Modeling Irrigation of Tomatoes with Saline Water in Semi-Arid Conditions Using Hydrus-1D
Highlights
- Hydrus-1D successfully simulated soil water and salt dynamics in a soil profile, with tomatoes irrigated with saline water.
- The multiplicative S-model was the best model for reproducing a decrease in tomato relative yield due to irrigation water salinity.
- An increase in seasonal temperature of 2°C had no significant effect on tomato yield.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Measurements
2.2. Hydrus-1D Model
2.2.1. Theory
2.2.2. Soil Hydraulic Properties and Solute Transport Parameters
2.2.3. Model Calibration and Validation
2.3. Statistical Evaluation of Modeling Results
3. Results and Discussion
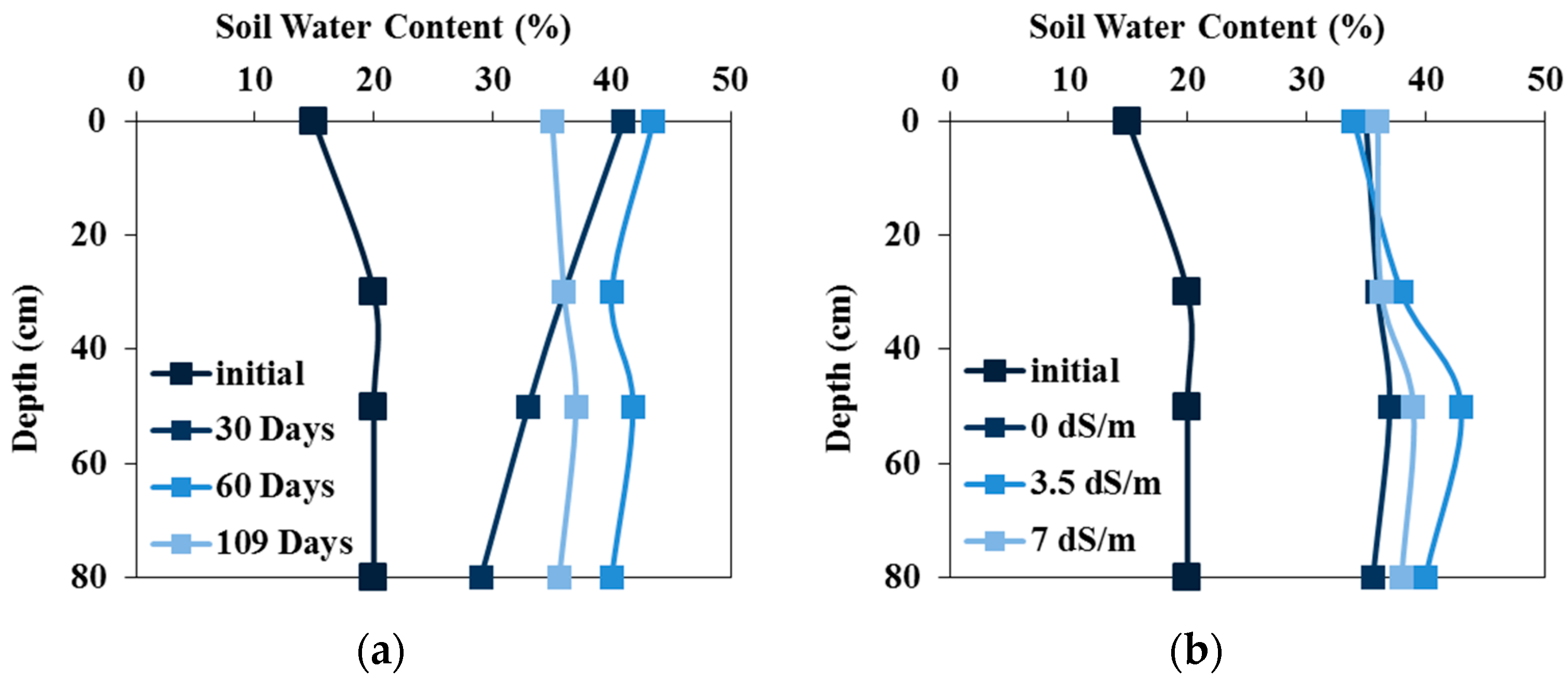
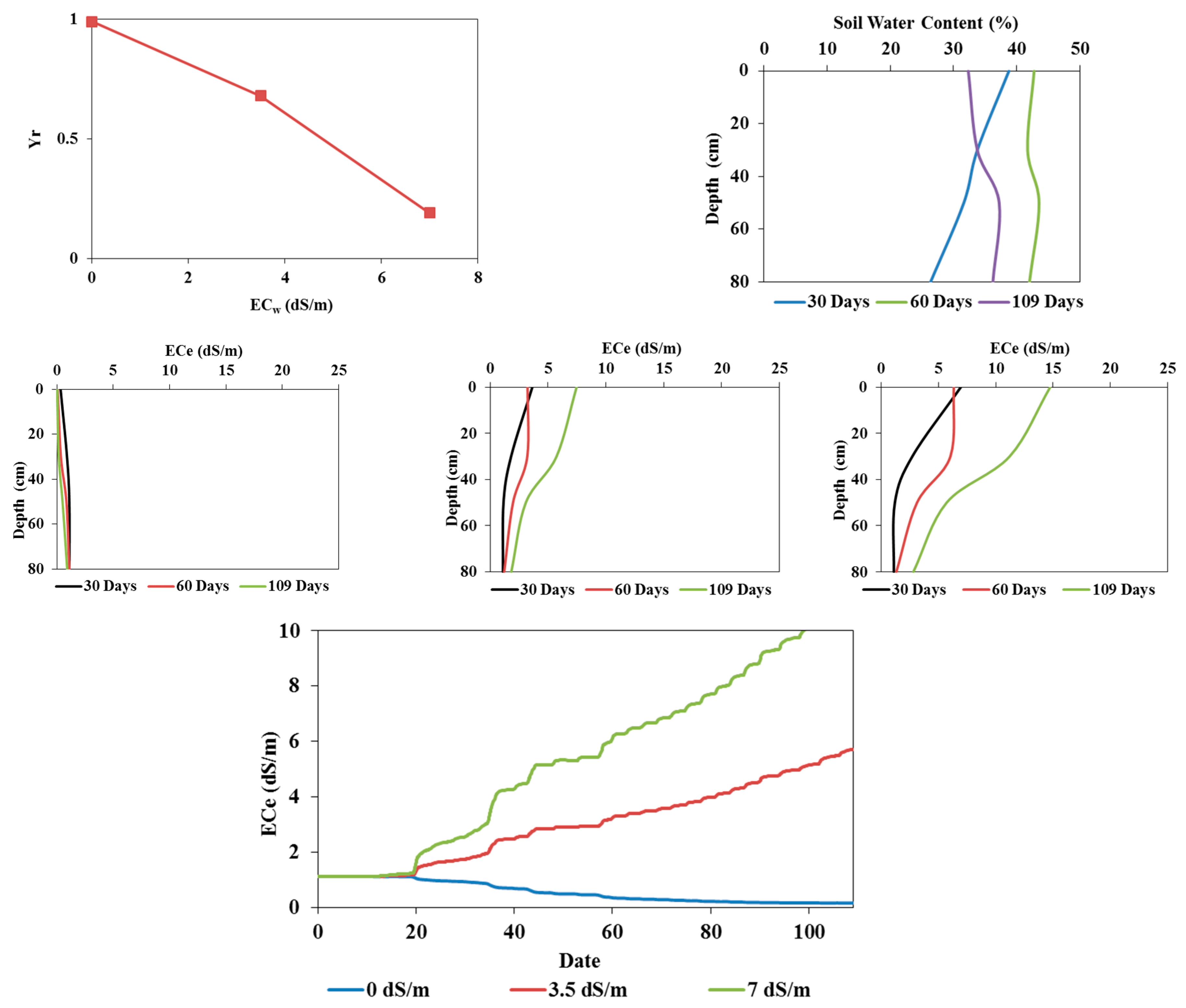
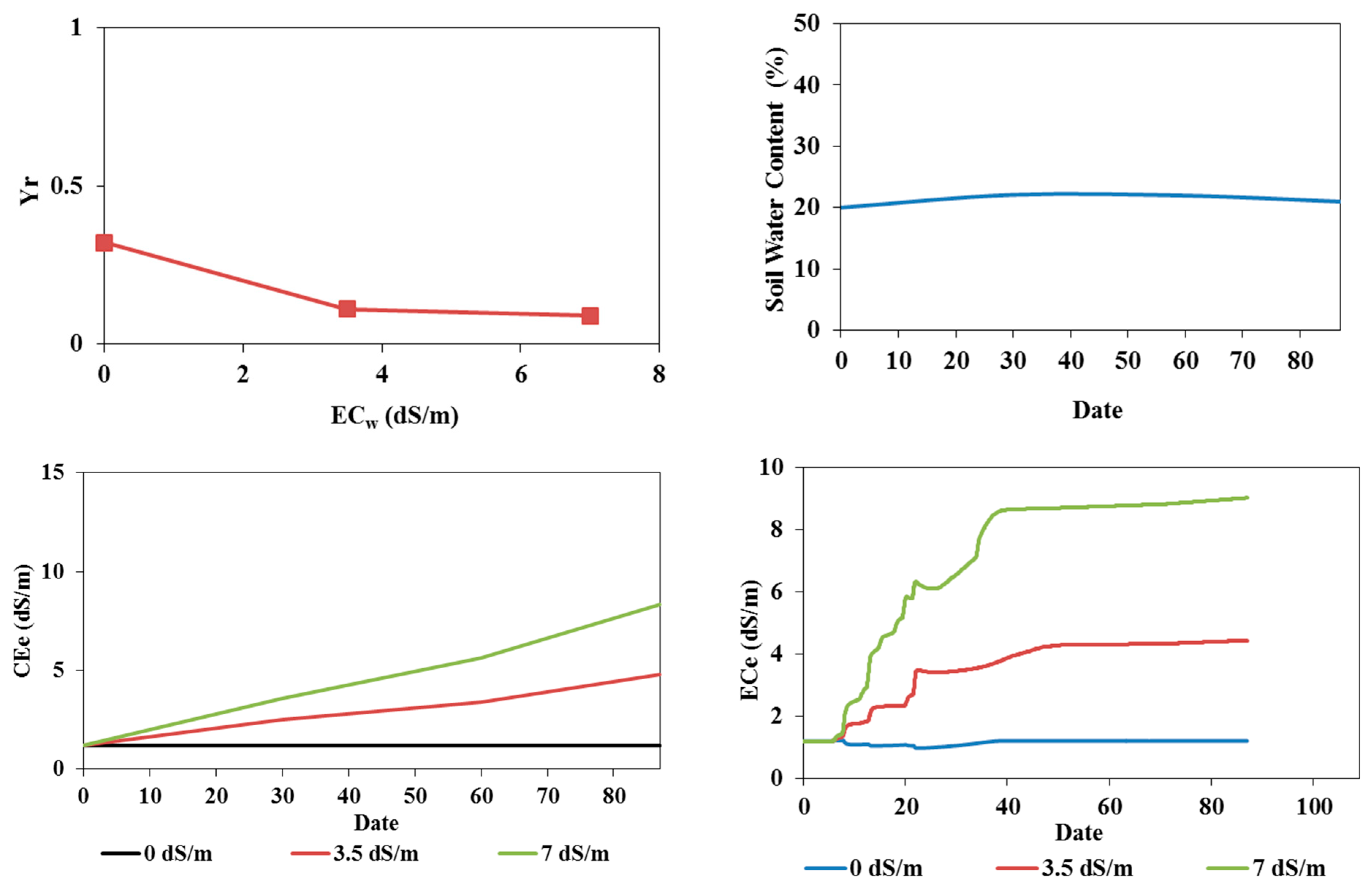
3.1. Soil Water Content Dynamics
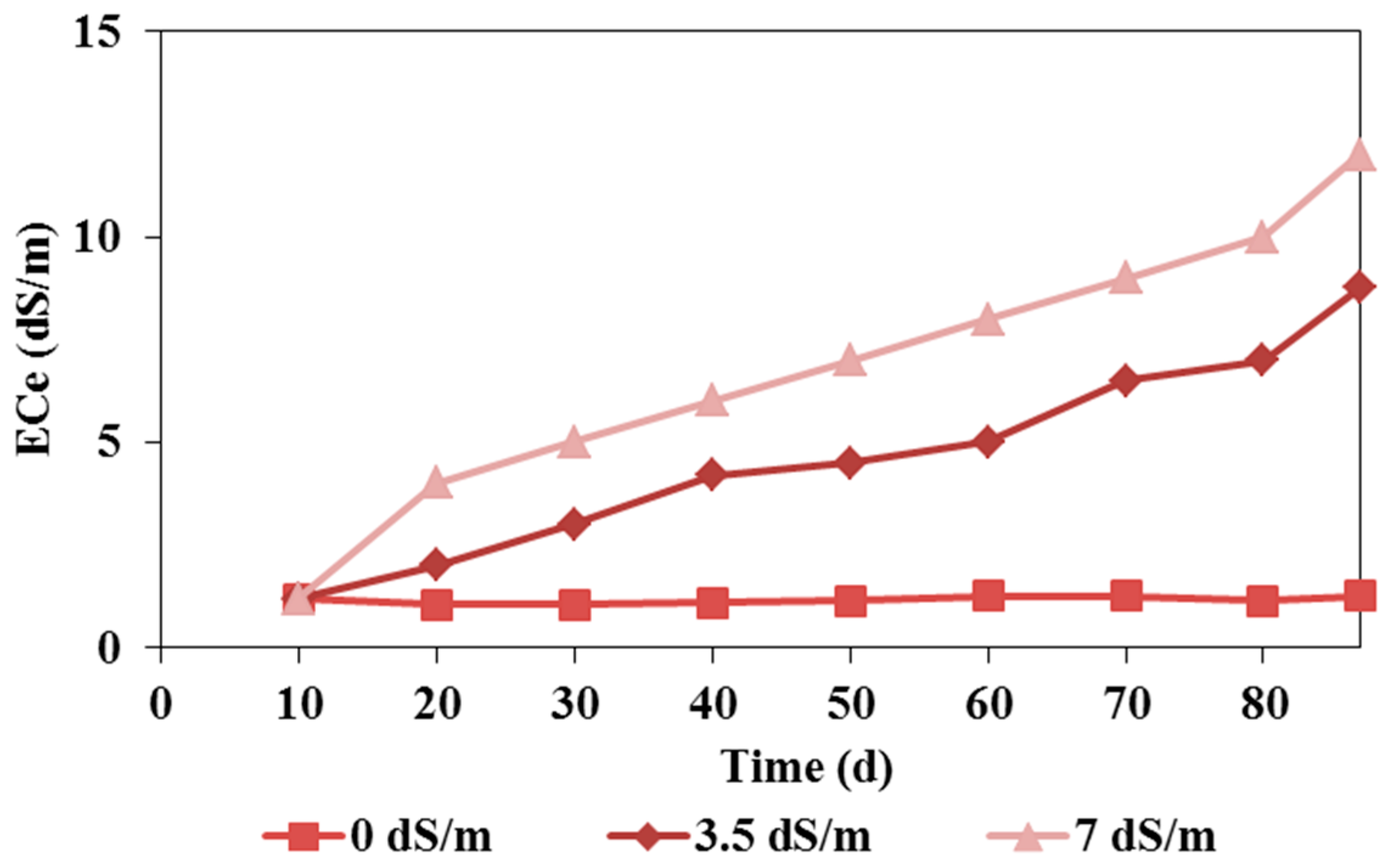
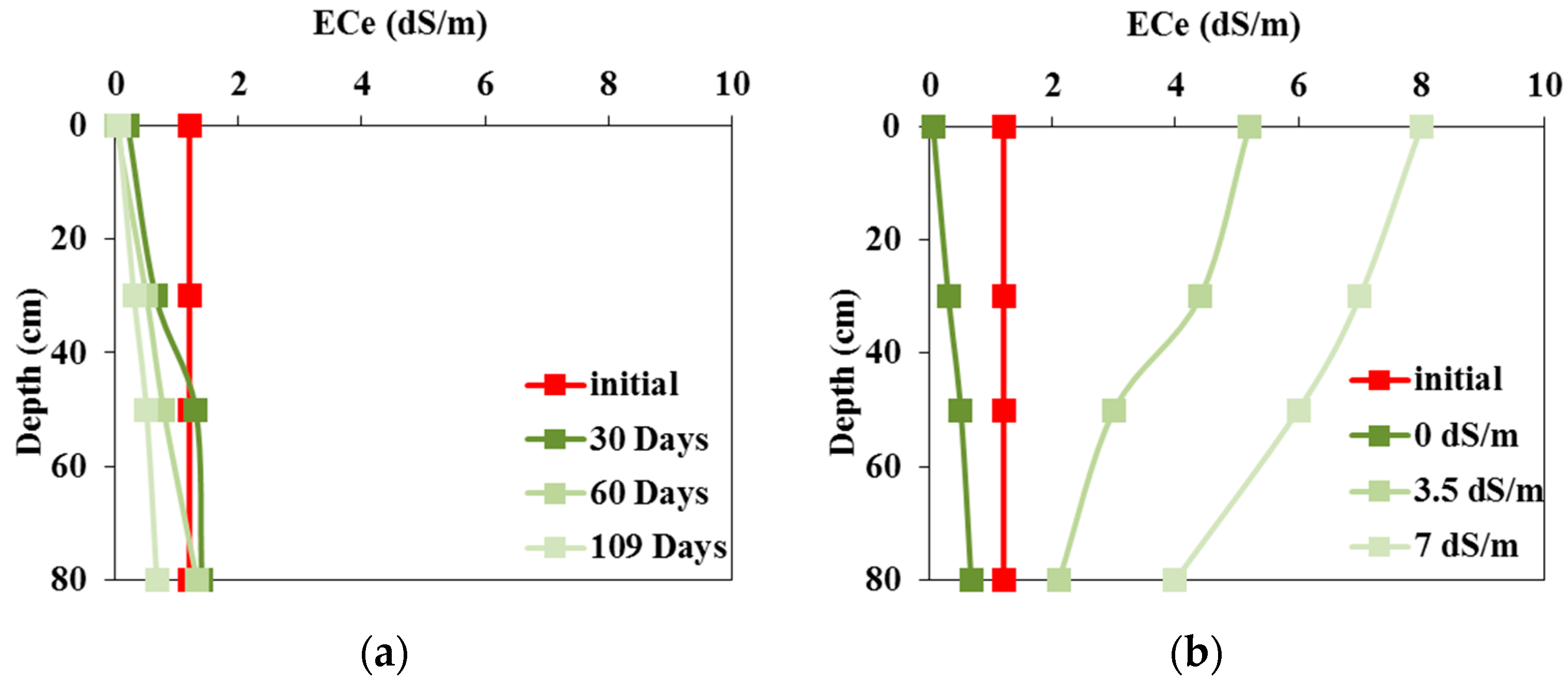
3.2. Soil Salinity Dynamics
3.3. Numerical Modeling of Water and Salt Dynamics with Root Water Uptake
3.3.1. Inputs to Hydrus-1D
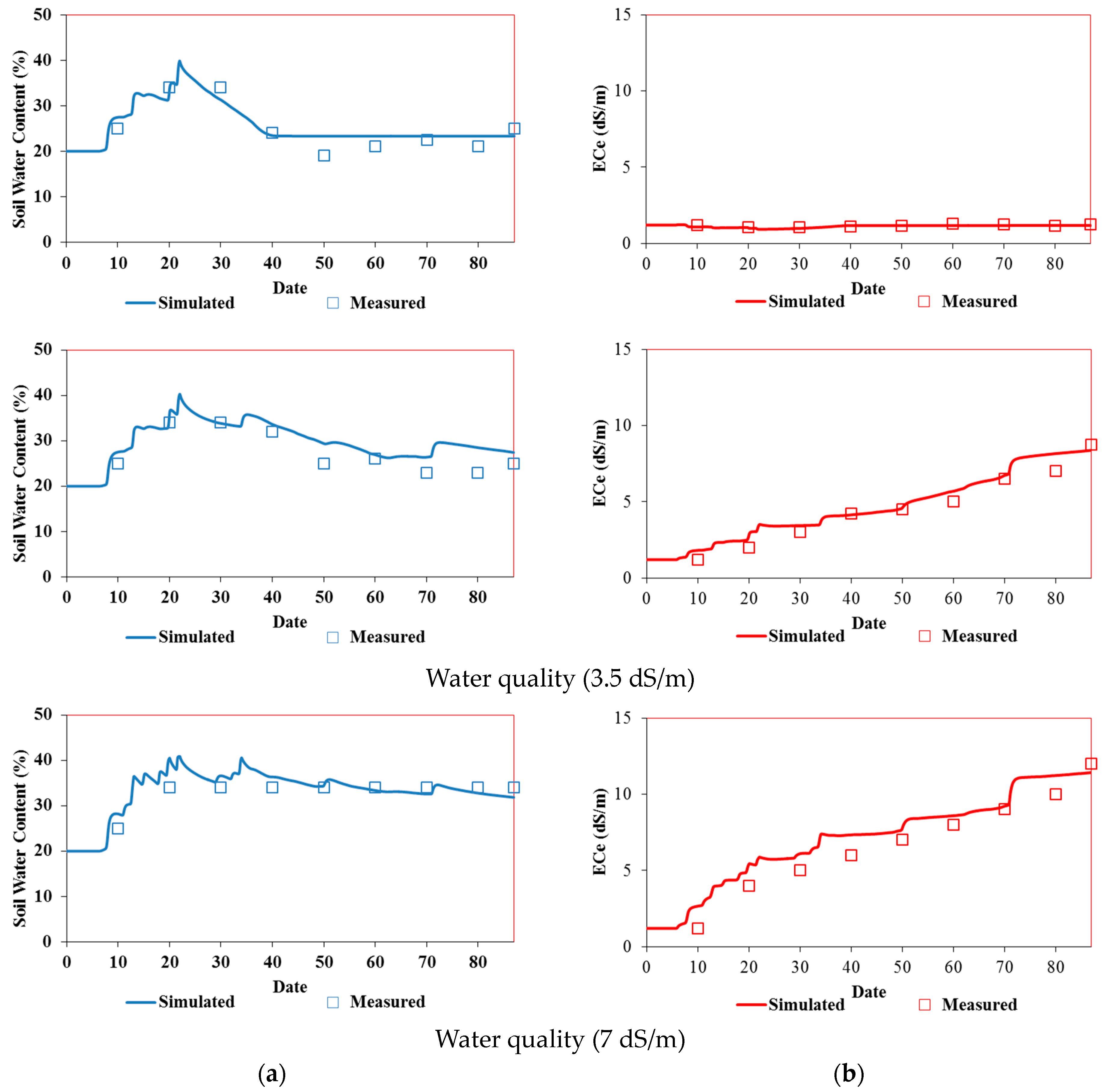
3.3.2. Simulation Results
Model Calibration
Model Validation
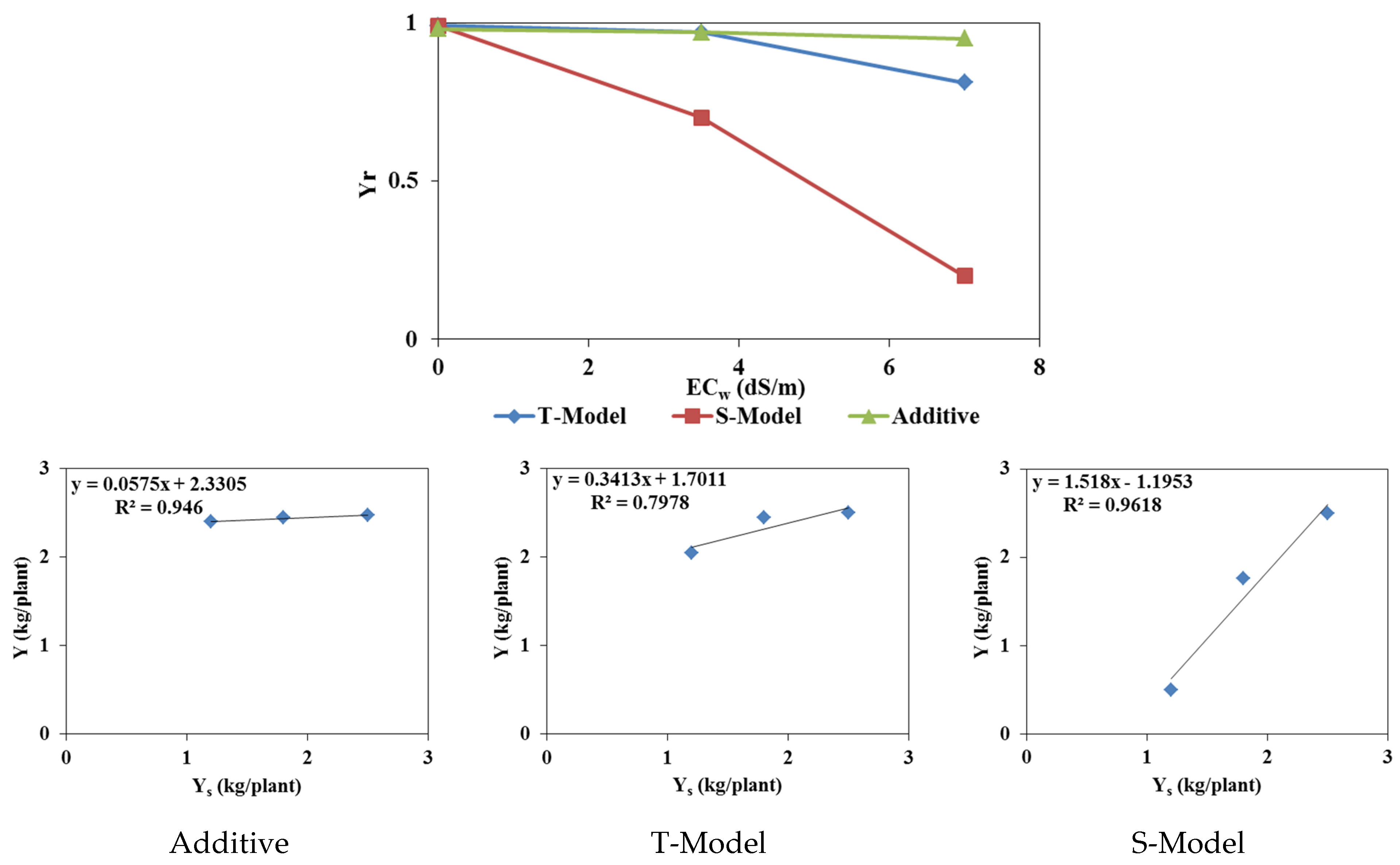
3.3.3. Crop Yield
3.4. Effect of Temperature Increase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Besser, H.; Dhaouadi, L.; Hadji, R.; Hamed, Y.; Jemmali, H. Ecologic and Economic Perspectives for Sustainable Irrigated Agriculture under Arid Climate Conditions: An Analysis Based on Environmental Indicators for Southern Tunisia. J. Afr. Earth Sci. 2021, 177, 104134. [Google Scholar] [CrossRef]
