A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal
Abstract
1. Introduction
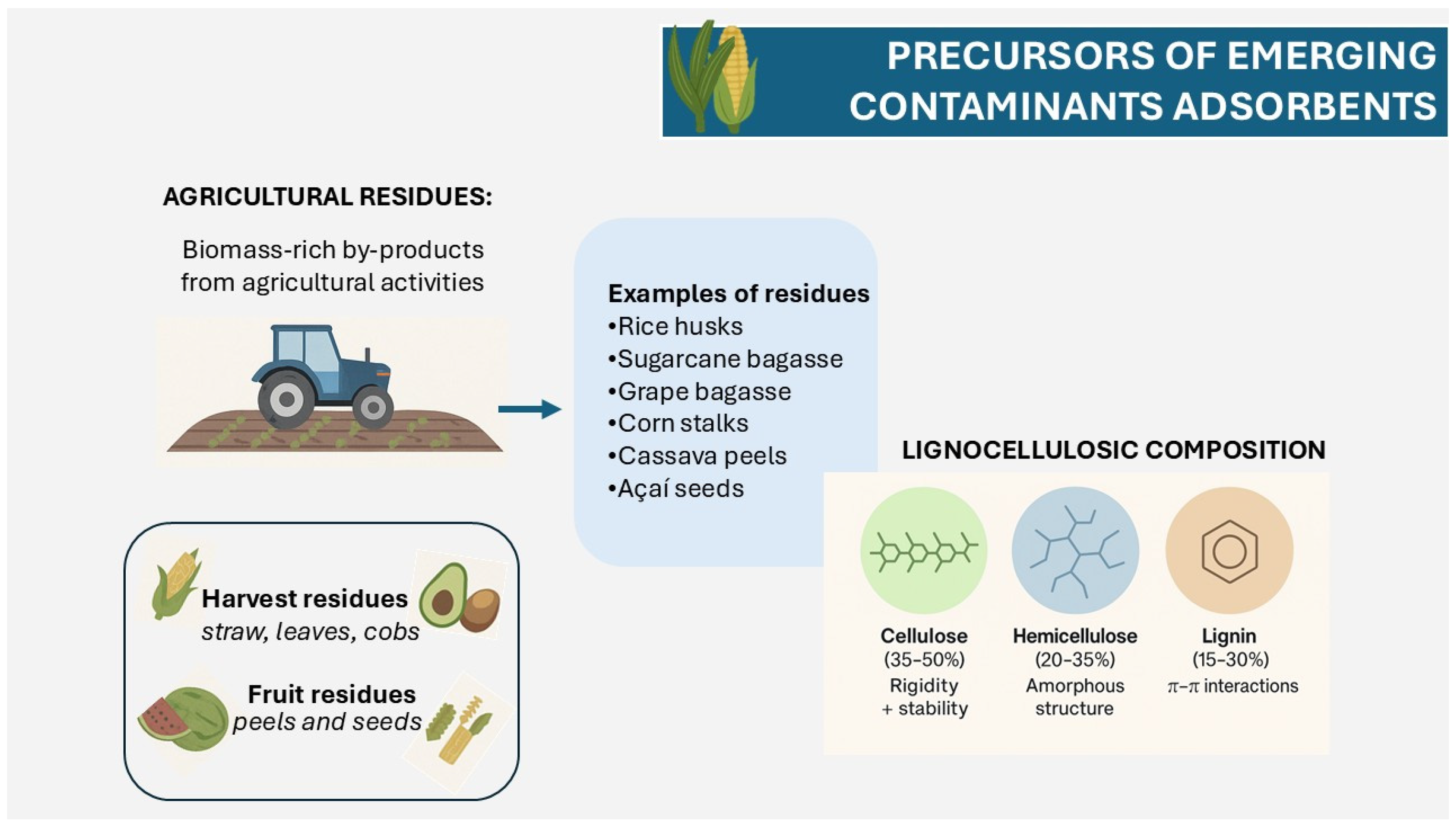
2. Agricultural Residues
3. Preparation and Modification of Adsorbents
3.1. Pyrolysis Techniques: Conventional Pyrolysis vs. Microwave-Assisted Pyrolysis
3.1.1. Key Influencing Factors
3.1.2. Energy Efficiency, Economic Feasibility, and Environmental Performance of MAP
3.2. Chemical Activation and Functionalization
3.3. Surface Engineering and Magnetization
4. Types of ECs and Adsorption Mechanism
4.1. Adsorption Mechanisms in ECS Removal
4.1.1. π–π Interactions and Hydrophobic Forces
4.1.2. Hydrogen Bonding and Surface Functional Groups
4.1.3. Electrostatic Interactions and pH Dependence
4.1.4. Ion Exchange and Metal–Ligand Complexation
4.1.5. Photocatalytic and Fenton-Assisted Hybrid Mechanisms
4.1.6. Influence of Ionic Strength and Competing Species
4.2. Adsorption Studies
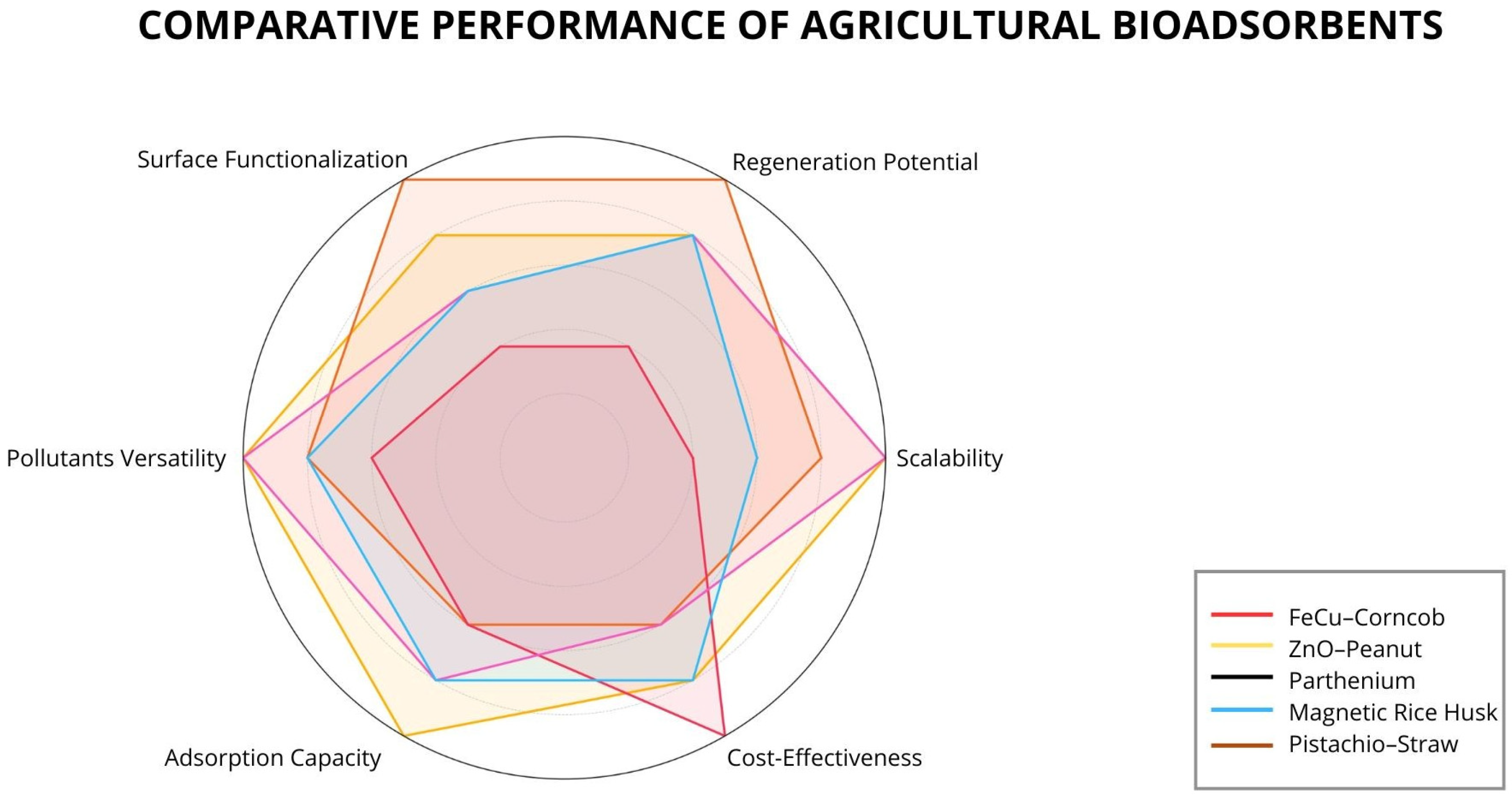
5. Application of Adsorbents from Agricultural Residues
| Type of Effluent | Target Adsorbate | Feedstock Type | Surface Modification | Maximum Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|
| Pharmaceuticals and Personal Care Products | Tetracycline (TC), ciprofloxacin (CIP), ibuprofen (IBP), and sulfamethoxazole (SMX) | Sunflower seed husk | H3PO4 | 429.3, 361.6, 251.3, and 251.1 for TC, CIP, SMX, and IBP, respectively | [84] |
| Tetracycline hydrochloride | Corn straw and wheat stalk | Lignin impregnation | 31.48 | [85] | |
| Sulfamethazine (SMT), oxytetracycline hydrochloride (OTC), and amoxicillin (AMX) | Rice straw | KOH | 6.47, 87.8, and 7.67 for SMT, OTC and AMX, respectively | [88] | |
| Ciprofloxacin | Tea waste | MnSO4 | 121.42 | [89] | |
| Chloroquine | Cassava residue | NaOH and microwave-assisted pyrolysis | 39 | [71] | |
| Methyl paraben (MPB), carbamazepine (CZP), ibuprofen (IBP), and triclosan (TCS) from simulated and real wastewater Ciprofloxacin from real wastewater | Empty palm bunch | H2SO4 | 60.2, 51.7, 38.8, and 35.4 for MPB, CZP, IBP, and TCS, respectively | [87] | |
| Pomegranate peels | H3PO4 | 142.86 | [90] | ||
| Pesticides | Chlorpyrifos | Pomegranate peels | H3PO4 | 100 | [91] |
| Imidacloprid | Sugarcane bagasse | KOH with iron/zinc | 313 | [92] | |
| Dyes | Methylene blue | Wheat straw | Ball-milled biochar with fly ash | 854.75 | [93] |
| Methylene blue | Rice straw | Ammonium phosphate | 156.36 | [94] | |
| Methylene blue | Walnut shell | Supercritical CO2 pretreatment and potassium hydroxide activation | 80.4 | [95] | |
| Methylene blue | Fronds and leaves from date palm | FeSO4.7H2O | 85.1 | [96] | |
| Food Red 17 and Acid Blue 9 | Cassava bagasse | NH4Cl | 131 and 150 for Food Red 17 and Acid Blue 9, respectively | [97] | |
| Reactive black 5 dye from simulated and industrial wastewater | Beta vulgaris leaves | Nitrogen and sulfur co-doping with copper zinc ferrite composite | 276.57 | [86] | |
| Inorganic Pollutants | Fluoride in simulated and glass wastewater | Coconut husks | MnFe2O4 magnetization/Al-La metal–organic framework in chitosan/β–cyclodextrin aerogel | 38.59 | [98] |
| Pb2+ and Cu2+ | Rice husk and corn cob | - | 61.07 and 17.35 for Pb2+ and Cu2+, respectively | [82] | |
| Cd2+ | Rice straw | Fe-Mn oxides | 120.77 | [99] | |
| Pb2+, Cd2+, and Cu2+ | Wheat straw | Microwave-assisted pyrolysis with activated carbon | 139.44, 52.92, and 31.25 for Pb2+, Cd2+, and Cu2+, respectively | [83] | |
| Cd2+ | Corn stalks | Amino modification | 375.58 | [100] | |
| Cd2+ | Sesame straw | Alkaline hydrogen peroxide pretreatment | 87.13 | [101] | |
| Cd2+ and As3+ | Pennisetum sp. straw | Fe-Mn oxides | 141.1 and 31.8 for Cd2+ and As3+, respectively | [102] | |
| Phosphate from simulated, river, and sewage wastewater | Corn straw | KHCO3 activation, Fe3O4 magnetization, and La(OH)3 loading | 116.08 | [103] | |
| Phosphate | Orange peels | CaCO3/ZnO | 52.96 | [104] | |
| Phosphate | Corncob, sugarcane bagasse, rice straw, and sawdust | Fe2+ and La3+ impregnation | 27.49 | [105] | |
| Phosphate | Papaya leaves | Lanthanum-organic framework coating | 47.5 | [106] | |
| Tris-(1-chloro-2-propyl) phosphate | Corn straw | - | 2.34 | [107] | |
| Emerging Micro-Pollutants | Microplastics | Corncob | Fe(NO3)3 and FeSO4 | 1737 | [108] |
6. Regeneration and Reusability
6.1. Regeneration of Spent Adsorbents
6.1.1. Chemical Regeneration
6.1.2. Thermal Regeneration
6.1.3. Microwave-Assisted Regeneration
6.1.4. Magnetic Separation and Recovery
6.2. Reuse and Final Disposal of Spent Adsorbents
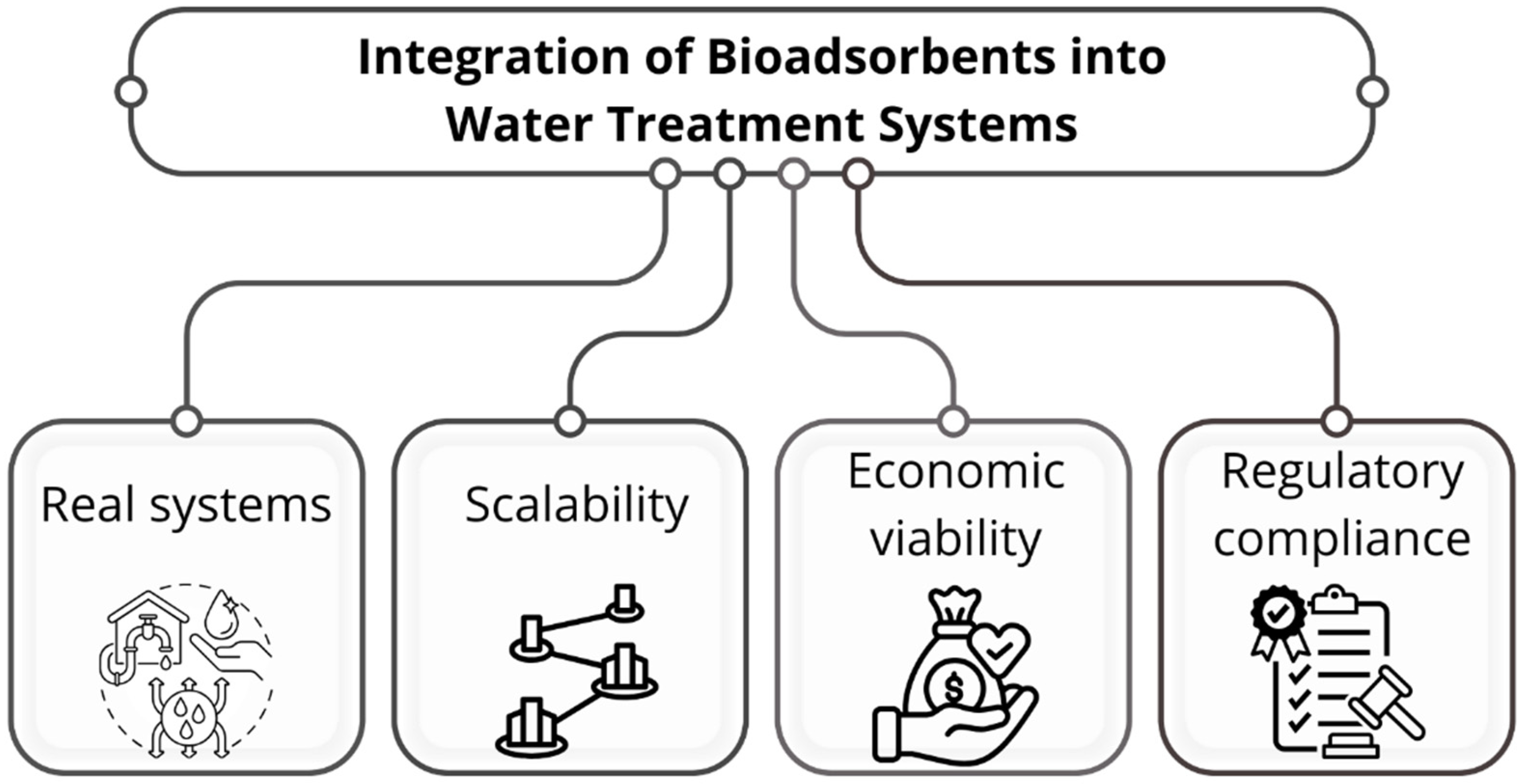
7. Integration of Bioadsorbents into Water Treatment Systems
7.1. Fixed-Bed and Continuous-Flow Systems
7.2. Hybrid and Integrated Technologies
| Technologies | Agricultural Residues | Composites | Degradation | Main Results | Reference |
|---|---|---|---|---|---|
| Photocatalytic–adsorptive systems | Peanut shell | ZnO/N,O-containing biochar | Dye (methylene blue) and antibiotics (tetracycline hydrochloride) | Removal efficiency of 96.0 and 97.1% and contact time of 70 and 140 min for methylene blue and tetracycline hydrochloride, respectively | [77] |
| Fallen sycamore leaves | Lamellar sycamore leaf TiO2/biochar | Dye (methyl orange) and antibiotics (ciprofloxacin) | Removal efficiency of 95.7 and 80.8% and degradation of 74.2 and 50.2% for methyl orange and Ciprofloxacin, respectively | [79] | |
| Pomelo peel | Sn quantum dot-loaded N- and O-containing biochar | Dye (methylene blue, malachite green, and rhodamine B) | Removal efficiency of >90% in 60 min after 5 cycles | [78] | |
| Corn plants | Corn plant biochar and manganese (Mn)-composited copper oxide (CuO) | Dye (Congo red and Eriochrome Black T) | Removal efficiency of 98 and 95%, and degradation of 92 and 88% for Congo red and Eriochrome Black T, respectively | [170] | |
| Mandarin peels | TiO2/mandarin waste peels (cellulose source) | Dye (methyl orange) | Removal efficiency of 98.9%, contact time of 30 min, and adsorption capacity of 104.2 mg/g | [169] | |
| Fenton-like processes | Pistachio shell | Biochar-based Fe3O4 nanoparticles and ascorbic acid | Dye (methylene blue) and pesticides (acetamiprid) | Adsorption capacity of 370.4 and 357.1 mg/g for methylene blue and acetamiprid, respectively | [74] |
| Corncob | Biochar-based magnetic Fe–Cu bimetallic | Antibiotic (ciprofloxacin) | Removal efficiency of 93.6%, contact time of 360 min, and degradation of 66% | [72] | |
| Rubber tree bark and coconut shell | Biochar with FeCl3 and H2O2 solutions | Dye (methylene blue) and hexavalent chromium (Cr (VI)) | Adsorption capacity of 335.6 and 258.1 mg/g for methylene blue and chromium, respectively | [171] | |
| Wheat straw | Iron tailings and wheat straw blends | Dye (methylene blue) | Removal efficiency of 84.0% | [172] | |
