Abstract
Rivers in tropical semi-arid regions face increasing anthropogenic pressures yet remain critically understudied despite their global importance. This study evaluated the aquatic macroinvertebrate community structure in the Ranchería River, Colombia, across three land use conditions: conserved zones (CZs), urban/agricultural zones (UAZs), and mining influence zones (MZs). Ten sampling stations were established, and macroinvertebrate communities were assessed alongside physical, chemical, and hydromorphological variables during the dry season (January–March 2021). A total of 9288 individuals from 84 genera across 16 orders were collected. Generalized Linear Models revealed significant differences among zones for 67 genera (79.8%), indicating strong community responses to land use gradients. Conserved zones exhibited the highest diversity according to the Hill numbers and were dominated by sensitive taxa, including Simulium, Smicridea, and Leptohyphes. Urban/agricultural zones showed the lowest richness (35 genera) and were characterized by disturbance-tolerant species, particularly Melanoides. Mining zones displayed intermediate diversity but exhibited severe habitat alterations. A redundancy analysis with variance partitioning revealed that land use types constituted the primary driver of community structure (a 24.1% pure effect), exceeding the physical and chemical variables (19.5%) and land cover characteristics (19.2%). The integrated model explained 63.5% of the total compositional variation, demonstrating that landscape-scale anthropogenic disturbances exert a greater influence on aquatic communities than local environmental conditions alone. Different anthropogenic activities create distinct environmental filters affecting macroinvertebrate assemblages, emphasizing the importance of land use planning for maintaining aquatic ecosystem integrity in semi-arid watersheds.
1. Introduction
Rivers constitute fundamental ecosystems that support critical ecological functions and provide essential services for both aquatic and terrestrial organisms [1]. However, these systems face increasing pressures from anthropogenic activities that alter environmental conditions and substantially impact functional diversity [2,3,4]. Human interventions including urbanization, intensive agriculture, and extractive industries contribute significantly to biodiversity loss in riverine ecosystems [5,6]. Concurrently, climate change exacerbates these anthropogenic pressures, intensifying ecosystem degradation globally [7].
Aquatic macroinvertebrates are effective bioindicators of environmental quality in riverine systems, accurately reflecting ecosystem health through their sensitivity to physical, chemical, and habitat modifications [8,9]. Community dynamics respond predictably to environmental changes, with alterations in taxonomic diversity, abundance, and species composition serving as early warning indicators of ecosystem degradation [10,11]. In neotropical rivers, macroinvertebrate assemblages have proven valuable for assessing anthropogenic impacts and environmental gradients [12,13,14,15,16]. Studies consistently demonstrate substantial negative effects of land use changes on freshwater biodiversity patterns [17].
The Sierra Nevada de Santa Marta (SNSM), a UNESCO Biosphere Reserve in northern Colombia, exemplifies the complex interactions between exceptional biodiversity and intense anthropogenic pressures in tropical semi-arid systems. This region shows a unique socioecological context where arid conditions intersect with diverse land uses, including conservation, mining, and agriculture. This provides an ideal natural laboratory for understanding ecosystem responses to multiple stressors with direct applicability to similar systems worldwide. Semi-arid ecosystems cover approximately 40% of the Earth′s land surface and are expanding under climate change scenarios. Insights from the SNSM are directly transferable to comparable regions across Mediterranean, African, Australian, and other global contexts. While research in temperate and humid tropical rivers has extensively documented the impacts of land use on aquatic communities [12,13,14,15,16], semi-arid systems remain critically understudied despite their distinct hydrological regimes, heightened vulnerability to disturbances, and growing global significance for conservation and management strategies [18].
The Ranchería River, located in La Guajira, Colombia, within the Sierra Nevada de Santa Marta region, represents a crucial aquatic ecosystem for the region due to its multiple ecosystem services. This watercourse has undergone various transformations that have generated negative impacts on regional biodiversity [19,20]. It has become the primary recipient of waste from socioeconomic activities and human settlements [21] while experiencing progressive deterioration in its ecological condition related to mining operations in the territory [22].
In a study focused on the Ranchería River, Granados-Martínez et al. [23] investigated the aquatic macroinvertebrate diversity along a gradient of human disturbance, from minimally disturbed reference sites to areas near urban centers and open-pit mining. Their results demonstrated environmental (conductivity, pH, temperature, dissolved oxygen) and community structure changes along the anthropogenic gradient, with sensitive taxa like Ephemeroptera and Coleoptera associated with pristine conditions, while tolerant species such as Rhagovelia and the invasive Melanoides dominated disturbed areas. While this research confirmed that human activities significantly influence macroinvertebrate assemblages, said study focused on broad disturbance gradients rather than examining how specific land use practices (mining, agriculture, urbanization) create distinct environmental filters affecting functional community organization.
Our study advances our understanding of the mechanisms through which different land use types affect the structure of macroinvertebrates. Unlike the previous gradient-based approach, our research examines three contrasting land use conditions—conserved zones, coal mining, and urban/agricultural practices—to understand how different anthropogenic activities selectively filter species. We aim to enhance the understanding of the factors determining the distribution patterns and taxonomic composition of benthic fauna in tropical fluvial systems subjected to varied anthropogenic pressures.
2. Materials and Methods
2.1. The Study Area
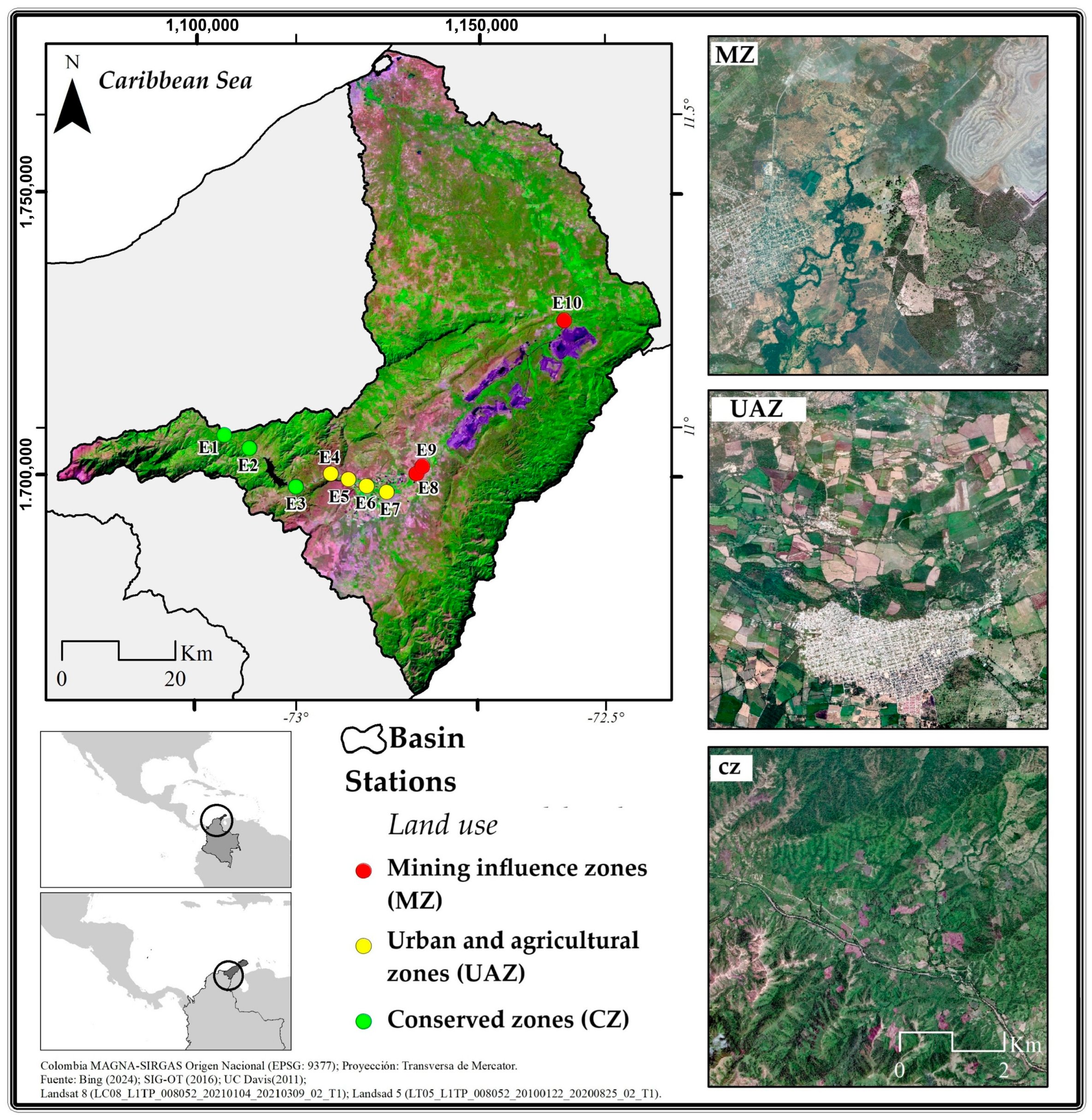
The Ranchera River basin (Figure 1) is located in the northernmost part of Colombia (11°33′ N, 72°54′ W), specifically in the Department of La Guajira. The river originates in the Chirigua páramo on the eastern slope of the Sierra Nevada de Santa Marta (SNSM) mountain range at an elevation of 3875 m above sea level (m.a.s.l.). The river basin has an area of 4070 km2 and extends for approximately 248 km until it flows into the Caribbean Sea near the city of Riohacha [24,25]. The region is considered the most arid zone in Colombia, with an annual precipitation regime ranging between 501 and 1000 mm, presenting a decreasing rainfall gradient from the headwaters toward the north [24].