- Srivastava, A.; Parida, V.K.; Majumder, A.; Gupta, B.; Gupta, A.K. Treatment of Saline Wastewater Using Physicochemical, Biological, and Hybrid Processes: Insights into Inhibition Mechanisms, Treatment Efficiencies and Performance Enhancement. J. Environ. Chem. Eng. 2021, 9, 105775. [Google Scholar] [CrossRef]
- Martínez-Alvarez, V.; González-Ortega, M.J.; Martin-Gorriz, B.; Soto-García, M.; Maestre-Valero, J.F. The Use of Desalinated Seawater for Crop Irrigation in the Segura River Basin (South-Eastern Spain). Desalination 2017, 422, 153–164. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and Irrigation Management Strategies for Saline-Sodic Soils and Waters Aimed at Environmentally Sustainable Agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil Salinity and Drought Tolerance: An Evaluation of Plant Growth, Productivity, Microbial Diversity, and Amelioration Strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Deeb, M.; Smagin, A.V.; Pauleit, S.; Fouché-Grobla, O.; Podwojewski, P.; Groffman, P.M. The Urgency of Building Soils for Middle Eastern and North African Countries: Economic, Environmental, and Health Solutions. Sci. Total Environ. 2024, 917, 170529. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; He, P.; Chen, D.; Dai, X.; Jin, Q.; Su, X. The Optimal Irrigation Water Salinity and Salt Component for High-Yield and Good-Quality of Tomato in Ningxia. Agric. Water Manag. 2022, 274, 107940. [Google Scholar] [CrossRef]