| Laurel leaves and watermelon peels | Hydrochars | Dye (anionic—reactive red 180; cationic—basic red 18) | Removal efficiency of 99.8 and 98.8% for reactive red 180 and basic red 18, respectively | [173] | |
| Membrane filtration pretreated with adsorption | Corn stover | Biochar and ceramic membrane filtration | Nitrogen, phosphorus, and organic matter | Removal efficiency of 91.42%, 91.49%, 89.54%, and 76.34% for total nitrogen, total ammonia nitrogen, total phosphorus, and soluble chemical oxygen demand, respectively | [174] |
8. Conclusions
Funding
Conflicts of Interest
References
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions—A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Hube, S.; Wu, B. Mitigation of Emerging Pollutants and Pathogens in Decentralized Wastewater Treatment Processes: A Review. Sci. Total Environ. 2021, 779, 146545. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Maillard, J.Y.; Simões, L.C.; Simões, M. Emerging Contaminants Affect the Microbiome of Water Systems—Strategies for Their Mitigation. NPJ Clean Water 2020, 3, 39. [Google Scholar] [CrossRef]
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging Contaminants as Global Environmental Hazards. A bibliometric analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Ajala, O.J.; Tijani, J.O.; Salau, R.B.; Abdulkareem, A.S.; Aremu, O.S. A Review of Emerging Micro-Pollutants in Hospital Wastewater: Environmental Fate and Remediation Options. Results Eng. 2022, 16, 100671. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, Seasonal Variations and Removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Tejedor, J.; Villamar-Ayala, C.A.; Vizuete, G. Transforming Waste into Solutions: Raw and Modified Bioadsorbents for Emerging Contaminant Removal. J. Environ. Chem. Eng. 2025, 13, 116720. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Alvez-Tovar, B.; Scalize, P.S.; Angiolillo-Rodríguez, G.; Albuquerque, A.; Ebang, M.N.; de Oliveira, T.F. Agro-Industrial Waste Upcycling into Activated Carbons: A Sustainable Approach for Dye Removal and Wastewater Treatment. Sustainability 2025, 17, 2036. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Sapag, K.; Rodríguez-Reinoso, F. Tailoring Biomass-Based Activated Carbon for CH4 Storage by Combining Chemical Activation with H3PO4 or ZnCl2 and Physical Activation with CO2. Carbon 2016, 110, 138–147. [Google Scholar] [CrossRef]
- González-García, P. Activated Carbon from Lignocellulosics Precursors: A Review of the Synthesis Methods, Characterization Techniques and Applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Das, O.; Babu, K.; Shanmugam, V.; Sykam, K.; Tebyetekerwa, M.; Neisiany, R.E.; Försth, M.; Sas, G.; Gonzalez-Libreros, J.; Capezza, A.J.; et al. Natural and Industrial Wastes for Sustainable and Renewable Polymer Composites. Renew. Sustain. Energy Rev. 2022, 158, 112054. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.V.; Aigbodion, V.S. Trends in the Development and Utilization of Agricultural Wastes as Heterogeneous Catalyst for Biodiesel Production. J. Energy Inst. 2021, 98, 244–258. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Sun, J.; Lu, G.; Chen, M.; Deng, F.; Dou, R.; Nie, L.; Zhong, Y. Synthesis of Silica-Composited Biochars from Alkali-Fused Fly Ash and Agricultural Wastes for Enhanced Adsorption of Methylene Blue. Sci. Total Environ. 2020, 729, 139055. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.L.; Nargotra, P.; Chen, C.W.; Sun, P.P.; Singhania, R.R.; Patel, A.K.; Dong, C. Di Journey of Lignin from a Roadblock to Bridge for Lignocellulose Biorefineries: A Comprehensive Review. Sci. Total Environ. 2023, 861, 160560. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V. Von Pretreatment Techniques for Agricultural Waste. Case Stud. Chem. Environ. Eng. 2022, 6, 100229. [Google Scholar] [CrossRef]
- Steigerwald, J.M.; Ray, J.R. Adsorption Behavior of Perfluorooctanesulfonate (PFOS) onto Activated Spent Coffee Grounds Biochar in Synthetic Wastewater Effluent. J. Hazard. Mater. Lett. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E.R.V. The Use of Carbon Adsorbents for the Removal of Perfluoroalkyl Acids from Potable Reuse Systems. Chemosphere 2017, 184, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Huo, S.; Feng, J.; Lu, X. Adsorption of Perfluorooctane Sulfonate (PFOS) on Corn Straw-Derived Biochar Prepared at Different Pyrolytic Temperatures. J. Taiwan Inst. Chem. Eng. 2017, 78, 265–271. [Google Scholar] [CrossRef]
- Liu, N.; Wu, C.; Lyu, G.; Li, M. Efficient Adsorptive Removal of Short-Chain Perfluoroalkyl Acids Using Reed Straw-Derived Biochar (RESCA). Sci. Total Environ. 2021, 798, 149191. [Google Scholar] [CrossRef] [PubMed]
- Mong, G.R.; Chong, C.T.; Chong, W.W.F.; Ng, J.H.; Ong, H.C.; Ashokkumar, V.; Tran, M.V.; Karmakar, S.; Goh, B.H.H.; Mohd Yasin, M.F. Progress and Challenges in Sustainable Pyrolysis Technology: Reactors, Feedstocks and Products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.C.; Zhang, Q.J.; Zhang, W.M.; Zhang, Q.X. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Azeta, O.; Ayeni, A.O.; Agboola, O.; Elehinafe, F.B. A Review on the Sustainable Energy Generation from the Pyrolysis of Coconut Biomass. Sci. Afr. 2021, 13, e00909. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Tejasvi, R. A Review on Role of Process Parameters on Pyrolysis of Biomass and Plastics: Present Scope and Future Opportunities in Conventional and Microwave-Assisted Pyrolysis Technologies. Process Saf. Environ. Prot. 2022, 162, 435–462. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Yek, P.N.Y.; Lock, S.S.M.; Chin, B.L.F.; Yiin, C.L.; Lan, J.C.W.; Lam, S.S. Microwave-Assisted Pyrolysis in Biomass and Waste Valorisation: Insights into the Life-Cycle Assessment (LCA) and Techno-Economic Analysis (TEA). Chem. Eng. J. 2024, 491, 151942. [Google Scholar] [CrossRef]
- Idris, S.S.; Zailan, M.I.; Azron, N.; Rahman, N.A. Sustainable Green Charcoal Briquette from Food Waste via Microwave Pyrolysis Technique: Influence of Type and Concentration of Binders on Chemical and Physical Characteristics. Int. J. Renew. Energy Dev. 2021, 10, 425–433. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Lock, S.S.M.; Chin, B.L.F.; Yiin, C.L.; Cheah, K.W.; Loy, A.C.M.; Yek, P.N.Y.; Chong, W.W.F.; Lam, S.S. Microwave Processing of Oil Palm Wastes for Bioenergy Production and Circular Economy: Recent Advancements, Challenges, and Future Prospects. Bioresour. Technol. 2023, 369, 128478. [Google Scholar] [CrossRef]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Microwave Pyrolysis of Sewage Biosolids: Dielectric Properties, Microwave Susceptor Role and Its Impact on Biochar Properties. J. Anal. Appl. Pyrolysis 2018, 129, 93–100. [Google Scholar] [CrossRef]
