Assessing an Optical Tool for Identifying Tidal and Associated Mangrove Swamp Rice Fields in Guinea-Bissau, West Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Key Characteristics of the Case Studies
2.2. Field Data Collection and Soil Laboratory Analysis
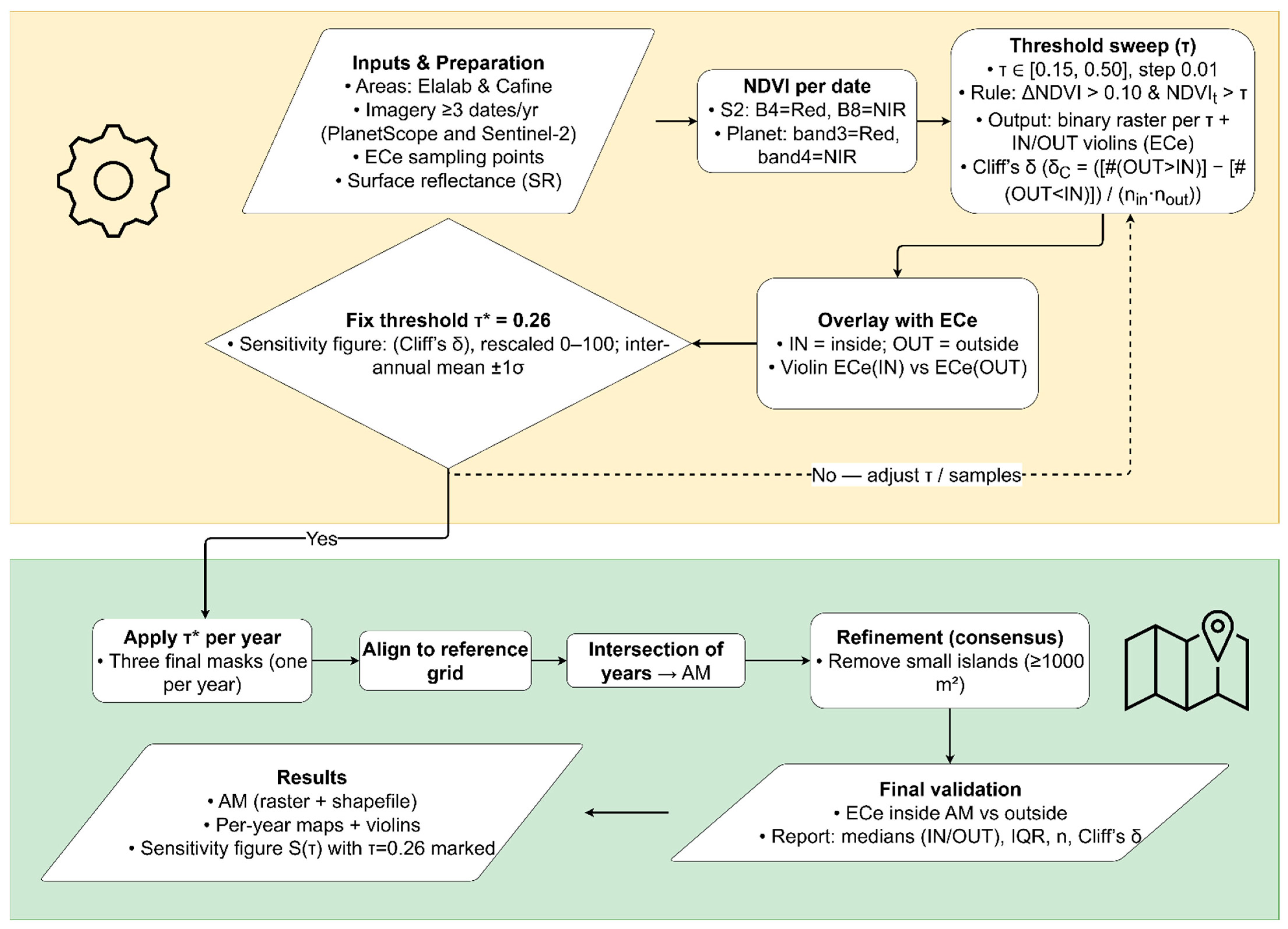
2.3. Methodology Overview
2.4. Remote Sensing Data and Calculations
- Image selection
- Vegetation index computation (NDVI)
- NDVI change detection
- Binary classification and vectorization
- Result validation
2.4.1. Image Selection
| Village | Provider | Temporal Resolution | Resolution (m) | Year | Date |
|---|---|---|---|---|---|
| Cafine-Cafal | Sentinel 2B | 6 days | 10 | 2019 | 05-30 |
| 06-14 | |||||
| 07-04 | |||||
| Planet Labs | 2.9 days | 3 | 2022 | 05-24 | |
| 06-02 | |||||
| 06-10 | |||||
| 06-26 | |||||
| 2023 | 05-16 | ||||
| 05-23 | |||||
| 06-04 | |||||
| 06-24 | |||||
| Elalab | Planet Labs | 2.9 days | 3 | 2021 | 06-24 |
| 07-04 | |||||
| 08-13 | |||||
| 2022 | 06-08 | ||||
| 06-22 | |||||
| 07-09 | |||||
| 08-01 | |||||
| 2024 | 05-31 | ||||
| 06-11 | |||||
| 06-16 | |||||
| 07-14 |
2.4.2. NDVI Computation
2.4.3. Change Detection Method for Early Appearance of Vegetation (Et)
- i.
- NDVI_t > τ (NDVI threshold), and
- ii.
- ΔNDVI_t > δ (minimum positive change).