- Khondoker, M.; Mandal, S.; Gurav, R.; Hwang, S. Freshwater Shortage, Salinity Increase, and Global Food Production: A Need for Sustainable Irrigation Water Desalination—A Scoping Review. Earth 2023, 4, 223–240. [Google Scholar] [CrossRef]
- Li, P.; Ren, L. Evaluating the Saline Water Irrigation Schemes Using a Distributed Agro-Hydrological Model. J. Hydrol. 2021, 594, 125688. [Google Scholar] [CrossRef]
- Yu, Q.; Kang, S.; Hu, S.; Zhang, L.; Zhang, X. Modeling Soil Water-Salt Dynamics and Crop Response under Severely Saline Condition Using WAVES: Searching for a Target Irrigation Volume for Saline Water Irrigation. Agric. Water Manag. 2021, 256, 107100. [Google Scholar] [CrossRef]
- Kanzari, S.; Jaziri, R.; Ali, K.B.; Daghari, I. Long-Term Evaluation of Soil Salinization Risks under Different Climate Change Scenarios in a Semi-Arid Region of Tunisia. Water Supply 2021, 21, 2463–2476. [Google Scholar] [CrossRef]
- Majeed, A.; Stockle, C.O.; King, L.G. Computer Model for Managing Saline Water for Irrigation and Crop Growth: Preliminary Testing with Lysimeter Data. Agric. Water Manag. 1994, 26, 239–251. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Recent Developments and Applications of the HYDRUS Computer Software Packages. Vadose Zone J. 2016, 15, 1–25. [Google Scholar] [CrossRef]
- Šimůnek, J.; Brunetti, G.; Jacques, D.; van Genuchten, M.T.; Šejna, M. Recent developments and applications of the HYDRUS computer software packages since 2016. Vadose Zone J. 2024, 23, e20310. [Google Scholar]
- van Dam, J.C.; Groenendijk, P.; Hendriks, R.F.A.; Kroes, J.G. Advances of Modeling Water Flow in Variably Saturated Soils with SWAP. Vadose Zone J. 2008, 7, 640–653. [Google Scholar] [CrossRef]
