Application of Infrared Spectroscopy Techniques for Identification of Ancient Vegetation and Soil Change on Loess Areas
Abstract
:1. Introduction
2. Materials and Methods
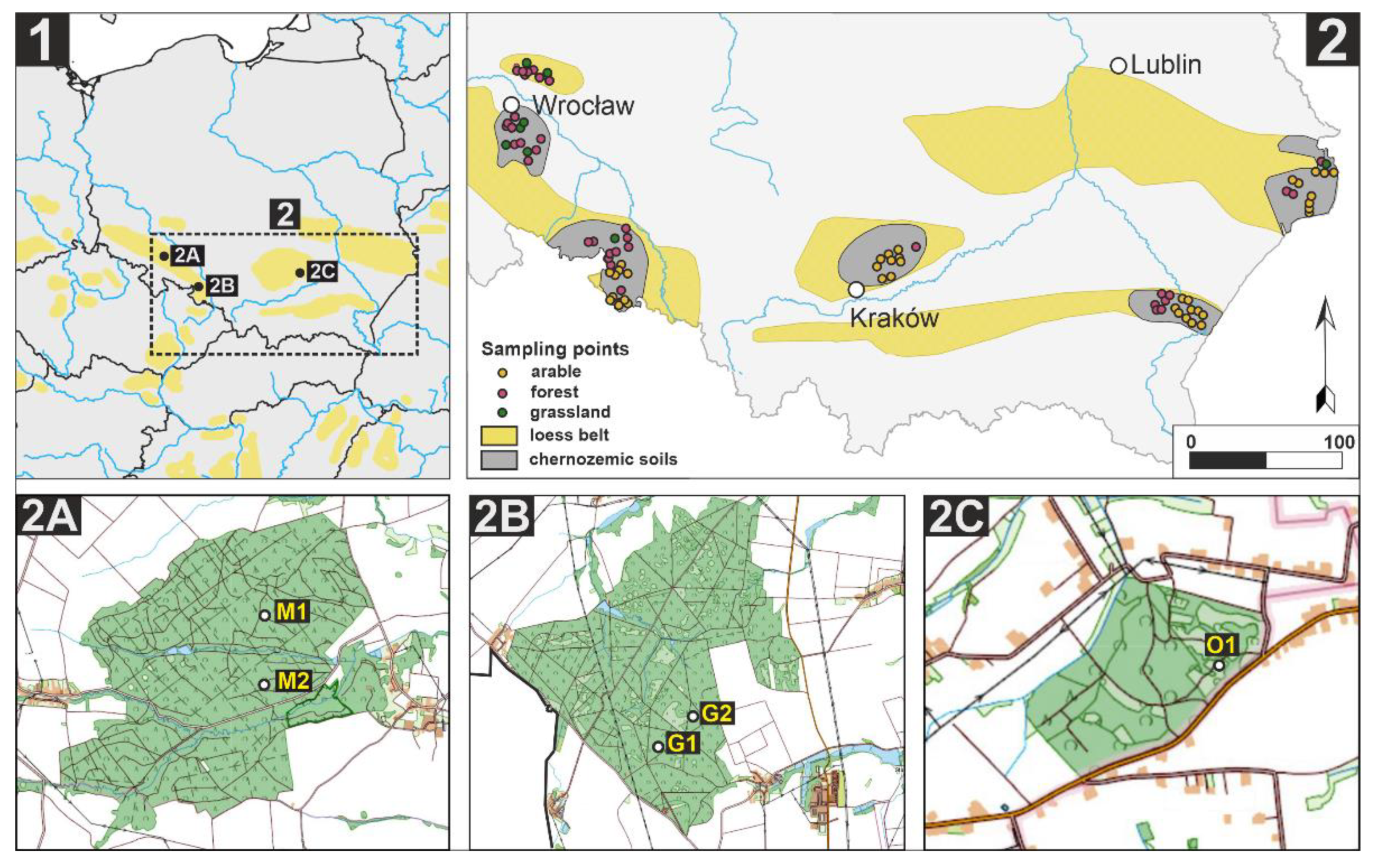
2.1. Soil Sampling
2.2. Sample Preparation and Statistical Analysis
2.3. Preparing the Reference Library of MIR/NIR Samples
3. Results and Discussion
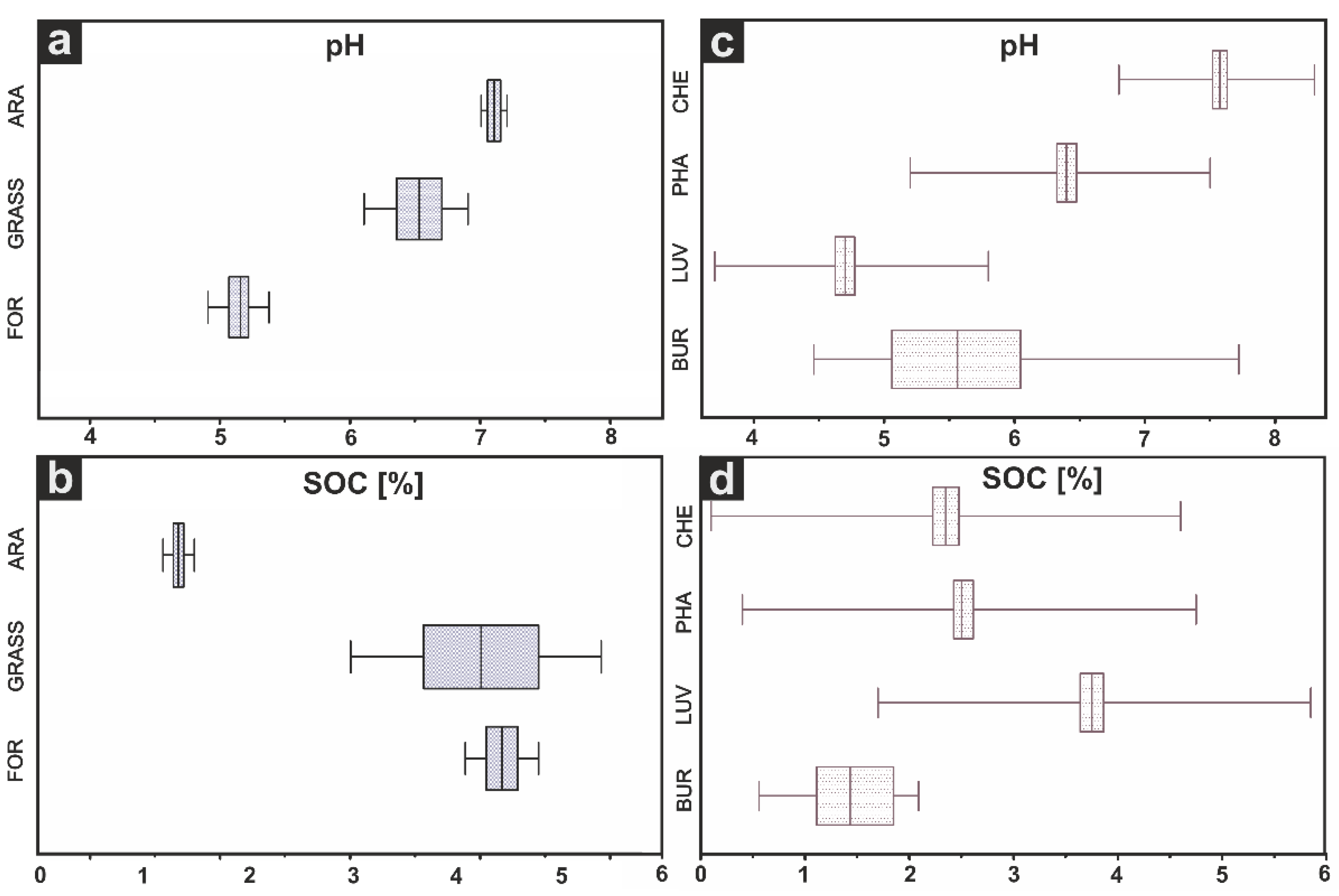
3.1. Standard Soil Properties
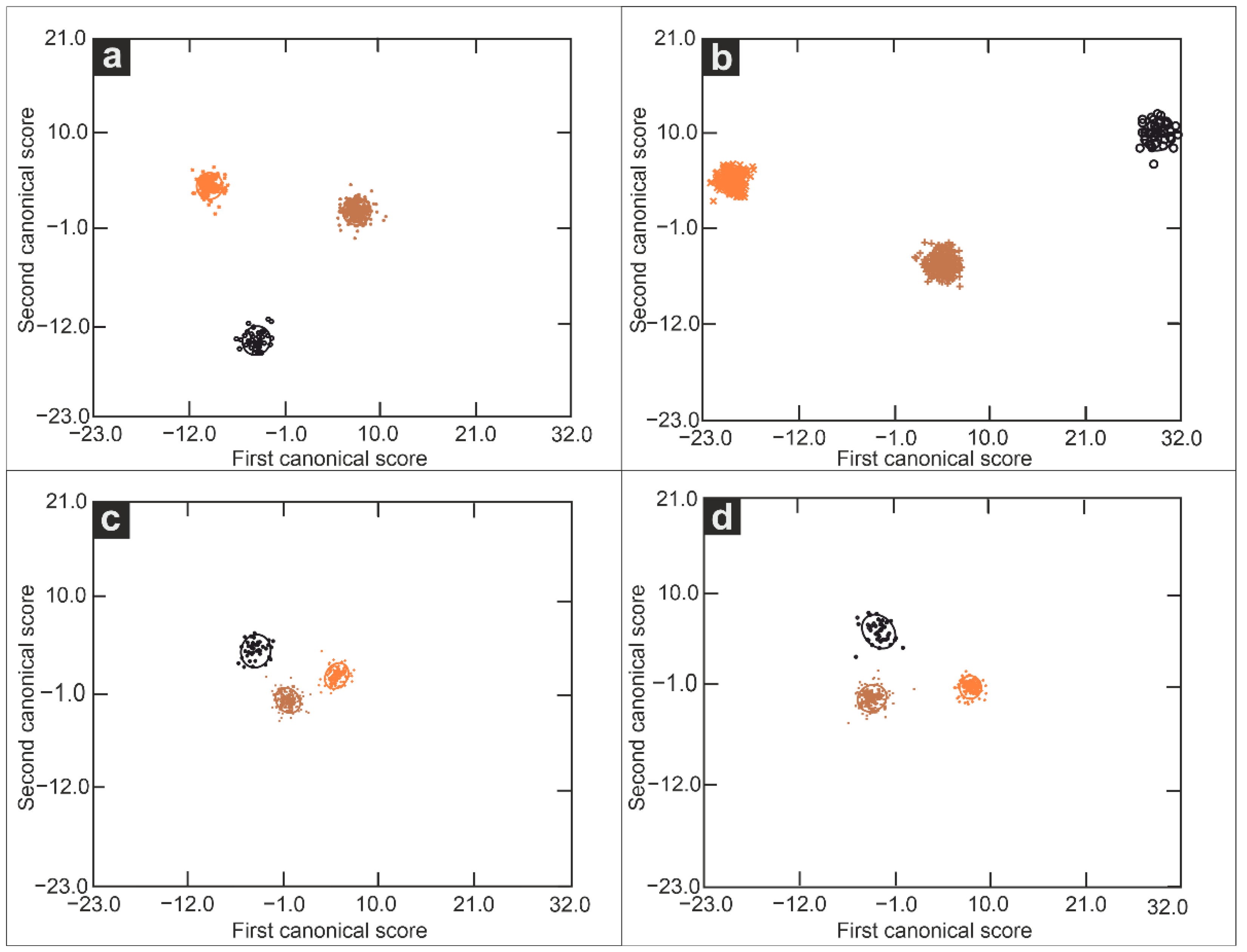
3.2. Mahalanobis Distances for NIR and MIR Spectral Range
3.3. Coefficient of Variation for NIR and MIR Spectral Range
3.4. Selection of Recommended Analytical Approach
3.5. Land Use of Buried Soils
3.6. Identified Type of Buried Soils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Managing Chernozem for Reducing Global Warming. In Regenerative Agriculture; Dent, D., Boincean, B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 81–93. ISBN 978-3-030-72223-4. [Google Scholar]
- Pozniak, S.P.; Havrysh, N.S. Soils in the Memory of World Nations. Pol. J. Soil Sci. 2019, 52, 13. [Google Scholar] [CrossRef]
- Vysloužilová, B.; Ertlen, D.; Schwartz, D.; Šefrna, L. Chernozem. From Concept to Classification: A Review. AUC Geogr. 2016, 51, 85–95. [Google Scholar] [CrossRef]
- Altermann, M.; Rinklebe, J.; Merbach, I.; Körschens, M.; Langer, U.; Hofmann, B. Chernozem—Soil of the Year 2005. J. Plant Nutr. Soil Sci. 2005, 168, 725–740. [Google Scholar] [CrossRef]
- Dreibrodt, S.; Hofmann, R.; Dal Corso, M.; Bork, H.-R.; Duttmann, R.; Martini, S.; Saggau, P.; Schwark, L.; Shatilo, L.; Videiko, M.; et al. Earthworms, Darwin and Prehistoric Agriculture-Chernozem Genesis Reconsidered. Geoderma 2022, 409, 115607. [Google Scholar] [CrossRef]
- Eckmeier, E.; Gerlach, R.; Gehrt, E.; Schmidt, M.W.I. Pedogenesis of Chernozems in Central Europe—A Review. Geoderma 2007, 139, 288–299. [Google Scholar] [CrossRef]
- Kabała, C.; Charzyński, P.; Czigány, S.; Novák, T.J.; Saksa, M.; Świtoniak, M. Suitability of World Reference Base for Soil Resources (WRB) to Describe and Classify Chernozemic Soils in Central Europe. Soil Sci. Annu. 2019, 70, 244–257. [Google Scholar] [CrossRef]
- Kondrateva, M.A.; Chashchin, A.N. Analysis and Assessment of the Use of Chernozems of the Kungur Forest-Steppe Based on Remote Sensing Data. IOP Conf. Ser. Earth Environ. Sci. 2021, 862, 012099. [Google Scholar] [CrossRef]
- Bulgakov, V.; Gadzalo, I.; Adamchuk, V.; Demydenko, O.; Velichko, V.; Nowak, J.; Ivanovs, S. Dynamics of the Humus Content under Different Chernozem Treatment Conditions. J. Ecol. Eng. 2022, 23, 118–128. [Google Scholar] [CrossRef]