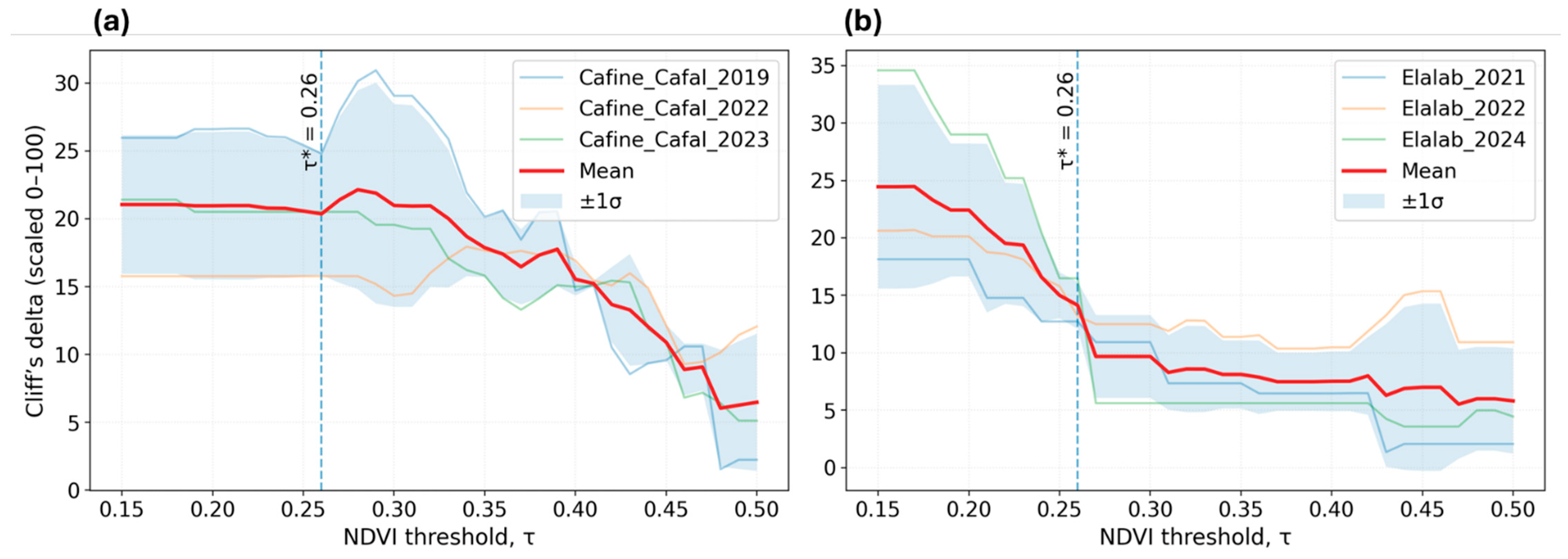
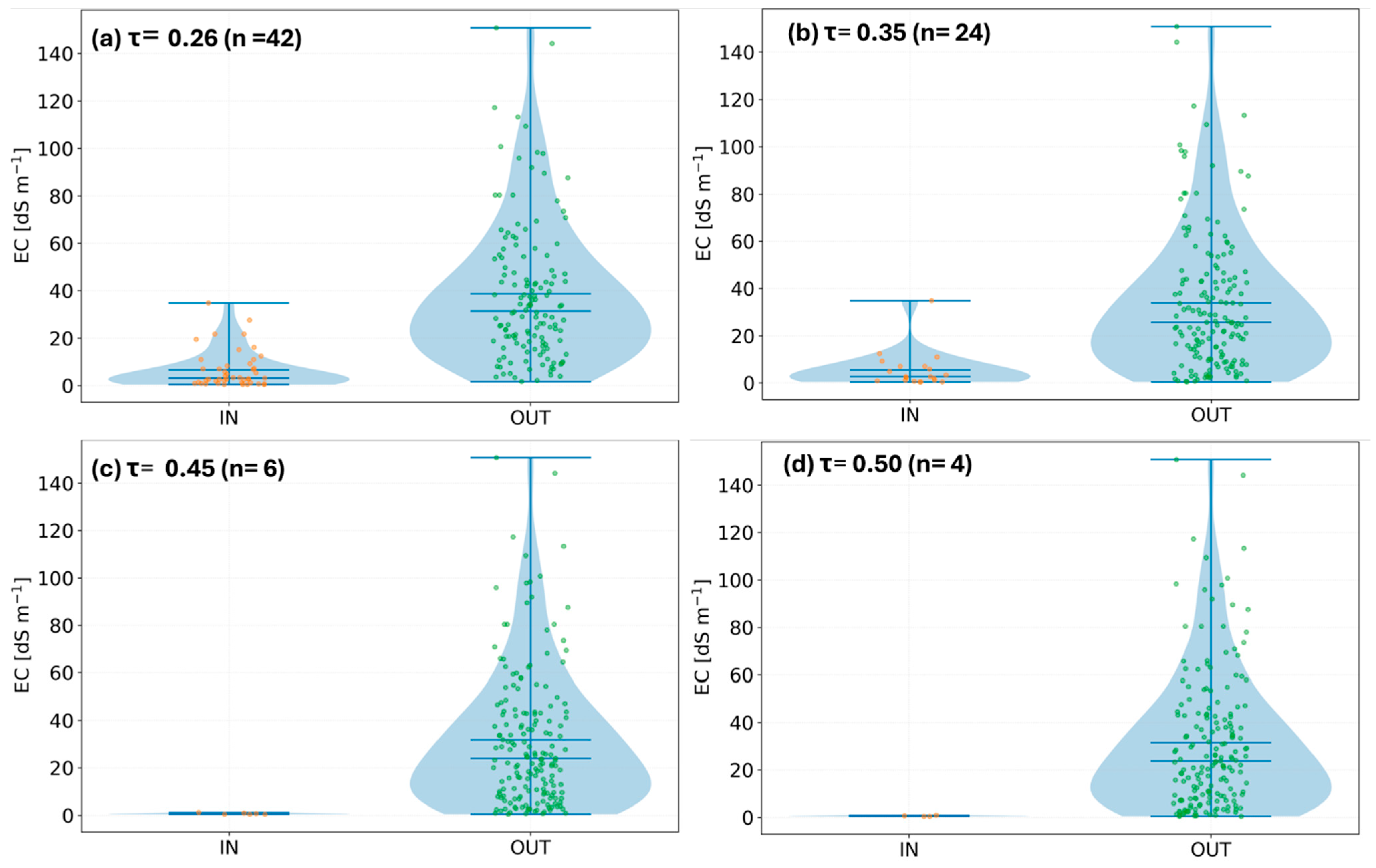
2.4.4. Per-Year Threshold Sweep and Diagnostics
2.4.5. Threshold Selection and Multi-Year Integration
2.5. Validation of the Result
2.6. Spatial Post-Processing
2.7. Statistical Analysis
3. Results
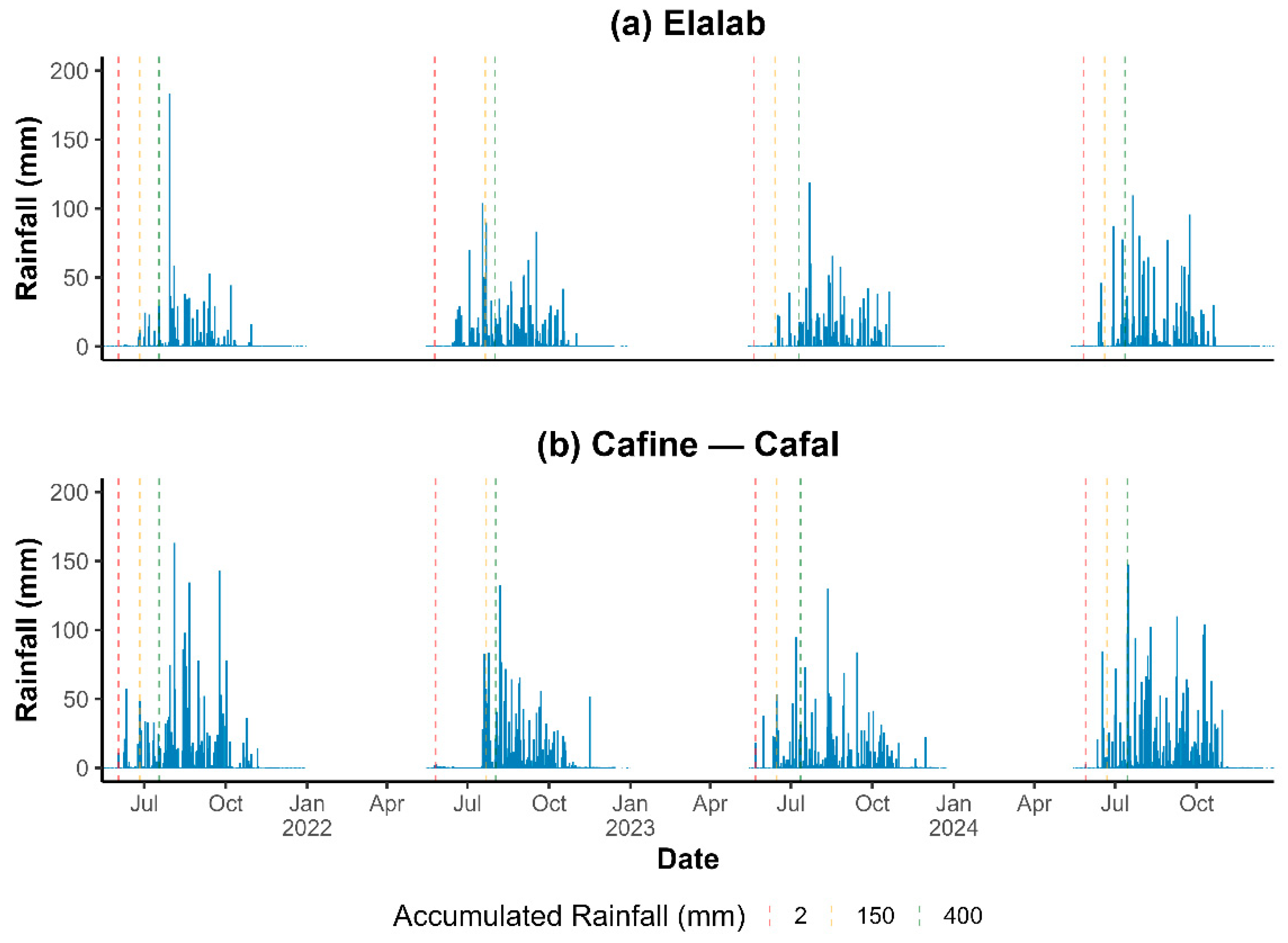
3.1. Rainfall Timing for NDVI Windows and Image Selection
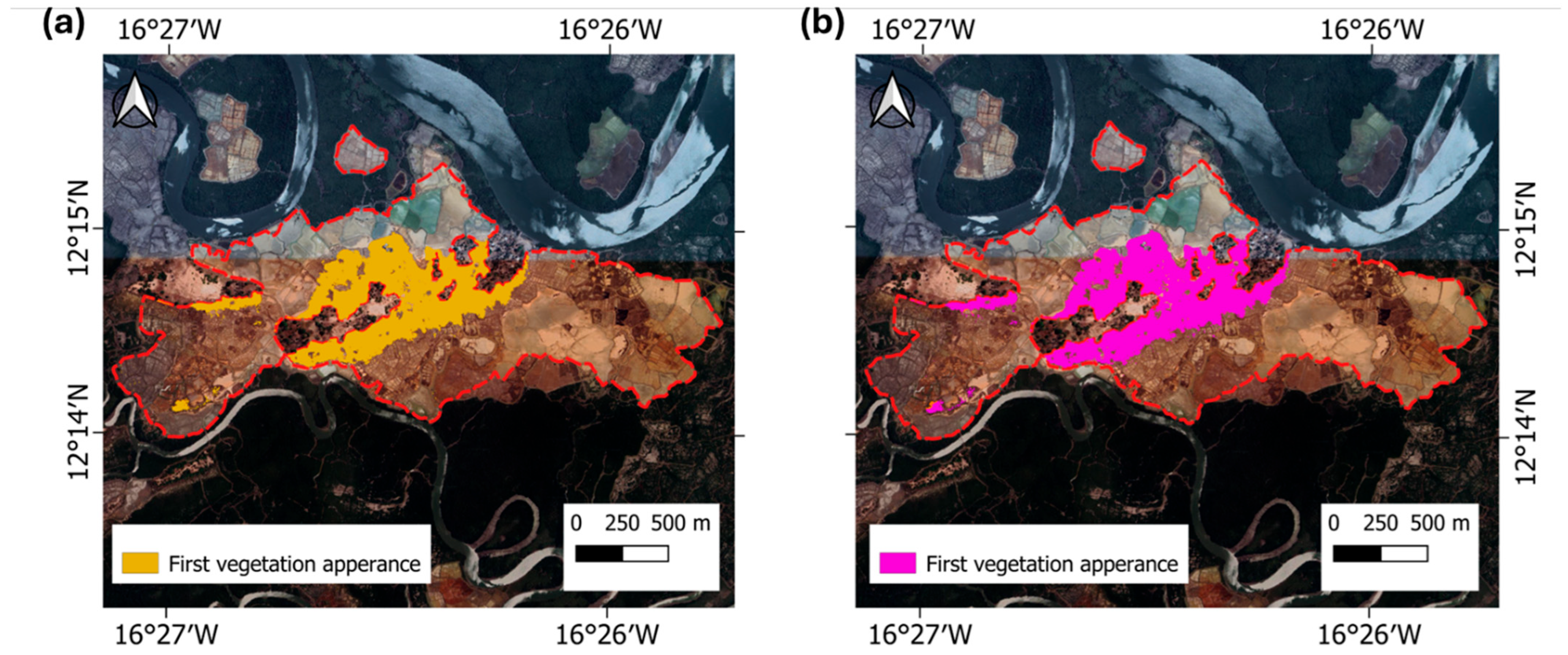
3.2. Validation of NDVI Thresholds for Early Vegetation, Salinity Zoning, and Site Classification
3.3. Comparison of the Sites’ Nutrients
4. Discussion
4.1. Rainfall Variability, Observation Windows, and Cloud Constraints
4.2. NDVI Usage and Threshold Calibration
4.3. Cross-Sensor Considerations
4.4. Management Implications for MSRP
4.5. Considerations Regarding the Model and Research Gaps
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temudo, M.P. Inovação e Mudança Em Sociedades Rurais Africanas Gestão de Recursos Naturais, Saber Local e Instituições de Desenvolvimento Induzido Estudo de Caso Na Guiné-Bissau. Ph.D. Thesis, Universidade Técnica de Lisboa, Lisbon, Portugal, 1998. [Google Scholar]
- Soullier, G.; Demont, M.; Arouna, A.; Lançon, F.; Mendez del Villar, P. The State of Rice Value Chain Upgrading in West Africa. Glob. Food Sec. 2020, 25, 100365. [Google Scholar] [CrossRef]
- Carney, J. Black Rice: The African Origins of Rice Cultivation in the Americas; Harvard University Press: Cambridge, MA, USA, 2001; Volume 1. [Google Scholar]
- Balasubramanian, V.; Sie, M.; Hijmans, R.J.; Otsuka, K. Increasing Rice Production in Sub-Saharan Africa: Challenges and Opportunities. Adv. Agron. 2007, 94, 55–133. [Google Scholar] [CrossRef]
- Sylla, M.; Stein, A.; van Breemen, N.; Fresco, L.O. Spatial Variability of Soil Salinity at Different Scales in the Mangrove Rice Agro-Ecosystem in West Africa. Agric. Ecosyst. Environ. 1995, 54, 1–15. [Google Scholar] [CrossRef]
- Garbanzo, G.; do Rosário Cameira, M.; Paredes, P.; Temudo, M.; Ramos, T.B. Modeling Soil Water and Salinity Dynamics in Mangrove Swamp Rice Production System of Guinea Bissau, West Africa. Agric. Water Manag. 2025, 313, 109494. [Google Scholar] [CrossRef]
- Temudo, M.P. Planting Knowledge, Harvesting Agro-Biodiversity: A Case Study of Southern Guinea-Bissau Rice Farming. Hum. Ecol. 2011, 39, 309–321. [Google Scholar] [CrossRef]
- Temudo, M.P.; Cabral, A.I. The Social Dynamics of Mangrove Forests in Guinea-Bissau, West Africa. Hum. Ecol. 2017, 45, 307–320. [Google Scholar] [CrossRef]
- Ullah Sarkar, M.I.; Jahan, A.; Hossain, A.T.M.S.; Sarker, M.A.B.S.; Islam, A.; Islam, M.R. Effect of Nutrient Omission on Rice Yield in a Wetland Double Rice Cropping System. J. Plant Nutr. 2023, 46, 312–320. [Google Scholar] [CrossRef]
- Tan, Z.X.; Lal, R.; Wiebe, K.D. Global Soil Nutrient Depletion and Yield Reduction. J. Sustain. Agric. 2005, 26, 123–146. [Google Scholar] [CrossRef]
- Martiarena, M.L.; Temudo, M.P. Endogenous Learning and Innovation in African Smallholder Agriculture: Lessons from Guinea-Bissau. J. Agric. Educ. Ext. 2024, 30, 161–179. [Google Scholar] [CrossRef]