- Miao, Q.; Rosa, R.D.; Shi, H.; Paredes, P.; Zhu, L.; Dai, J.; Gonçalves, J.M.; Pereira, L.S. Modeling Water Use, Transpiration and Soil Evaporation of Spring Wheat–Maize and Spring Wheat–Sunflower Relay Intercropping Using the Dual Crop Coefficient Approach. Agric. Water Manag. 2016, 165, 211–229. [Google Scholar] [CrossRef]
- Kumar, P.; Sarangi, A.; Singh, D.K.; Parihar, S.S.; Sahoo, R.N. Simulation of Salt Dynamics in the Root Zone and Yield of Wheat Crop under Irrigated Saline Regimes Using SWAP Model. Agric. Water Manag. 2015, 148, 72–83. [Google Scholar] [CrossRef]
- Kanzari, S.; Daghari, I.; Šimůnek, J.; Younes, A.; Ilahy, R.; Ben Mariem, S.; Rezig, M.; Ben Nouna, B.; Bahrouni, H.; Ben Abdallah, M.A. Simulation of Water and Salt Dynamics in the Soil Profile in the Semi-Arid Region of Tunisia—Evaluation of the Irrigation Method for a Tomato Crop. Water 2020, 12, 1594. [Google Scholar] [CrossRef]
- Ploeg, D.V.; Heuvelink, E. Influence of Sub-Optimal Temperature on Tomato Growth and Yield: A Review. J. Hortic. Sci. Biotechnol. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Bhandari, R.; Neupane, N.; Adhikari, D.P. Climatic Change and Its Impact on Tomato (Lycopersicum esculentum L.) Production in Plain Area of Nepal. Environ. Chall. 2021, 4, 100129. [Google Scholar] [CrossRef]
- Petrović, I.; Savić, S.; Gricourt, J.; Causse, M.; Jovanović, Z.; Stikić, R. Effect of Long-Term Drought on Tomato Leaves: The Impact on Metabolic and Antioxidative Response. Physiol. Mol. Biol. Plants 2021, 27, 2805–2817. [Google Scholar] [CrossRef]