- Drewnik, M.; Żyła, M. Properties and Classification of Heavily Eroded Post-Chernozem Soils in Proszowice Plateau (Southern Poland). Soil Sci. Annu. 2019, 70, 225–233. [Google Scholar] [CrossRef]
- Baltruschat, H.; Santos, V.M.; da Silva, D.K.A.; Schellenberg, I.; Deubel, A.; Sieverding, E.; Oehl, F. Unexpectedly High Diversity of Arbuscular Mycorrhizal Fungi in Fertile Chernozem Croplands in Central Europe. Catena 2019, 182, 104135. [Google Scholar] [CrossRef]
- Vysloužilová, B.; Danková, L.; Ertlen, D.; Novák, J.; Schwartz, D.; Šefrna, L.; Delhon, C.; Berger, J.-F. Vegetation History of Chernozems in the Czech Republic. Veg. Hist. Archaeobotany 2014, 23, 97–108. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E.; Łyszczarz, S.; Sadowy, A. Forest Habitats and Forest Types on Chernozems in South-Eastern Poland. Soil Sci. Annu. 2019, 70, 234–243. [Google Scholar] [CrossRef]
- Łabaz, B.; Kabała, C.; Waroszewski, J.; Dudek, M.; Bogacz, A.; Gruszka, D.; Młynek, S. Medium-Term Transformation of Chernozems under Broadleaf Forests in the Temperate Climate of South-East Poland. Geoderma Reg. 2022, 30, 00535. [Google Scholar] [CrossRef]
- Łabaz, B.; Waroszewski, J.; Dudek, M.; Bogacz, A.; Kabała, C. Persistence of Arable Chernozems and Chernic Rendzic Phaeozems in the Eroded Undulating Loess Plateau in Central Europe. Catena 2022, 216, 106417. [Google Scholar] [CrossRef]
- Kabała, C. Chernozem (Czarnoziem)—Soil of the Year 2019 in Poland. Origin, Classification and Use of Chernozems in Poland. Soil Sci. Annu. 2019, 70, 184–192. [Google Scholar] [CrossRef]
- Łabaz, B.; Musztyfaga, E.; Waroszewski, J.; Bogacz, A.; Jezierski, P.; Kabała, C. Landscape-Related Transformation and Differentiation of Chernozems—Catenary Approach in the Silesian Lowland, SW Poland. Catena 2018, 161, 63–76. [Google Scholar] [CrossRef]
- Messager, E.; Nomade, S.; Wilhelm, B.; Joannin, S.; Scao, V.; Von Grafenstein, U.; Martkoplishvili, I.; Ollivier, V.; Mgeladze, A.; Dumoulin, J.-P.; et al. New Pollen Evidence from Nariani (Georgia) for Delayed Postglacial Forest Expansion in the South Caucasus. Quat. Res. 2017, 87, 121–132. [Google Scholar] [CrossRef]
- Joannin, S.; Ali, A.A.; Ollivier, V.; Roiron, P.; Peyron, O.; Chevaux, S.; Nahapetyan, S.; Tozalakyan, P.; Karakhanyan, A.; Chataigner, C. Vegetation, Fire and Climate History of the Lesser Caucasus: A New Holocene Record from Zarishat Fen (Armenia): Paleoenvironment and Paleoclimate in Armenia. J. Quat. Sci. 2014, 29, 70–82. [Google Scholar] [CrossRef]