- Leunda Martiarena, M.; Céspedes, J.; Varanda, M.; Merkohasanaj, M.; dos Santos, B.A.; Temudo, M.P. The Role and Drivers of Cooperation in Managing Hydraulic Infrastructures for Sustainable Mangrove Rice Production in Guinea-Bissau. Sustainability 2024, 17, 136. [Google Scholar] [CrossRef]
- Garbanzo, G.; Céspedes, J.; Sandoval, J.; Temudo, M.; Paredes, P.; Cameira, M. do R. Moving toward the Biophysical Characterization of the Mangrove Swamp Rice Production System in Guinea Bissau: Exploring Tools to Improve Soil- and Water-Use Efficiencies. Agronomy 2024, 14, 335. [Google Scholar] [CrossRef]
- Garbanzo, G.; Cameira, M.; Paredes, P. The Mangrove Swamp Rice Production System of Guinea-Bissau: Identification of the Main Constraints Associated with Soil Salinity and Rainfall Variability. Agronomy 2024, 14, 468. [Google Scholar] [CrossRef]
- Chuvieco, E. Fundamentals of Satellite Remote Sensing: An Environmental Approach; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780429506482. [Google Scholar]
- Karnieli, A.; Shachak, M.; Tsoar, H.; Zaady, E.; Kaufman, Y.; Danin, A.; Porter, W. The Effect of Microphytes on the Spectral Reflectance of Vegetation in Semiarid Regions. Remote Sens. Environ. 1996, 57, 88–96. [Google Scholar] [CrossRef]
- Shi, S.; Yang, P.; van der Tol, C. Spatial-Temporal Dynamics of Land Surface Phenology over Africa for the Period of 1982–2015. Heliyon 2023, 9, e16413. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.; Meroni, M.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Zurita-Milla, R.; Oosterbeek, K.; O’Connor, B.; Paganini, M. Vegetation Phenology from Sentinel-2 and Field Cameras for a Dutch Barrier Island. Remote Sens. Environ. 2018, 215, 517–529. [Google Scholar] [CrossRef]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Nagai, S.; Nasahara, K.N.; Muraoka, H.; Akiyama, T.; Tsuchida, S. Field Experiments to Test the Use of the Normalized-Difference Vegetation Index for Phenology Detection. Agric. For. Meteorol. 2010, 150, 152–160. [Google Scholar] [CrossRef]
- Nagol, J.R.; Vermote, E.F.; Prince, S.D. Effects of Atmospheric Variation on AVHRR NDVI Data. Remote Sens. Environ. 2009, 113, 392–397. [Google Scholar] [CrossRef]
- Feng, D.; Yang, H.; Gao, K.; Jin, X.; Li, Z.; Nie, C.; Zhang, G.; Fang, L.; Zhou, L.; Guo, H.; et al. Time-Series NDVI and Greenness Spectral Indices in Mid-to-Late Growth Stages Enhance Maize Yield Estimation. Field Crops Res. 2025, 333, 110069. [Google Scholar] [CrossRef]
- Kamble, M.V.; Ghosh, K.; Rajeevan, M.; Samui, R.P. Drought Monitoring over India through Normalized Difference Vegetation Index (NDVI). MAUSAM 2010, 61, 537–546. [Google Scholar] [CrossRef]
- Balaghi, R.; Tychon, B.; Eerens, H.; Jlibene, M. Empirical Regression Models Using NDVI, Rainfall and Temperature Data for the Early Prediction of Wheat Grain Yields in Morocco. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 438–452. [Google Scholar] [CrossRef]
- Cheng, K.S.; Wei, C.; Chang, S.C. Locating Landslides Using Multi-Temporal Satellite Images. Adv. Space Res. 2004, 33, 296–301. [Google Scholar] [CrossRef]