- Hadiya, V.; Popat, K.; Vyas, S.; Varjani, S.; Vithanage, M.; Kumar Gupta, V.; Núñez Delgado, A.; Zhou, Y.; Loke Show, P.; Bilal, M.; et al. Biochar Production with Amelioration of Microwave-Assisted Pyrolysis: Current Scenario, Drawbacks and Perspectives. Bioresour. Technol. 2022, 355, 127303. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Sunarso, J.; Li, C.; Pham, G.H.; Phan, C.; Liu, S. Microwave-Assisted Catalytic Methane Reforming: A Review. Appl. Catal. A Gen. 2020, 599, 117620. [Google Scholar] [CrossRef]
- Palla, S.; Surya, D.V.; Pritam, K.; Puppala, H.; Basak, T.; Palla, V.C.S. A Critical Review on the Influence of Operating Parameters and Feedstock Characteristics on Microwave Pyrolysis of Biomass. Environ. Sci. Pollut. Res. 2024, 31, 57570–57593. [Google Scholar] [CrossRef]
- Durán-Jiménez, G.; Rodriguez, J.; Stevens, L.; Kostas, E.T.; Dodds, C. Microwave Pyrolysis of Waste Biomass and Synthesis of Micro-Mesoporous Activated Carbons: The Role of Textural Properties for CO2 and Textile Dye Adsorption. Chem. Eng. J. 2024, 488, 150926. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, F.; Ling, Z.; Zhan, M.; Zhang, G.; Yuan, D. Comparative Study by Microwave Pyrolysis and Conventional Pyrolysis of Pharmaceutical Sludge: Resourceful Disposal and Antibiotic Adsorption. J. Hazard. Mater. 2024, 468, 133867. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yek, P.N.Y.; Foong, S.Y.; Liew, R.K.; Ge, S.; Lam, S.S. Single-Mode Microwave Pyrolysis of Engineered Biochar from Shrimp Shell Waste for Landfill Wastewater Treatment. J. Ind. Eng. Chem. 2025, 90, 1536–1544. [Google Scholar] [CrossRef]
- Omar, R.; Idris, A.; Yunus, R.; Khalid, K.; Aida Isma, M.I. Characterization of Empty Fruit Bunch for Microwave-Assisted Pyrolysis. Fuel 2011, 90, 1536–1544. [Google Scholar] [CrossRef]
- Ke, L.; Zhou, N.; Wu, Q.; Zeng, Y.; Tian, X.; Zhang, J.; Fan, L.; Ruan, R.; Wang, Y. Microwave Catalytic Pyrolysis of Biomass: A Review Focusing on Absorbents and Catalysts. NPJ Mater. Sustain. 2024, 2, 24. [Google Scholar] [CrossRef]
- Li, B.; Fan, X.; Yu, S.; Xia, H.; Nong, Y.; Bian, J.; Sun, M.; Zi, W. Microwave Heating of Biomass Waste Residues for Sustainable Bioenergy and Biomass Materials Preparation: A Parametric Simulation Study. Energy 2023, 274, 127347. [Google Scholar] [CrossRef]
- Lin, J.; Sun, S.; Xu, D.; Cui, C.; Ma, R.; Luo, J.; Fang, L.; Li, H. Microwave Directional Pyrolysis and Heat Transfer Mechanisms Based on Multiphysics Field Stimulation: Design Porous Biochar Structure via Controlling Hotspots Formation. Chem. Eng. J. 2022, 429, 132195. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Sridevi, V.; Ramesh, P.; Sankar Rao, C.; Tukarambai, M.; Kamireddi, D.; Gautam, R.; Dharaskar, S.A.; Pritam, K. Synthesis of Sustainable Chemicals from Waste Tea Powder and Polystyrene via Microwave-Assisted in-Situ Catalytic Co-Pyrolysis: Analysis of Pyrolysis Using Experimental and Modeling Approaches. Bioresour. Technol. 2022, 362, 127813. [Google Scholar] [CrossRef] [PubMed]
- Neha, S.; Rajput, P.; Remya, N. Biochar from Microwave Co-Pyrolysis of Food Waste and Polyethylene Using Different Microwave Susceptors—Production, Modification and Application for Metformin Removal. Environ. Res. 2022, 210, 112922. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.R.; Hoff, R.; Mărculescu, C.; Boldor, D. Investigation of Microwave-Assisted Pyrolysis of Biomass with Char in a Rectangular Waveguide Applicator with Built-in Phase-Shifting. Appl. Energy 2020, 259, 114217. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Y.; Zhang, D.; Chu, H. Microwave-Assisted Pyrolysis of Biomass to High-Value Products: Factors Assessment, Mechanism Analysis, and Critical Issues Proposal. Chem. Eng. J. 2024, 498, 155362. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, J.; Dai, L.; Guo, F.; Wang, Y.; Li, H.; Deng, W.; Lei, H.; Chen, P.; Liu, Y.; et al. Syngas Production from Biomass Pyrolysis in a Continuous Microwave Assisted Pyrolysis System. Bioresour. Technol. 2020, 314, 123756. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.; Zhou, N.; Fan, L.; Zhang, Y.; Peng, P.; Anderson, E.; Ding, K.; Wang, Y.; Liu, Y.; et al. Development and Application of a Continuous Fast Microwave Pyrolysis System for Sewage Sludge Utilization. Bioresour. Technol. 2018, 256, 295–301. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, H.; Ao, W.; Li, J.; Liu, G.; Chen, X.; Fu, J.; Ran, C.; Liu, Y.; Kang, Q.; et al. Microwave Pyrolysis of Textile Dyeing Sludge in a Continuously Operated Auger Reactor: Condensates and Non-Condensable Gases. Environ. Pollut. 2017, 228, 331–343. [Google Scholar] [CrossRef]
- Mao, X.; Kang, Q.; Liu, Y.; Siyal, A.A.; Ao, W.; Ran, C.; Fu, J.; Deng, Z.; Song, Y.; Dai, J. Microwave-Assisted Pyrolysis of Furfural Residue in a Continuously Operated Auger Reactor: Biochar Characterization and Analysis. Energy 2019, 168, 573–584. [Google Scholar] [CrossRef]
- Haeldermans, T.; Campion, L.; Kuppens, T.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. A Comparative Techno-Economic Assessment of Biochar Production from Different Residue Streams Using Conventional and Microwave Pyrolysis. Bioresour. Technol. 2020, 318, 124083. [Google Scholar] [CrossRef]
- Putra, N.R.; Zaini, M.A.A.; Zaini, A.S.; Airlangga, B.; Rizkiyah, D.N.; Kusuma, H.S.; Agustini, S.; Abdullah, S.; Yustisia, Y.; Khairullah, I.; et al. Evaluating the Efficacy of Palm Waste in Adsorption Processes for Wastewater Treatment: A Review. Can. J. Chem. Eng. 2024, 103, 2088–2106. [Google Scholar] [CrossRef]
- Momin, S.C.; Nath, J.; Mahana, A.; Pradhan, R.B.; Mehta, S.K. Chemical Activation of Microcystis Aeruginosa Biomass: A Promising Approach for Enhanced Diclofenac Sorption and Water Treatment. J. Chem. Technol. Biotechnol. 2024, 99, 149–163. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A Review on Application of Activated Carbons for Carbon Dioxide Capture: Present Performance, Preparation, and Surface Modification for Further Improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef]
- Başakçılardan Kabakcı, S.; Karakurt Çevik, B.; Borand, M.N.; Al, K. Hydrochar-Derived Activated Carbons from Poplar and Spruce Sawdust: Synthesis, Characteristics and Carbon Adsorption Performance. Adsorption 2024, 30, 2083–2098. [Google Scholar] [CrossRef]
- Alahabadi, A.; Singh, P.; Raizada, P.; Anastopoulos, I.; Sivamani, S.; Dotto, G.L.; Landarani, M.; Ivanets, A.; Kyzas, G.Z.; Hosseini-Bandegharaei, A. Activated Carbon from Wood Wastes for the Removal of Uranium and Thorium Ions through Modification with Mineral Acid. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125516. [Google Scholar] [CrossRef]
- Shang, S.; Che, W.; Li, Y. Removal of Cr(VI) from Water Using Microalgae-Based Modified Biochar: Adsorption Performance, Mechanisms, and Life Cycle Assessment. Algal Res. 2025, 85, 103819. [Google Scholar] [CrossRef]