- Krupski, M.; Mackiewicz, M.; Kabała, C.; Ehlert, M.; Cendrowska, M. Earthen Mounds in the Głubczyce Forest (SW Poland)—Are They Prehistoric Long-Barrows? Geoarchaeology of the Silesian Soil Record and Human-Environment Interplay in the Holocene. Praehistorische. Z. 2021, 96, 413–433. [Google Scholar] [CrossRef]
- Kabała, C.; Przybył, A.; Krupski, M.; Łabaz, B.; Waroszewski, J. Origin, Age and Transformation of Chernozems in Northern Central Europe—New Data from Neolithic Earthen Barrows in SW Poland. Catena 2019, 180, 83–102. [Google Scholar] [CrossRef]
- Krupski, M.; Kruczkowska, B.; Kittel, P.; Jakubczak, M.; Skrzyński, G.; Golyeva, A.; Niedziółka, K.; Urbańczyk, P. Evidence of Prehistoric and Early Medieval Agriculture and Its Impact on Soil and Land Relief Transformation in the Białowieża Natural Forest (NE Poland). Geoderma 2022, 410, 115668. [Google Scholar] [CrossRef]
- Strouhalová, B.; Ertlen, D.; Šefrna, L.; Novák, T.J.; Virágh, K.; Schwartz, D. Assessing the Vegetation History of European Chernozems through Qualitative near Infrared Spectroscopy. Quaternaire 2019, 30, 227–241. [Google Scholar] [CrossRef]
- Räsänen, S.; Suutari, H.; Nielsen, A.B. A Step Further towards Quantitative Reconstruction of Past Vegetation in Fennoscandian Boreal Forests: Pollen Productivity Estimates for Six Dominant Taxa. Rev. Palaeobot. Palynol. 2007, 146, 208–220. [Google Scholar] [CrossRef]
- Vrydaghs, L.; Ball, T.B.; Devos, Y. Beyond Redundancy and Multiplicity. Integrating Phytolith Analysis and Micromorphology to the Study of Brussels Dark Earth. J. Archaeol. Sci. 2016, 68, 79–88. [Google Scholar] [CrossRef]
- Zhao, D.; Arshad, M.; Li, N.; Triantafilis, J. Predicting Soil Physical and Chemical Properties Using Vis-NIR in Australian Cotton Areas. Catena 2021, 196, 104938. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, X.; Khongnawang, T.; Arshad, M.; Triantafilis, J. A Vis-NIR Spectral Library to Predict Clay in Australian Cotton Growing Soil. Soil Sci. Soc. Am. J. 2018, 82, 1347–1357. [Google Scholar] [CrossRef]
- Zhao, D.; Arshad, M.; Wang, J.; Triantafilis, J. Soil Exchangeable Cations Estimation Using Vis-NIR Spectroscopy in Different Depths: Effects of Multiple Calibration Models and Spiking. Comput. Electron. Agric. 2021, 182, 105990. [Google Scholar] [CrossRef]