- Ji, Z.; Pan, Y.; Zhu, X.; Zhang, D.; Wang, J. A Generalized Model to Predict Large-Scale Crop Yields Integrating Satellite-Based Vegetation Index Time Series and Phenology Metrics. Ecol. Indic. 2022, 137, 108759. [Google Scholar] [CrossRef]
- Tan, K.C.; Lim, H.S.; MatJafri, M.Z.; Abdullah, K. A Comparison of Radiometric Correction Techniques in the Evaluation of the Relationship between LST and NDVI in Landsat Imagery. Environ. Monit. Assess. 2012, 184, 3813–3829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yano, T.; Nishiyama, S.; Kimura, R. Radiometric Correction for Linear Change-detection Techniques: Analysis in Bi-temporal Space. Int. J. Remote Sens. 2007, 28, 5143–5157. [Google Scholar] [CrossRef]
- Temudo, M.P.; Cabral, A.I.R. Climate Change as the Last Trigger in a Long-Lasting Conflict: The Production of Vulnerability in Northern Guinea-Bissau, West Africa. J. Peasant Stud. 2023, 50, 315–338. [Google Scholar] [CrossRef]
- Temudo, M.P.; Cabral, A.I.R.; Reis, P. The Sea Swallowed Our Houses and Rice Fields: The Vulnerability to Climate Change of Coastal People in Guinea-Bissau, West Africa. Hum. Ecol. 2022, 50, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Garbanzo, G.; Céspedes, J.; Temudo, M.; Cameira, M.d.R.; Paredes, P.; Ramos, T. Advances in Soil Salinity Diagnosis for Mangrove Swamp Rice Production in Guinea Bissau, West Africa. Sci. Remote Sens. 2025, 11, 100231. [Google Scholar] [CrossRef]
- Mendes, O.; Fragoso, M. Recent Changes in Climate Extremes in Guinea-Bissau. Afr. Geogr. Rev. 2024, 44, 1–19. [Google Scholar] [CrossRef]
- Mendes, O.; Correia, E.; Fragoso, M. Variability and Trends of the Rainy Season in West Africa with a Special Focus on Guinea-Bissau. Theor. Appl. Climatol. 2025, 156, 242. [Google Scholar] [CrossRef]
- Sultan, B.; Janicot, S. The West African Monsoon Dynamics. Part II: The “Preonset” and “Onset” of the Summer Monsoon. J. Clim. 2003, 16, 3407–3427. [Google Scholar] [CrossRef]
- Garbanzo, G.; Céspedes, J.; Temudo, M.; Ramos, T.B.; Cameira, M.d.R.; Pereira, L.S.; Paredes, P. Addressing Weather Data Gaps in Reference Crop Evapotranspiration Estimation: A Case Study in Guinea-Bissau, West Africa. Hydrology 2025, 12, 161. [Google Scholar] [CrossRef]
- Harris, S.A. Comments on the Application of the Holdridge System for Classification of World Life Zones as Applied to Costa Rica. Arct. Alp. Res. 2014, 5, 187–191. [Google Scholar] [CrossRef]
- Holdridge, L.R. Determination of World Plant Formations from Simple Climatic Data. Science 1947, 105, 367–368. [Google Scholar] [CrossRef]
- Merkohasanaj, M.; Cortez, N.; Goulao, L.; Andreetta, A. Characterisation of Physical-Chemical and Fertility Dynamics of Mangrove Soils from Guinea-Bissau in Different Agroecological Conditions Underlying Paddy Rice Cultivation Ao Cultivo Do Arroz. Rev. Cienc. Agrar. 2022, 45, 267–271. [Google Scholar] [CrossRef]
