Life History Traits of the Stygophilous Amphipod Synurella ambulans in the Hyporheic Zone of the Lower Reaches of the Upper Sava River (Croatia)
Abstract
:1. Introduction
1.1. Distribution Patterns and Ecology of Synurella ambulans
1.2. Amphipod Life History Strategy in the Subterranean Environment
1.3. Methodology for Life History Analyses
1.4. The Aims of the Research
2. Materials and Methods
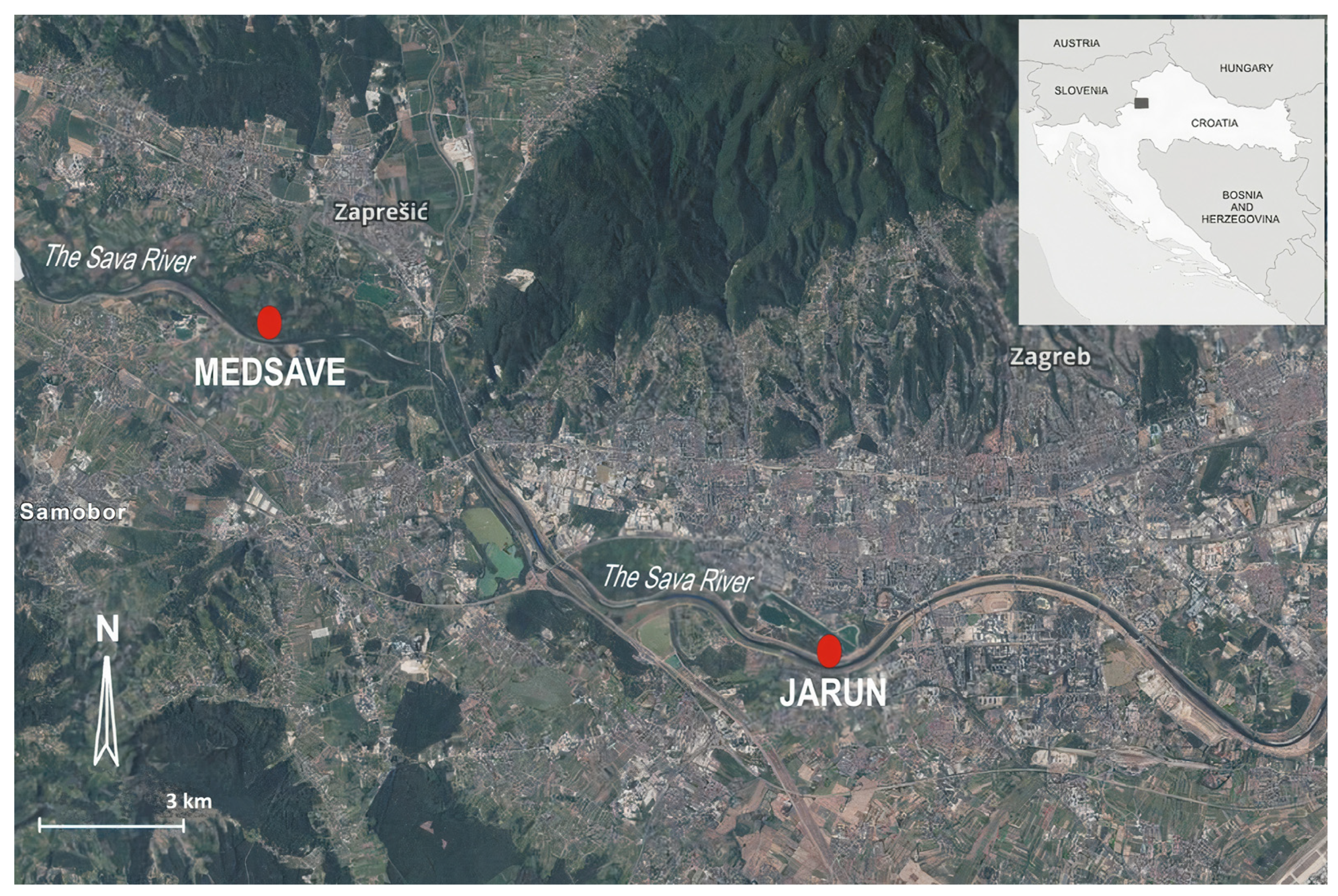
2.1. Study Area
2.2. Sample Collection and Field Measurements
2.3. Laboratory Measurements
2.3.1. Determination of Environmental Parameters
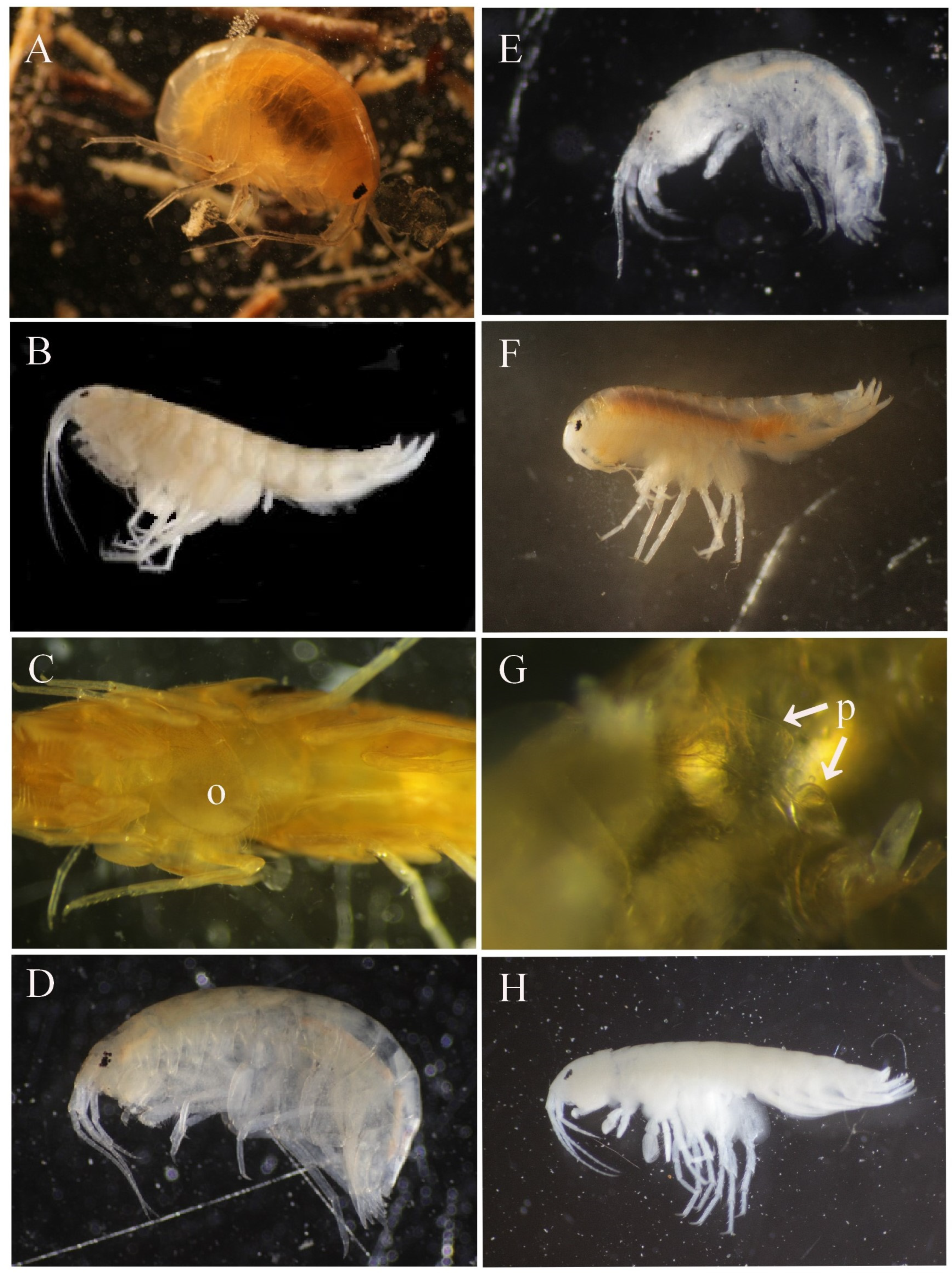
2.3.2. Amphipod Measurements
2.4. Data Analysis
2.4.1. Cohort and Growth Analyses
2.4.2. Mortality and Longevity
2.4.3. Principal Component Analysis (PCA) and Canonical Correspondence Analysis (CCA)
3. Results