- Vysloužilová, B.; Ertlen, D.; Šefrna, L.; Novák, T.; Virágh, K.; Rué, M.; Campaner, A.; Dreslerová, D.; Schwartz, D. Investigation of Vegetation History of Buried Chernozem Soils Using Near-Infrared Spectroscopy (NIRS). Quat. Int. 2015, 365, 203–211. [Google Scholar] [CrossRef]
- Dudek, M.; Kabała, C.; Łabaz, B.; Mituła, P.; Bednik, M.; Medyńska-Juraszek, A. Mid-Infrared Spectroscopy Supports Identification of the Origin of Organic Matter in Soils. Land 2021, 10, 215. [Google Scholar] [CrossRef]
- Palmborg, C.; Nordgren, A. Partitioning the Variation of Microbial Measurements in Forest Soils into Heavy Metal and Substrate Quality Dependent Parts by Use of near Infrared Spectroscopy and Multivariate Statistics. Soil Biol. Biochem. 1996, 28, 711–720. [Google Scholar] [CrossRef]
- Ertlen, D.; Schwartz, D.; Trautmann, M.; Webster, R.; Brunet, D. Discriminating between Organic Matter in Soil from Grass and Forest by Near-Infrared Spectroscopy. Eur. J. Soil Sci. 2010, 61, 207–216. [Google Scholar] [CrossRef]
- Ng, W.; Minasny, B.; Jeon, S.H.; McBratney, A. Mid-Infrared Spectroscopy for Accurate Measurement of an Extensive Set of Soil Properties for Assessing Soil Functions. Soil Secur. 2022, 6, 100043. [Google Scholar] [CrossRef]
- Waruru, B.K.; Shepherd, K.D.; Ndegwa, G.M.; Sila, A.; Kamoni, P.T. Application of Mid-Infrared Spectroscopy for Rapid Characterization of Key Soil Properties for Engineering Land Use. Soils Found. 2015, 55, 1181–1195. [Google Scholar] [CrossRef]
- Dudek, M.; Łabaz, B.; Kabała, C. Comparison of Sequential Extraction Techniques and Fractional Composition of Humic Substances in Chernozems and Phaeozems of Poland—A Review. Soil Sci. Annu. 2021, 72, 132235. [Google Scholar] [CrossRef]
- Dudek, M.; Łabaz, B.; Bednik, M.; Medyńska-Juraszek, A. Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland. Agronomy 2022, 12, 733. [Google Scholar] [CrossRef]
- Zhang, Y.; Hartemink, A.E.; Huang, J. Spectral Signatures of Soil Horizons and Soil Orders—An Exploratory Study of 270 Soil Profiles. Geoderma 2021, 389, 114961. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soil and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; ISBN 9789251083697. [Google Scholar]
- Blażejczyk, K. Climate and Bioclimate of Poland. In Natural and Human Environment of Poland. A geographical Overview; Degórski, M., Ed.; Polish Academy of Sciences: Warsaw, Poland, 2006; Volume 19, pp. 31–48. [Google Scholar]