- Merkohasanaj, M.; Cortez, N.; Cunha-Queda, C.; Andreetta, A.; Cossa, V.; Martín-Peinado, F.J.; Temudo, M.P.; Goulao, L.F. Linking Soil Fertility and Production Constraints with Local Knowledge and Practices for Two Different Mangrove Swamp Rice Agroecologies, Guinea-Bissau, West Africa. Agronomy 2025, 15, 342. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Estimation of the Colloidal Material in Soils. Science 1926, 64, 362. [Google Scholar] [CrossRef] [PubMed]
- Day, P.R. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 2015; pp. 545–567. [Google Scholar]
- Soil Survey Staff. Kellogg Soil Survey Laboratory Methods Manual, Version 6; U.S. Department of Agriculture: Lincoln, NE, USA, 2022. [Google Scholar]
- Chapman, H.D. Cation-Exchange Capacity. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 2016; pp. 891–901. ISBN 9780891182047. [Google Scholar]
- Lee, R.; Taylor, M.; Daly, B.; Reynolds, J. The Extraction of Al, Fe and Si from a Range of New Zealand Soils by Hydroxylamine and Ammonium Oxalate Solutions. Soil Res. 1989, 27, 377. [Google Scholar] [CrossRef]
- McKeague, J.A.; Day, J.H. Dithionite and Oxalate Extractable Fe and Al as Aids in Differentiating Various Classes of Soils. Can. J. Soil Sci. 1966, 46, 13–22. [Google Scholar] [CrossRef]
- Zhang, H.; Hardy, D.; Mylavarapy, R.; Wang, J. Mehlich-3. In Soil Test Methods from the Southeastern United States; Sikora, F., Moore, K., Eds.; Southern Cooperative Series Bulletin N.419; Southern Association of Agricultural Experiment Station Directors (SAAESD): Raleigh, NC, USA, 2014; p. 101. ISBN 1-58161-419-5. [Google Scholar]
- Wilschefski, S.; Baxter, M. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, S.; Buyuktas, D.; Okturen, F.; Citak, S. Assessment of Different Soil to Water Ratios (1:1, 1:2.5, 1:5) in Soil Salinity Studies. Geoderma 2008, 144, 361–369. [Google Scholar] [CrossRef]
- Cliff, N. Answering Ordinal Questions with Ordinal Data Using Ordinal Statistics. Multivar. Behav. Res. 1996, 31, 331–350. [Google Scholar] [CrossRef]
- Balata, D.; Gama, I.; Domingos, T.; Proença, V. Using Satellite NDVI Time-Series to Monitor Grazing Effects on Vegetation Productivity and Phenology in Heterogeneous Mediterranean Forests. Remote Sens. 2022, 14, 2322. [Google Scholar] [CrossRef]
- Macbeth, G.; Razumiejczyk, E.; Ledesma, R.D. Cliff’s Delta Calculator: A Non-Parametric Effect Size Program for Two Groups of Observations. Univ. Psychol. 2010, 10, 545–555. [Google Scholar] [CrossRef]
- Tan, J.; Ding, J.; Han, L.; Ge, X.; Wang, X.; Wang, J.; Wang, R.; Qin, S.; Zhang, Z.; Li, Y. Exploring Planet Scope Satellite Capabilities for Soil Salinity Estimation and Mapping in Arid Regions Oases. Remote Sens. 2023, 15, 1066. [Google Scholar] [CrossRef]
- Planet Labs PBC Planet Application Program Interface: In Space for Life on Earth. Available online: https://api.planet.com/ (accessed on 28 March 2024).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Rouse, R.W.H.; Haas, J.A.W.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Reserves Technology Satellite Symposium, Washington, DC, USA, 10–14 December 1973; NASA SP-351. NASA: Greenbelt, MD, USA, 1974; p. 317. [Google Scholar]