- Delgado-Vargas, V.A.; Ayala-Garay, O.J.; Arévalo-Galarza, M.d.L.; Gautier, H. Increased Temperature Affects Tomato Fruit Physicochemical Traits at Harvest Depending on Fruit Developmental Stage and Genotype. Horticulturae 2023, 9, 212. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration: Guide-lines for computing crop water requirements. In FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998; 300p. [Google Scholar]
- Hide, J.C. Diagnosis and Improvement of Saline and Alkali Soils. U.S. Salinity Laboratory Staff; L. A. Richards, Ed. U.S. Dept. of Agriculture, Washington, D.C., Rev. Ed., 1954. Vii + 160 Pp. Illus. $2. (Order from Supt. of Documents, GPO, Washington 25, D.C.). Science 1954, 120, 800. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Mualem, Y. A New Model for Predicting the Hydraulic Conductivity of Unsaturated Porous Media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of Field Water Use And Crop Yield. In Simulation Monographs; Pudoc: Wageningen, The Netherlands, 1978; p. 189. [Google Scholar]
- Maas, E.V. Crop salt tolerance. In Agricultural Salinity Assessment and Management; Tanji, K.K., Ed.; ASCE Manuals and Reports on Engineering Practice: New York, NY, USA, 1990; Volume 71. [Google Scholar]
- van Genuchten, M.T. A Numerical Model for Water and Solute Movement in and Below the Root Zone, Unpublished Research Report; U.S. Salinity Laboratory, USDA, ARS: Riverside, CA, USA, 1987. [Google Scholar]
- Kanzari, S.; Rezig, M.; Ben Nouna, B. Estimating Hydraulic Properties of Unsaturated Soil Using a Single Tensiometer. Am. J. Geophys. Geochem. Geosystems 2017, 3, 1–4. [Google Scholar]
