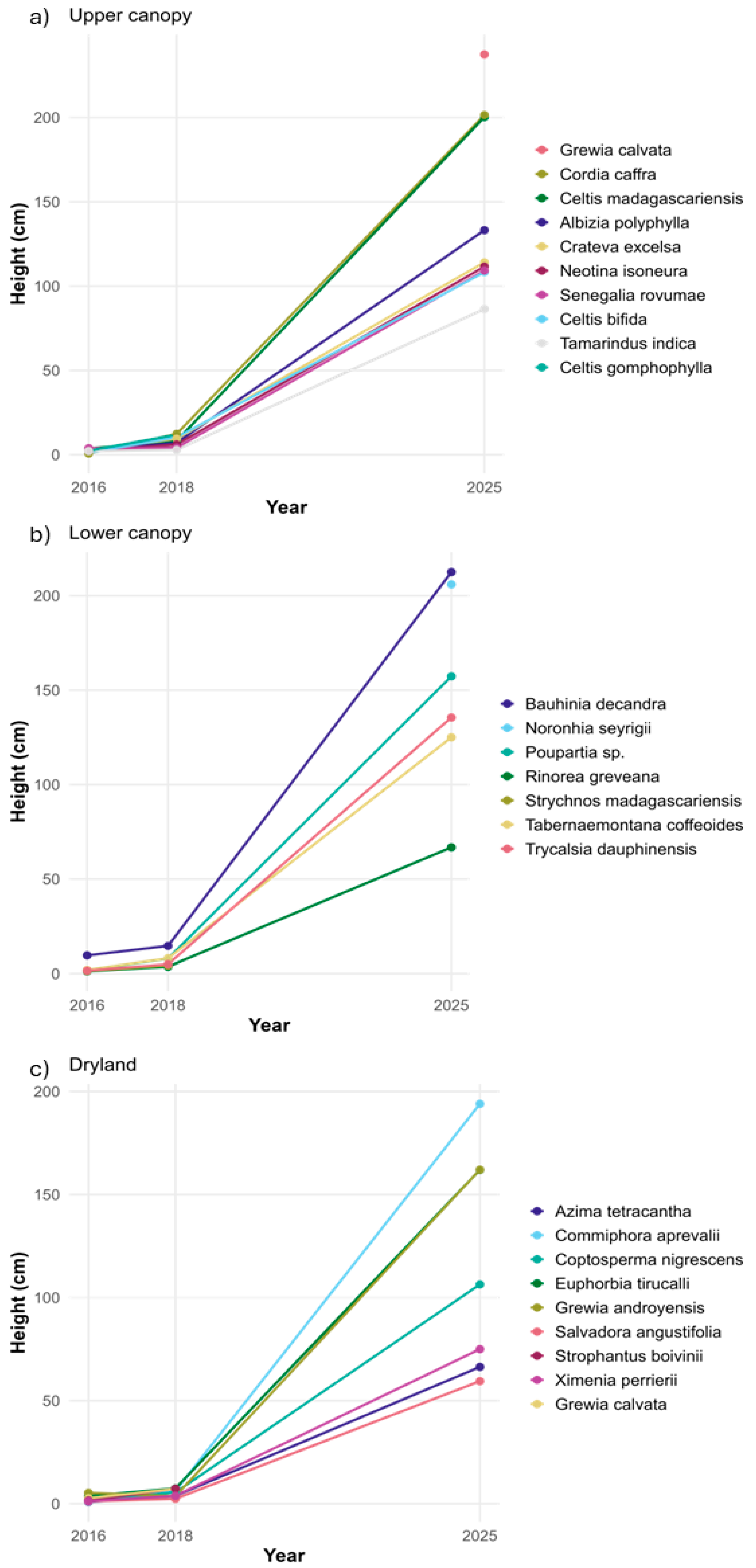
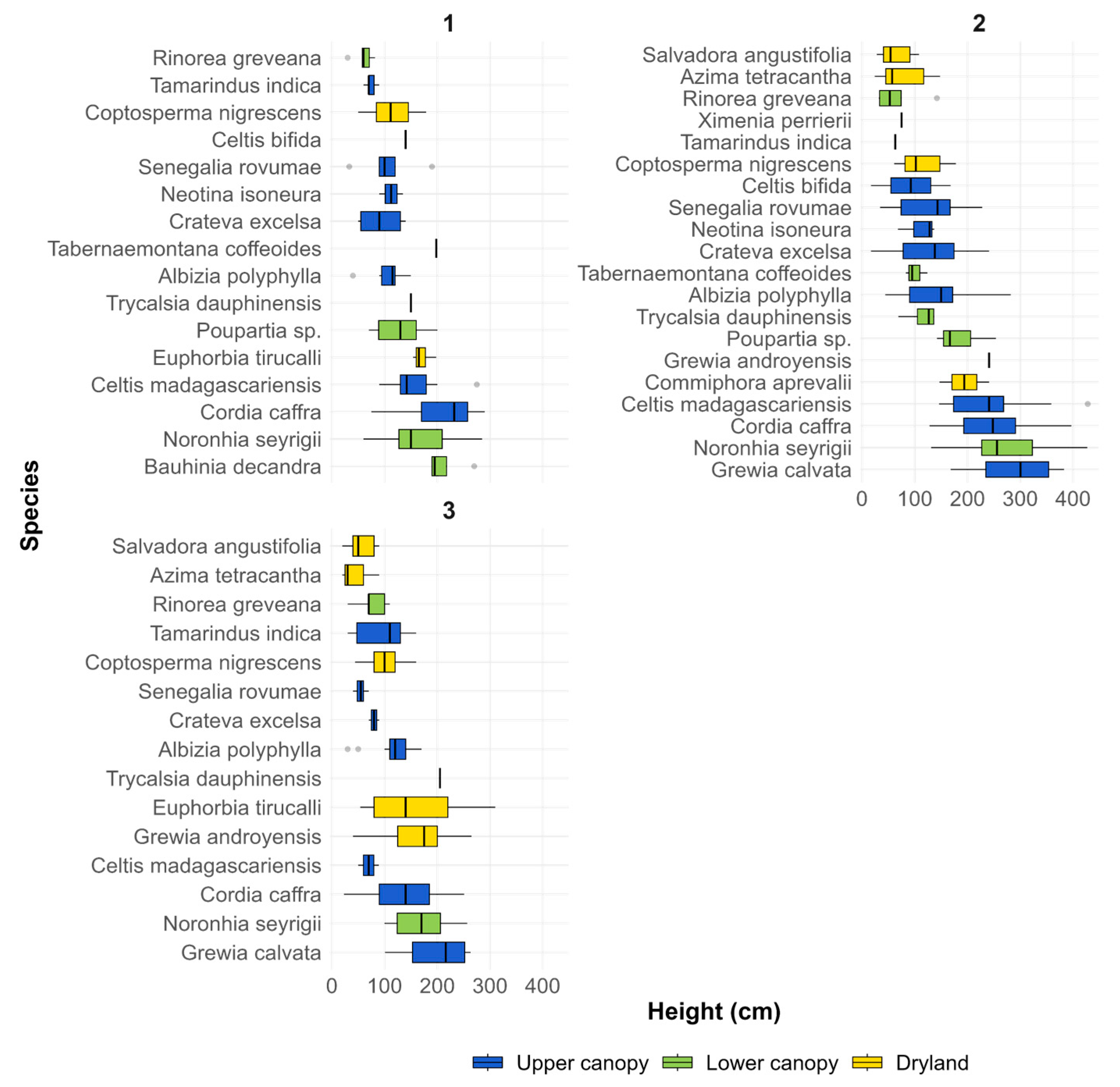
3.1. Growth Variations
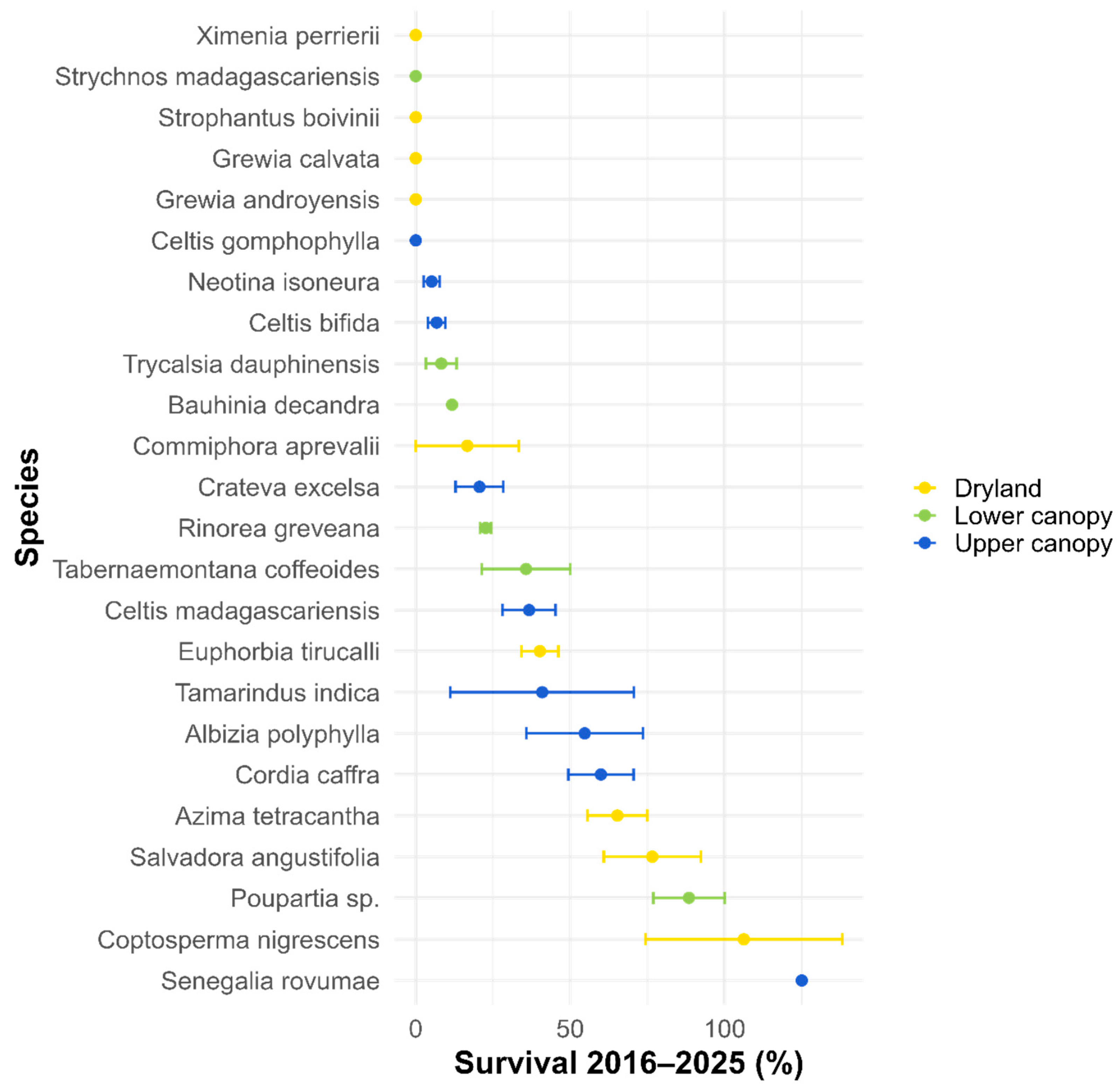
3.2. Survival and Mortality
3.3. Recruitment from the Forest 2017–18
3.4. Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| FAST GROWERS | |||||
| Plot | 1 | 2A | 3 | ||
| Cordia caffra * | U | Grewia calvata | D | Grewia calvata | D |
| Bauhinia decandra | L | Commiphora sp. | D | Commiphora sp. | D |
| Euphorbia sp. | D | Noronhia seyrigii | L | Noronhia seyrigii | L |
| Nhronhia seyrigii | L | Cordia caffra * | U | Cordia caffra * | U |
| Celtis madagascariensis | U | Celtis madagascariensis | U | ||
| SLOW GROWERS | |||||
| Coptosperma nigrescens | D | Tamarindus indica | U | Celtis madagascariensis | U |
| Senegalia rovumae ** | U | Azima tetracantha | D | Crateva excelsa | U |
| Tamarindus indica | U | Rinorea greveana | L | Senegalia rovumae ** | U |
| Rinorea greveana | L | Salvadora angustifolia | D | Salvadora angustifolia | D |
References