- Gandhi, G.M.; Parthiban, S.; Thummalu, N.; Christy, A. Ndvi: Vegetation Change Detection Using Remote Sensing and Gis—A Case Study of Vellore District. Procedia Comput. Sci. 2015, 57, 1199–1210. [Google Scholar] [CrossRef]
- Blanton, R.; Hossain, A.K.M.A. Mapping the Recovery Process of Vegetation Growth in the Copper Basin, Tennessee Using Remote Sensing Technology. GeoHazards 2020, 1, 31–43. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009; ISBN 1441412697. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 30 March 2024).
- Purnamasari, L.; Rostaman, T.; Widowati, L.R.; Anggria, L. Comparison of Appropriate Cation Exchange Capacity (CEC) Extraction Methods for Soils from Several Regions of Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012209. [Google Scholar] [CrossRef]
- Ciric, V.; Prekop, N.; Seremesic, S.; Vojnov, B.; Pejic, B.; Radovanovic, D.; Marinkovic, D. The Implication of Cation Exchange Capacity (CEC) Assessment for Soil Quality Management and Improvement. J. Agric. For. 2023, 69, 113–134. [Google Scholar] [CrossRef]
- Cheng, Y.; Vrieling, A.; Fava, F.; Meroni, M.; Marshall, M.; Gachoki, S. Phenology of Short Vegetation Cycles in a Kenyan Rangeland from PlanetScope and Sentinel-2. Remote Sens. Environ. 2020, 248, 112004. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mishra, N.; Shukla, A.K.; Yadav, A.; Rao, G.S.; Bhanumurthy, V. Satellite Data Planning for Flood Mapping Activities Based on High Rainfall Events Generated Using TRMM, GEFS and Disaster News. Ann. GIS 2017, 23, 131–140. [Google Scholar] [CrossRef]
- Peña-Luque, S.; Ferrant, S.; Cordeiro, M.C.R.; Ledauphin, T.; Maxant, J.; Martinez, J.-M. Sentinel-1&2 Multitemporal Water Surface Detection Accuracies, Evaluated at Regional and Reservoirs Level. Remote Sens. 2021, 13, 3279. [Google Scholar] [CrossRef]
- Karlsen, S.R.; Elvebakk, A.; Tømmervik, H.; Belda, S.; Stendardi, L. Changes in Onset of Vegetation Growth on Svalbard, 2000–2020. Remote Sens. 2022, 14, 6346. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fan, H.; Yang, L.; Jiang, Y.; Sun, Z.; Zhang, Y. NDVI-Based Spatial and Temporal Vegetation Trends and Their Response to Precipitation and Temperature Changes in the Mu Us Desert from 2000 to 2019. Water Sci. Technol. 2023, 88, 430–442. [Google Scholar] [CrossRef]
- Lan, S.; Dong, Z. Incorporating Vegetation Type Transformation with NDVI Time-Series to Study the Vegetation Dynamics in Xinjiang. Sustainability 2022, 14, 582. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, J.; Li, Y.; Fan, J.; Wu, H. Characteristics of NDVI Changes in the Altay Region from 1981 to 2018 and Their Relationship to Climatic Factors. Land 2023, 12, 564. [Google Scholar] [CrossRef]
