Chemical and Isotopic Investigation of Abiotic Oxidation of Lactate Substrate in the Presence of Varied Electron Acceptors and Under Circumneutral Anaerobic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Media Composition
2.2. Media Preparation
2.3. Chemical Analyses
2.4. Stable Carbon Isotope Analyses
3. Results and Discussion
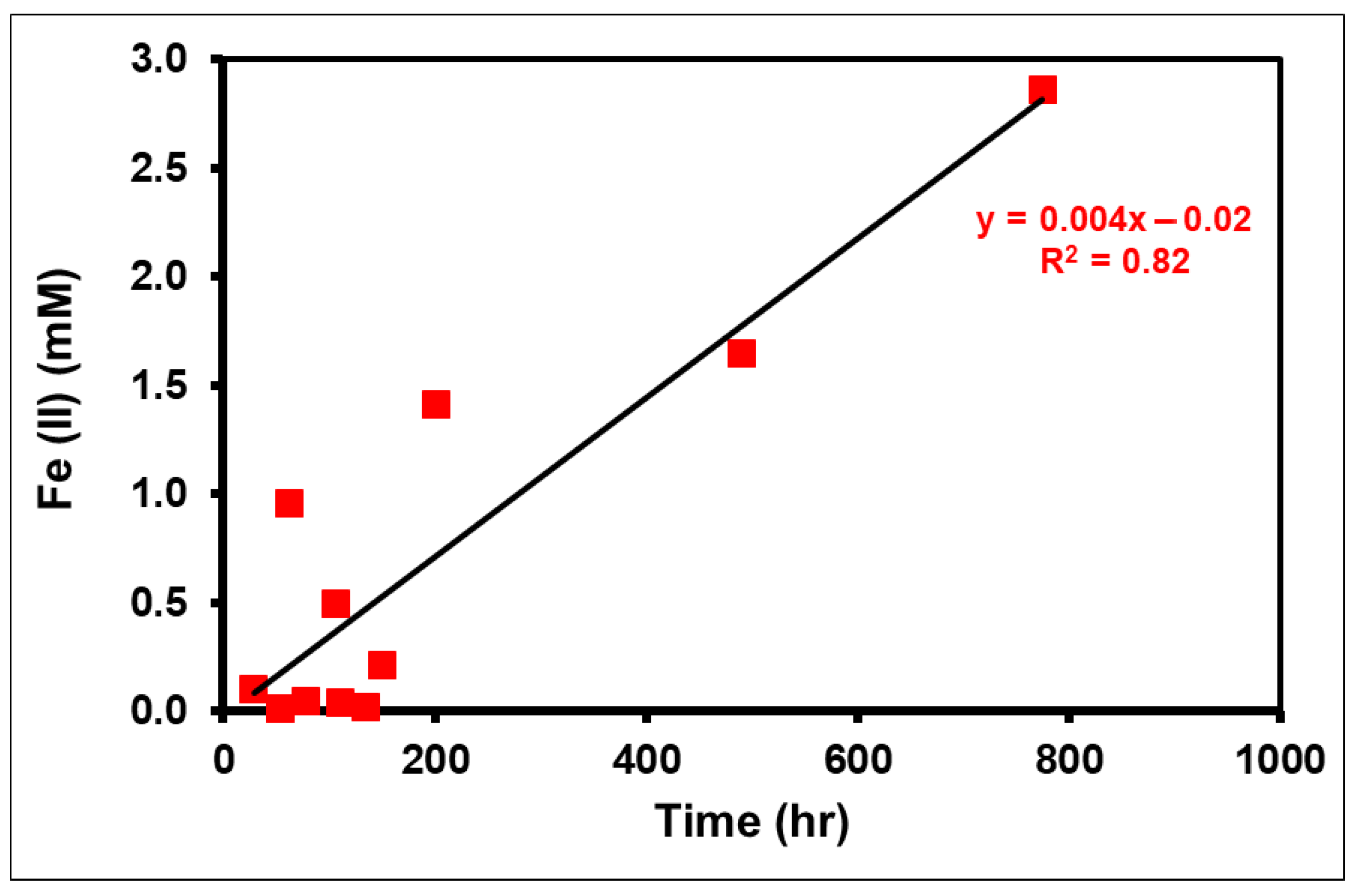
3.1. Extent of Iron Reduction and Organic Compound Oxidation
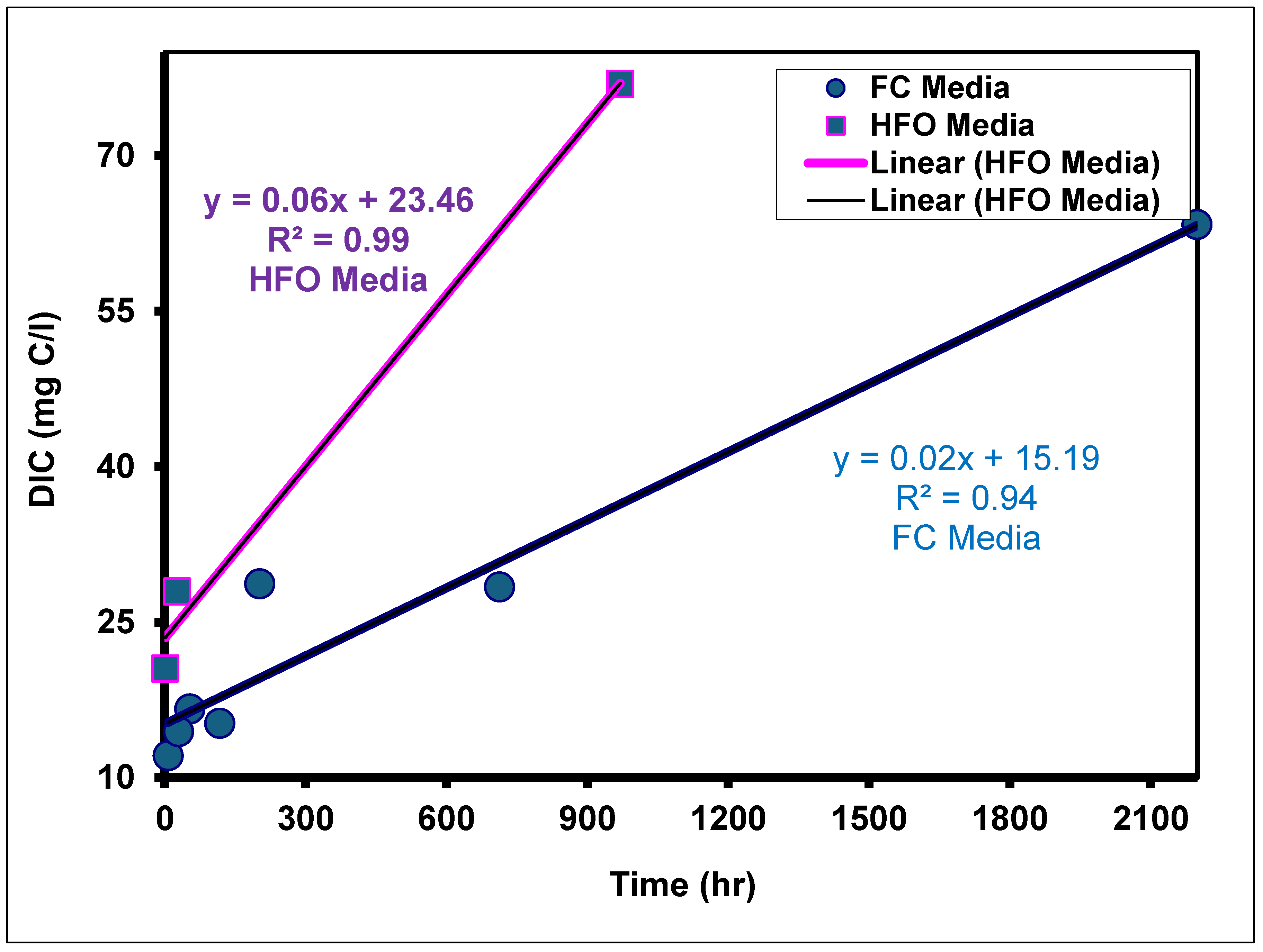
3.2. Effects of Media Composition
3.3. Effect of Fe(III)-Containing Compounds
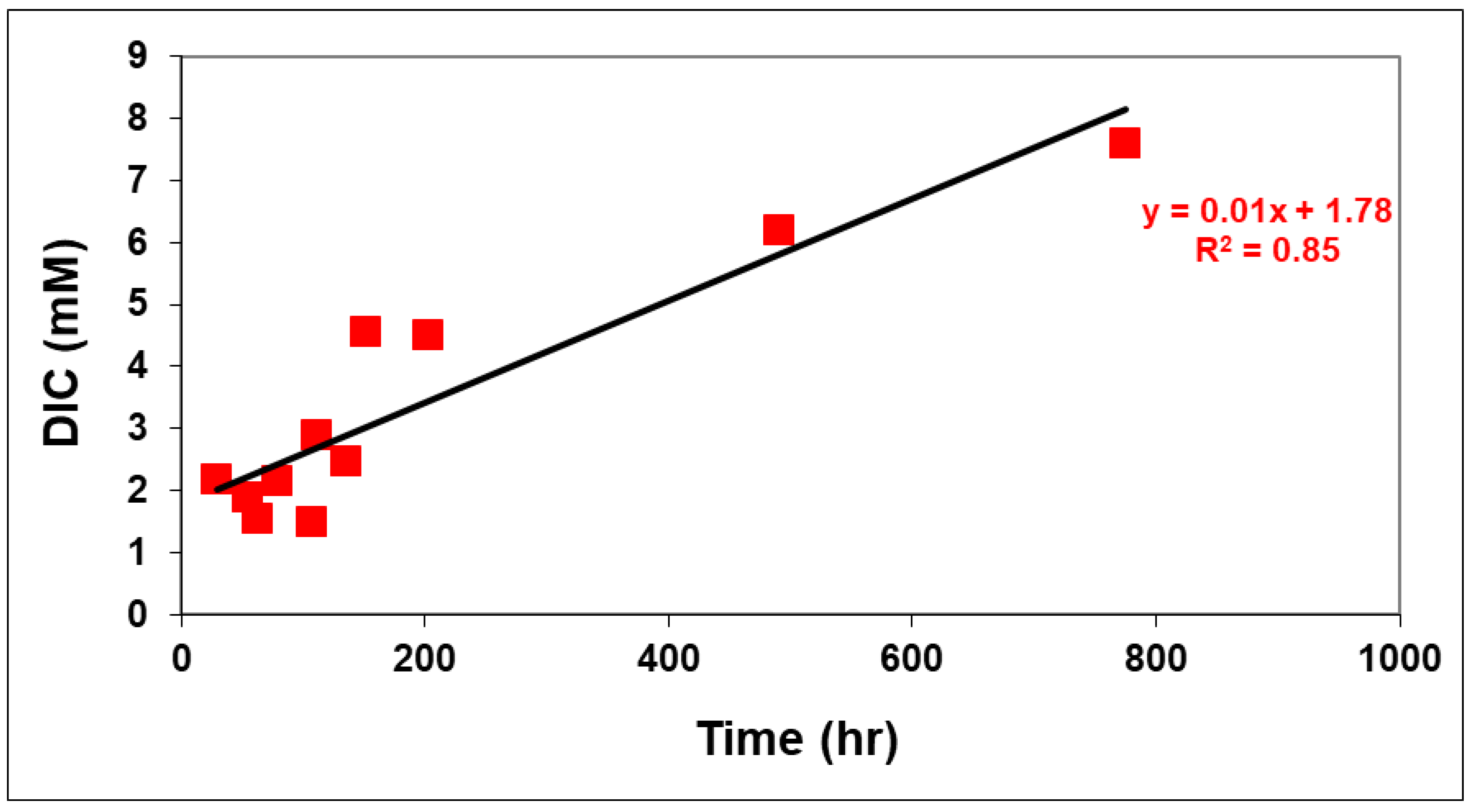
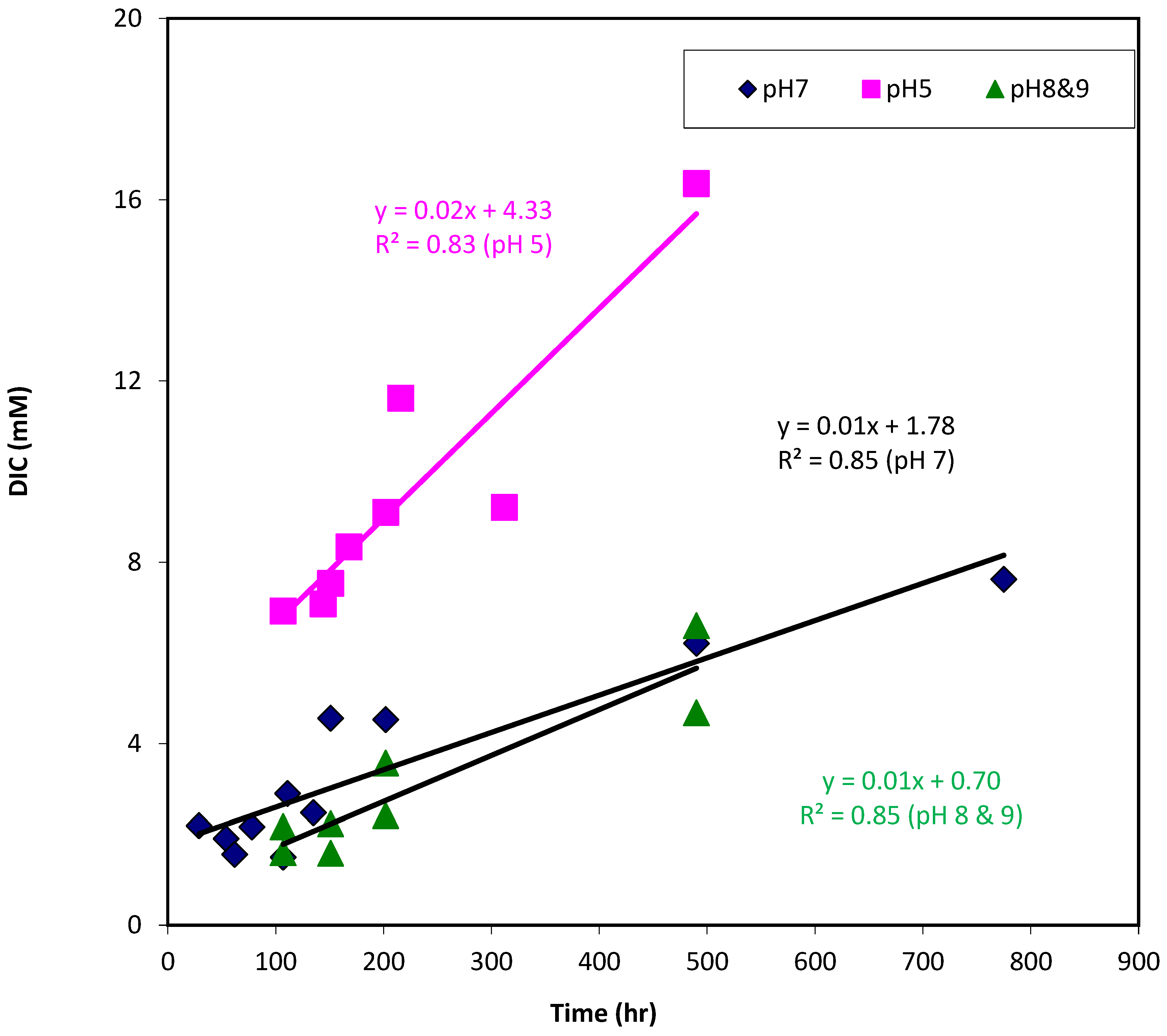
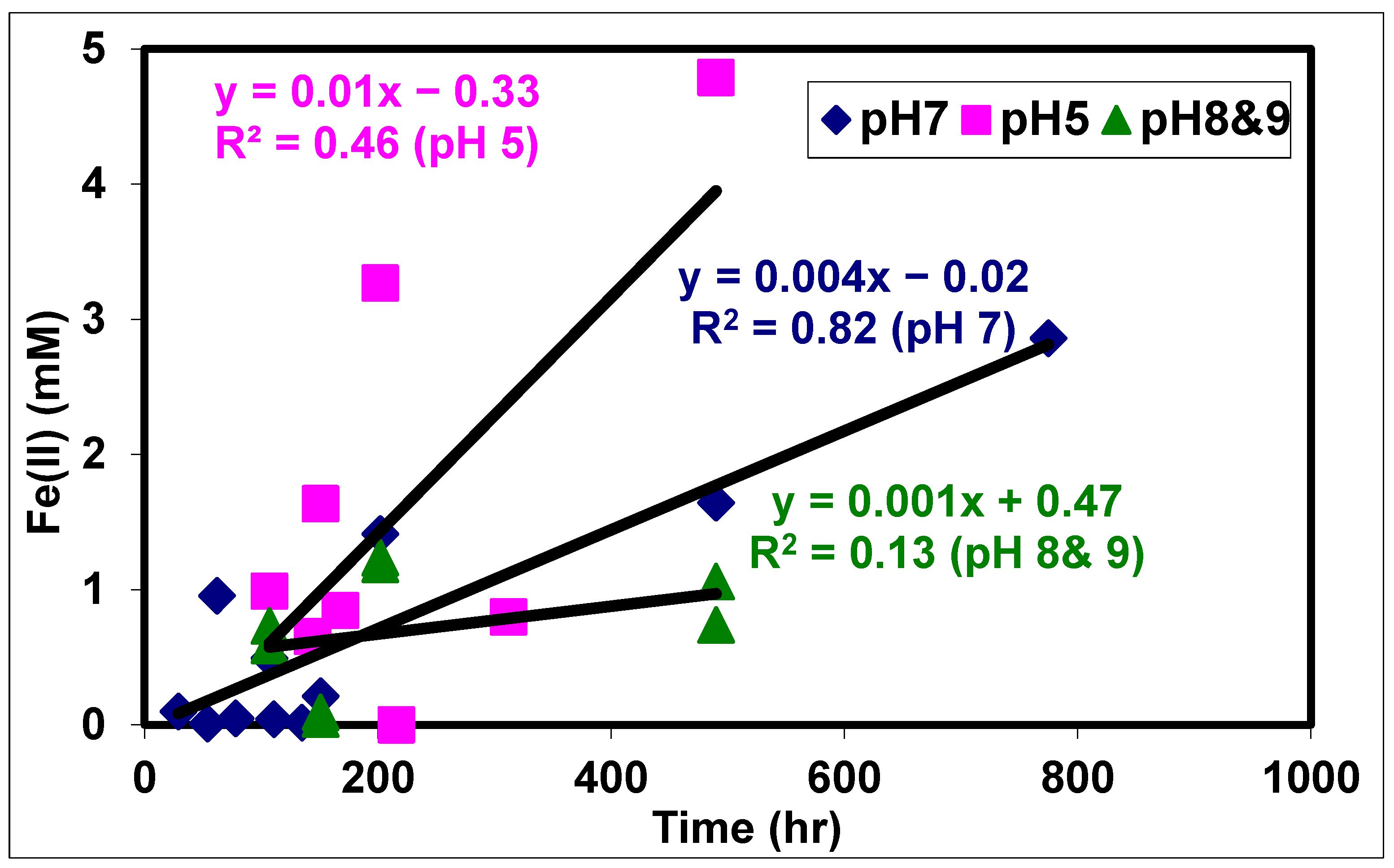
3.4. Effect of pH
3.5. Effect of Light Under Acidic and Neutral Conditions
3.6. Stable Carbon Isotopes in Abiotic Systems

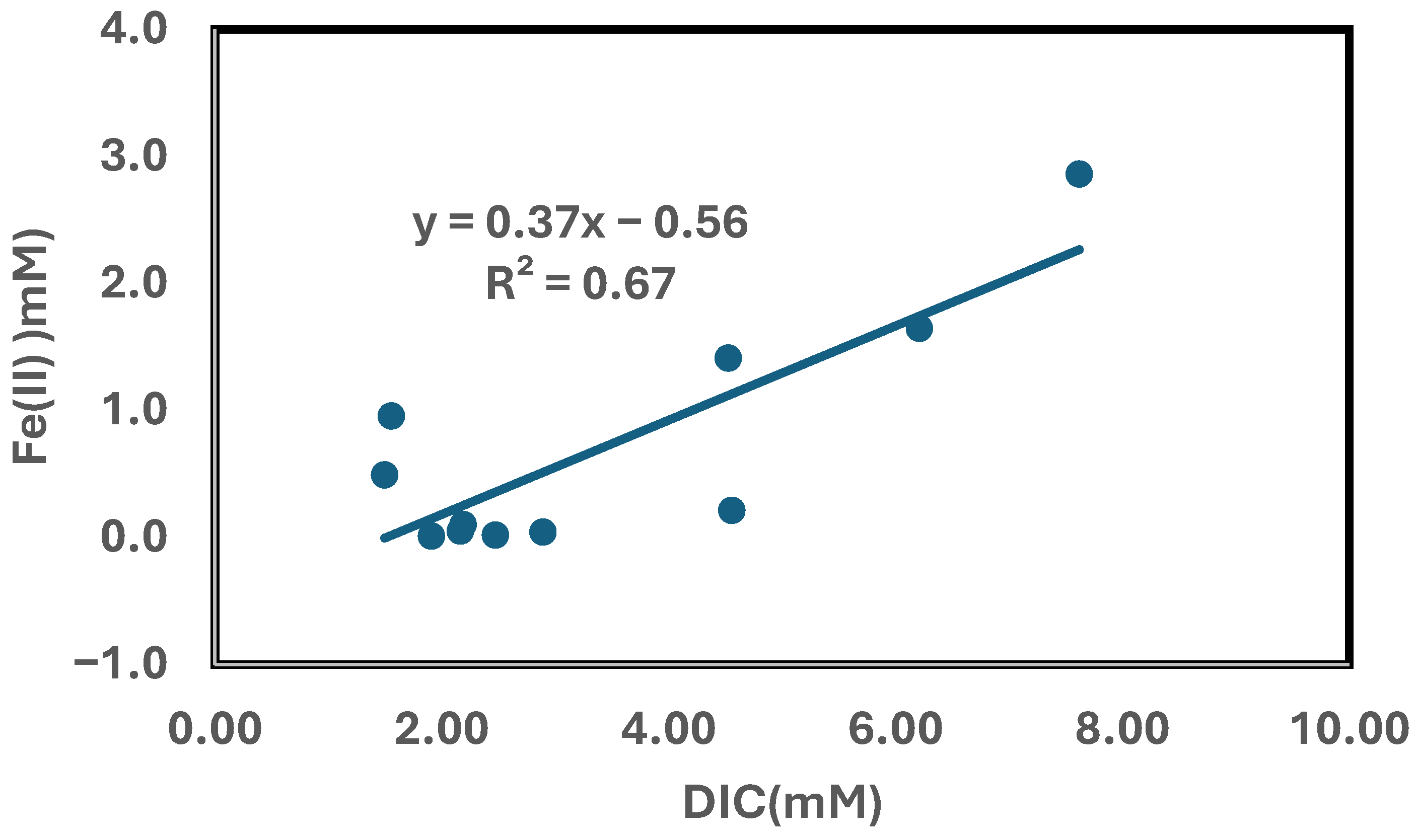
3.7. Chemical Factors Controlling Redox Processes
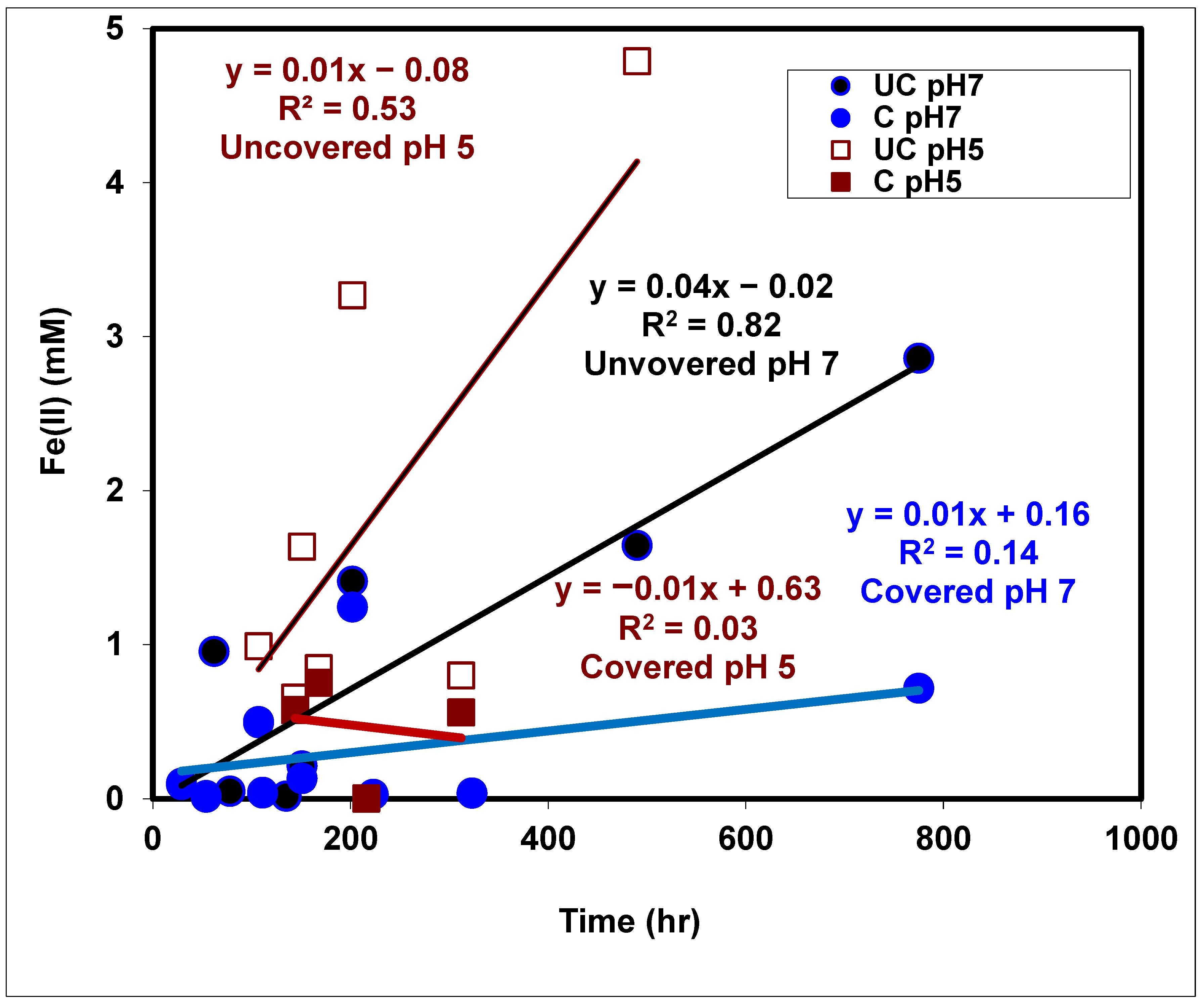