- Kaya, N.; Yıldız Uzun, Z.; Altuncan, C.; Uzun, H. Adsorption of Congo Red from Aqueous Solution onto KOH-Activated Biochar Produced via Pyrolysis of Pine Cone and Modeling of the Process Using Artificial Neural Network. Biomass Convers. Biorefin. 2022, 12, 5293–5315. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Zhao, X.; Cao, J. High-Efficient Removal and Adsorption Mechanism of Organic Dyes in Wastewater by KOH-Activated Biochar from Phenol-Formaldehyde Resin Modified Wood. Sep. Purif. Technol. 2024, 330, 125542. [Google Scholar] [CrossRef]
- Basri, A.H.H.; Abdulhameed, A.S.; Jawad, A.H.; Wu, R.; ALOthman, Z.A.; Algburi, S. Reed Straw-Based Activated Carbon Produced via Microwave Method-Assisted-ZnCl2 for the Removal of Crystal Violet Dye: Multivariate Modeling and Optimization. Biomass Convers. Biorefin. 2024, 15, 10475–10487. [Google Scholar] [CrossRef]
- Astuti, W.; Sulistyaningsih, T.; Prastiyanto, D.; Rusiyanto; Lanjar; Riayanti, F.I.; Astuti, A.W.; Wibowo, W.T.; Handayani, A.D.; Wulandari, D.A. Influence of Lignocellulosic Composition in Biomass Waste on the Microstructure and Dye Adsorption Characteristics of Microwave-Assisted ZnCl2 Activated Carbon. Biomass Convers. Biorefin. 2024, 14, 16681–16697. [Google Scholar] [CrossRef]
- Qu, J.; Meng, Q.; Peng, W.; Shi, J.; Dong, Z.; Li, Z.; Hu, Q.; Zhang, G.; Wang, L.; Ma, S.; et al. Application of Functionalized Biochar for Adsorption of Organic Pollutants from Environmental Media: Synthesis Strategies, Removal Mechanisms and Outlook. J. Clean. Prod. 2023, 423, 138690. [Google Scholar] [CrossRef]
- Allou, N.B.; Tigori, M.A.; Koffi, A.A.; Halidou, M.; Eroi, N.S.; Atheba, P.; Trokourey, A. Methylene Blue Magnetic Adsorption Separation Process from Aqueous Solution Using Corn Cob. Sci. Afr. 2023, 21, e01828. [Google Scholar] [CrossRef]
- Srinivas, C.; Ranjith Kumar, E.; Tirupanyam, B.V.; Singh Meena, S.; Bhatt, P.; Prajapat, C.L.; Chandrasekhar Rao, T.V.; Sastry, D.L. Study of Magnetic Behavior in Co-Precipitated Ni–Zn Ferrite Nanoparticles and Their Potential Use for Gas Sensor Applications. J. Magn. Magn. Mater. 2020, 502, 166534. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Soltys, L.; Macyk, W. Magnetic Adsorbents for Removal of Pharmaceuticals: A Review of Adsorption Properties. J. Mol. Liq. 2023, 384, 122174. [Google Scholar] [CrossRef]
- Mitra, A.; Barick, B.; Mohapatra, J.; Sharma, H.; Meena, S.S.; Aslam, M. Large Tunneling Magnetoresistance in Octahedral Fe3O4 Nanoparticles. AIP Adv 2016, 6, 055007. [Google Scholar] [CrossRef]
- Hao, J.; Cui, Z.; Liang, J.; Ma, J.; Ren, N.; Zhou, H.; Xing, D. Sustainable Efficient Utilization of Magnetic Porous Biochar for Adsorption of Orange G and Tetracycline: Inherent Roles of Adsorption and Mechanisms. Environ. Res. 2024, 252, 19634. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Z.; Wang, Z.; Zhou, F.; Yu, J.; Chi, R.; Xiao, C. Adsorption of Pb2+ in Solution by Phosphate-Solubilizing Microbially Modified Biochar Loaded with Fe3O4. J. Taiwan Inst. Chem. Eng. 2024, 156, 105363. [Google Scholar] [CrossRef]
- Duan, X.; Chen, X.; Shi, L.; Cao, Y.; Liang, Y.; Wang, T.; Huang, C.; Cao, Y. Functionality-Dependent Removal Efficiency and Mechanisms of Polystyrene Microplastics by a Robust Magnetic Biochar. J. Environ. Chem. Eng. 2025, 13, 115509. [Google Scholar] [CrossRef]
- Srinadh, R.V.; Remya, N. Congo Red Removal Using Microwave Assisted Pyrolysis Derived Magnetic Hemp Hurd Biochar. Chem. Eng. Res. Des. 2024, 212, 321–331. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; De Farias, B.S.; Rios, E.C.; Ribeiro, A.C.; Acosta, K.D.R.; Gomes, C.P.W.; Cadaval, J.; Oliveira Gonçalves, J.; Silva De Farias, B.; Rios, E.C.; et al. Sustainable Removal of Chloroquine from Aqueous Solutions Using Microwave-Activated Cassava Biochar Derived from Agricultural Waste. Sustainability 2024, 16, 9854. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, X.; Zheng, X.; Feng, X.; Yu, A. Adsorption and Fenton-like Degradation of Ciprofloxacin Using Corncob Biochar-Based Magnetic Iron–Copper Bimetallic Nanomaterial in Aqueous Solutions. Nanomaterials 2022, 12, 579. [Google Scholar] [CrossRef]
- Christ-Ribeiro, A.; Maciel, J.V.; Bier, E.M.; Pinto, J.S.; Dias, D. Application of Electrochemical Sensors in the Determination of Synthetic Dyes in Foods or Beverages and Their Toxicological Effects on Human Health: A Review. Food Anal. Methods 2022, 15, 2394–2413. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Islami, M.R. Bioadsorbent from Magnetic Activated Carbon Hybrid for Removal of Dye and Pesticide. ChemistrySelect 2020, 5, 8814–8822. [Google Scholar] [CrossRef]
- Fatima, M.; Kelso, C.; Hai, F. Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater. Water 2025, 17, 1401. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Nguyen, H.M.; Ahmed, J.; Jeyapaul, A. Bio-Sorbents Derived from Agricultural Biomass for the Removal of Emerging Pollutants and Its Adsorption Mechanisms. J. Iran. Chem. Soc. 2023, 20, 2457–2470. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, J.; Ren, Y.; Yang, Z.; Zhong, M.; Feng, X.; Su, B.; Lei, Z. Excellent Adsorptive-Photocatalytic Performance of Zinc Oxide and Biomass Derived N, O-Contained Biochar Nanocomposites for Dyes and Antibiotic Removal. Chem. Eng. J. 2023, 451, 138959. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Gao, W.; Liu, Y.; Chen, J.; Zhong, M.; Li, W.; Su, B.; Lei, Z. Adsorptive-Photocatalytic Removal of Organic Dyes via Biomass-Derived Nitrogen, Oxygen-Containing Biochar-Embedded Tin Quantum Dots Catalyst. J. Environ. Chem. Eng. 2025, 13, 116331. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.; Gao, X.; Gai, L.; Lu, X.; Ma, H. Leaf-like Networks Consisting of TiO2/Biochar Composite for Enhanced Adsorptive-Photocatalytic Treatment of Antibiotic and Dye. Surf. Interfaces 2024, 51, 104615. [Google Scholar] [CrossRef]
- Samanth, A.; Vinayagam, R.; Varadavenkatesan, T.; Selvaraj, R. Fixed Bed Column Adsorption Systems to Remove 2,4-Dichlorophenoxyacetic Acid Herbicide from Aqueous Solutions Using Magnetic Activated Carbon. Environ. Res. 2024, 261, 119696. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Rakshitha, D.; Meghashree, M.; Kumaraswamy, G.P.; Nayanathara, O.S. Parthenium Hysterophorus Invasive Weed Valorization into Biochar for Removal of Pharmaceuticals and Personal Care Products: Competitive Adsorption Analysis via Batch and Fixed–Bed Column Systems. J. Water Process. Eng. 2024, 68, 106578. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of Different Biomass Species and Pyrolysis Temperatures on Heavy Metal Adsorption, Stability and Economy of Biochar. Ind. Crops Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Chang, S.; Wang, H.; Wang, M.; Xiang, W.; Gao, B. Microwave Biochar Produced with Activated Carbon Catalyst: Characterization and Adsorption of Heavy Metals. Environ. Res. 2023, 216, 114732. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.T.; Nguyen, T.B.; Chen, W.H.; Chen, C.W.; Kumar Patel, A.; Bui, X.T.; Chen, L.; Singhania, R.R.; Dong, C. Di Phosphoric Acid-Activated Biochar Derived from Sunflower Seed Husk: Selective Antibiotic Adsorption Behavior and Mechanism. Bioresour. Technol. 2023, 371, 128593. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, X.; Luo, J.; Li, Y.; Guo, T.; Gao, B. Performance of Lignin Impregnated Biochar on Tetracycline Hydrochloride Adsorption: Governing Factors and Mechanisms. Environ. Res. 2022, 215, 114339. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, A.M.; Fawzy, M.; El-Khouly, M.E.; Eltaweil, A.S. Nitrogen and Sulfur-Doped Biochar Supported Magnetic CuZnFe2O4 as a Sustainable Adsorbent for Efficient Reactive Black Dye 5 Removal from Industrial Wastewater. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Choudhary, V.; Philip, L. Sustainability Assessment of Acid-Modified Biochar as Adsorbent for the Removal of Pharmaceuticals and Personal Care Products from Secondary Treated Wastewater. J. Environ. Chem. Eng. 2022, 10, 107592. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Liang, Y.; Wu, T.; Chen, Y.; Yan, J.; Zhu, Y.; Ding, D. Adsorptive Decontamination of Antibiotics from Livestock Wastewater by Using Alkaline-Modified Biochar. Environ. Res. 2023, 226, 115676. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, S.; Bi, C.; Peng, C.; Wang, Y.; Li, Y.; Tao, E. Modulation of Ciprofloxacin Adsorption Conjugation Effect by Specific Conformation of Manganese-Nitrogen Co-Doped Biochar and Its Mechanistic Study. Sep. Purif. Technol. 2025, 363, 132304. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A. New Nanostructured Activated Biochar for Effective Removal of Antibiotic Ciprofloxacin from Wastewater: Adsorption Dynamics and Mechanisms. Environ. Res. 2022, 210, 112929. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A. Nanostructured Modified Biochar for Effective Elimination of Chlorpyrifos from Wastewater: Enhancement, Mechanisms and Performance. J. Water Process Eng. 2022, 47, 102703. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, Y.; Yang, L.; Wu, L.; Li, P.; Gao, F.; Qi, X.; Zhang, Z. Adsorptive Removal of Imidacloprid by Potassium Hydroxide Activated Magnetic Sugarcane Bagasse Biochar: Adsorption Efficiency, Mechanism and Regeneration. J. Clean. Prod. 2021, 292, 126005. [Google Scholar] [CrossRef]
- Li, H.; Kong, J.; Zhang, H.; Gao, J.; Fang, Y.; Shi, J.; Ge, T.; Fang, T.; Shi, Y.; Zhang, R.; et al. Mechanisms and Adsorption Capacities of Ball Milled Biomass Fly Ash/Biochar Composites for the Adsorption of Methylene Blue Dye from Aqueous Solution. J. Water Process Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, M.; Luo, J.; Li, W.; Fan, X.; Zhou, N.; Xu, H.; Shi, Y. Facile Preparation of N/P Co-Doped Mesoporous Biochar for Efficient Removal of Methylene Blue from Aqueous Solutions: A 2D-FTIR-COS, Adsorption Mechanism Analysis, and Fixed-Bed Column Study. J. Water Process Eng. 2025, 72, 107479. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, Y.; Wei, W.; Shi, J.; Jin, H. Preparation of Biochar Adsorption Material from Walnut Shell by Supercritical CO2 Pretreatment. Biochar 2024, 6, 11. [Google Scholar] [CrossRef]
- Eniola, J.O.; Sizirici, B.; Khaleel, A.; Yildiz, I. Fabrication of Engineered Biochar-Iron Oxide from Date Palm Frond for the Effective Removal of Cationic Dye from Wastewater. J. Water Process Eng. 2023, 54, 104046. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; Crispim, M.M.; Rios, E.C.; Silva, L.F.; de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Nawaz, A.; Manoharadas, S.; Dotto, G.L. New and Effective Cassava Bagasse-Modified Biochar to Adsorb Food Red 17 and Acid Blue 9 Dyes in a Binary Mixture. Environ. Sci. Pollut. Res. 2024, 31, 5209–5220. [Google Scholar] [CrossRef]
- Foroutan, R.; Tutunchi, A.; Foroughi, M.; Ramavandi, B. Efficient Fluoride Removal from Water and Industrial Wastewater Using Magnetic Chitosan/β-Cyclodextrin Aerogel Enhanced with Biochar and MOF Composites. Sep. Purif. Technol. 2025, 363, 132128. [Google Scholar] [CrossRef]
- Tan, W.T.; Zhou, H.; Tang, S.F.; Zeng, P.; Gu, J.F.; Liao, B.H. Enhancing Cd(II) Adsorption on Rice Straw Biochar by Modification of Iron and Manganese Oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, H.; Zheng, X.; Zhao, B.; Diao, J.; Jiang, Y. Enhanced Adsorption of Cadmium from Aqueous Solution by Amino Modification Biochar and Its Adsorption Mechanism Insight. J. Environ. Chem. Eng. 2023, 11, 109747. [Google Scholar] [CrossRef]
- Liu, B.; Chen, T.; Wang, B.; Zhou, S.; Zhang, Z.; Li, Y.; Pan, X.; Wang, N. Enhanced Removal of Cd2+ from Water by AHP-Pretreated Biochar: Adsorption Performance and Mechanism. J. Hazard. Mater. 2022, 438, 129467. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Chen, X.; Sarkar, B.; Bolan, N.S.; Wei, T.; Zhou, H.; Wang, H. Co-Adsorption Mechanisms of Cd(II) and As(III) by an Fe-Mn Binary Oxide Biochar in Aqueous Solution. Chem. Eng. J. 2023, 466, 143199. [Google Scholar] [CrossRef]
- Zhang, Y.; Akindolie, M.S.; Tian, X.; Wu, B.; Hu, Q.; Jiang, Z.; Wang, L.; Tao, Y.; Cao, B.; Qu, J. Enhanced Phosphate Scavenging with Effective Recovery by Magnetic Porous Biochar Supported La(OH)3: Kinetics, Isotherms, Mechanisms and Applications for Water and Real Wastewater. Bioresour. Technol. 2021, 319, 124232. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Huang, Y.; Song, L.; Chen, H.; Zhu, S.; Tang, C. Enhanced Adsorption of Phosphate on Orange Peel-Based Biochar Activated by Ca/Zn Composite: Adsorption Efficiency and Mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129728. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Wang, Y.; Xu, Z.; Diao, Z. Lanthanum/Iron Co-Modified Biochar for Highly Efficient Adsorption of Low-Concentration Phosphate from Aqueous Solution. J. Environ. Chem. Eng. 2024, 12, 111876. [Google Scholar] [CrossRef]
- Thomas, S.S.; Viswanathan, N.; Periyasamy, S.; Al-Asbahi, B.A. Enriched Phosphate Adsorption Using Lanthanum Organic Frameworks Decorated Papaya Biochar Supported Alginate Composite. J. Mol. Liq. 2025, 426, 127286. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, X.; Li, Y.; Deng, Y.; He, Q.; Dai, W. Aging Alters the Physicochemical Properties of Biochar, Enhances Its Adsorption Performance for Tris-(1-Chloro-2-Propyl) Phosphate, and Changes the Adsorption Mechanism. Environ. Technol. Innov. 2025, 37, 104053. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yu, S.; Cui, M. Removal of Pristine and Aged Microplastics from Water by Magnetic Biochar: Adsorption and Magnetization. Sci. Total Environ. 2023, 875, 162647. [Google Scholar] [CrossRef]
- Momina; Shahadat, M.; Isamil, S. Regeneration Performance of Clay-Based Adsorbents for the Removal of Industrial Dyes: A Review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef]