Figure 1.
Sampling points across different land use areas in 10 stations of the Ranchería River, 2021.
A total of 10 sampling stations were classified into three categories according to the predominant land use: conserved zones (CZs; n = 3), urban and agricultural zones (UAZs; n = 4), and mining influence zones (MZs; n = 3) (Figure 1). The selection of the sampling sites followed a stratified random design: (1) first, the river was divided into three segments based on the predominant land use through a GIS analysis of 2021 Landsat imagery; (2) within each segment, potential sampling points were identified at approximately equal intervals (every 8–10 km); and (3) the final selection considered accessibility and the representation of the land use category. Site independence was ensured by maintaining a minimum distance of 2 km between consecutive sampling points, exceeding the average width of the river (15–60 m) by more than 30 times, thus minimizing potential spatial autocorrelation [25].
Land cover characterization was conducted by analyzing an area of influence of 3 km around each sampling point, following the established methodologies for tropical fluvial systems. This buffer distance was selected based on previous studies [26,27,28,29] that have demonstrated its effectiveness for capturing the primary anthropogenic influences on river ecosystems while minimizing local point source effects. The land use classification was performed using ArcGIS 10.8 with a supervised classification algorithm (maximum likelihood) applied to 2021 Landsat 8 OLI imagery (30 m resolution), validated using 50 ground control points (overall accuracy = 89%). The delimitation of the 3 km influence area was based on studies that have demonstrated the effectiveness of this spatial scale for evaluating the impacts of land use on fluvial systems. Allan [26] and Leal et al. [27] analyzed the land cover within a 2–5 km radius and effectively identified patterns that influenced aquatic ecosystems. This scale has been validated by Sponseller et al. [28] and Jimenez-Valencia et al. [29].
2.2. Field Methods
A 100 m reach was selected at each sampling station, where five replicates were collected using a Surber net (area: 0.09 m2; mesh size: 250 μm), following the methodology of Char [30]. Three sampling campaigns were conducted monthly during the dry season (January–March 2021). The samples integrated all substrate types present in each reach. Collected specimens were preserved in 96% ethanol for subsequent dietary analysis and were identified to the most specific taxonomic level possible using stereoscopic microscopy and specialized taxonomic keys [31,32,33,34]. Physical and chemical variables were measured in situ during each sampling campaign (three times per site) using a calibrated Multi 350i WTW multiparameter probe, which recorded the temperature (°C), pH, electrical conductivity (μS/cm), total dissolved solids (mg/L), and dissolved oxygen (mg/L). Additionally, water samples were collected in sterilized polyethylene bottles for laboratory analysis, following the standardized APHA protocols [35]. Nutrients, including nitrites (NO2), nitrates (NO3), phosphates (PO4), and chlorides (Cl), were analyzed, as was the total hardness and alkalinity (expressed as CaCO3 mg/L). The microbiological analyses included total and fecal coliforms (MPN/100 mL). Hydromorphological characterization was conducted through transects every 20 m along the 100 m reach (5 transects per site), where the bankfull width, depth (measured at 3 points per transect), and discharge were measured using the float method [36]. River habitat characteristics were evaluated by quantifying the flow types (riffles, slow currents, and pools), dominant substrates (rocks, sand), and the presence of macrophytes. Substrate composition was quantified using a modified Wentworth scale with 10 categories (bedrock, boulder, cobble, pebble, gravel, sand, silt, clay, organic matter, and macrophytes), with 100 particles randomly selected and measured along each transect. Riparian zone characterization included estimating the cover percentage of forest, shrubs, grasses, crops, urban areas, livestock, and mining activities in a 3 km buffer on each side of the channel using ArcGIS 10.8 software and Landsat satellite imagery from 2021.
2.3. Statistical Analysis
Statistical analyses were conducted to evaluate the differences in the physical, chemical, hydromorphological, and biological variables across land use zones (conserved zones—CZs; urban/agricultural zones—UAZs; mining zones—MZs). Generalized Linear Models (GLMs) were employed to account for non-normal data distributions, with zone as a fixed factor [37]. The model selection was based on the Akaike Information Criterion (AIC) values, using Poisson or negative binomial distributions for count data and Gaussian distributions for continuous variables. Post hoc pairwise comparisons were performed using Tukey′s Honest Significant Difference (HSD) tests [38].
The diversity profiles were analyzed using Hill numbers (q = 0, 1, 2) and sample-size-based rarefaction and extrapolation curves [39]. The sampling completeness was assessed through sample coverage. The 95% confidence intervals were obtained through bootstrapping with 1000 replications [40].
The relationships between environmental variables and macroinvertebrate assemblages were examined through a Redundancy Analysis (RDA) [41,42] with variance partitioning. The RDA was selected over a Canonical Correspondence Analysis because the gradient length in the preliminary Detrended Canonical Correspondence Analysis was <3 SD units (0.59 SD), indicating linear species–environment relationships. Environmental variables were organized into three ecologically meaningful groups: (1) physical and chemical variables (pH and temperature); (2) land cover variables (bare soil, crops, riparian grass, riparian forest, riparian shrubs, and urban areas); and (3) land use types (conserved zones, mining zones, and urban/agricultural zones).
Prior to the analysis, the collinearity was assessed using Variance Inflation Factors (VIFs > 10), and stepwise selection (using the ordistep() function) identified the most parsimonious variable set based on the AIC. Variance partitioning (using the varpart() function) decomposed the total explained variance into the pure effects of each variable group, shared effects among groups, and unexplained variance. The model significance and pure effects of each group were evaluated through permutation tests (999 permutations), with the performance assessed using adjusted R2 values [42].
All of the statistical analyses were performed in R software version 4.3.3 [43] using the following packages: vegan [44] for the RDA and VIF calculations; ade4 [45] for the multivariate analyses; iNEXT [46] and iNEXT.4 steps [47] for the diversity analysis and rarefaction curves; ggplot2 [48] for data visualization; ggrepel [49] for plot labeling; and dplyr [50] for data manipulation.
3. Results
3.1. Physical and Chemical Parameters
The GLM analysis showed significant spatial differentiation in the basic water chemistry parameters across the land use zones. The mineral content indicators (TDS, conductivity, and total hardness) showed consistently elevated levels in conserved zones compared to those in urban/agricultural areas (p ≤ 0.04), with mining zones displaying intermediate values. The analysis identified no significant cross-zone variations in the temperature regimes, dissolved oxygen concentrations, or pH levels (all p > 0.35). The nutrient parameters (nitrites, nitrates, phosphates) and microbiological indicators (total and fecal coliforms) similarly showed no statistically significant spatial differences despite numerical variations among zones (all p > 0.15) (Table 1)

Table 1.
Physical, chemical, and microbiological parameters (mean ± standard deviation) in different land use zones of the Ranchería River. CZ: Conserved Zone; UAZ: Urban and Agricultural Zone; MZ: Mining Influence Zone. TCs: Total Coliforms; FCs: Fecal Coliforms. Differences among zones were analyzed using Generalized Linear Models (GLMs), followed by Tukey’s HSD post hoc test. Different letters (a, b) indicate significant differences (* p < 0.05).
3.2. Hydromorphological Variables
The GLM analysis showed significant effects of land use on both the riparian vegetation structure and the channel’s morphological characteristics. Riparian vegetation exhibited a clear gradient across the land use zones, with the forest cover decreasing progressively from conserved to urban to mining areas (p = 0.027), while anthropogenic vegetation types including crops, urban areas, and livestock pastures dominated the urban/agricultural zones (p = 0.027 for all categories) (Table 2).

Table 2.