- Zaitunah, A.; Samsuri; Ahmad, A.G.; Safitri, R.A. Normalized Difference Vegetation Index (Ndvi) Analysis for Land Cover Types Using Landsat 8 Oli in Besitang Watershed, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012112. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, B.; Liang, L.; Huang, Y.; Matocha, C.; Lee, C.D.; Sama, M.; Masri, B.E.; Ren, W. Detecting Recent Crop Phenology Dynamics in Corn and Soybean Cropping Systems of Kentucky. Remote Sens. 2021, 13, 1615. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J.; Ni, P.; Zhang, J.; Wang, H. Monitoring Paddy Rice Phenology Using Time Series Modis Data over Jiangxi Province, China. Int. J. Agric. Biol. Eng. 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Chandler, C.J.; van der Heijden, G.M.F.; Boyd, D.S.; Foody, G.M. Detection of Spatial and Temporal Patterns of Liana Infestation Using Satellite-Derived Imagery. Remote Sens. 2021, 13, 2774. [Google Scholar] [CrossRef]
- Zhao, Q.; Qu, Y. The Retrieval of Ground NDVI (Normalized Difference Vegetation Index) Data Consistent with Remote-Sensing Observations. Remote Sens. 2024, 16, 1212. [Google Scholar] [CrossRef]
- Gozdowski, D.; Stępień, M.; Panek, E.; Varghese, J.; Bodecka, E.; Rozbicki, J.; Samborski, S. Comparison of Winter Wheat NDVI Data Derived from Landsat 8 and Active Optical Sensor at Field Scale. Remote Sens. Appl. 2020, 20, 100409. [Google Scholar] [CrossRef]
- Ji, L.; Peters, A.J. Lag and Seasonality Considerations in Evaluating AVHRR NDVI Response to Precipitation. Photogramm. Eng. Remote Sens. 2005, 71, 1053–1061. [Google Scholar] [CrossRef]
- Dardel, C.; Kergoat, L.; Hiernaux, P.; Mougin, E.; Grippa, M.; Tucker, C.J. Re-Greening Sahel: 30years of Remote Sensing Data and Field Observations (Mali, Niger). Remote Sens. Environ. 2014, 140, 350–364. [Google Scholar] [CrossRef]
- Thiam, S.; Villamor, G.B.; Faye, L.C.; Sène, J.H.B.; Diwediga, B.; Kyei-Baffour, N. Monitoring Land Use and Soil Salinity Changes in Coastal Landscape: A Case Study from Senegal. Environ. Monit. Assess. 2021, 193, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, B.; Liu, C.; Lin, Y.; Fu, Q.; Li, N.; Li, H. Soil Fertility, Enzyme Activity, and Microbial Community Structure Diversity among Different Soil Textures under Different Land Use Types in Coastal Saline Soil. J. Soils Sediments 2021, 21, 2240–2252. [Google Scholar] [CrossRef]
- Lal, R. Principles of Sustainable Soil Management in Agroecosystems; Lal, R., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781466513471. [Google Scholar]
- Amanullah; Inamullah; Alkahtani, J.; Elshikh, M.S.; Alwahibi, M.S.; Muhammad, A.; Ahmad, M.; Khalid, S. Phosphorus and Zinc Fertilization Influence Crop Growth Rates and Total Biomass of Coarse vs. Fine Types Rice Cultivars. Agronomy 2020, 10, 1356. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B. Dry-Matter and Grain Yield, Nutrient Uptake, and Phosphorus Use-Efficiency of Lowland Rice as Influenced by Phosphorus Fertilization. Commun. Soil Sci. Plant Anal. 2007, 38, 1289–1297. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Li, M.; Sun, H.; Pei, X.; Mi, X.; Zheng, L. Development of NDVI Measurement Device Based on Active Light Source; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2015. [Google Scholar]
- Bhaveshkumar, K.I.; Sharma, L.K. Dual Polarimetric Radar Vegetation Index for Assessing Mangrove Phenological Shifts under Eco-Climatic Stress. In Proceedings of the 2024 IEEE India Geoscience and Remote Sensing Symposium (InGARSS), Goa, India, 2–5 December 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–4. [Google Scholar]