- Mesa-Sierra, N.; De La Peña-Domene, M.; Campo, J.; Giardina, C.P. Restoration of Tropical Dry Forest: An Analysis of Constraints and Successes across a Highly Threatened Biome. Front. Environ. Sci. 2025, 12, 1458613. [Google Scholar] [CrossRef]
- Viani, R.A.G.; Barreto, T.E.; Farah, F.T.; Rodrigues, R.R.; Brancalion, P.H.S. Monitoring Young Tropical Forest Restoration Sites: How Much to Measure? Trop. Conserv. Sci. 2018, 11, 1940082918780916. [Google Scholar] [CrossRef]
- Gatica-Saavedra, P.; Echeverría, C.; Nelson, C.R. Ecological Indicators for Assessing Ecological Success of Forest Restoration: A World Review. Restor. Ecol. 2017, 25, 850–857. [Google Scholar] [CrossRef]
- Evangelista De Oliveira, R.; Lex Engel, V.; De Paula Loiola, P.; Fernando Duarte De Moraes, L.; De Souza Vismara, E. Top 10 Indicators for Evaluating Restoration Trajectories in the Brazilian Atlantic Forest. Ecol. Indic. 2021, 127, 107652. [Google Scholar] [CrossRef]
- Díaz-Triana, J.E.; Torres-Rodríguez, S.; Muñoz-P, L.; Avella-M, A. Monitoreo de La Restauración Ecológica En Un Bosque Seco Tropical Interandino (Huila, Colombia): Programa y Resultados Preliminares. Caldasia 2019, 41, 60–77. [Google Scholar] [CrossRef]