Hydromorphological variables (mean ± standard deviation) in different land use zones of the Ranchería River. Differences were analyzed using Generalized Linear Models (GLMs) with Tukey’s HSD post hoc test. Different letters (a, b, c) indicate significant differences (* p < 0.05).
The channel morphology demonstrated distinct zone-specific patterns. Conserved zones were characterized by riffle-dominated flow regimes (76.7% versus 13.3% in mining zones; p = 0.027) and predominantly a rocky substrate composition (83.3% versus 8.3% in mining zones; p = 0.027). Conversely, mining zones exhibited a prevalence of slow-flowing currents (83.3%) and pool formations (76.7%; both p < 0.03). Urban/agricultural zones displayed intermediate morphological characteristics but were distinguished by extensive macrophyte colonization (71.3%; p = 0.026).
In contrast, the riparian corridor width, bare soil coverage, and overall substrate composition showed no significant variations across the land use zones (all p > 0.05), suggesting that these parameters are less sensitive to anthropogenic modifications in this watershed system.
3.3. Macroinvertebrates
A total of 9288 individuals (UAZs = 3342, MZs = 2887, and CZs = 3059) were collected, distributed across 16 orders, 49 families, and 84 genera (Appendix A). Among the orders, Ephemeroptera, Trichoptera, Coleoptera, and Diptera were the most diverse in terms of families and genera. The Generalized Linear Models revealed significant differences among zones for 67 genera (79.8%), indicating strong responses of the macroinvertebrate communities to the land use gradients. The urban and agricultural zones (UAZs) and mining influence zones (MZs) were characterized by a high abundance of disturbance-tolerant taxa, particularly Melanoides (131.82 ± 159.91 and 138.33 ± 150.36 ind/m2, respectively) and Pyrgophorus (7.80 ± 9.59 and 6.67 ± 3.19 ind/m2), both of which were absent from the conserved zones (CZs). Conversely, conserved zones exhibited a significantly higher abundance of sensitive taxa, including Simulium (100.38 ± 92.94 ind/m2), Smicridea (63.67 ± 73.94 ind/m2), Leptohyphes (51.38 ± 51.15 ind/m2), and Anacroneuria (25.20 ± 44.21 ind/m2). Several genera showed zone-specific distributions: Lachlania (25.83 ± 44.13 ind/m2), Atopsyche (1.50 ± 0.71 ind/m2), and multiple Odonata genera, Phyllogomphoides, Progomphus, and Limnogonus, were exclusive to conserved zones, while pollution indicator taxa such as Caenis, Acanthagrion, and Chironomus were found only in urban/agricultural areas. Mining zones showed distinctive patterns compared to other land use categories, with significantly higher abundance of genera of the Elmidae family (Cylloepus: 39.00 ± 50.91 ind/m2; Stenhelmoides: 23.00 ± 0.00 ind/m2) and the exclusive occurrence of taxa like Atya (11.50 ± 4.95 ind/m2) and several Gastropoda genera (Marisa, Ancylidae, Physa) in this zone typeThe EPT index components showed contrasting responses: while Ephemeroptera and Trichoptera were generally more abundant in conserved areas, Plecoptera (Anacroneuria) showed the strongest conservation signal (p = 0.038). Taxa exhibiting no significant differences across zones included generalist species such as Tricorythodes (p = 0.690), Austrolimnius (p = 0.364), and Thraulodes (p = 0.456), suggesting their broad environmental tolerance.
3.4. Diversity Profiles
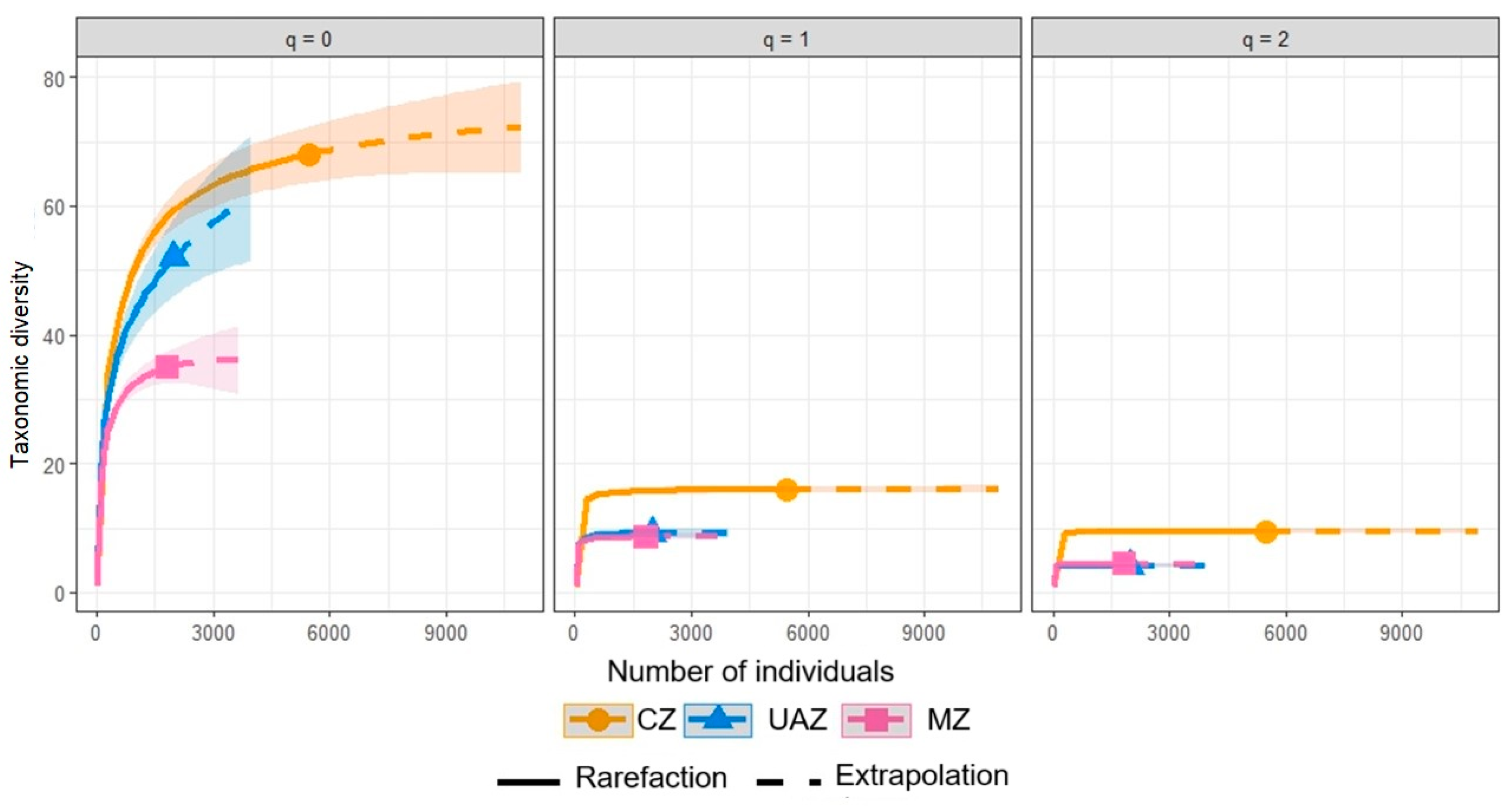
The diversity analyses using Hill numbers (q = 0, 1, 2) revealed consistent patterns among different land uses. Conserved zones (CZs) showed the highest species richness (68 observed, 74.12 ± 11.39 estimated) and effective number of species for Shannon diversity (Shannon: 16.07 ± 0.30; Simpson: 9.53 ± 0.17), with a sampling completeness of 92%. Mining influence zones (MZs) presented intermediate values for richness (52 observed, 68.89 ± 15.57 estimated) and effective number of species for Shannon diversity (9.35 ± 0.33) but with the lowest sampling completeness (75%), suggesting the potential to discover more species. Urban and agricultural zones (UAZs) had the lowest species richness (35 observed, 36.12 ± 5.92 estimated) but the highest sampling completeness (97%) and Simpson values (4.38 ± 0.16), slightly higher than those for MZs (4.14 ± 0.17), indicating a less diverse but more equitable and completely sampled community. The sample coverage curves (Figure 2) illustrate these patterns for orders q = 0, 1, and 2, where q = 0 represents species richness. These curves confirm that CZs maintain the highest diversity in all orders, with the potential for additional species, while UAZs reach an asymptote more quickly, consistent with the high sampling completeness.
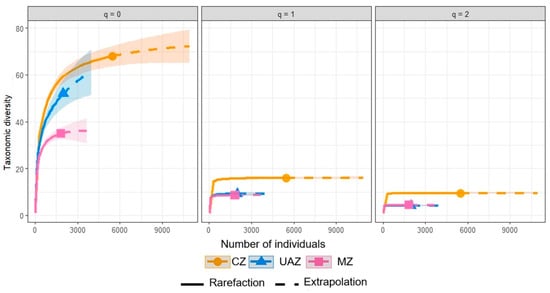
Figure 2.