- Vanclooster, M.; Mallants, D.; Diels, J.; Feyen, J. Determining Local-Scale Solute Transport Parameters Using Time Domain Reflectometry (TDR). J. Hydrol. 1993, 148, 93–107. [Google Scholar] [CrossRef]
- Mallants, D.; Vanclooster, M.; Meddahi, M.; Feyen, J. Estimating Solute Transport in Undisturbed Soil Columns Using Time-Domain Reflectometry. J. Contam. Hydrol. 1994, 17, 91–109. [Google Scholar] [CrossRef]
- Toride, N.; Leij, F.J.; van Genuchten, M.T. The CXTFIT Code for Estimating Transport Parameters from Laboratory or Field Tracer Experiment. Research Report N°137; US Salinity Laboratory: Riverside, CA, USA, 1999; p. 119. [Google Scholar]
- Carucci, F.; Gagliardi, A.; Giuliani, M.M.; Gatta, G. Irrigation Scheduling in Processing Tomato to Save Water: A Smart Approach Combining Plant and Soil Monitoring. Appl. Sci. 2023, 13, 7625. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, L.; Zhu, C.; Li, W.; Jing, D.; Zhang, L.; Liu, Y.; Li, T.; Li, J. Evaluating the Irrigation Schedules of Greenhouse Tomato by Simulating Soil Water Balance under Drip Irrigation. Agric. Water Manag. 2023, 283, 108323. [Google Scholar] [CrossRef]
- Shan, G.; Sun, Y.; Cheng, Q.; Wang, Z.; Zhou, H.; Wang, L.; Xue, X.; Chen, B.; Jones, S.B.; Lammers, P.S.; et al. Monitoring Tomato Root Zone Water Content Variation and Partitioning Evapotranspiration with a Novel Horizontally-Oriented Mobile Dielectric Sensor. Agric. For. Meteorol. 2016, 228–229, 85–94. [Google Scholar] [CrossRef]
- Gassmann, M.; Gardiol, J.; Serio, L. Performance Evaluation of Evapotranspiration Estimations in a Model of Soil Water Balance. Meteorol. Appl. 2011, 18, 211–222. [Google Scholar] [CrossRef]