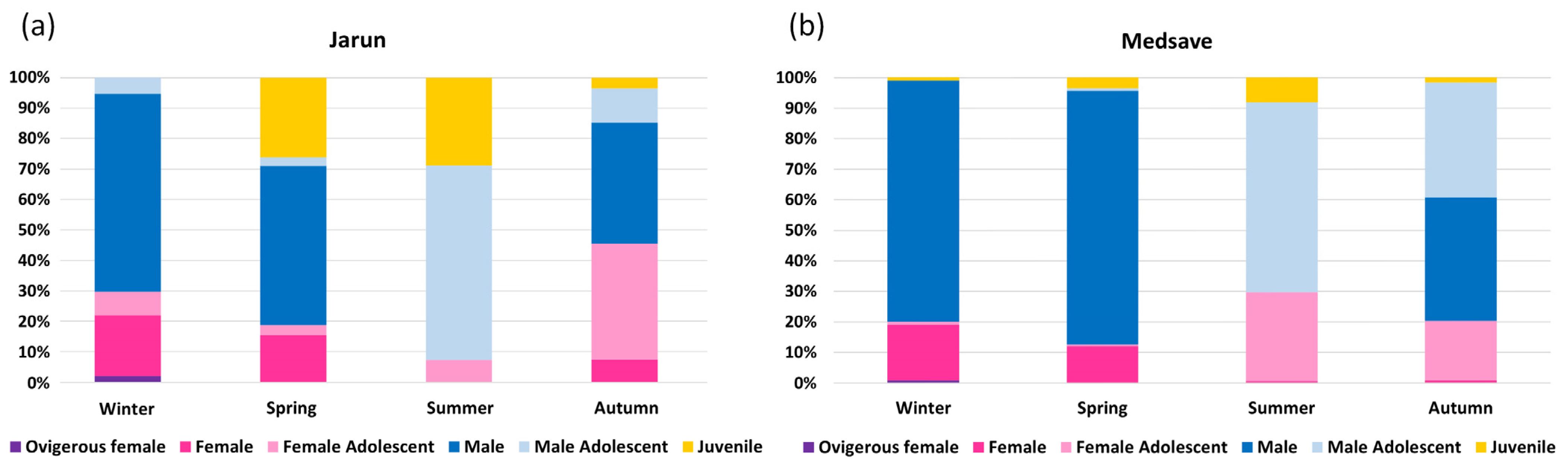
3.1. Population Structure
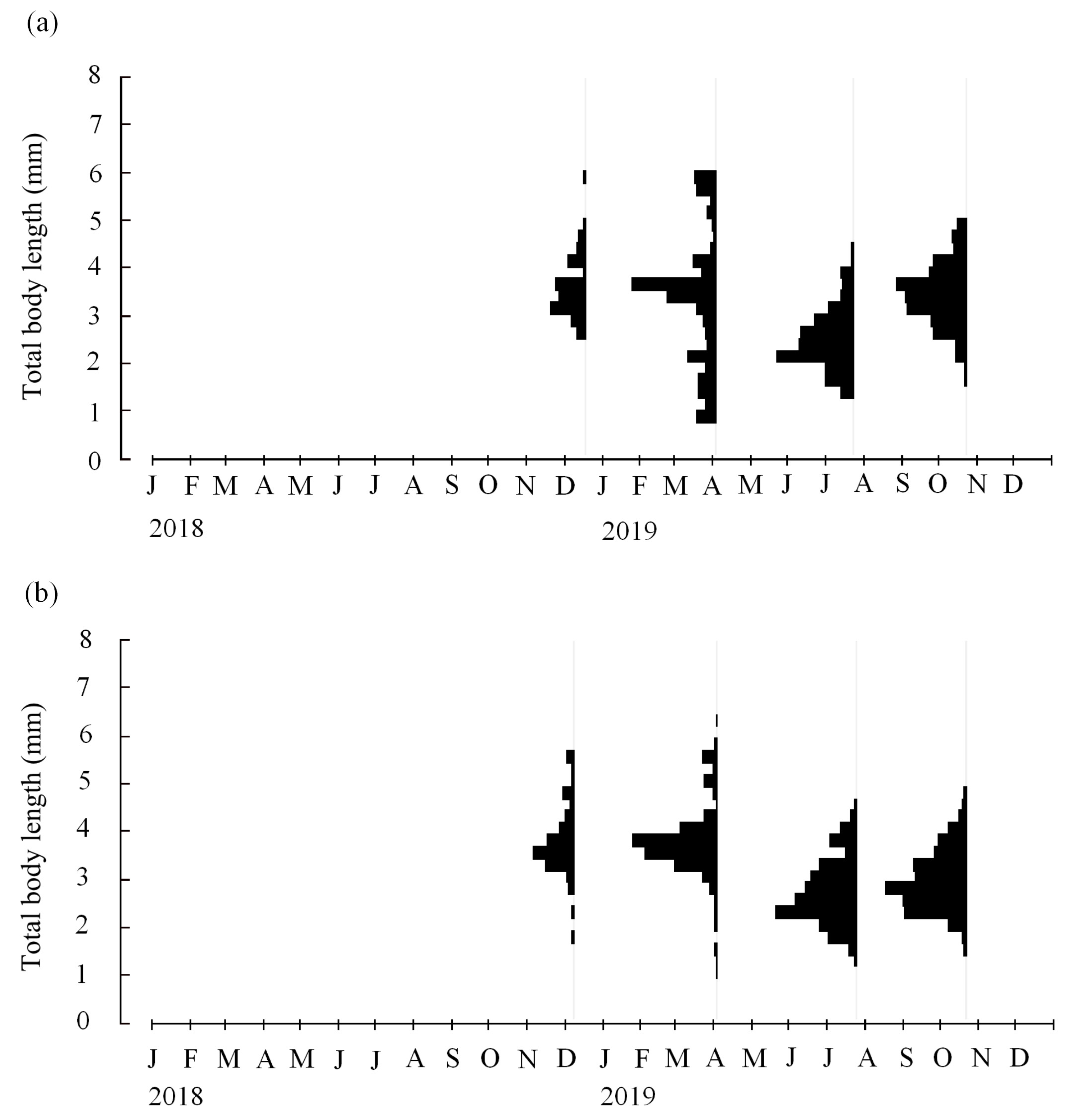
3.2. Length-Frequency Analysis
3.3. Cohorts and Growth
3.4. Mortality
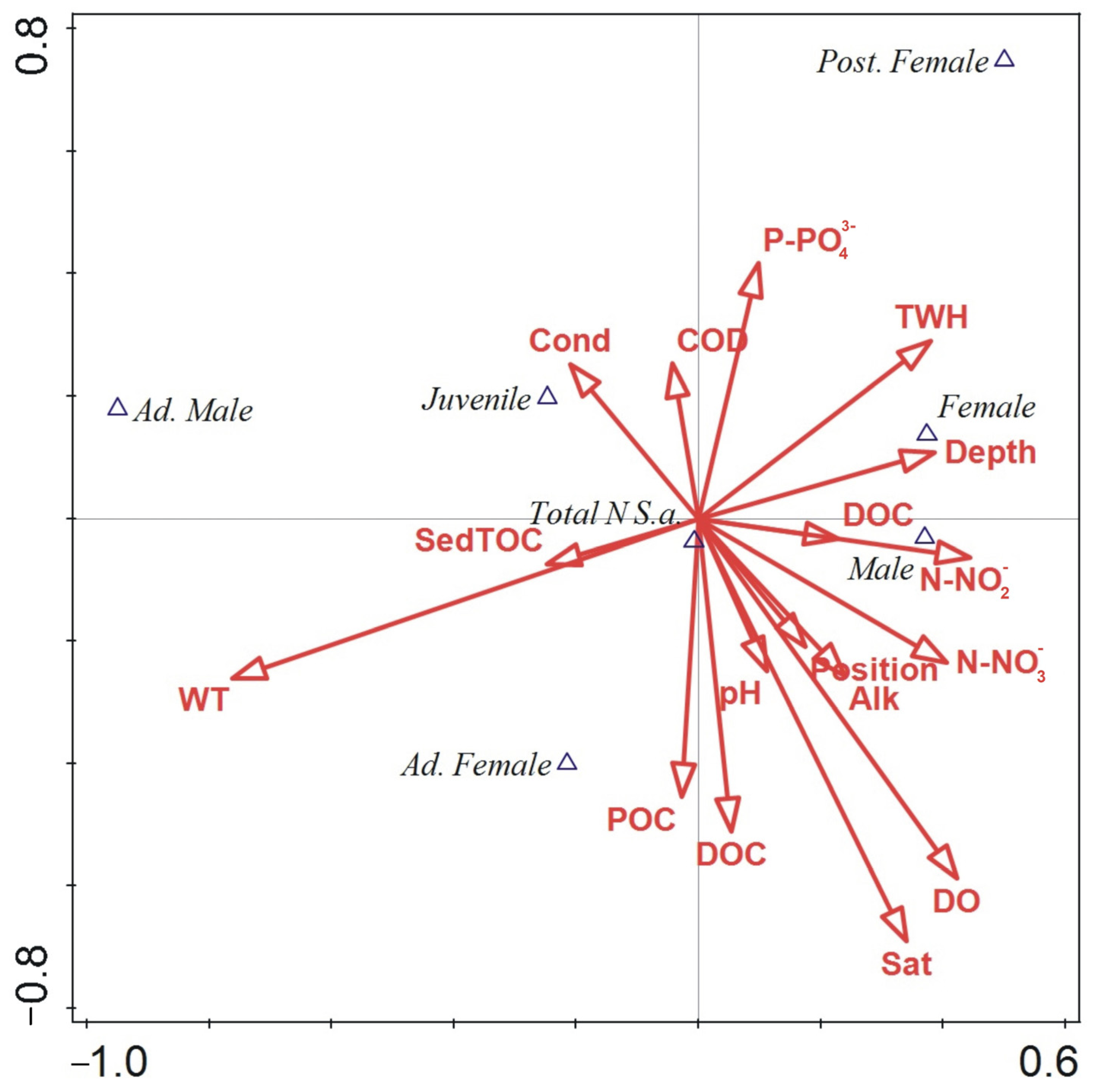
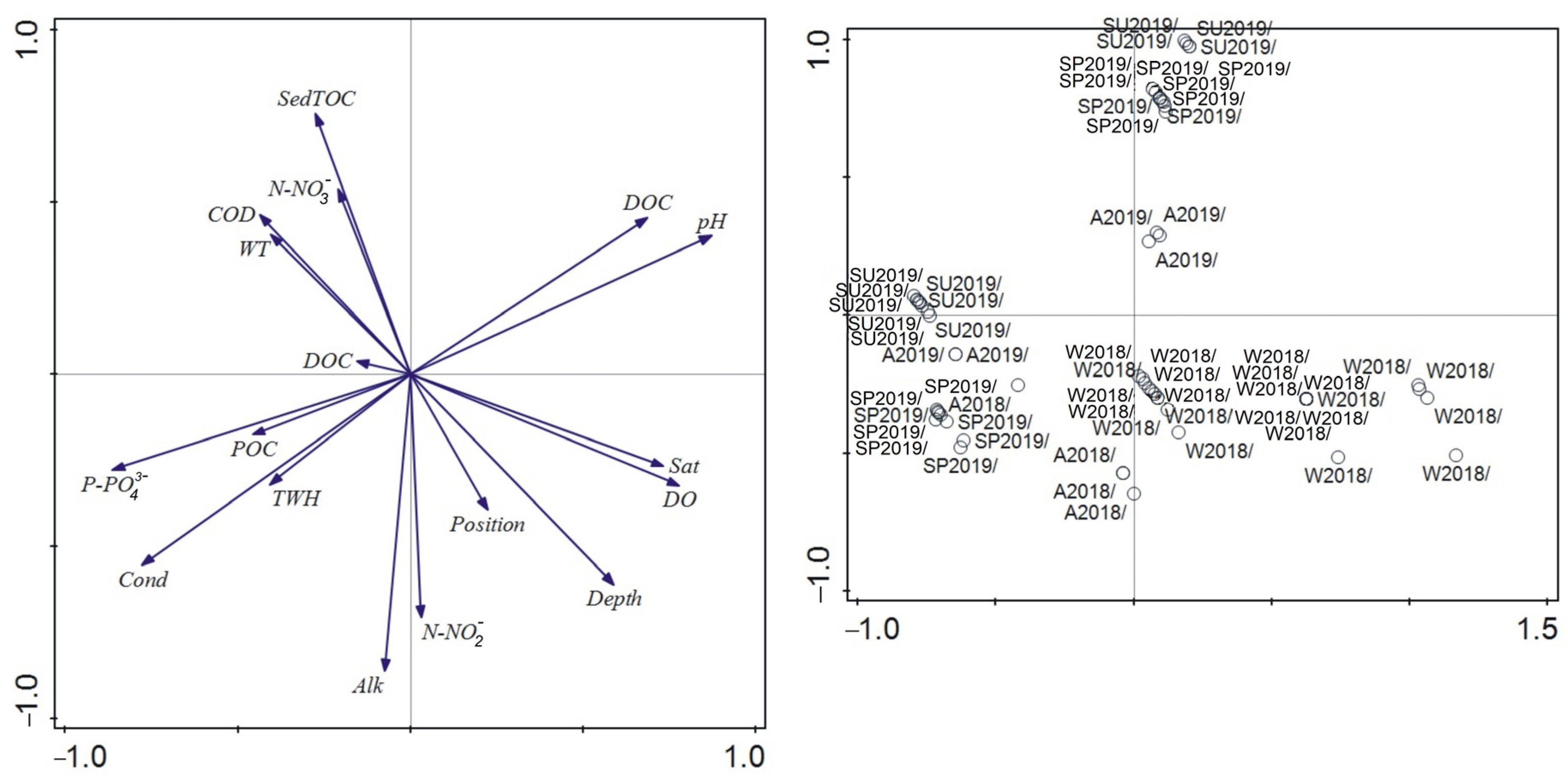
3.5. Relationship between Environmental Parameters and Gender/Ontogeny Classes’ Abundance
4. Discussion
4.1. Life Cycle
4.2. Growth and Mortality
4.3. Environmental Factors and Life History Traits of S. ambulans
4.4. Advantages and Limitations of Length-Based Methods for Amphipod Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arfianti, T.; Wilson, S.; Costello, M.J. Progress in the Discovery of Amphipod Crustaceans. PeerJ 2018, 6, e5187. [Google Scholar] [CrossRef]
- Benito, J.B.; Porter, M.L.; Niemiller, M.L. Comparative Mitogenomic Analysis of Subterranean and Surface Amphipods (Crustacea, Amphipoda) with Special Reference to the Family Crangonyctidae. bioRxiv 2023, preprint. [Google Scholar] [CrossRef]
- Horton, T.; De Broyer, C.; Bellan-Santini, D.; Copilaș-Ciocianu, D.; Corbari, L.; Daneliya, M.; Dauvin, J.-C.; Desiderato, A.; Fišer, C.; Grabowski, M.; et al. World Amphipoda Database. Available online: https://www.marinespecies.org/amphipoda (accessed on 29 July 2023).