The rarefaction and extrapolation curves for the three orders of diversity (q = 0, 1, 2) by land use.
3.5. The Relationship Between Environmental Variables, Land Use, and Macroinvertebrates
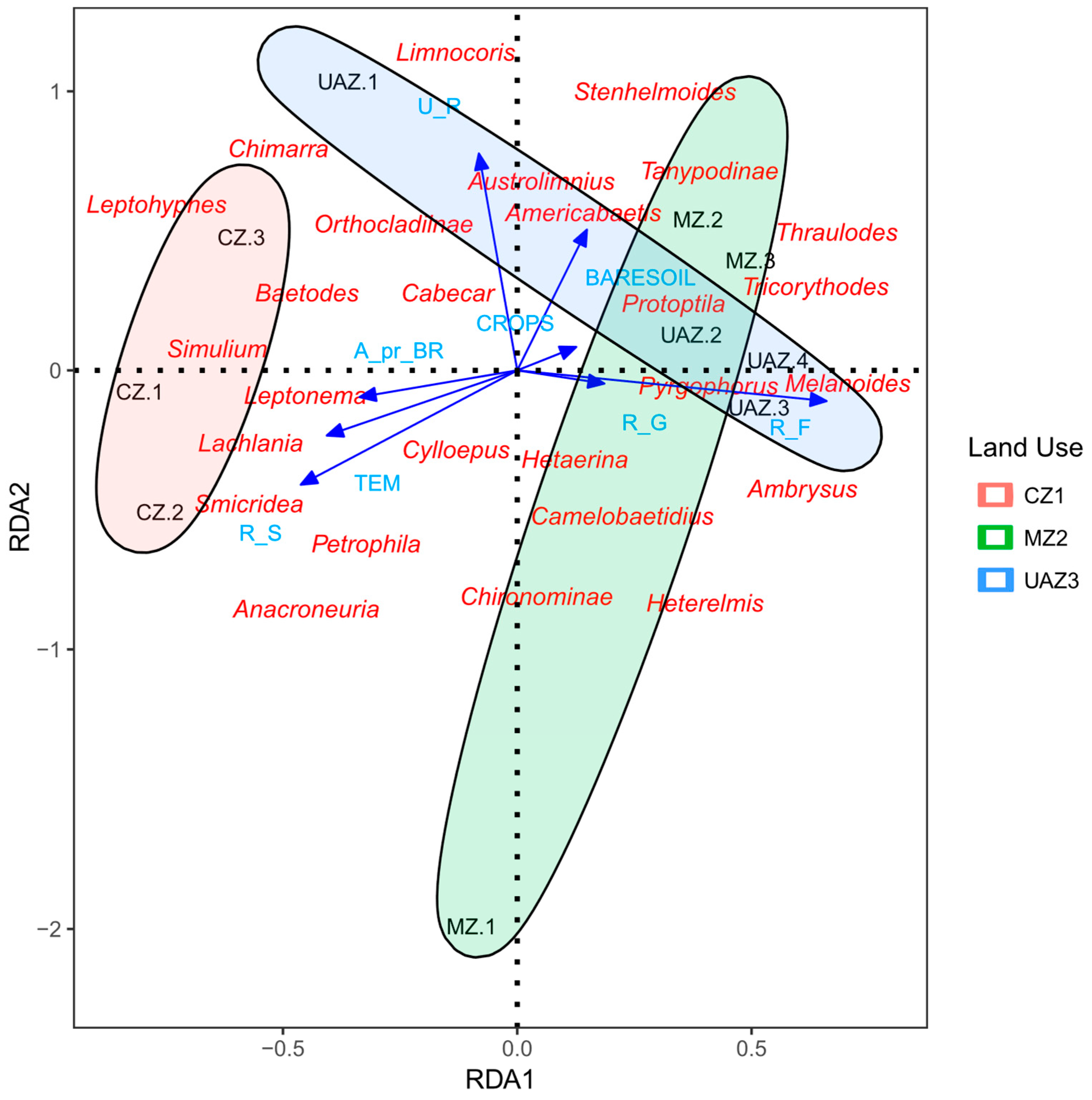
The Redundancy Analysis (RDA) showed significant relationships between environmental variables, land use types, and macroinvertebrate community composition in the Ranchería River basin (Figure 3). Following stepwise variable selection to address multicollinearity issues (a VIF < 10), the parsimonious model included six key environmental variables—bare soil coverage (BARESOIL), agricultural crops (CROPS), riparian grass (R_G), riparian forest (R_F), riparian shrubs (R_S), and urban areas (U_R)—along with the physical and chemical parameters of pH and temperature (TEM). The integrated RDA model proved statistically significant (p < 0.05), explaining 63.5% of the total variation in macroinvertebrate community composition (adjusted R2 = 0.635).
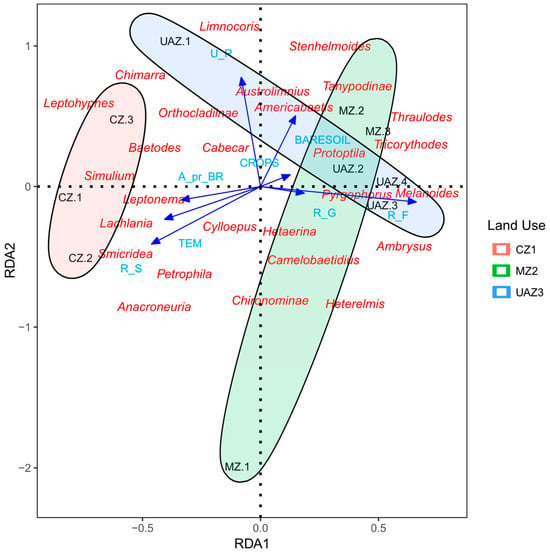
Figure 3.
The redundancy analysis (RDA) between environmental variables, land use, and macroinvertebrates in the Ranchería River basin, La Guajira, Colombia, 2021. Land use zones: CZ (conserved zone), MZ (mining influence zone), UAZ (urban and agricultural zone). Environmental variables: TEM (temperature), R_S (riparian shrubs), R_G (riparian grasses), R_F (riparian forest), BARESOIL (bare soil). Blue triangles indicate sampling sites, red text shows macroinvertebrate taxa, blue text shows environmental variables, and ellipses represent 95% confidence intervals.
The variance partitioning analysis revealed that land use types constituted the primary driver of community structure, explaining 24.1% of the pure variance in species composition, exceeding the individual contributions of the physical and chemical variables (19.5%, p = 0.089) and the land cover characteristics (19.2%, p > 0.1). Shared effects among variable groups accounted for an additional 8.4% of the explained variance, while 67.4% remained unexplained, likely reflecting unmeasured local habitat factors and stochastic processes. The first canonical axis (RDA1) captured 46.2% of the explained variance, representing a primary gradient of the intensity of anthropogenic disturbance, while the second axis (RDA2) explained an additional 14.6%, reflecting the habitat heterogeneity and riparian conditions.
As shown in Figure 3, conserved zones (CZs) clustered primarily in the negative quadrants of the ordination space, characterized by an association with sensitive genera such as Leptohyphes, Anacroneuria, and Simulium. These sites presented lower pH values (8.17 ± 0.21) and moderate temperatures (27.17 ± 1.65 °C), with higher riparian forest coverage. In contrast, urban and agricultural zones (UAZs) were positioned in the positive quadrant of RDA1, showing a strong association with pollution-tolerant genera, including Melanoides and Pyrgophorus. These disturbed sites were characterized by higher pH values (8.30 ± 0.15), increased bare soil coverage, and agricultural land use intensity.
Mining zones (MZs) displayed intermediate positioning along the RDA ordination, distributed primarily along the negative RDA2 axis (Figure 3). These sites presented intermediate pH levels (8.43 ± 0.40) and variable temperatures (24.83 ± 2.38 °C), supporting macroinvertebrate assemblages with moderate disturbance characteristics. Genera such as Americabaetis, Tricorythodes, and Heterelmis showed a particular association with mining-influenced areas, suggesting intermediate tolerance to anthropogenic perturbation.
The environmental variable vectors in Figure 3 demonstrate that pH and bare soil coverage were the strongest drivers along RDA1 (coefficients = −21.80 and 6.18, respectively), while temperature variation influenced the secondary gradient represented by RDA2 (coefficient = −135.06). Riparian forest cover (R_F) and shrub coverage (R_S) showed contrasting responses, with forest coverage being negatively associated with the disturbance intensity (coefficient = 3.23), while shrub dominance correlated with intermediate disturbance levels (coefficient = −8.36).