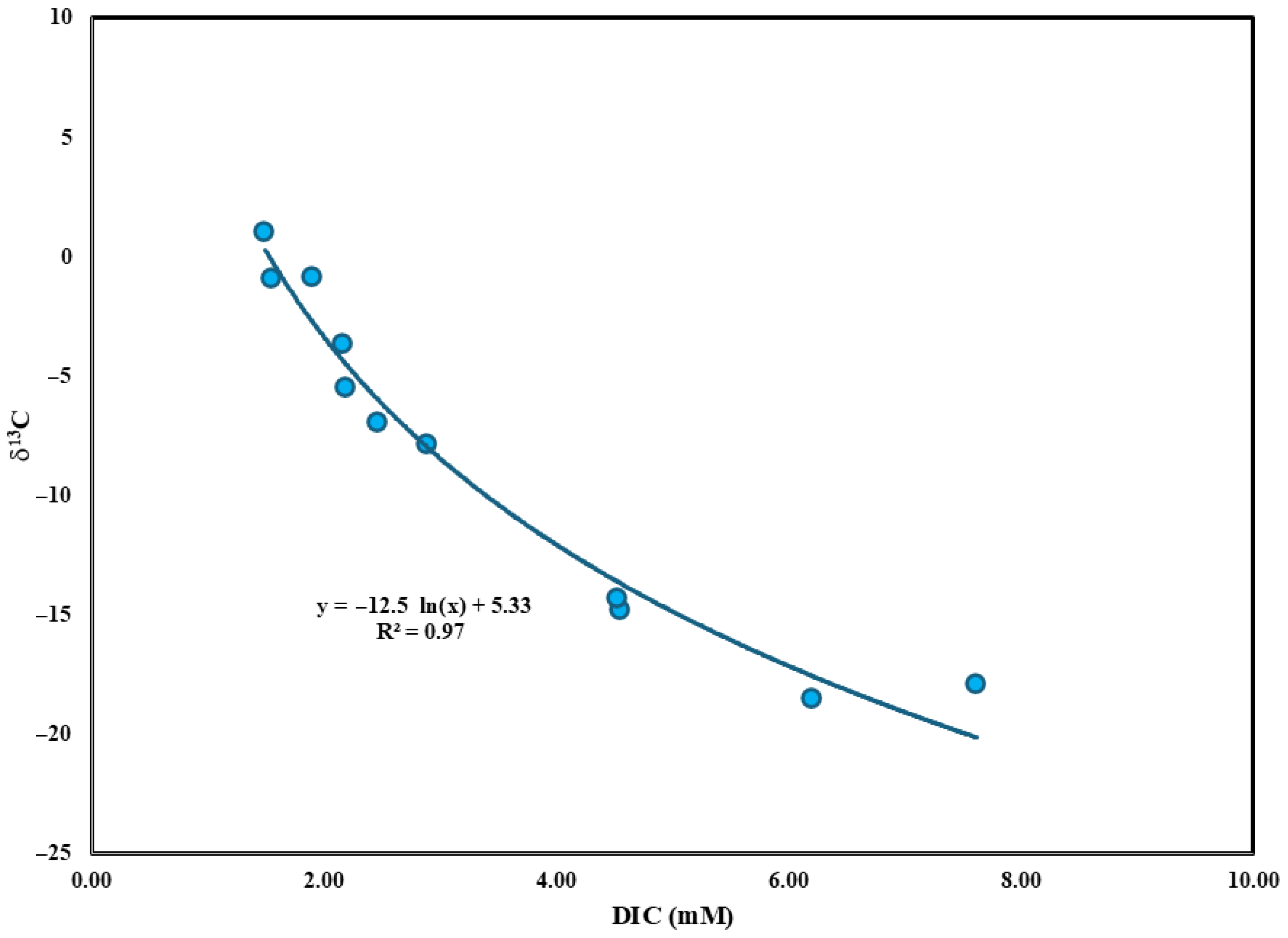
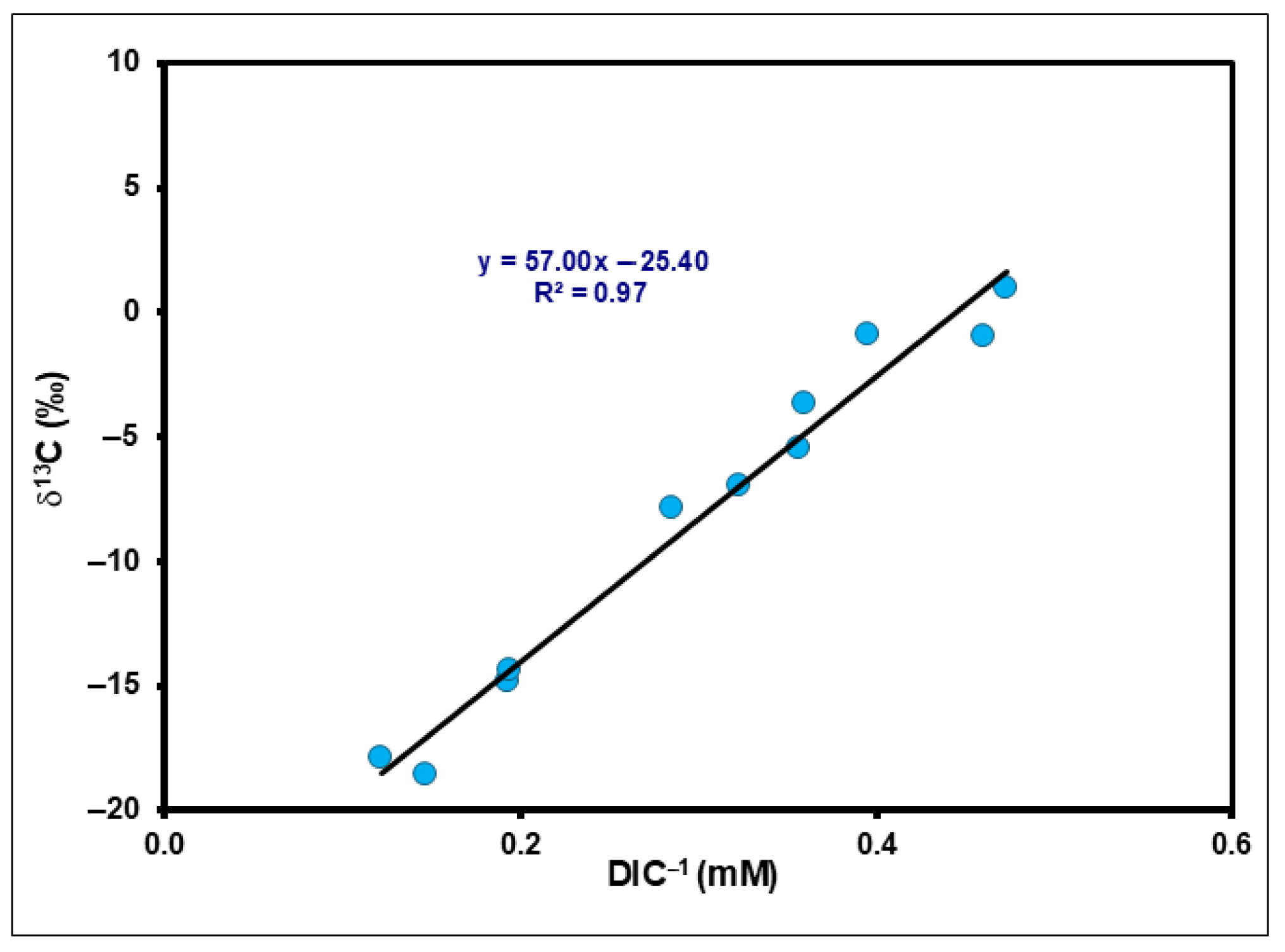
3.8. Isotopic Factors Controlling Redox Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grundl, T.J.; Sparks, D.I. Kinetics and mechanisms of reactions at the mineral–water interface: An overview. In Mineral–Water Interfacial Reactions: Kinetics and Mechanisms; Sparks, D.L., Grundl, T.J., Eds.; American Chemical Society: Washington, DC, USA, 1998; pp. 2–13. [Google Scholar]
- Li, F.B.; Chen, J.J.; Liu, C.S.; Dong, J.; Liu, T. Effect of iron oxides and carboxylic acids on photochemical degradation of bisphenol A. Biol. Fertil. Soils 2006, 42, 409–417. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffamnn, M.R. Environmental photochemistry: Is iron oxide (Hematite) an active photocatalyst? A Comparative study: Fe2O3, ZnO, TiO2. J. Photochem. Photobiol. A Chem. 1989, 48, 161–169. [Google Scholar] [CrossRef]