- Altermatt, F.; Alther, R.; Fišer, C.; Jokela, J.; Konec, M.; Küry, D.; Mächler, E.; Stucki, P.; Westram, A.M. Diversity and Distribution of Freshwater Amphipod Species in Switzerland (Crustacea: Amphipoda). PLoS ONE 2014, 9, e110328. [Google Scholar] [CrossRef] [PubMed]
- Giari, L.; Fano, E.A.; Castaldelli, G.; Grabner, D.; Sures, B. The Ecological Importance of Amphipod-Parasite Associations for Aquatic Ecosystems. Water 2020, 12, 2429. [Google Scholar] [CrossRef]
- Väinölä, R.; Witt, J.D.S.; Grabowski, M.; Bradbury, J.H.; Jazdzewski, K.; Sket, B. Global Diversity of Amphipods (Amphipoda; Crustacea) in Freshwater. Hydrobiologia 2008, 595, 241–255. [Google Scholar] [CrossRef]
- Copilaş-Ciocianu, D.; Boroş, B.V. Contrasting Life History Strategies in a Phylogenetically Diverse Community of Freshwater Amphipods (Crustacea: Malacostraca). Zoology 2016, 119, 21–29. [Google Scholar] [CrossRef]
- Copilaş-Ciocianu, D.; Sidorov, D.; Gontcharov, A. Adrift across Tectonic Plates: Molecular Phylogenetics Supports the Ancient Laurasian Origin of Old Limnic Crangonyctid Amphipods. Org. Divers. Evol. 2019, 19, 191–207. [Google Scholar] [CrossRef]
- Copilaş-Ciocianu, D.; Grabowski, M.; Pârvulescu, L.; Petrusek, A. Zoogeography of Epigean Freshwater Amphipoda (Crustacea) in Romania: Fragmented Distributions and Wide Altitudinal Variability. Zootaxa 2014, 3893, 243–260. [Google Scholar] [CrossRef]
- Meijering, M.P.D.; Jazdzewski, K.; Köhn, J. Ecotypes of Amphipoda in Central European Inland Waters. Pol. Arch. Hydrobiol. 1995, 42, 527–536. [Google Scholar]
- Konopacka, A.; Błażewicz-Paszkowycz, M. Life History of Synurella ambulans (F. Müller, 1846) (Amphipoda, Crangonyctidae) from Central Poland. Pol. Arch. Hydrobiol. 2000, 47, 597–605. [Google Scholar]
- Sidorov, D.; Palatov, D. Taxonomy of the Spring Dwelling Amphipod Synurella ambulans (Crustacea: Crangonyctidae) in West Russia: With Notes on Its Distribution and Ecology. Eur. J. Taxon. 2012, 23, 1–19. [Google Scholar] [CrossRef]
- Holsinger, J.R. Holartic Crangonyctid Amphipods. In Stygofauna Mundi. A Faunistic, Distributional, and Ecological Synthesis of the World Fauna Inhabiting Subterranean Waters; Backhuys, W., Ed.; E.J. Brill: Leiden, The Netherlands, 1986; pp. 535–549. [Google Scholar]
- Pljakić, M. Die Variabilität Der Synurella—Populationen an Verschiedenen Jugoslawischen Standorten. Verh. Deutsch. Zool. Gesell. Graz. 1957, 20, 494–505. [Google Scholar]
- Karaman, G.S. Contribution to the Knowledge of the Amphipoda. Genus Synurella Wrzes. in Yugoslavia with Remarks on Its All World Known Species, Their Synonymy, Bibliography and Distribution (Fam. Gammaridae). Poljopr. Šumarstvo 1974, 20, 83–133. [Google Scholar]
- Heckes, U.; Hess, M.; Burmeister, E.-G. Ein Vorkommen von Synurella ambulans F. Müller 1846 (Amphipoda: Crangonyctidae) in Südbayern (On the Occurence of Synurella ambulans F. Müller 1846 (Amphipoda: Crangonyctidae) in Southern Bavaria). Lauterbornia 1996, 25, 95–105. [Google Scholar]
- Arbačiauskas, K. Synurella ambulans (F. Müller, 1846), A New Native Amphipod Species of Lithuanian Waters. Acta Zool Litu 2008, 18, 66–68. [Google Scholar] [CrossRef]
- Borutzky, E. VII.—On the Occurrence of the Amphipod Synurella ambulans in Russia. In Annals and Magazine of Natural History: Series 9; Taylor & Francis: Abingdon, UK, 1927; Volume 20, pp. 63–66. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T.; Gottstein, S. Hypotelminorheic—A Unique Freshwater Habitat. Subterr. Biol. 2006, 4, 1–7. [Google Scholar]
- Sket, B.; Stoch, F. Recent Fauna of the Cave Križna Jama in Slovenia. Mitt. Komm. Quartärforsch. Österr. Akad. Wiss. 2014, 21, 45–55. [Google Scholar]
- Andrikovics, S.; Forro, L.; Metz, H. The Occurence of Synurella ambulans (Müller, 1846) (Crustacea, Amphipoda) in Neusiedlersee. Sitzungsber. Osterr. Akad. Wiss. 1982, 1, 139–141. [Google Scholar]