- Abou Ali, A.; Bouchaou, L.; Er-Raki, S.; Hssaissoune, M.; Brouziyne, Y.; Ezzahar, J.; Khabba, S.; Chakir, A.; Labbaci, A.; Chehbouni, A. Assessment of Crop Evapotranspiration and Deep Percolation in a Commercial Irrigated Citrus Orchard under Semi-Arid Climate: Combined Eddy-Covariance Measurement and Soil Water Balance-Based Approach. Agric. Water Manag. 2023, 275, 107997. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Q.; Ren, D.; Xu, X.; Xiong, Y.; Huang, G. Soil Evaporation and Its Impact on Salt Accumulation in Different Landscapes under Freeze–Thaw Conditions in an Arid Seasonal Frozen Region. Vadose Zone J. 2021, 20, e20098. [Google Scholar] [CrossRef]
- Mai, J. Ten Strategies towards Successful Calibration of Environmental Models. J. Hydrol. 2023, 620, 129414. [Google Scholar] [CrossRef]
- Oster, J.D.; Letey, J.; Vaughan, P.; Wu, L.; Qadir, M. Comparison of Transient State Models That Include Salinity and Matric Stress Effects on Plant Yield. Agric. Water Manag. 2012, 103, 167–175. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]







| Layer (cm) | θr (cm3·cm−3) | θs (cm3·cm−3) | α (cm−1) | n (-) | Ks (cm·d−1) |
|---|---|---|---|---|---|
| Pot experiment | |||||
| - | 0.1 | 0.41 | 0.27 | 1.11 | 6.41 |
| Field experiment | |||||
| 0–20 cm | 0.078 | 0.546 | 0.07 | 1.067 | 8.87 |
| 20–40 cm | 0.078 | 0.544 | 0.07 | 1.079 | 8.87 |
| 40–60 cm | 0.078 | 0.445 | 0.10 | 1.073 | 12.6 |
| 60–80 cm | 0.078 | 0.443 | 0.03 | 1.078 | 12.5 |
| Variable | Experiment | Date 1 | Date 2 | Date 3 |
|---|---|---|---|---|