- Abrahamson, H.B.; Rezvani, A.B.; Brushmiller, J.G. Photochemical and spectroscopic studies of complexes of iron(III) with citric acid and other carboxylic acids. Inorganica Chim. Acta 1994, 226, 117–127. [Google Scholar] [CrossRef]
- Deng, N.S.; Wu, F.; Luo, F.; Xiao, M. Ferric citrate induced photodegradation of dyes in aqueous solutions. Chemosphere 1998, 36, 3101–3112. [Google Scholar] [CrossRef]
- Wu, F.; Deng, N.S. Photochemistry of hydrolytic iron (III) species and photoinduced degradation of organic compounds, A mini-review. Chemosphere 2000, 41, 1137–1147. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Minero, C.; Lucchiari, M.; Vione, D.; Maurino, V. Fe III-Enhanced Sonochemical Degradation of Methylene Blue in Aqueous Solution. Environ. Sci. Technol. 2005, 39, 8936–8942. [Google Scholar] [CrossRef] [PubMed]
- Meeroff, D.E.; Englehardt, J.D.; Echegoyen, L.A.; Shibata, T. Iron-Mediated Aeration: Evaluation of Energy-Assisted Enhancement for In Situ Subsurface Remediation. J. Environ. Eng. 2006, 132, 747–757. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E.; Harrison, M.A.J.; Olariu, R.I.; Arsene, C. Photochemical reactions in the tropospheric aqueous phase and on particulate matter. Chem. Soc. Rev. 2006, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Wu, F.; Deng, N. Quantitation of Hydroxyl Radicals from Photolysis of Fe(III)-Citrate Complexes in Aerobic Water. Environ. Sci. Pollut. Res. 2006, 13, 156–160. [Google Scholar] [CrossRef]
- Howsawkeng, J.; Watts, R.; Washington, D.; Teel, A.; Hess, T.; Crawford, R. Evidence for simultaneous abiotic-biotic oxidations in a microbial-Fenton’s system. Environ. Sci. Technol. 2001, 35, 2961–2966. [Google Scholar] [CrossRef]
- Ndjou’ou, A.C.; Cassidy, D. Surfactant production accompanying the modified Fenton oxidation of hydrocarbons in soil. Chemosphere 2006, 65, 1611–1615. [Google Scholar] [CrossRef]
- Northup, A.; Cassidy, D. Evidence for the production of surfactants accompanying the chemical oxidation of hydrocarbons in soils. J. Adv. Oxid. Technol. 2007, 10, 137–143. [Google Scholar]
- Calza, P.; Maurino, V.; Minero, C.; Pelizzetti, E.; Sega, M.; Vincenti, M. Photoinduced halophenol formation in the presence of iron(III) species or cadmium sulfide. J. Photochem. Photobiol. A Chem. 2005, 170, 61–67. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Calza, P.; Pelizzetti, E. Phenol chlorination and photochlorination in the presence of chloride ions in homogeneous aqueous solution. Environ. Sci. Technol. 2005, 39, 5066–5075. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory, Preparation and Characterization; Wile-VCH: Weinheim, Germany, 2000; p. 188. [Google Scholar]
- Adamson, A.W.; Waltz, W.L.; Zinato, E.; Watts, D.W.; Fleischauer, P.D.; Lindholm, R.D. Photochemistry of transition-metal coordination compounds. Chem. Rev. 1968, 68, 541–543. [Google Scholar] [CrossRef]