- Gerhardt, R.; Farias, B.S.; Moura, J.M.; de Almeida, L.S.; da Silva, A.R.; Dias, D.; Cadaval, T.R.S.; Pinto, L.A.A. Development of Chitosan/Spirulina Sp. Blend Films as Biosorbents for Cr6+ and Pb2+ Removal. Int. J. Biol. Macromol. 2020, 155, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.M.; Farias, B.S.; Rodrigues, D.A.S.; Moura, C.M.; Dotto, G.L.; Pinto, L.A.A. Preparation of Chitosan with Different Characteristics and Its Application for Biofilms Production. J. Polym. Environ. 2015, 23, 470–477. [Google Scholar] [CrossRef]
- Gkika, D.A.; Tolkou, A.K.; Katsoyiannis, I.A.; Kyzas, G.Z. The Adsorption-Desorption-Regeneration Pathway to a Circular Economy: The Role of Waste-Derived Adsorbents on Chromium Removal. Sep. Purif. Technol. 2025, 368, 132996. [Google Scholar] [CrossRef]
- Cadaval, T.R.S.; Dotto, G.L.; Seus, E.R.; Mirlean, N.; de Almeida Pinto, L.A. Vanadium Removal from Aqueous Solutions by Adsorption onto Chitosan Films. Desalination Water Treat. 2016, 57, 16583–16591. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Synthesis of Palm Kernel Shells-Biochar Adsorbent for Removal of Methylene Blue and Then Reused for Latent Fingerprint Detection Using Spent Adsorbent. Green. Anal. Chem. 2025, 13, 100259. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohammad, A.W. The Removal of Anionic and Cationic Dyes from an Aqueous Solution Using Biomass-Based Activated Carbon. Sci. Rep. 2021, 11, 8623. [Google Scholar] [CrossRef]
- El-Bendary, N.; El-Etriby, H.K.; Mahanna, H. Reuse of Adsorption Residuals for Enhancing Removal of Ciprofloxacin from Wastewater. Environ. Technol. 2022, 43, 4438–4454. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.S. Agricultural Waste of Sugarcane Bagasse as Efficient Adsorbent for Lead and Nickel Removal from Untreated Wastewater: Biosorption, Equilibrium Isotherms, Kinetics and Desorption Studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwary, R.K.; Yadav, M.; Singh, K.M.P. Nonlinear regression analysis and response surface modeling of Cr (VI) removal from synthetic wastewater by an agro-waste Cocos Nucifera: Box-Behnken Design (BBD). Int. J. Phytoremediat. 2020, 23, 791–808. [Google Scholar] [CrossRef]
- Mahanty, B.; Mondal, S. Synthesis of Magnetic Biochar Using Agricultural Waste for the Separation of Cr(VI) From Aqueous Solution. Arab. J. Sci. Eng. 2021, 46, 10803–10818. [Google Scholar] [CrossRef]
- Manzoor, Q.; Sajid, A.; Hussain, T.; Iqbal, M.; Abbas, M.; Nisar, J. Efficiency of Immobilized Zea Mays Biomass for the Adsorption of Chromium from Simulated Media and Tannery Wastewater. J. Mater. Res. Technol. 2019, 8, 75–86. [Google Scholar] [CrossRef]
- Rzig, B.; Guesmi, F.; Sillanpää, M.; Hamrouni, B. Biosorption Potential of Olive Leaves as a Novel Low-Cost Adsorbent for the Removal of Hexavalent Chromium from Wastewater. Biomass Convers. Biorefin. 2024, 14, 12961–12979. [Google Scholar] [CrossRef]
- Shakya, A.; Núñez-Delgado, A.; Agarwal, T. Biochar Synthesis from Sweet Lime Peel for Hexavalent Chromium Remediation from Aqueous Solution. J. Environ. Manag. 2019, 251, 109570. [Google Scholar] [CrossRef]
- Rezvani, B.; Hallajisani, A.; Tavakoli, O. Super-Effective Biochar Adsorbents from Co-Pyrolysis of Rice Husk and Sewage Sludge: Adsorption Performance, Advanced Regeneration, and Economic Analysis. Bioresour. Technol. Rep. 2025, 29, 102046. [Google Scholar] [CrossRef]
- Gomase, V.; Rathi, T.; Muley, A.; Saravanan, D.; Jugade, R. Soybean Biochar as Highly Efficient Adsorbent for Ofloxacin from Aqueous and CO2 from Gaseous Phase: Mathematical Modelling and Regeneration Studies. Clean. Chem. Eng. 2025, 11, 100154. [Google Scholar] [CrossRef]
- Zeng, S.; Choi, Y.K.; Kan, E. Iron-Activated Bermudagrass-Derived Biochar for Adsorption of Aqueous Sulfamethoxazole: Effects of Iron Impregnation Ratio on Biochar Properties, Adsorption, and Regeneration. Sci. Total Environ. 2021, 750, 141691. [Google Scholar] [CrossRef] [PubMed]
- Cusioli, L.F.; de Souza, R.M.; Beltran, L.B.; Bergamasco, R. Use of Low-Cost Adsorbent Functionalized with Iron Oxide Nanoparticles for Ivermectin Removal. S. Afr. J. Chem. Eng. 2024, 47, 142–149. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, N.; Dalal, A.; Panghal, P.; Sharma, A.K.; Kumar, S. Cutting-Edge Regeneration Technologies for Saturated Adsorbents: A Systematic Review on Pathways to Circular Wastewater Treatment System. Environ. Monit. Assess. 2025, 197, 215. [Google Scholar] [CrossRef]
- Márquez, P.; Benítez, A.; Chica, A.F.; Martín, M.A.; Caballero, A. Evaluating the Thermal Regeneration Process of Massively Generated Granular Activated Carbons for Their Reuse in Wastewater Treatments Plants. J. Clean. Prod. 2022, 366, 132685. [Google Scholar] [CrossRef]
- Liu, X.; Huang, P.; Ma, W.; Jin, F.; Tai, L. Unveiling Adsorption Mechanisms and Regeneration Challenges of Durian Peel Biochar for Ciprofloxacin Removal: Batch Experiments and DFT Study. J. Water Process Eng. 2025, 75, 107991. [Google Scholar] [CrossRef]
- Durán-Jiménez, G.; Stevens, L.A.; Hodgins, G.R.; Uguna, J.; Ryan, J.; Binner, E.R.; Robinson, J.P. Fast Regeneration of Activated Carbons Saturated with Textile Dyes: Textural, Thermal and Dielectric Characterization. Chem. Eng. J. 2019, 378, 121774. [Google Scholar] [CrossRef]
- Qin, Y.; Chai, B.; Wang, C.; Yan, J.; Fan, G.; Song, G. Removal of Tetracycline onto KOH-Activated Biochar Derived from Rape Straw: Affecting Factors, Mechanisms and Reusability Inspection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128466. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, L.; Guo, X.; Yang, M.; Lu, Y.; Wang, Y.; Zheng, Y.; Wei, G. Adsorption of Cationic Dye from Water Using an Iron Oxide/Activated Carbon Magnetic Composites Prepared from Sugarcane Bagasse by Microwave Method. Environ. Technol. 2021, 42, 337–350. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Sahroni, I.; Wijayana, A.; Faraswati, M.; Dwi Putri, A.; Oh, W.C.; Doong, R.-a. Magnetically-Separable Photocatalyst of Magnetic Biochar from Snake Fruit Peel for Rhodamine B Photooxidation. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100669. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Shu, K.; Zheng, T. Simulation of Moisture Removal in Microwave Regeneration of Carbon Adsorbents: Comparing Conventional Heating and Multiple Microwave Frequencies with Economic Implications. Chem. Eng. J. 2025, 503, 158629. [Google Scholar] [CrossRef]
- Zhang, X.N.; Mao, G.Y.; Jiao, Y.B.; Shang, Y.; Han, R.P. Adsorption of Anionic Dye on Magnesium Hydroxide-Coated Pyrolytic Bio-Char and Reuse by Microwave Irradiation. Int. J. Environ. Sci. Technol. 2014, 11, 1439–1448. [Google Scholar] [CrossRef]
- Li, K.; Zhang, B.; Ma, R.; Li, S. Magnetic Composite Adsorbents for Radioactive Cs+/Sr2+ Removal: Progress in Material Design and Enhanced Performance. Sep. Purif. Technol. 2025, 374, 133683. [Google Scholar] [CrossRef]