- Huang, J.; Rinnan, Å.; Bruun, T.B.; Engedal, T.; Bruun, S. Identifying the Fingerprint of Permanganate Oxidizable Carbon as a Measure of Labile Soil Organic Carbon Using Fourier Transform Mid-infrared Photoacoustic Spectroscopy. Eur. J. Soil Sci. 2021, 72, 1831–1841. [Google Scholar] [CrossRef]
- Ertlen, D.; Schwartz, D.; Brunet, D.; Trendel, J.-M.; Adam, P.; Schaeffer, P. Qualitative near Infrared Spectroscopy, a New Tool to Recognize Past Vegetation Signature in Soil Organic Matter. Soil Biol. Biochem. 2015, 82, 127–134. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis. In Chemical Methods, Part 3; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; ISBN 978-0-89118-825-4. [Google Scholar]
- Kobierski, M.; Kondratowicz-Maciejewska, K.; Banach-Szott, M.; Wojewódzki, P.; Peñas Castejón, J.M. Humic Substances and Aggregate Stability in Rhizospheric and Non-Rhizospheric Soil. J. Soils Sediments 2018, 18, 2777–2789. [Google Scholar] [CrossRef]
- Bojko, O.; Kabała, C. Loss-on-Ignition as an Estimate of Total Organic Carbon in the Mountain Soils. Pol. J. Soil Sci. 2014, 47, 71–79. [Google Scholar]
- De Maesschalck, R.; Jouan-Rimbaud, D.; Massart, D.L. The Mahalanobis Distance. Chemom. Intell. Lab. Syst. 2000, 50, 1–18. [Google Scholar] [CrossRef]
- Webster, R.; Oliver, M.A.; Webster, R. Statistical Methods in Soil and Land Resource Survey. In Spatial Information Systems; Oxford University Press: Oxford, UK; New York, NY, USA, 1990; ISBN 978-0-19-823317-6. [Google Scholar]
- Chodorowski, J.; Bartmiński, P.; Plak, A.; Dębicki, R. Chernozems of Lubelszczyzna (Eastern Poland). Soil Sci. Annu. 2019, 70, 258–269. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Methods in Soil and Land Resource Survey. Technometrics 1992, 34, 497–498. [Google Scholar] [CrossRef]
- Leone, A.P.; Viscarra-Rossel, R.A.; Amenta, P.; Buondonno, A. Prediction of Soil Properties with PLSR and Vis-NIR Spectroscopy: Application to Mediterranean Soils from Southern Italy. Curr. Anal. Chem. 2012, 8, 283–299. [Google Scholar] [CrossRef]
- Viscarra, R.R.A.; Behrens, T.; Ben-Dor, E.; Brown, D.J.; Demattê, J.A.M.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A Global Spectral Library to Characterize the World’s Soil. Earth-Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef]
- Ložek, V. Středoevropské Bezlesí v Čase a Prostoru III. Historie Lesa a Bezlesí v Kvartéru. Ochr. Přírody 2004, 59, 71–79. [Google Scholar]
- Błońska, E.; Lasota, J.; Gruba, P. Effect of Temperate Forest Tree Species on Soil Dehydrogenase and Urease Activities in Relation to Other Properties of Soil Derived from Loess and Glaciofluvial Sand. Ecol. Res. 2016, 31, 655–664. [Google Scholar] [CrossRef]