| Soil Water Profile | Field | 9.30 | 7.30 | 6.40 |
| Pot | 10.30 | 11.10 | 8.30 | |
| Soil Salinity Profile | Field | 5.30 | 2.10 | 4.60 |
| Pot | 2.00 | 4.50 | 1.70 |
| Variable | Experiment | Irrigation Water Quality | RMSE (%) (on the Final Day) |
|---|---|---|---|
| Soil Water Profile | Field | 3.5 dS/m | 9.10 |
| 7 dS/m | 1.10 | ||
| Soil Water Profile | Pot | 3.5 dS/m | 3.00 |
| 7 dS/m | 1.10 | ||
| Soil Salinity Profile | Field | 3.5 dS/m | 10.20 |
| 7 dS/m | 5.70 | ||
| Soil Salinity Profile | Pot | 3.5 dS/m | 5.20 |
| 7 dS/m | 3.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanzari, S.; Šimůnek, J.; Daghari, I.; Younes, A.; Ali, K.B.; Mariem, S.B.; Ghannem, S. Modeling Irrigation of Tomatoes with Saline Water in Semi-Arid Conditions Using Hydrus-1D. Land 2024, 13, 739. https://doi.org/10.3390/land13060739
Kanzari S, Šimůnek J, Daghari I, Younes A, Ali KB, Mariem SB, Ghannem S. Modeling Irrigation of Tomatoes with Saline Water in Semi-Arid Conditions Using Hydrus-1D. Land. 2024; 13(6):739. https://doi.org/10.3390/land13060739
Chicago/Turabian StyleKanzari, Sabri, Jiří Šimůnek, Issam Daghari, Anis Younes, Khouloud Ben Ali, Sana Ben Mariem, and Samir Ghannem. 2024. "Modeling Irrigation of Tomatoes with Saline Water in Semi-Arid Conditions Using Hydrus-1D" Land 13, no. 6: 739. https://doi.org/10.3390/land13060739
APA StyleKanzari, S., Šimůnek, J., Daghari, I., Younes, A., Ali, K. B., Mariem, S. B., & Ghannem, S. (2024). Modeling Irrigation of Tomatoes with Saline Water in Semi-Arid Conditions Using Hydrus-1D. Land, 13(6), 739. https://doi.org/10.3390/land13060739