- Labuto, G.; Cardona, D.S.; Debs, K.B.; Imamura, A.R.; Bezerra, K.C.H.; Carrilho, E.N.V.M.; Haddad, P.S. Low-Cost Agroindustrial Biomasses and Ferromagnetic Bionanocomposites to Cleanup Textile Effluents. Desalination Water Treat. 2018, 112, 80–89. [Google Scholar] [CrossRef]
- Mohammadi, R.; Eshaq, G.; Winkler, M.K.H.; Pihlajamäki, A. Magnetic Biochar Aluminum Cross-Linked Composite Beads for Preferential Phosphate Separation from Phosphate-Rich Effluents. J. Environ. Chem. Eng. 2025, 13, 116815. [Google Scholar] [CrossRef]
- Ye, M.; Li, Z.; Wen, L.; Duan, F.; Zhang, L. Enhanced Cr(VI) Removal via Surfactant-Tailored Magnetic Functionalized-Biochar: Synergistic Alkyl Grafting and Iron Dispersion for Broad-Spectrum PH Adaptability. J. Environ. Chem. Eng. 2025, 13, 117877. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Huang, S.; Deng, X.; Wu, J.; Wu, S. Magnetic Biochar Derived from Penicillin Fermentation Residue for Efficient Penicillin G Sodium Adsorption: Kinetics, Degradation Mechanism, and Simulations. Bioresour. Technol. 2025, 435, 132885. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Peng, Y.; Fang, Z. Molybdate-Loaded Magnetic Biochar Activates Persulfate for Efficient Degradation of Sulfamethazine. Sep. Purif. Technol. 2025, 362, 131911. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Onotu, O.P.; Tywabi-Ngeva, Z. Advantages of the Reuse of Spent Adsorbents and Potential Applications in Environmental Remediation: A Review. Green. Anal. Chem. 2024, 11, 100156. [Google Scholar] [CrossRef]
- Priya, E.; Sharma, J.; Sarkar, S.; Maji, P.K. Phosphate Recovery from Wastewater by Cellulose-Based Adsorbent Derived from Agricultural Waste and Its Reuse as a Slow-Release Fertilizer. J. Environ. Chem. Eng. 2025, 13, 116716. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Cd2+ Ion Adsorption and Re-Use of Spent Adsorbent with N-Doped Carbon Nanoparticles Coated on Cerium Oxide Nanorods Nanocomposite for Fingerprint Detection. Chem. Phys. Impact 2022, 5, 100083. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Pillay, K.; Tywabi-Ngeva, Z. Novel Development of Zinc Oxide–Coated Carbon Nanoparticles from Pineapple Leaves Using Sol Gel Method for Optimal Adsorption of Cu2+ and Reuse in Latent Fingerprint Application. Environ. Sci. Pollut. Res. 2024, 31, 38801–38820. [Google Scholar] [CrossRef] [PubMed]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, H.; He, T.; Gao, S. Review on the Effect Collision between Hazardous Metal Ions and Geopolymer as Adsorbents or in Situ Stabilization/Solidification. Appl. Clay Sci. 2024, 249, 107258. [Google Scholar] [CrossRef]
- Pranudta, A.; Patra, S.; Amonpattaratkit, P.; Klysubun, W.; Saiyasombat, C.; El-Moselhy, M.M.; Nguyen, T.T.; Padungthon, S. Immobilization of Arsenic in Wastewater from Regeneration of Fixed-Bed Adsorbent by Co-Precipitation with Zirconium Nano-Sludge for Disposal in Landfills. J. Environ. Chem. Eng. 2022, 10, 107756. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Factors Affecting Hazardous Waste Solidification/Stabilization: A Review. J. Hazard. Mater. 2006, 137, 267–276. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, C.; Chen, P.; Ning, S.; Chen, L.; Xie, Y.; Wu, Y.; Wu, H.; Wei, Y.; Shi, W.; et al. Treatment of Cs-Sorbed Zeolite as Spent Adsorbent by Cold Sintering for Stabilization and Solidification of Secondary Wastes. Chem. Eng. J. 2024, 499, 156626. [Google Scholar] [CrossRef]
- Sayago, U.F.C. Design and Development of a Pilot-Scale Industrial Wastewater Treatment System with Plant Biomass and EDTA. Water 2023, 15, 3484. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Rodríguez-Calvo, A.; Robledo-Mahón, T.; Aranda, E.; González-López, J.; Calvo, C. Design of Bio-Absorbent Systems for the Removal of Hydrocarbons from Industrial Wastewater: Pilot-Plant Scale. Toxics 2021, 9, 162. [Google Scholar] [CrossRef]
- Hedayati Marzbali, M.; Hakeem, I.G.; Ngo, T.; Surapaneni, A.; Shah, K. Innovative Chemical Functionalisation of Biosolids for Removing Heavy Metals and Enhancing Ammonium Recovery from Wastewater. Int. J. Environ. Sci. Technol. 2024, 22, 6665–6680. [Google Scholar] [CrossRef]
- Samuel Olugbenga, O.; Goodness Adeleye, P.; Blessing Oladipupo, S.; Timothy Adeleye, A.; Igenepo John, K. Biomass-Derived Biochar in Wastewater Treatment—A Circular Economy Approach. Waste Manag. Bull. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Juela, D.; Vera, M.; Cruzat, C.; Astudillo, A.; Vanegas, E. A New Approach for Scaling up Fixed-Bed Adsorption Columns for Aqueous Systems: A Case of Antibiotic Removal on Natural Adsorbent. Process. Saf. Environ. Prot. 2022, 159, 953–963. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; González-Delgado, Á.D. Application of Techno-Economic and Sensitivity Analyses as Decision-Making Tools for Assessing Emerging Large-Scale Technologies for Production of Chitosan-Based Adsorbents. ACS Omega 2020, 5, 17601–17610. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Giannakoudakis, D.A.; Frontistis, Z.; Zorpas, A.A.; Pashalidis, I.; Triantafyllidis, K. Biomass-Derived Adsorbents: Universal Database Development for Their Synthesis and Remediation Efficiency as a Necessary Step to Move from Laboratory- to Pilot-Scale Applications. Curr. Opin. Green Sustain. Chem. 2024, 47, 100902. [Google Scholar] [CrossRef]
- Coelho, R.S.; Soares, L.C.; Adarme, O.F.H.; Maia, L.C.; Costa, C.S.D.; Guibal, E.; Gurgel, L.V.A. A Review on Advances in the Use of Raw and Modified Agricultural Lignocellulosic Residues in Mono- and Multicomponent Continuous Adsorption of Inorganic Pollutants for Upscaling Technologies. Polymers 2025, 17, 953. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Äkräs, L.; Paganelli, Z.; Dammann, N.; Abidnejad, R.; Lipponen, S.; Silvenius, F.; Vahvaselkä, M.; Ilvesniemi, H.; Seppälä, J.; et al. Can Biochar Fillers Advance the Properties of Composites? Early-Stage Characterization and Life Cycle Assessment of Novel Polyamide/Biochar Biocomposites. Environ. Res. 2025, 275, 121446. [Google Scholar] [CrossRef]
- Kakati, U.; Aier, I.; Anand, A.; Kaushal, P. Thermo-Kinetic and Techno-Economic Analysis of Biochar Conversion of Pine Needles for Clean Fuel Production in the Himalayan Region of India. Bioresour. Technol. Rep. 2025, 30, 102104. [Google Scholar] [CrossRef]
- Ferreira Junior, J.E.G.; Maia, L.C.; dos Santos, G.R.; Soares, L.C.; Gurgel, L.V.A. An Affordable Bioadsorbent System to Treat Arsenic-Contaminated Drinking Water in the Developing World: Prototyping and Economic Assessment. J. Environ. Chem. Eng. 2023, 11, 111199. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. National Primary Drinking Water Regulations; Office of the Federal Register: Washington, DC, USA, 2000.
- EU Directive (EU) 2020/2184 of the European Parliment and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020, 2019, L 435/1.