- Gebrehiwet, T.A. Laboratory Based Chemical and Isotopic Investigations on Biotic (Microbial) and Abiotic Reduction of Iron and Oxidation of Organic Compounds. Ph.D. Dissertation, Western Michigan University, Kalamazoo, MI, USA, 2007. [Google Scholar]
- Gebrehiwet, T.A.; Krishnamurthy, R.V. Isotopic and chemical investigations of Fe (III) reduction by Shewanella putrefaciens strain 200R. Geobiology 2007, 5, 391–399. [Google Scholar] [CrossRef]
- Haas, J.R.; DiChristina, T.J.; Wade, R., Jr. Thermodynamics of U(VI) sorption onto Shewanella putrefaciens. Chem. Geol. 2001, 180, 33–54. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Krishnamurthy, R.V.; Atekwana, E.A.; Guha, H. A simple, Inexpensive Carbonate-Phosphoric Acid Reaction Method for the Analysis of Carbon and Oxygen Isotopes of Carbonates. Anal. Chem. 1997, 69, 4256–4258. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Krishnamurthy, R.V. Investigations of inorganic carbon cycling of som natural waters using a modified gas evolution technique. J. Hydrol. 1998, 205, 265–278. [Google Scholar] [CrossRef]
- Fischer, M.; Warneck, P. Photodecomposition of nitrite and undissociated nitrous acid in aqueous solution. J. Phys. Chem. 1996, 100, 18749–18756. [Google Scholar] [CrossRef]
- Minero, C.; Bono, F.; Rubertelli, F.; Dvino, D.; Maurino, V.; Pelizzetti, E.; Vione, D. On the effect of pH in aromatic photonitration upon nitrate photolysis. Chemosphere 2007, 66, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.; EAtekwana, A.; Krishnamurthy, R.V. Concentrations and isotopes of dissolved inorganic carbon in denitrifying environments. Geophys. Res. Lett. 1997, 24, 1511–1514. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebrehiwet, T.A.; Krishnamurthy, R.V. Chemical and Isotopic Investigation of Abiotic Oxidation of Lactate Substrate in the Presence of Varied Electron Acceptors and Under Circumneutral Anaerobic Conditions. Water 2025, 17, 2308. https://doi.org/10.3390/w17152308
Gebrehiwet TA, Krishnamurthy RV. Chemical and Isotopic Investigation of Abiotic Oxidation of Lactate Substrate in the Presence of Varied Electron Acceptors and Under Circumneutral Anaerobic Conditions. Water. 2025; 17(15):2308. https://doi.org/10.3390/w17152308
Chicago/Turabian StyleGebrehiwet, Tsigabu A., and R. V. Krishnamurthy. 2025. "Chemical and Isotopic Investigation of Abiotic Oxidation of Lactate Substrate in the Presence of Varied Electron Acceptors and Under Circumneutral Anaerobic Conditions" Water 17, no. 15: 2308. https://doi.org/10.3390/w17152308
APA StyleGebrehiwet, T. A., & Krishnamurthy, R. V. (2025). Chemical and Isotopic Investigation of Abiotic Oxidation of Lactate Substrate in the Presence of Varied Electron Acceptors and Under Circumneutral Anaerobic Conditions. Water, 17(15), 2308. https://doi.org/10.3390/w17152308