- Culbertson, K.A.; Treuer, T.L.H.; Mondragon-Botero, A.; Ramiadantsoa, T.; Reid, J.L. The Eco-Evolutionary History of Madagascar Presents Unique Challenges to Tropical Forest Restoration. Biotropica 2022, 54, 1081–1102. [Google Scholar] [CrossRef]
- Winchester, V.; Hardwick, K.; Rasamimanana, H.; Raharison, S.; Mertl-Millhollen, A.; Gärtner, H.; McCrae, J. Berenty Reserve—A Gallery Forest in Decline in Dry Southern Madagascar—Towards Forest Restoration. Land 2018, 7, 8. [Google Scholar] [CrossRef]
- Mondragón-Botero, A.; Riera, B.; Rasamimanana, H.; Razafindrakoto, S.; Warme, E.; Powers, J.S. Structural and Compositional Differences in Gallery and Spiny Forests of Southern Madagascar: Implications for Conservation of Lemur and Tree Species. PLoS ONE 2024, 19, e0307907. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld-Jones, K.; Randriamboavonjy, T.M.; Williams, G.; Mertl-Millhollen, A.S.; Pinkus, S.; Rasamimanana, H. Tamarind Recruitment and Long-Term Stability in the Gallery Forest at Berenty, Madagascar. In Ringtailed Lemur Biology. Developments in Primatology: Progress and Prospect; Jolly, A., Sussman, R.W., Rasamimanana, H., Eds.; Springer: Boston, MA, USA, 2006; pp. 69–85. [Google Scholar]
- Rambeloarivony, H.; Jolly, A. Berenty Reserve: Past, Present and Future. In Leaping Ahead: Advances in Prosimian Biology; Masters, J., Gamba, M., Génin, F., Eds.; Springer: New York, NY, USA, 2013; pp. 353–359. ISBN 978-1-4614-4511-1. [Google Scholar]
- Jolly, A. Berenty Reserve, Madagascar: A Long Time in a Small Space. In Long-Term Field Studies of Primates; Kappeler, P.M., Watts, D.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 21–44. ISBN 978-3-642-22514-7. [Google Scholar]
- Mondragón-Botero, A.; Powers, J.S. How Dry Is Dead? Evaluating the Impact of Desiccation on the Viability of the Invasive Species Cissus quadrangularis. Plant-Environ. Interact. 2024, 5, e70011. [Google Scholar] [CrossRef]
- Sagar, R.; Mondragon-Botero, A.; Dolins, F.; Morgan, B.; Vu, T.P.; McCrae, J.; Winchester, V. Forest Restoration at Berenty Reserve, Southern Madagascar: A Pilot Study of Tree Growth Following the Framework Species Method. Land 2021, 10, 1041. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 8 December 2025).