- CONAMA. Resolução CONAMA 430/2011. Diário Oficial da União, 16 May 2011. [Google Scholar]
- Mohd Ali Jinnah, S.N.H.; Md Ali, U.F.; Gopinath, S.C.; Ibrahim, N.; Ahmad, R.; Mohamed Zuki, F. Oil Palm Waste-Derived Reduced Graphene Oxide (RGO) for Dynamic Adsorption of Dye in a Fixed-Bed System. Desalination Water Treat. 2024, 317, 100019. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Zhu, C.; Sun, W.; Wang, H.; Liu, D.; Huang, B.; Fan, P. Lignin-Derived Porous Carbon with Interconnected Pores for Bulky Dye Adsorption in Fixed-Bed Systems. Chem. Eng. Sci. 2024, 298, 120345. [Google Scholar] [CrossRef]
- Ramola, S.; Rawat, N.; Shankhwar, A.K.; Srivastava, R.K. Fixed Bed Adsorption of Pb and Cu by Iron Modified Bamboo, Bagasse and Tyre Biochar. Sustain. Chem. Pharm. 2021, 22, 100486. [Google Scholar] [CrossRef]
- Loganathan, P.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Submerged Membrane/Adsorption Hybrid Process in Water Reclamation and Concentrate Management—A Mini Review. Environ. Sci. Pollut. Res. 2023, 30, 42738–42752. [Google Scholar] [CrossRef]
- Ezzine, M.; El-Shafie, A.S.; Youssef, K.M.; El-Azazy, M. Bifunctional TiO2—Cellulose Based Nanocomposites for Synergistic Adsorptive-Photocatalytic Removal of Methyl Orange: Response Modelling and Optimization. Int. J. Biol. Macromol. 2025, 306, 141753. [Google Scholar] [CrossRef]
- Ullah, F.; Ul Haq Khan, Z.; Sabahat, S.; Aftab, M.; Sun, J.; Samad Shah, N.; Rahim, A.; Abdullah, M.M.S.; Imran, M. Synergistic Degradation of Toxic Azo Dyes Using Mn-CuO@Biochar: An Efficient Adsorptive and Photocatalytic Approach for Wastewater Treatment. Chem. Eng. Sci. 2025, 302, 120844. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Yin, Z.; Liu, S.; Bian, S.; Zhang, Y. A Highly Efficient Strategy for Enhancing the Adsorptive and Magnetic Capabilities of Biochar Using Fenton Oxidation. Bioresour. Technol. 2020, 315, 123797. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Li, S.; Cao, Y. Highly Active Fenton-like Catalyst Derived from Solid Waste-Iron Ore Tailings Using Wheat Straw Pyrolysis. Environ. Sci. Pollut. Res. 2022, 29, 31567–31576. [Google Scholar] [CrossRef]
- Eskikaya, O.; Isik, Z.; Arslantas, C.; Yabalak, E.; Balakrishnan, D.; Dizge, N.; Rao, K.S. Preparation of Hydrochar Bio-Based Catalyst for Fenton Process in Dye-Containing Wastewater Treatment. Environ. Res. 2023, 216, 114357. [Google Scholar] [CrossRef]
- Lin, S.; Lyu, T.; Pan, M.; Hou, Y.; Guo, C.; Chen, Z.; Dong, R.; Liu, S. Exploration of Ammonia Stripping Coupled Adsorption-Membrane Filtration Process for Treating Kitchen Waste Biogas Slurry. Environ. Res. 2025, 274, 121318. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, H.; Wei, X. Enhanced Fenton-like Rate and Anti-Interference through Designing Materials with Specific Excellent Sorption to Pollutant. Chem. Eng. J. 2024, 502, 158046. [Google Scholar] [CrossRef]
- Aldana, J.C.; Agudelo, C.; Álvarez, P.M.; Acero, J.L. Removal of Micropollutants in Water Reclamation by Membrane Filtration: Impact of Pretreatments and Adsorption. Membranes 2024, 14, 146. [Google Scholar] [CrossRef]
- Wang, X.; Tao, Y.; Chang, C.; Zhou, M.; Li, J.; Fang, J.; Wang, Q.; He, A. Synergistic Removal of Ultra-Fine Coal Particles and Ca2+/Mg2+ from Mine Wastewater via Dual-Stage Adsorption Prior to Membrane Filtration. Sep. Purif. Technol. 2025, 360, 130944. [Google Scholar] [CrossRef]
- Mu, H.; Qiu, Q.; Cheng, R.; Qiu, L.; Xie, K.; Gao, M.; Liu, G. Adsorption-Enhanced Ceramic Membrane Filtration Using Fenton Oxidation for Advanced Treatment of Refinery Wastewater: Treatment Efficiency and Membrane-Fouling Control. Membranes 2021, 11, 651. [Google Scholar] [CrossRef]
| Criterion | Conventional Pyrolysis (CP) | Microwave-Assisted Pyrolysis (MAP) |
|---|---|---|
| Heating mode | External, by conduction or radiation | Volumetric and selective (direct interaction with biomass molecules) |
| Heating rate | Slow | Fast |
| Heating uniformity | Lower uniformity (temperature gradients) | More uniform heating |
| Energy efficiency | Lower efficiency | High energy efficiency |
| Residence time | Long | Short |
| Process control | Simpler control, less precise | Easier automation and precise control |
| Interaction with material | Independent of dielectric constant | Dependent on dielectric constant (requires suitable biomass or absorber) |
| Need for physical contact | Yes (heat transferred via contact with hot surfaces) | No (contactless heating) |
| Main products | Biochar (high stability), bio-oil, non-condensable gases; lower syngas yield, higher tar formation | Biochar with higher surface area, less tar, H2-rich gas |
| Industrial application | Broad application and well-established technology | Still limited due to equipment cost and dielectric restrictions |
| Parameter | Conventional Pyrolysis (CP) | Microwave-Assisted Pyrolysis (MAP) | General Effects |
|---|---|---|---|
| Biomass composition | Less sensitive to dielectric constant; cellulose and lignin yield more stable biochar [35] | Highly dependent on dielectric permittivity; affects energy absorption [43] | Biomass rich in cellulose and lignin results in more aromatic and stable biochar |
| Particle size | Larger particles (>0.25 mm) require more time for complete carbonization [29] | Smaller particles (<0.25 mm) favor uniform heating and avoid hotspots [40] | Smaller particle size enhances conversion efficiency and increases surface area |
| Temperature | Gradual increase (up to >600 °C); favors biochar graphitization and carbonization [37] | Rapid and intense; forms micropores and aromatic compounds in minutes (>600 °C) [36] | Higher temperatures reduce biochar yield while increasing porosity and production of gases and bio-oil |
| Residence time | Long (minutes to hours); progressive carbonization [28] | Short (seconds to minutes); risk of hotspots [44] | Longer residence time improves structural stability but decreases yield |
| Atmosphere | Less sensitive; commonly uses N2; CO2 can act as mild activating agent [40] | Atmosphere affects microwave absorption by changing the dielectric permittivity; vapors (e.g., steam or CO2) can alter thermal field and promote mild physical activation of biochar [28,38] | Atmospheric conditions influence oxidation, activation mechanisms, and product composition |
| Pyrolysis power | Indirectly applicable via temperature and residence time [40] | Defines heating intensity; directly linked to hotspot formation [45,46] | Increased power accelerates reaction rate but increases risk of thermal collapse |
| Absorbers/catalysts | Less common use; catalysts like Fe and Ni applied to control temperature and increase combustible gas production | Essential for low-dielectric-constant biomass; includes dielectric loss absorbers (e.g., silicon carbide, graphite, charcoal) and magnetic loss absorbers (e.g., ferrites and metal particles like Fe, Ni, Co); influences yield, activation, and selectivity [40] | Catalysts and absorbers enhance product yield, activation efficiency, and selectivity |
| Adsorbent | Adsorbate | Solvent | Removal Efficiency (%) | Number of Effective Regeneration Cycles | Reference |
|---|---|---|---|---|---|
| Palm kernel shell biochar | Methylene blue | 0.1 N HCl and 0.1 M NaOH solutions | 97.5 | 4 | [114] |
| Corncob and luffa sponge activated carbons | Ciprofloxacin | Methanol | 80.7% and 78.3% | 5 | [116] |
| Activated carbon made from rubber seed and rubber seed shell | Congo red and methylene blue | NaCl (0.01–0.3 mol/L) | 97–98% | 7 | [115] |
| Sugarcane bagasse | Pb (II) and Ni (II) | 0.1 M HNO3, HCl, and NaOH | 45–55% for NaOH; 75–79% for HCl; 90–96% for HNO3 | - | [117] |
| Coconut shell and coir | CR (IV) | Solutions of HCl, H2SO4, NaOH (0.2 N), and distilled water | 60–100% | 4 | [118] |
| Peanut husk with iron oxide biochar | Cr(IV) | 0.1 M NaOH | 56–96% | 4 | [119] |
| Corncob | CR (III) and Cr (IV) | 0.1 M NaOH | 80–96% | 5 | [120] |
| Olive leaves | Cr (IV) | NaOH (0.1 mol/L), NaCl (0.1 mol/L), water | 70–100% for NaOH; 60–100% for NaCl; and 30–90% for water | 5 | [121] |
| Sweet lime peel biochar | Cr(IV) | 1 M HCl, 1 M NaOH, and 0.1 M HCl | 80–95% for 1 M HCl; 30–80% for NaOH; 15–80% for 0.1 M HCl | 3 | [122] |
| Biochar from rice husk and sewage sludge | Alizarin Red S | Acetone, ethanol, methanol, and subcritical ethanol | 60–80% for acetone, ethanol, and methanol; 80–100% for subcritical ethanol | 5 | [123] |
| Soybean biochar | Ofloxacin | Ethanol | 60–85% | 6 | [124] |
| Bermda-grass-derived biochar | Sulfamethoxazole | 0.1 M NaOH | 50–100% | 4 | [125] |
| Seed hulls with iron nanoparticles | Ivermectin | - | 83–100% | 5 | [126] |
| Cassava-derived activated carbon | Chloroquine | Hydrochloric acid solution (0.2 mol/L) | 70–100% | 5 | [71] |
| Cassava-derived biochar | Food Red 17 and Acid Blue 9 dyes | NaOH (2 mol/L) | 66–100% for Acid Blue 9; 20–100% for Food Red 17 | 12 | [97] |
| Adsorbent | Adsorbate | T (°C) | Time (h) | Gas Flow | Removal Efficiency (%) | Number of Regeneration Cycles | Reference |
|---|---|---|---|---|---|---|---|
| Durian peel biochar | Ciprofloxacin | 400 | 2 | Air | 33–72% | 3 | [129] |
| Rape straw biochar | Tetracycline | 400 | 1 | Air | 97% | 6 | [131] |
| Bermuda-grass-derived biochar | Sulfamethoxazole | 275 | 3 | Air | 50–100% | 4 | [125] |
| Coconut shell activated carbon | Basic Blue 9 and Acid Blue 93 | 500 | 0.5 | Nitrogen | 42–100% | 4 | [130] |
| Magnetic sugarcane bagasse activated carbon | Methylene blue | 300 | 0.5 | Air | 85–100% | 4 | [132] |
| Magnetic biochar from snake fruit peel | Rhodamine B | 200 | - | Air | 99% | 5 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.O.; Leones, A.R.; de Farias, B.S.; da Silva, M.D.; Jaeschke, D.P.; Fernandes, S.S.; Ribeiro, A.C.; Cadaval, T.R.S., Jr.; Pinto, L.A.d.A. A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal. Water 2025, 17, 2141. https://doi.org/10.3390/w17142141
Gonçalves JO, Leones AR, de Farias BS, da Silva MD, Jaeschke DP, Fernandes SS, Ribeiro AC, Cadaval TRS Jr., Pinto LAdA. A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal. Water. 2025; 17(14):2141. https://doi.org/10.3390/w17142141
Chicago/Turabian StyleGonçalves, Janaína Oliveira, André Rodríguez Leones, Bruna Silva de Farias, Mariele Dalmolin da Silva, Débora Pez Jaeschke, Sibele Santos Fernandes, Anelise Christ Ribeiro, Tito Roberto Santanna Cadaval, Jr., and Luiz Antonio de Almeida Pinto. 2025. "A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal" Water 17, no. 14: 2141. https://doi.org/10.3390/w17142141
APA StyleGonçalves, J. O., Leones, A. R., de Farias, B. S., da Silva, M. D., Jaeschke, D. P., Fernandes, S. S., Ribeiro, A. C., Cadaval, T. R. S., Jr., & Pinto, L. A. d. A. (2025). A Comprehensive Review of Agricultural Residue-Derived Bioadsorbents for Emerging Contaminant Removal. Water, 17(14), 2141. https://doi.org/10.3390/w17142141