- Moog, O.; Konar, M.; Humpesch, U.H. The Macrozoobenthos of the River Danube in Austria. Lauterbornia 1994, 15, 25–51. [Google Scholar]
- Giginyak, Y.G.; Moroz, M.D. Ecological and Biotopical Features of the Relict Amphipod Synurella ambulans from Springs of Belarus (in Russian). Dokl. Natl. Acad. Sci. Belarus. 2000, 44, 81–83. [Google Scholar]
- Boets, P.; Lock, K.; Goethals, P.L.M. First Record of Synurella ambulans (Müller 1846) (Amphipoda: Crangonictidae) in Belgium. Belg. J. Zool. 2010, 140, 244–245. [Google Scholar]
- Gottstein, S.; Mihaljević, Z.; Perović, G.; Kerovec, M. The Distribution of Amphipods (Crustacea) in Different Habitats along the Mura and Drava River Systems in Croatia. Int. Assoc. Danub. Res. 2000, 33, 231–236. [Google Scholar]
- Straškraba, M. Amphipoden Der Tschechoslowakei Nach Den Sammlungen von Prof. Hrabe. Acta Soc. Zool. Bohemoslov. 1962, 26, 117–145. [Google Scholar]
- Berezina, N.A.; Ďuriš, Z. First Record of the Invasive Species Dikerogammarus villosus (Crustacea: Amphipoda) in the Vltava River (Czech Republic). Aquat. Invasions 2008, 3, 455–460. [Google Scholar] [CrossRef]
- Tempelman, D.; Arbačiauskas, K.; Grudule, N. First Record of Synurella ambulans (Crustacea: Amphipoda) in Estonia and Its Distribution in the Baltic States. Lauterbornia 2010, 69, 21–27. [Google Scholar]
- Nesemann, H. Zur Verbreitung von Niphargus (Phaenogammarus) Dudich 1941 Und Synurella Wrzesniowski 1877 in Der Ungarischen Tiefebene (Crustacea, Amphipoda) (The Distribution of Niphargus (Phaenogammarus) Dudich 1941 and Synurella Wrzesniowski 1877 in Hungarian Lowlands (Crustacea, Amphipoda). Lauterbornia 1993, 13, 61–71. [Google Scholar]
- Muskó, I.B. Occurrence of Amphipoda in Hungary since 1853. Crustaceana 1994, 66, 144–152. [Google Scholar]
- Ruffo, S. Il Genere Synurella Wrzesn. in Anatolia, Descrizione Di Una Nuova Specie e Considerazioni Su Lyurella hyrcana Dersh. (Crustacea, Amphipoda, Gammaridae). Mem. Mus. Civ. Stor. Nat. 1974, 20, 389–404. [Google Scholar]
- Casellato, S.; Masiero, L.; La Piana, G.; Gigliotti, F. The Alien Amphipod Crustacean Dikerogammarus villosus in Lake Garda (N-Italy): The Invasion Continues. In Biological Invasions—From Ecology to Conservation; Rabitsch, W., Essl, F., Klingenstein, F., Eds.; NEOBIOTA: Vienna, Austria, 2008; Volume 7, pp. 115–122. [Google Scholar]
- Schellenberg, A. Krebstiere Oder Crustacea. IV: Flohkrebse Oder Amphipoda. In Die Tierwelt Deutschlands; Verlag von Gustav Fischer: Jena, Germany, 1942; p. 40. [Google Scholar]
- Tretjakova, R.; Paidere, J.; Brakovska, A. Assessment by Macroinvertebrates of the Ecological Quality of Shallow Lake with Rich Sapropel Sediments. Environment Technologies Resources. In Proceedings of the International Scientific and Practical Conference, Rezekne, Latvia, 15–16 June 2023; Volume 1, pp. 228–234. [Google Scholar] [CrossRef]
- Mrdak, D.; Petrović, D.; Katnić, A.; Erceg, M. Integrated Study to Support the Designation of the Trans-Boundary Lake/Shkodra as Biosphere Reserve; University of Montenegro, Faculty of Sciences and Mathematics: Podgorica, Montenegro, 2011. [Google Scholar]
- Konopacka, A.; Sobocinska, V. Uwagi Na Temat Wystepowania Skorupiaka Synurella ambulans (Müll.) (Amphipoda, Crangonyctidae) w Polsce. Przegląd Zool. 1992, 36, 123–131. [Google Scholar]
- Copilaş-Ciocianu, D.; Pârvulescu, L. Faunistic Overview upon the Aquatic Malacostracans (Crustacea, Malacostraca) of Cefa Nature Park (Crişana, Romania). Transylv. Rev. Syst. Ecol. Res. 2012, 13, 99–106. [Google Scholar]
- Copilaș-Ciocianu, D.; Fišer, C.; Borza, P.; Balázs, G.; Angyal, D.; Petrusek, A. A Comparative Study of Two Epigean Niphargus Species: Phylogenetic Relationships, Phylogeography, Morphology and Ecology. In Proceedings of the The 2nd Central European Symposium for Aquatic Macroinvertebrate Research, Pecs, Hungary, 3–8 July 2016. [Google Scholar]