- Błońska, E.; Kacprzyk, M.; Spólnik, A. Effect of Deadwood of Different Tree Species in Various Stages of Decomposition on Biochemical Soil Properties and Carbon Storage. Ecol. Res. 2017, 32, 193–203. [Google Scholar] [CrossRef]
- Baumann, W.; Fritzsche, C.; Coblenz, W.; Fiedler, H.J.; Brückner, H.P. Stratigraphische Befunde Zur Schnurkeramik in Einem Grabhügel Bei Werben. Kr. Leipz. Ausgrab. Funde 1983, 28, 1–10. [Google Scholar]
- Hejcman, M.; Součková, K.; Krištuf, P.; Peška, J. What Questions Can Be Answered by Chemical Analysis of Recent and Paleosols from the Bell Beaker Barrow (2500–2200 BC), Central Moravia, Czech Republic? Quat. Int. 2013, 316, 179–189. [Google Scholar] [CrossRef]
- Jamrichová, E.; Hédl, R.; Kolář, J.; Tóth, P.; Bobek, P.; Hajnalová, M.; Procházka, J.; Kadlec, J.; Szabó, P. Human Impact on Open Temperate Woodlands during the Middle Holocene in Central Europe. Rev. Palaeobot. Palynol. 2017, 245, 55–68. [Google Scholar] [CrossRef]
- Moskal-del Hoyo, M.; Mueller-Bieniek, A.; Alexandrowicz, W.P.; Wilczyński, J.; Wędzicha, S.; Kapcia, M.; Przybyła, M.M. The Continuous Persistence of Open Oak Forests in the Miechów Upland (Poland) in the Second Half of the Holocene. Quat. Int. 2017, 458, 14–27. [Google Scholar] [CrossRef]
- Vostrovská, I.; Bíšková, J.; Lukšíková, H.; Kočár, P.; Kočárová, R. The Environment and Subsistence of the Early Neolithic Settlement Area at Těšetice-Kyjovice, Czech Republic. Environ. Archaeol. 2019, 24, 248–262. [Google Scholar] [CrossRef]
- Johnson, D.L.; Schaetzl, R.J. Differing Views of Soil and Pedogenesis by Two Masters: Darwin and Dokuchaev. Geoderma 2015, 237–238, 176–189. [Google Scholar] [CrossRef]
- Kleber, M.; Röner, J.; Chenu, C.; Glaser, B.; Knicker, H.; Jahn, R. Prehistoric Alteration of Soil Properties in A Central German Chernozemic Soil: In Search of Pedologic Indicators for Prehistoric Activity. Soil Sci. 2003, 168, 292–306. [Google Scholar] [CrossRef]
- Lorz, C.; Saile, T. Anthropogenic Pedogenesis of Chernozems in Germany?—A Critical Review. Quat. Int. 2011, 243, 273–279. [Google Scholar] [CrossRef]
- Jeleńska, M.; Hasso-Agopsowicz, A.; Kądziałko-Hofmokl, M.; Sukhorada, A.; Bondar, K.; Matviishina, Z. Magnetic Iron Oxides Occurring in Chernozem Soil from Ukraine and Poland as Indicators of Pedogenic Processes. Stud. Geophys. Geod. 2008, 52, 255–270. [Google Scholar] [CrossRef]