- Werden, L.K.; Alvarado, J.P.; Zarges, S.; Calderón, M.E.; Schilling, E.M.; Gutiérrez, L.M.; Powers, J.S. Using Soil Amendments and Plant Functional Traits to Select Native Tropical Dry Forest Species for the Restoration of Degraded Vertisols. J. Appl. Ecol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef]
- Laughlin, D.C. Applying Trait-Based Models to Achieve Functional Targets for Theory-Driven Ecological Restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef]
- Brouwer, R.; Peña-Claros, M.; Bongers, F.; Poorter, L.; Guillemot, J.; Almeida, D.R.A.; De Almeida, C.T.; Resende, A.F.; Simões, L.H.P.; Ivanauskas, N.M.; et al. Functional Recovery of Tropical Forests: The Role of Restoration Methods and Environmental Conditions. Biol. Conserv. 2025, 309, 111269. [Google Scholar] [CrossRef]
- Brancalion, P.H.S.; Holl, K.D. Guidance for Successful Tree Planting Initiatives. J. Appl. Ecol. 2020, 57, 2349–2361. [Google Scholar] [CrossRef]
- Kremer, K.; Jonsson, B.; Tavares, M.F.; Bauhus, J. Effects of Planting Density on the Performance of Reforestation and Afforestation Plantings in Temperate and Boreal Forests: A Systematic Review. Restor. Ecol. 2025, 33, e70103. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, F.; Lin, Q.; Guo, S.; Li, S.; Zhang, Y.; Feng, Z.; Wang, X.; Rensing, C.; Cao, G.; et al. Effects of Different Stand Densities on the Composition and Diversity of Soil Microbiota in a Cunninghamia Lanceolata Plantation. Plants 2025, 14, 98. [Google Scholar] [CrossRef]
- Elliott, S.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Selecting Framework Tree Species for Restoring Seasonally Dry Tropical Forests in Northern Thailand Based on Field Performance. For. Ecol. Manag. 2003, 184, 177–191. [Google Scholar] [CrossRef]
- Forrester, D.I. The Spatial and Temporal Dynamics of Species Interactions in Mixed-Species Forests: From Pattern to Process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Elliott, S.D.; Blakesley, D.; Hardwick, K. Restoring Tropical Forests: A Practical Guide; Kew Publishing, Royal Botanic Gardens: London, UK, 2013; ISBN 978-1-84246-442-7. [Google Scholar]
- Chazdon, R.; Brancalion, P. Restoring Forests as a Means to Many Ends. Science 2019, 365, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Pennington, R.T.; Baker, T.R. Plants, People and Long-term Ecological Monitoring in the Tropics. Plants People Planet 2021, 3, 222–228. [Google Scholar] [CrossRef]
- Wright, P.C. For the Love of Lemurs: My Life in the Wilds of Madagascar; Lantern Books, a Division of Booklight Inc.: New York, NY, USA, 2014; ISBN 978-1-59056-445-5. [Google Scholar]
- D’Antonio, C.M.; August-Schmidt, E.; Fernandez-Going, B. Invasive Species and Restoration Challenges. In Foundations of Restoration Ecology; Palmer, M.A., Zedler, J.B., Falk, D.A., Eds.; Island Press/Center for Resource Economics: Washington, DC, USA, 2016; pp. 216–244. ISBN 978-1-61091-828-2. [Google Scholar]
- Chazdon, R.L.; Norden, N.; Colwell, R.K.; Chao, A. Monitoring Recovery of Tree Diversity during Tropical Forest Restoration: Lessons from Long-Term Trajectories of Natural Regeneration. Phil. Trans. R. Soc. B 2023, 378, 20210069. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Mondragon-Botero, A.; Winchester, V. Monitoring Forest Restoration in Berenty Reserve, Southern Madagascar. Land 2026, 15, 30. https://doi.org/10.3390/land15010030
Mondragon-Botero A, Winchester V. Monitoring Forest Restoration in Berenty Reserve, Southern Madagascar. Land. 2026; 15(1):30. https://doi.org/10.3390/land15010030
Chicago/Turabian StyleMondragon-Botero, Ariadna, and Vanessa Winchester. 2026. "Monitoring Forest Restoration in Berenty Reserve, Southern Madagascar" Land 15, no. 1: 30. https://doi.org/10.3390/land15010030
APA StyleMondragon-Botero, A., & Winchester, V. (2026). Monitoring Forest Restoration in Berenty Reserve, Southern Madagascar. Land, 15(1), 30. https://doi.org/10.3390/land15010030