4. Discussion
This comparative analysis of the Ranchería River revealed pronounced differences in both the water quality and macroinvertebrate communities among the three land use zones, demonstrating a clear environmental degradation gradient associated with anthropogenic activities [26]. Conserved zones consistently exhibited superior ecological conditions, characterized by high macroinvertebrate diversity, the dominance of sensitive taxa, and the maintenance of natural hydrochemical processes within structurally complex habitats [51]. Urban and agricultural zones represented the opposite extreme, showing a severely compromised ecosystem integrity with markedly reduced biodiversity, the proliferation of disturbance-tolerant species, and clear chemical signatures of human contamination, alongside extensive habitat homogenization [52]. Mining influence zones displayed an intermediate position in terms of their biological diversity but demonstrated the most severe physical habitat alterations, combining moderate species richness with dramatic geomorphological changes and distinctive hydrochemical conditions [53]. These zone-specific patterns reflect how different types of anthropogenic pressures generate distinct environmental filters that selectively favor different biological communities, creating a mosaic of ecosystem conditions across the watershed [54]. The magnitude of these differences underscores the critical importance of land use planning in maintaining aquatic ecosystem integrity in tropical semi-arid regions.
The water quality assessment revealed that only the mineral content parameters showed significant spatial differentiation among the land use zones, while the conventional pollution indicators remained statistically invariant across the watershed. The significantly elevated levels of total dissolved solids, conductivity, and total hardness in the conserved zones (p ≤ 0.04) compared to those in urban/agricultural areas likely reflect natural hydrogeochemical processes rather than anthropogenic contamination. These patterns can be attributed to longer water residence times and enhanced mineral dissolution in undisturbed geological settings, where natural weathering processes proceed without anthropogenic interference [55]. The intermediate values observed in mining zones suggest that while mining activities may alter some geochemical processes, they do not necessarily increase the overall mineralization beyond natural levels.
Notably, traditional water quality indicators including pH, dissolved oxygen, nutrients (nitrites, nitrates, phosphates), and microbiological parameters (total and fecal coliforms) showed no significant differences among zones (all p > 0.15), despite numerical variations. This absence of significant contamination gradients across land use types is particularly noteworthy in a watershed experiencing diverse anthropogenic pressures. The lack of pH variations (p = 0.36) suggests that the region′s predominant limestone geology provides a consistent buffering capacity across all zones, effectively neutralizing potential acidification from both agricultural inputs and mining activities [56,57]. Similarly, the uniform dissolved oxygen levels (p = 0.49) and the absence of significant nutrient enrichment patterns indicate that conventional water quality parameters may be less sensitive indicators of the impacts of land use in this semi-arid system compared to biological and habitat structure variables.
The uniform microbiological conditions across zones (p = 0.68) suggest that coliform bacteria sources are more influenced by regional climatic and hydrological factors than by specific land use activities. This finding contrasts with the expectations from temperate regions, where agricultural and urban areas typically show elevated bacterial contamination, but aligns with semi-arid system dynamics where episodic precipitation events and high solar radiation may standardize microbial communities across the watershed [58,59]. These results highlight the importance of using multiple indicator types when assessing the ecosystem integrity in tropical semi-arid regions, as traditional water quality metrics may not capture the full spectrum of anthropogenic impacts.
The distinct macroinvertebrate community patterns observed among zones reflect species-specific responses to environmental filtering processes, with the variance partitioning analysis revealing that land use types constitute the primary driver of community structure (24.1% pure effect), exceeding the individual contributions of physical and chemical variables (19.5%) and land cover characteristics (19.2%). The integrated model explained 63.5% of the total compositional variation, demonstrating that landscape-scale anthropogenic disturbances exert a greater influence on aquatic communities than local environmental conditions alone [60]. The dominance of sensitive taxa such as Simulium and Smicridea in the conserved zones can be attributed to their requirements for high water quality, heterogeneous rocky substrates, and well-oxygenated conditions, which are maintained in structurally complex habitats with intact riparian vegetation [61,62]. The exclusive presence of indicator genera like Lachlania and Atopsyche in these zones further reflects their narrow ecological tolerance and dependence on specific microhabitat conditions that are disrupted by anthropogenic activities [63]. Conversely, the overwhelming dominance of Melanoides in disturbed zones represents a classic ecological response to environmental stress, as this invasive gastropod possesses broad environmental tolerance and thrives in organically enriched, chemically altered waters with a simplified habitat structure [64,65]. The intermediate diversity patterns in mining zones likely result from the complex interplay between moderate chemical alterations and severe physical habitat modifications, creating environmental conditions that exclude the most sensitive species while still supporting some moderately tolerant taxa [66]. The strong association between community structure and the hydrogeomorphological variables reflects how habitat heterogeneity serves as a template for species assembly, with flow regime alterations, substrate modifications, and riparian vegetation loss acting as the primary drivers of community simplification [67,68]. These biological responses demonstrate how macroinvertebrate communities function as integrated indicators of ecosystem health, reflecting the cumulative effects of multiple anthropogenic stressors on aquatic habitat quality [60,62]. Notably, these patterns show remarkable consistency with those from previous assessments conducted in the same watershed in 2010, indicating the persistence of disturbance–diversity relationships over time and confirming the stability of these ecological responses to anthropogenic pressure gradients in the Ranchería River system [23].
5. Conclusions
This study demonstrates that different land uses significantly influence the ecological integrity of the Ranchería River in the semi-arid region of La Guajira, Colombia. Our integrated approach revealed that biological and habitat structure indicators are more sensitive to anthropogenic impacts than conventional water quality parameters are in tropical semi-arid systems.
- The physical and chemical parameters showed limited sensitivity to land use impacts. Only the mineral content indicators (TDS, conductivity, total hardness) exhibited significant differences among zones (p ≤ 0.04), while pH, dissolved oxygen, nutrients, and the microbiological parameters showed no significant variations (all p > 0.15) despite diverse anthropogenic pressures.
- The hydromorphological characteristics were significantly altered by land use. Conserved zones maintained their natural features (riparian forest: 100%; riffles: 76.67%; rocky substrates: 83.33%), while urban/agricultural zones showed extensive transformation (crops: 75%; slow currents: 65%; macrophytes: 71.3%), and mining zones exhibited the most severe alterations (pools: 76.67%; reduced riparian forest: 31.67%).
- Macroinvertebrate communities responded strongly to the land use gradients. Of 84 genera collected (9288 individuals), 67 genera (79.8%) showed significant differences among zones. Conserved zones supported the highest diversity (68 species, Shannon index: 16.07), with sensitive taxa (Simulium, Smicridea), while the disturbed zones were dominated by tolerant species (Melanoides, Pyrgophorus).
- Land use was the primary driver of community structure. The multivariate analysis explained 63.5% of the variation in the macroinvertebrate community composition, with the land use types accounting for 24.1% of the pure variance, exceeding the contributions of the physical and chemical (19.5%) and land cover (19.2%) characteristics.
For watershed management, we recommend (1) the protection and restoration of riparian forest; (2) the implementation of buffer zones; (3) the prioritization of biological monitoring over traditional water quality assessments; and (4) land use policies that consider the vulnerability of fluvial ecosystems in semi-arid regions.
This research demonstrates that biological indicators provide more robust ecosystem health assessments than conventional physical and chemical parameters in tropical semi-arid rivers, emphasizing the need for integrated ecological monitoring approaches in similar regions worldwide.
Author Contributions
Conceptualization: C.G.-M., M.G.-M., E.L.-L. and J.R.R.; methodology: C.G.-M., M.G.-M., E.L.-L. and J.R.R.; software: C.G.-M.; validation: C.G.-M., M.G.-M., E.L.-L. and J.R.R.; formal analysis: C.G.-M.; investigation: C.G.-M.; resources: M.G.-M., E.L.-L. and J.R.R.; data curation: C.G.-M.; writing—original draft preparation: C.G.-M.; writing—review and editing: M.G.-M., E.L.-L. and J.R.R.; visualization: C.G.-M.; supervision: M.G.-M., E.L.-L. and J.R.R.; project administration: C.G.-M.; funding acquisition: M.G.-M., E.L.-L. and J.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This research received no external funding. The APC was funded by the Instituto Politécnico Nacional through the Secretaría de Investigación y Posgrado (SIP).
Data Availability Statement
The data presented in this study can be made available on request by the corresponding author.