- Gerlach, R.; Baumewerd-Schmidt, H.; van den Borg, K.; Eckmeier, E.; Schmidt, M.W.I. Prehistoric Alteration of Soil in the Lower Rhine Basin, Northwest Germany—Archaeological, 14C and Geochemical Evidence. Geoderma 2006, 136, 38–50. [Google Scholar] [CrossRef]
- Strouhalová, B.; Gebhardt, A.; Ertlen, D.; Šefrna, L.; Flašarová, K.; Kolařík, P.; Schwartz, D. From Chernozem to Luvisol or from Luvisol to Chernozem? A Discussion about the Relationships and Limits of the Two Types of Soils. A Case Study of the Soil Catena of Hrušov, Czechia. Geografie 2020, 125, 473–500. [Google Scholar] [CrossRef]

| MIR 1st Derivative | MIR 2nd Derivative | NIR 1st Derivative | NIR 2nd Derivative | |||||
|---|---|---|---|---|---|---|---|---|
| Canonical Scores | Score 1 | Score 2 | Score 1 | Score 2 | Score 1 | Score 2 | Score 1 | Score 2 |
| Land Use | ||||||||
| Arable-grassland | 20.6 | 23.1 | 37.3 | 35.9 | 3.3 | 9.4 | 12.0 | 10.9 |
| Arable-forest | 25.7 | 2.0 | 21.9 | 14.0 | 7.6 | 1.0 | 9.6 | 2.2 |
| Forest-grassland | 5.1 | 25.1 | 59.2 | 21.9 | 4.5 | 9.4 | 2.4 | 13.1 |
| Soil type | ||||||||
| Chernozem-Phaeozem | 12.5 | 16.2 | 25.0 | 15.0 | 4.4 | 6.3 | 0.9 | 8.3 |
| Chernozem-Luvisol | 5.9 | 19.4 | 49.2 | 5.5 | 11.0 | 3.1 | 11.3 | 6.9 |
| Luvisol-Phaeozem | 18.4 | 3.2 | 24.2 | 9.5 | 6.6 | 3.2 | 12.2 | 1.4 |
| MIR 1st Derivative | MIR 2nd Derivative | NIR 1st Derivative | NIR 2nd Derivative | |||||
|---|---|---|---|---|---|---|---|---|
| Canonical Scores | Score 1 | Score 2 | Score 1 | Score 2 | Score 1 | Score 2 | Score 1 | Score 2 |
| Arable | 9.3 | 243 | 15 | 14 | 30 | 197 | 22 | 369 |
| Forest | 6.3 | 37 | 6.3 | 14 | 23 | 195 | 20 | 52 |
| Grassland | 11 | 4.5 | 2.5 | 2.8 | 774 | 13 | 10 | 7.1 |
| Chernozem | 21 | 6.8 | 3.7 | 12 | 20 | 28 | 22 | 369 |
| Luvisol | 14 | 81 | 24 | 20 | 65 | 51 | 20 | 52 |
| Phaeozem | 8.4 | 22 | 4.7 | 20 | 18 | 75 | 10 | 7.1 |
| Horizon | Depth (cm) | Position in Barrow | Vegetation Cover Canonical Scores | Soil Type Canonical Scores | Predicted Vegetation | Predicted Soil Type | ||
|---|---|---|---|---|---|---|---|---|
| Score 1 | Score 2 | Score 1 | Score 2 | |||||
| Głubczyce Forest G1 | ||||||||
| A(E)b | 60–70 | barrow mound | 18.0 | 7.2 | 1.3 | 16.4 | FOR/GRASS | PHA/LUV |
| Ab1 | 70–80 | buried soil | 18.1 | 6.8 | −29.4 | 7.6 | FOR/GRASS | LUV |
| Ab2 | 80–90 | −9.2 | 31.0 | 19.8 | 21.1 | GRASS | PHA | |
| Ab3 | 90–100 | 5.8 | 38.4 | 35.0 | 5.0 | GRASS | PHA | |
| AB1 | 100–110 | −16.7 | 49.0 | −48.2 | 14.6 | n.i. | n.i. | |
| AB2 | 110–123 | −15.0 | −22.7 | 18.4 | 7.6 | ARA | PHA | |