- Yang, S.; Wang, L.; Shi, C.; Lu, Y. Evaluating the Relationship between the Photochemical Reflectance Index and Light Use Efficiency in a Mangrove Forest with Spartina Alterniflora Invasion. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 778–785. [Google Scholar] [CrossRef]
- Rezvan, H.; Valadan Zoej, M.J.; Youssefi, F.; Ghaderpour, E. Automated Rice Seedling Segmentation and Unsupervised Health Assessment Using Segment Anything Model with Multi-Modal Feature Analysis. Sensors 2025, 25, 5546. [Google Scholar] [CrossRef] [PubMed]

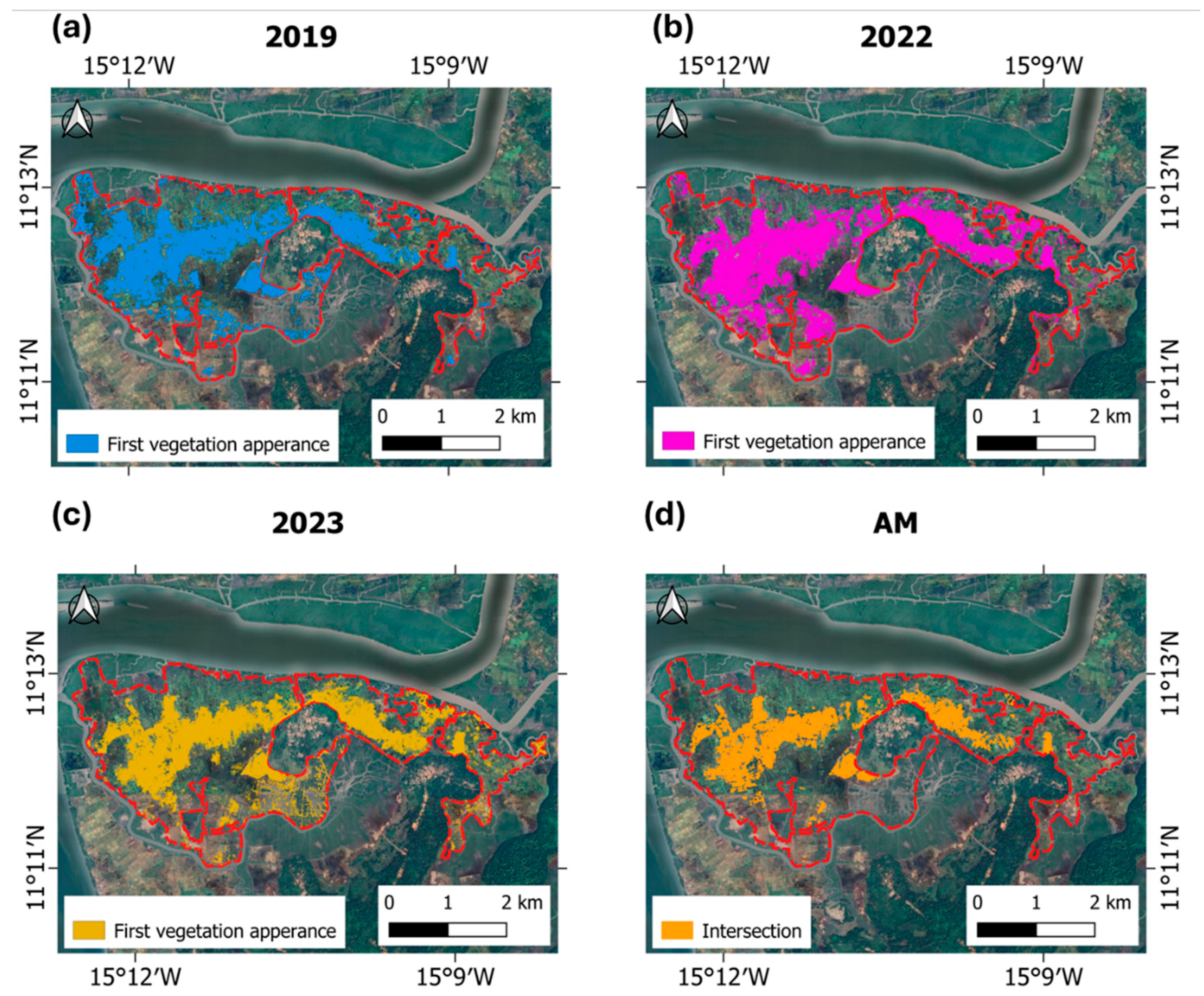



| Village | Variables | Units | Mean TM | Mean AM | W | p Value |
|---|---|---|---|---|---|---|
| Elalab n = 99 | ECe | dS m−1 | 106.53 | 9.09 | 1096.5 | <0.001 |
| Sand | Index | 0.56 | 0.86 | 172.0 | <0.001 | |
| Clay | 0.12 | 0.08 | 890.0 | 0.003 | ||
| Silt | 0.32 | 0.06 | 1014.5 | <0.001 | ||
| CEC+ | cmol(+) Kg−1 | 9.94 | 3.15 | 905.0 | 0.002 | |
| Fe * | % | 0.24 | 0.08 | 903.0 | 0.002 | |
| P | mg L−1 | 15.24 | 14.36 | 770.0 | 0.079 | |
| Zn | 1.04 | 0.84 | 752.5 | 0.114 | ||
| Cu | 1.33 | 0.94 | 818.5 | 0.006 | ||
| Fe_m3 | 309.31 | 277.71 | 666.5 | 0.476 | ||
| Mn | 3.42 | 1.44 | 914.0 | 0.001 | ||
| B | 6.30 | 0.78 | 1103.5 | <0.001 | ||
| S | 740.14 | 81.43 | 1081.0 | <0.001 | ||
| Cafine-Cafal n = 183 | ECe | dS m−1 | 38.16 | 6.02 | 4682.5 | <0.001 |
| Sand | Index | 0.26 | 0.27 | 2664.5 | 0.793 | |
| Clay | 0.27 | 0.33 | 2024.5 | 0.045 | ||
| Silt | 0.47 | 0.40 | 3698.5 | <0.001 | ||
| CEC+ | cmol(+) Kg−1 | 24.90 | 24.78 | 2307.0 | 0.316 | |
| Fe * | % | 0.82 | 0.63 | 3460.0 | 0.002 | |
| P | mg L−1 | 17.05 | 8.63 | 4205.5 | <0.001 | |
| Zn | 2.66 | 1.79 | 3566.5 | 0.001 | ||
| Cu | 0.96 | 0.83 | 2971.5 | 0.036 | ||
| Fe_m3 | 485.53 | 446.97 | 3031.5 | 0.118 | ||
| Mn | 19.53 | 15.46 | 2871.5 | 0.318 | ||
| B | 2.91 | 1.45 | 4493.0 | <0.001 | ||
| S | 395.99 | 165.09 | 4090.0 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Céspedes, J.; Garbanzo-León, J.; Temudo, M.; Garbanzo, G. Assessing an Optical Tool for Identifying Tidal and Associated Mangrove Swamp Rice Fields in Guinea-Bissau, West Africa. Land 2025, 14, 2144. https://doi.org/10.3390/land14112144
Céspedes J, Garbanzo-León J, Temudo M, Garbanzo G. Assessing an Optical Tool for Identifying Tidal and Associated Mangrove Swamp Rice Fields in Guinea-Bissau, West Africa. Land. 2025; 14(11):2144. https://doi.org/10.3390/land14112144
Chicago/Turabian StyleCéspedes, Jesus, Jaime Garbanzo-León, Marina Temudo, and Gabriel Garbanzo. 2025. "Assessing an Optical Tool for Identifying Tidal and Associated Mangrove Swamp Rice Fields in Guinea-Bissau, West Africa" Land 14, no. 11: 2144. https://doi.org/10.3390/land14112144
APA StyleCéspedes, J., Garbanzo-León, J., Temudo, M., & Garbanzo, G. (2025). Assessing an Optical Tool for Identifying Tidal and Associated Mangrove Swamp Rice Fields in Guinea-Bissau, West Africa. Land, 14(11), 2144. https://doi.org/10.3390/land14112144