Acknowledgments
We thank the Universidad de La Guajira (Colombia) for the logistical support through the Faculty of Basic and Applied Sciences, the Biology program, and the Research Center. We also thank the Instituto Politécnico Nacional and the SIP (Secretaría de Investigación y Posgrado) for funding the publication of this research. During the preparation of this manuscript, the authors used Claude Sonnet (Anthropic, version available on May 20, 2024) as an assistive tool for reference identification and formatting. All references were manually reviewed, verified, and selected by the authors, who take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Abundance (ind/m2) of aquatic macroinvertebrates in different land use zones of the Ranchería River.
Table A1.
Abundance (ind/m2) of aquatic macroinvertebrates in different land use zones of the Ranchería River.
| Order | Family | Genus | CZ | UAZ | MZ | p-Value |
|---|---|---|---|---|---|---|
| Neotaenioglossa | Thiaridae | Melanoides | 0.0 ± 0.0 b | 131.82 ± 159.91 a | 138.33 ± 150.36 a | <0.001 |
| Hydrobiidae | Pyrgophorus | 0.0 ± 0.0 b | 7.80 ± 9.59 a | 6.67 ± 3.19 a | 0.003 | |
| Architaenioglossa | Ampullariidae | Pomacea | 0.0 ± 0.0 c | 1.25 ± 0.58 a | 10.00 ± 5.66 b | <0.001 |
| Basommatophora | Planorbidae | Marisa | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 2.50 ± 0.71 b | 0.012 |
| Gyraulus | 2.67 ± 0.58 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.001 | ||
| Ancylidae | Ancylidae | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 5.00 ± 0.00 b | 0.001 | |
| Physidae | Physa | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.032 | |
| Sphaeriida | Sphaeriidae | Pisidium | 2.00 ± 0.00 a | 2.33 ± 0.58 a | 13.00 ± 0.00 b | 0.023 |
| Ephemeroptera | Baetidae | Americabaetis | 4.25 ± 0.84 a | 4.30 ± 2.50 a | 4.86 ± 3.31 a | 0.872 |
| Camelobaetidius | 3.00 ± 1.00 a | 4.80 ± 2.77 a | 5.43 ± 7.94 a | 0.752 | ||
| Baetodes | 20.25 ± 8.62 a | 14.00 ± 0.00 a | 1.00 ± 0.00 b | 0.031 | ||
| Leptohyphidae | Tricorythodes | 22.33 ± 16.76 a | 25.15 ± 18.71 a | 31.67 ± 34.19 a | 0.690 | |
| Leptohyphes | 51.38 ± 51.15 b | 13.30 ± 13.62 a | 13.50 ± 12.45 a | 0.019 | ||
| Cabecar | 9.00 ± 6.00 c | 0.0 ± 0.0 a | 20.00 ± 0.00 b | 0.041 | ||
| Leptophlebiidae | Thraulodes | 16.00 ± 0.00 a | 9.50 ± 5.68 a | 20.00 ± 24.14 a | 0.456 | |
| Farrodes | 6.00 ± 2.83 a | 7.00 ± 0.00 a | 0.0 ± 0.0 b | 0.023 | ||
| Oligoneuriidae | Lachlania | 25.83 ± 44.13 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.002 | |
| Caenidae | Caenis | 0.0 ± 0.0 b | 3.00 ± 0.00 a | 0.0 ± 0.0 b | 0.001 | |
| Odonata | Coenagrionidae | Argia | 3.67 ± 1.15 b | 1.60 ± 1.41 a | 1.00 ± 0.00 a | 0.030 |
| Ischnura | 0.0 ± 0.0 b | 6.50 ± 7.78 a | 3.00 ± 0.00 a | 0.048 | ||
| Acanthagrion | 0.0 ± 0.0 b | 2.00 ± 0.00 a | 0.0 ± 0.0 b | 0.001 | ||
| Libellulidae | Brechmorhoga | 4.00 ± 0.00 a | 6.33 ± 5.03 a | 0.0 ± 0.0 b | 0.034 | |
| Dythemis | 3.67 ± 1.30 c | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.039 | ||
| Elasmothemis | 0.0 ± 0.0 c | 6.00 ± 0.00 a | 2.00 ± 0.00 b | 0.041 | ||
| Macrodiplax | 0.0 ± 0.0 b | 1.00 ± 0.00 a | 0.0 ± 0.0 b | 0.032 | ||
| Macrothemis | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.032 | ||
| Calopterygidae | Hetaerina | 3.00 ± 1.00 ab | 6.67 ± 5.13 a | 1.00 ± 0.00 b | 0.045 | |
| Gomphidae | Phyllogomphoides | 1.00 ± 0.00 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.032 | |
| Progomphus | 1.00 ± 0.00 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.032 | ||
| Hemiptera | Naucoridae | Limnocoris | 2.75 ± 1.50 a | 1.50 ± 0.58 a | 2.60 ± 2.16 a | 0.322 |
| Ambrysus | 1.00 ± 0.00 a | 1.20 ± 0.50 a | 6.00 ± 6.14 b | 0.039 | ||
| Cryphocricos | 0.0 ± 0.0 c | 1.00 ± 0.00 a | 3.00 ± 0.00 b | 0.001 | ||
| Pelocoris | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 2.00 ± 0.00 b | 0.001 | ||
| Veliidae | Rhagovelia | 9.00 ± 0.00 a | 6.50 ± 7.78 a | 2.00 ± 0.00 a | 0.354 | |
| Microvelia | 9.00 ± 0.00 c | 1.00 ± 0.00 a | 0.0 ± 0.0 b | 0.041 | ||
| Gerridae | Limnogonus | 3.67 ± 0.58 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.001 | |
| Belostomatidae | Belostoma | 0.0 ± 0.0 b | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 0.048 | |
| Hidrometridae | Hydrometra | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 6.00 ± 0.00 b | 0.001 | |
| Trichoptera | Hydroptilidae | Oxyethira | 8.00 ± 0.00 b | 1.50 ± 0.71 a | 2.00 ± 0.00 a | 0.023 |
| Neotrichia | 7.00 ± 0.00 b | 0.0 ± 0.0 a | 4.75 ± 6.66 b | 0.038 | ||
| Betrichia | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.032 | ||
| Hydroptilidae | Mayatrichia | 2.00 ± 0.00 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.032 | |
| Philopotamidae | Chimarra | 14.83 ± 11.58 b | 4.71 ± 3.95 a | 6.50 ± 6.98 a | 0.028 | |
| Hydropsychidae | Smicridea | 63.67 ± 73.94 b | 24.33 ± 18.33 a | 37.80 ± 39.00 ab | 0.049 | |
| Leptonema | 17.40 ± 15.39 b | 3.50 ± 2.12 a | 1.00 ± 0.00 a | 0.016 | ||
| Glossosomatidae | Protoptila | 2.50 ± 2.12 a | 8.00 ± 4.24 a | 5.17 ± 4.03 a | 0.152 | |
| Culoptila | 0.0 ± 0.0 b | 2.00 ± 0.00 a | 0.0 ± 0.0 b | 0.001 | ||
| Helicopsychidae | Helicopsyche | 0.0 ± 0.0 c | 3.00 ± 0.00 a | 5.00 ± 0.00 b | 0.001 | |
| Calamoceratidae | Phylloicus | 2.00 ± 1.41 a | 3.00 ± 0.00 a | 0.0 ± 0.0 b | 0.045 | |
| Leptoceridae | Oecetis | 4.00 ± 1.63 b | 0.0 ± 0.0 a | 4.00 ± 0.00 b | 0.001 | |
| Nectopsyche | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 3.00 ± 0.00 b | 0.001 | ||
| Atanatolica | 1.00 ± 0.00 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.032 | ||
| Odontoceridae | Marilia | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.032 | |
| Hydrobiosidae | Atopsyche | 1.50 ± 0.71 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.023 | |
| Coleoptera | Elmidae | Heterelmis | 10.20 ± 6.38 ab | 18.00 ± 11.15 a | 6.00 ± 5.66 b | 0.039 |
| Austrolimnius | 35.50 ± 49.86 a | 22.14 ± 19.86 a | 14.50 ± 11.53 a | 0.364 | ||
| Cylloepus | 8.50 ± 0.00 c | 0.0 ± 0.0 a | 39.00 ± 50.91 b | 0.034 | ||
| Hexanchorus | 3.75 ± 1.92 a | 1.50 ± 0.71 a | 2.50 ± 1.73 a | 0.152 | ||
| Macrelmis | 2.00 ± 0.00 c | 1.00 ± 0.00 a | 0.0 ± 0.0 b | 0.001 | ||
| Microcylloepus | 7.00 ± 0.00 c | 0.0 ± 0.0 a | 2.00 ± 0.00 b | 0.041 | ||
| Phanocerus | 1.50 ± 0.71 a | 2.00 ± 0.00 a | 0.0 ± 0.0 b | 0.045 | ||
| Stegoelmis | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.032 | ||
| Stenelmis | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 2.00 ± 0.00 b | 0.001 | ||