| Głubczyce Forest G2 | ||||||||
| A | 110–120 | barrow mound | 16.6 | 62.0 | −22.5 | 16.2 | n.i. | LUV |
| AB | 120–134 | 15.5 | 39.1 | 20.0 | −7.8 | GRASS | PHA/CHER | |
| Ab1 | 140–150 | buried soil | 42.4 | 26.4 | 26.9 | 27.3 | GRASS | PHA |
| Ab2 | 150–160 | −15.0 | −5.5 | 8.8 | −3.7 | ARA | PHA | |
| Ab2/Abw | 160–170 | −17.9 | −8.5 | 56.5 | 11.8 | ARA | n.i. | |
| ABw | 170–180 | 12.2 | 30.7 | 64.5 | 75.8 | GRASS | n.i. | |
| BA | 180–190 | 29.0 | 18.3 | 16.7 | −13.7 | GRASS | PHA/CHER | |
| Muszkowice Forest M1 | ||||||||
| BC | 52–54 | barrow mound | 31.3 | 57.6 | −7.4 | 6.0 | n.i. | LUV |
| Ab | 54–75 | buried soil | −22.4 | −25.3 | −10.1 | −13.7 | ARA | LUV/CHER |
| ABw1 | 75–84 | 2.2 | 8.9 | 9.8 | 13.2 | ARA/GRASS | PHA | |
| ABw2 | 84–94 | −17.7 | −5.3 | −8.6 | 4.7 | ARA | LUV | |
| Muszkowice Forest M2 | ||||||||
| Abt1 | 88–100 | buried soil | 9.4 | 25.5 | 57.5 | 24.3 | GRASS | n.i. |
| Abt2 | 100–110 | −1.8 | −4.0 | 1.4 | 14.1 | ARA | PHA/LUV | |
| ABbt1 | 110–115 | 3.4 | 26.8 | 37.9 | 10.2 | GRASS | n.i. | |
| ABbt2 | 115–120 | 10.7 | 14.4 | 15.6 | 7.9 | GRASS/FOR | PHA | |
| BAbt1 | 120–125 | −40.5 | 0.7 | 9.5 | −6.0 | n.i. | PHA/CHER | |
| Ostrów O1 | ||||||||
| Ah2 | 30–45 | barrow mound | 41.0 | 38.0 | 34.9 | 50.5 | GRASS | n.i. |
| Ahb1 | 50–70 | buried soil | −36.9 | −14.2 | −64.8 | −10.4 | n.i. | n.i. |
| Ahb2 | 70–85 | −25.5 | 15.1 | 48.4 | −48.5 | GRASS/ARA | n.i. | |
| ABwk | 85–100 | −27.9 | 1.9 | −35.3 | −24.1 | ARA | CHER | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, M.; Kabała, C.; Łabaz, B.; Krupski, M. Application of Infrared Spectroscopy Techniques for Identification of Ancient Vegetation and Soil Change on Loess Areas. Land 2022, 11, 1294. https://doi.org/10.3390/land11081294
Dudek M, Kabała C, Łabaz B, Krupski M. Application of Infrared Spectroscopy Techniques for Identification of Ancient Vegetation and Soil Change on Loess Areas. Land. 2022; 11(8):1294. https://doi.org/10.3390/land11081294
Chicago/Turabian StyleDudek, Michał, Cezary Kabała, Beata Łabaz, and Mateusz Krupski. 2022. "Application of Infrared Spectroscopy Techniques for Identification of Ancient Vegetation and Soil Change on Loess Areas" Land 11, no. 8: 1294. https://doi.org/10.3390/land11081294
APA StyleDudek, M., Kabała, C., Łabaz, B., & Krupski, M. (2022). Application of Infrared Spectroscopy Techniques for Identification of Ancient Vegetation and Soil Change on Loess Areas. Land, 11(8), 1294. https://doi.org/10.3390/land11081294