| Stenhelmoides | 0.0 ± 0.0 c | 8.00 ± 0.00 a | 23.00 ± 0.00 b | 0.041 | ||
| Psephenidae | Psephenops | 1.00 ± 0.00 b | 4.50 ± 4.95 a | 9.00 ± 0.00 a | 0.045 | |
| Dryopidae | Pelonomus | 2.00 ± 1.41 ab | 4.00 ± 0.00 a | 1.00 ± 0.00 b | 0.039 | |
| Scirtidae | Elodes | 0.0 ± 0.0 b | 2.00 ± 0.00 a | 0.0 ± 0.0 b | 0.001 | |
| Staphylinidae | Staphylinidae | 0.0 ± 0.0 b | 4.00 ± 0.00 a | 0.0 ± 0.0 b | 0.001 | |
| Diptera | Chironomidae | Chironomus | 0.0 ± 0.0 b | 1.00 ± 0.00 a | 0.0 ± 0.0 b | 0.032 |
| Tanypodinae | 2.33 ± 1.15 ab | 4.20 ± 0.00 a | 1.83 ± 1.50 b | 0.029 | ||
| Orthocladiinae | 10.67 ± 9.17 ab | 32.80 ± 39.59 a | 2.00 ± 1.26 b | 0.041 | ||
| Chironominae | 8.50 ± 6.81 a | 16.40 ± 14.11 a | 4.50 ± 0.00 a | 0.152 | ||
| Simuliidae | Simulium | 100.38 ± 92.94 b | 29.50 ± 57.95 a | 3.50 ± 1.95 a | 0.010 | |
| Tabanidae | Tabanus | 2.00 ± 0.00 c | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.001 | |
| Tipulidae | Tipula | 1.00 ± 0.00 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.032 | |
| Ceratopogonidae | Bezzia | 0.0 ± 0.0 b | 1.00 ± 0.00 a | 0.0 ± 0.0 b | 0.032 | |
| Psychodidae | Psychodidae | 1.00 ± 0.00 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.032 | |
| Lepidoptera | Crambidae | Petrophila | 14.29 ± 14.36 a | 7.86 ± 4.65 a | 2.00 ± 1.26 b | 0.021 |
| Plecoptera | Perlidae | Anacroneuria | 25.20 ± 44.21 b | 2.00 ± 2.12 a | 4.00 ± 0.00 a | 0.038 |
| Megaloptera | Corydalidae | Corydalus | 1.67 ± 1.15 ab | 3.00 ± 0.00 a | 1.00 ± 0.00 b | 0.039 |
| Decapoda | Palaemonidae | Macrobrachium | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 5.67 ± 4.04 b | 0.029 |
| Atyidae | Atya | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 11.50 ± 4.95 b | 0.001 | |
| Trichodactylidae | Trichodactylus | 2.00 ± 0.00 c | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.001 | |
| Hirudinida | Glossiphoniidae | Glossiphoniidae | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.00 ± 0.00 b | 0.032 |
Note: CZ: Conserved Zone; UAZ: Urban and Agricultural Zone; MZ: Mining Influence Zone. Generalized Linear Models (GLMs) were fitted to assess the differences among zones, with zone as a fixed factor. Distribution families were selected based on the AICs (Poisson/negative binomial). Letters (a, b, c) indicate significant differences (p < 0.05) found via Tukey’s HSD post hoc test.
References
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Etter, A.; Wyngaarden, V. Patterns of Landscape Transformation in Colombia, with Emphasis in the Andean Region. Ambio 2000, 29, 412–439. [Google Scholar] [CrossRef]
- Ostrom, E. A general framework for analyzing sustainability of social-ecological systems. Science 2009, 325, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, I.P.; Gotelli, N.J. Water quality improvements offset the climatic debt for stream macroinvertebrates over twenty years. Nat. Commun. 2019, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Van Klink, R.; Sagouis, A.; Petsch, D.K.; Abong’o, D.A.; Alahuhta, J.; Chase, J.M. FreshLanDiv: A Global Database of Freshwater Biodiversity Across Different Land Uses. Glob. Ecol. Biogeogr. 2024, 33, e13917. [Google Scholar] [CrossRef]
- Pérez, J.I.; Nardini, A.G.; Galindo, A.A. Análisis Comparativo de Índices de Calidad del Agua Aplicados al Río Ranchería, La Guajira-Colombia. Inf. Tecnol. 2018, 29, 47–58. [Google Scholar] [CrossRef]
- Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Gaddam, M.K.R.; Velusamy, P.; Gomathi, R.; Muniasamy, S.K. Impact of climate change and anthropogenic activities on aquatic ecosystem—A review. Environ. Res. 2023, 238, 117233. [Google Scholar] [CrossRef]
- Springer, M. Biomonitoreo acuático. Rev. Biol. Trop. 2010, 58, 53–59. [Google Scholar]
- Nuñez, J.C.; Fragoso-Castilla, P.J. Uso de macroinvertebrados acuáticos como bioindicadores de contaminación del agua de la Ciénaga Mata de Palma (Colombia). Inf. Tecnol. 2019, 30, 319–330. [Google Scholar] [CrossRef]
- Sun, C.; Xia, L.; Zhang, M.; He, Q.; Yu, N.; Xiang, H.; Yang, H. The impacts of different seasons on macroinvertebrate community structure and functional diversity in the Jingui River, China. Glob. Ecol. Conserv. 2024, 51, e02876. [Google Scholar] [CrossRef]
- Duarte, C.; Antão, L.H.; Magurran, A.E.; de Deus, C.P. Shifts in fish community composition and structure linked to seasonality in a tropical river. Freshw. Biol. 2022, 67, 1789–1800. [Google Scholar] [CrossRef]
- Molina-Bolivar, G.; Jiménez-Pitré, I.; Bastidas-Barrannco, M. Distribution of Benthic Macro Invertebrates in the Estuarine Ecosystem the Riito, Riohacha-Colombian Guajira. Indian J. Sci. Technol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Vásquez-Ramos, J.M.; Guevara, G.; Reinoso-Flórez, G. Environmental factors associated with habitat preferences by caddisfly larvae in tropical dry forest watersheds (Tolima, Colombia). Rev. Biol. Trop. 2014, 62, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Rodríguez, J.F.; Pinilla Agudelo, G.A.; Moncada Álvarez, L.I. Estructura de la comunidad de dípteros acuáticos en tramos de los cursos altos de los ríos Fucha y Bogotá. Acta Biol. Colomb. 2021, 26, 147–159. [Google Scholar] [CrossRef]
- Tobias, S.R.; Molina-Bolívar, G.; Jiménez-Pitre, I. Comprehensive analysis of water quality in the middle and lower basin of the Marquesote River Colombian. Data Metadata 2023, 2, 54. [Google Scholar] [CrossRef]
- Romero-Murillo, P.; Gallego, J.L.; Leignel, V. Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean. Toxics 2023, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bao, S.; Heino, J.; Liu, Z.; Xie, P.; Zhong, X.; Zhou, Q. Interactive effects of aridity and local environmental factors on the functional trait composition and diversity of macroinvertebrate assemblages in dryland rivers. Sci. Total Environ. 2024, 954, 176671. [Google Scholar] [CrossRef] [PubMed]
- López-López, E. Editorial: Freshwater Ecosystems in Arid and Semiarid Zones Facing Multiple Stressors: Human Disturbances, Climate Change, and Dryland River Conservation. Front Environ Sci. 2021, 9, 814225. [Google Scholar] [CrossRef]
- Corpoguajira. Plan de Ordenación y Manejo de la Cuenca del río Ranchería. Corporación Autónoma Regional de La Guajira, 2012. Available online: https://www.corpoguajira.gov.co/web/attachments_Joom/article/897/Resolucion%201725%20del%2018%20de%20Diciembre%20de%202012.pdf (accessed on 15 January 2025).
- González-Martínez, M.D.; Huguet, C.; Pearse, J.; McIntyre, N.; Camacho, L.A. Assessment of potential contamination of Paramo soil and downstream water supplies in a coal-mining region of Colombia. Appl. Geochem. 2019, 108, 104382. [Google Scholar] [CrossRef]
- Argumedo, C.D.; Aguas, L.J.V. Fuentes terrestres de contaminación en la zona costera de La Guajira, Colombia. Rev. Investig. Agrar. Ambient. 2016, 7, 123–138. [Google Scholar] [CrossRef]
- Díaz Chávez, L.; Espinosa Romero, A.P.; Molina Bolivar, G. Salud ambiental del río Ranchería a través de macroinvertebrados acuáticos en el área de influencia del complejo carbonífero El Cerrejón. Tecnura 2020, 24, 49–63. [Google Scholar] [CrossRef]
- Granados-Martínez, C.; Guevara-Mora, M.; Rincón, J.; Herrera, E. Effects of human disturbance gradient on aquatic macroinvertebrate diversity: A study in a river of the Sierra Nevada de Santa Marta. Ambiente Água 2025, 20, e3019. [Google Scholar] [CrossRef]
- Barletta, M.; Melo, R.C.; Whitfield, A.K. Past and present conservation of south American estuaries. Estuar. Coast. Shelf Sci. 2023, 295, 108542. [Google Scholar] [CrossRef]
- Malits, A.; Ibarbalz, F.M.; Martín, J.; Flombaum, P. Higher biotic than abiotic natural variability of the plankton ecosystem revealed by a time series along a subantarctic transect. J. Mar. Syst. 2023, 238, 103843. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Leal, C.G.; Pompeu, P.S.; Gardner, T.A.; Leitão, R.P.; Hughes, R.M.; Kaufmann, P.R.; Barlow, J. Multi-scale assessment of human-induced changes to Amazonian instream habitats. Landsc. Ecol. 2016, 31, 1725–1745. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Benfield, E.F.; Valett, H.M. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshw. Biol. 2001, 46, 1409–1424. [Google Scholar] [CrossRef]
- Jiménez-Valencia, J.; Kaufmann, P.R.; Sattamini, A.; Mugnai, R.; Baptista, D.F. Assessing the ecological condition of streams in a southeastern Brazilian basin using a probabilistic monitoring design. Environ. Monit. Assess. 2014, 186, 4685–4695. [Google Scholar] [CrossRef] [PubMed]
- Chará, J. Manual para la Evaluación Biológica de Ambientes Acuáticos en Microcuencas Ganaderas, 2nd ed.; CIPAV: Cali, Colombia, 2004. [Google Scholar]
- Domínguez, E.; Molineri, C.; Pescador, M.L.; Hubbard, M.D.; Nieto, C. Ephemeroptera of South America; Pensoft Publishers: Sofia, Moscow, 2006; 646p. [Google Scholar]
- Posada-García, J.A. Clave ilustrada para la identificación de los géneros de larvas de Trichoptera en el nor-occidente de Sudamérica. Caldasia 2003, 25, 169–192. [Google Scholar]
- Roldán, G. Guía para el estudio de los macroinvertebrados acuáticos del Departamento de Antioquia; Fondo FEN Colombia-Colciencias-Universidad de Antioquia, Editorial Presencia Ltda: Bogotá, Colombia, 1988; pp. 1–217. [Google Scholar]
- Hamada, N.; Nessimian, J.L.; Querino, R.B. (Eds.) Insetos Aquáticos na Amazônia Brasileira: Taxonomia, Biologia e Ecologia, 1st ed.; Editora do INPA: Manaus, Brazil, 2014; 724p. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 January 2025).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, R package version 2.6-10; CRAN: Vienna, Austria, 2024; Available online: https://vegandevs.github.io/vegan/ (accessed on 15 January 2025).
- Dray, S.; Dufour, A. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: Interpolation and Extrapolation for Species Diversity. R package version 3.0.1. 2024. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed on 15 January 2025).
- Chao, A.; Hsieh, T.C. iNEXT.4steps: Four-Step Biodiversity Analysis Based on iNEXT [software]. Version 1.0.1. CRAN: Vienna, Austria. Available online: https://cran.r-project.org/web/packages/iNEXT.4steps/index.html (accessed on 12 July 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 15 January 2025)ISBN 978-3-319-24277-4.
- Slowikowski, K. ggrepel: Automatically Position Non-Overlapping Text Labels with ‘ggplot2’. R package version 0.9.3. 2023. Available online: https://CRAN.R-project.org/package=ggrepel (accessed on 15 January 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R package version 1.1.2. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 January 2025).
- Larson, D.M.; Dodds, W.K.; Veach, A.M. Removal of woody riparian vegetation substantially altered a stream ecosystem in an otherwise undisturbed Grassland watershed. Ecosystems 2019, 22, 64–76. [Google Scholar] [CrossRef]
- Giglou, K.S.; Nazari, R.; Karimi, M.; Museru, M.L.; Opare, K.N.; Nikoo, M.R. Future eco-hydrological dynamics: Urbanization and climate change effects in a changing landscape: A case study of Birmingham’s river basin. J. Clean. Prod. 2024, 447, 141320. [Google Scholar] [CrossRef]
- Macklin, M.G.; Brewer, P.A.; Hudson-Edwards, K.A.; Bird, G.; Coulthard, T.J.; Dennis, I.A. A geomorphological approach to the management of rivers contaminated by metal mining. Geomorphology 2006, 79, 423–447. [Google Scholar] [CrossRef]
- López-Giraldo, A.T.; Ríos-Pulgarín, M.I.; Gil-Guarín, I.C. Características del hábitat que regulan la estructura de las comunidades de macroinvertebrados en ríos tropicales de montaña (Antioquía, Colombia). Rev. Biol. Trop. 2023, 71, 52093. [Google Scholar] [CrossRef]
- Wohl, E.; Kramer, N.; Ruiz-Villanueva, V.; Scott, D.N.; Comiti, F.; Gurnell, A.M.; Piégay, H.; Lininger, K.B.; Jaeger, K.L.; Walters, D.M.; et al. The natural wood regime in rivers. BioScience 2019, 69, 259–273. [Google Scholar] [CrossRef]
- Kaden, S.; Schramm, M. Control model Spree/Schwarze Elster—A tool to optimise rehabilitation of water resources in the Lusatian mining district. Landsc. Urban Plan. 2000, 51, 101–108. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Soller, J.; Bartrand, T.; Ravenscroft, J.; Molina, M.; Whelan, G.; Schoen, M.; Ashbolt, N. Estimated human health risks from recreational exposures to stormwater runoff containing animal faecal material. Environ. Modell. Softw. 2015, 72, 21–32. [Google Scholar] [CrossRef]
- Parris, K. Impact of Agriculture on Water Pollution in OECD Countries: Recent Trends and Future Prospects. Int. J. Water Resour. Dev. 2011, 27, 33–52. [Google Scholar] [CrossRef]
- Chaves, L.R.B.; Franco, G.M.R. Diversidad de macroinvertebrados acuáticos en dos ecosistemas lóticos en El Doncello, Caquetá. Rev. Fac. Cienc. Básicas 2021, 17, 57–72. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Peters, K.; Kiesel, J.; Welti, E.A.; Gillmann, S.M.; Lorenz, A.W.; Haase, P. Stream macroinvertebrate communities in restored and impacted catchments respond differently to climate, land-use, and runoff over a decade. Sci. Total Environ. 2024, 929, 172659. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.A.; Molineri, C.; Reynaga, M.C.; Basualdo, C. Which index is the best to assess stream health? Ecol Indic. 2011, 11, 582–589. [Google Scholar] [CrossRef]
- Akamagwuna, F.C.; Ntloko, P.; Edegbene, A.O.; Odume, O.N. Are Ephemeroptera, Plecoptera and Trichoptera traits reliable indicators of semi-urban pollution in the Tsitsa River, Eastern Cape Province of South Africa? Environ. Monit. Assess. 2021, 193, 309. [Google Scholar] [CrossRef] [PubMed]
- Aljahdali, M.O.; Alhassan, A.B. Spatial variation of metallic contamination and its ecological risk in sediment and freshwater mollusk: Melanoides tuberculata (Müller, 1774) (Gastropoda: Thiaridae). Water 2020, 12, 206. [Google Scholar] [CrossRef]
- Purnama, M.F.; Salwiyah, S. Short Communication: Invasive mollusks Melanoides tuberculata, and Achatina fulica in Southeast Sulawesi, Indonesia. Biodiversitas 2022, 23, d230944. [Google Scholar] [CrossRef]
- Alonso-EguíaLis, P.; Mora, J.M.; Campbell, B.; Springer, M. Diversidad, Conservación y Uso de Los Macroinvertebrados Dulceacuícolas de México, Centroamérica, Colombia, Cuba y Puerto Rico; IMTA Press: Jiutepec, México, 2014. [Google Scholar]
- Dedieu, N.; Vigouroux, R.; Cerdan, P.; Céréghino, R. Invertebrate communities delineate hydro-ecoregions and respond to anthropogenic disturbance in East-Amazonian streams. Hydrobiologia 2015, 742, 95–105. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Tapia, J.; Hauenstein, E.; Peña-Cortés, F.; Vergara, C.; Cerna, C.; Vargas-Chacoff, L. Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages. Sci. Total Environ. 2017, 609, 724–734. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).