- Necpálová, K.; Stloukal, E. A Contribution to the Knowledge of Amphipoda Distribution in the National Parks Included in the All Taxa Biodiversity Inventory Project (in Slovak). Folia Faun. Slovaca 2011, 16, 191–200. [Google Scholar]
- Prevorčnik, S.; Remškar, A.; Fišer, C.; Sket, B.; Bračko, G.; Delić, T.; Mori, N.; Brancelj, A.; Zagmajster, M. Interstitial Fauna of the Sava River in Eastern Slovenia. Nat. Slov. 2019, 21, 13–23. [Google Scholar]
- Ruffo, S.; Vonk, R. Ingolfiella beatricis, New Species (Amphipoda: Ingolfiellidae) from Subterranean Waters of Slovenia. J. Crustac. Biol. 2001, 21, 484–491. [Google Scholar] [CrossRef]
- Mürle, U.; Weber, B.; Ortlepp, J. On the Occurrence of Synurella ambulans (Amphipoda: Crangonictidae) in the River Aare, Catchment Area of the River Rhine, Switzerland. Lauterbornia 2003, 48, 61–66. [Google Scholar]
- Özbek, M. An Overview on the Distribution of Synurella Genus in Turkey (Crustacea: Amphipoda). Ege J. Fish. Aquat. Sci. 2018, 35, 111–114. [Google Scholar] [CrossRef]
- Fedonenko, O.; Yakovenko, V.; Ananieva, T.; Sharamok, T.; Yesipova, N.; Marenkov, O. Fishery and Environmental Situation Assessment of Water Bodies in the Dnipropetrovsk Region of Ukraine. World Sci. News 2018, 92, 1–138. [Google Scholar]
- Žutinić, P.; Petrić, I.; Gottstein, S.; Gligora Udovič, M.; Kralj Borojević, K.; Kamberović, J.; Kolda, A.; Plenković-Moraj, A.; Ternjej, I. Microbial Mats as Shelter Microhabitat for Amphipods in an Intermittent Karstic Spring. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 1–13. [Google Scholar] [CrossRef]
- Gottstein Matočec, S.; Bakran-Petricioli, T.; Bedek, J.; Bukovec, D.; Buzjak, S.; Franičević, M.; Jalžić, B.; Kerovec, M.; Kletečki, E.; Kralj, J.; et al. An Overview of the Cave and Interstitial Biota of Croatia. Nat. Croat. 2002, 11, 1–112. [Google Scholar]
- Redžović, Z.; Erk, M.; Gottstein, S.; Sertić Perić, M.; Dautović, J.; Fiket, Ž.; Brkić, A.L.; Cindrić, M. Metal Bioaccumulation in Stygophilous Amphipod Synurella ambulans in the Hyporheic Zone: The Influence of Environmental Factors. Sci. Total Environ. 2023, 866, 161350. [Google Scholar] [CrossRef]
- Konopacka, A.; Hupało, K.; Rewicz, T.; Grabowski, M. Species Inventory and Distribution Patterns of Freshwater Amphipods in Moldova. North West J. Zool. 2014, 10, 382–392. [Google Scholar]
- Fišer, C.; Keber, R.; Kereži, V.; Moškrič, A.; Palandančić, A.; Petkovska, V.; Potočnik, H.; Sket, B. Coexistence of Species of Two Amphipod Genera: Niphargus timavi (Niphargidae) and Gammarus fossarum (Gammaridae). J. Nat. Hist. 2007, 41, 2641–2651. [Google Scholar] [CrossRef]
- Fišer, C.; Zagmajster, M.; Zakšek, V. Coevolution of Life History Traits and Morphology in Female Subterranean Amphipods. Oikos 2013, 122, 770–778. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Galassi, D.M.P.; Tabilio Di Camillo, A.; Pop, M.M.; Iepure, S.; Piccini, L. Life-History Traits and Acclimation Ability of a Copepod Species from the Dripping Waters of the Corchia Cave (Apuan Alps, Tuscany, Italy). Water 2023, 15, 1356. [Google Scholar] [CrossRef]
- Mayer, G.; Maas, A.; Dieter, W. Mouthpart Morphology of Synurella ambulans (F. Müller, 1846) (Amphipoda, Crangonyctidae). Spixiana 2015, 38, 219–229. [Google Scholar]
- Sket, B. Gegenseitige Beeinflussung Der Wasserpollution Und Das Hohlenmilieus. Proc. Int. Congr. Speleol. 1973, 5, 253–262. [Google Scholar]
- Hutchinson, G.E. An Introduction to Population Ecology; Yale University Press: New Haven, CT, USA, 1978. [Google Scholar]
- Bach, L.; Forbes, V.E.; Dahllöf, I. The Amphipod Orchomenella pinguis—A Potential Bioindicator for Contamination in the Arctic. Mar. Pollut. Bull. 2009, 58, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Sainte-Marie, B. A Review of the Reproductive Bionomics of Aquatic Gammaridean Amphipods: Variation of Life History Traits with Latitude, Depth, Salinity and Superfamily. Hydrobiologia 1991, 223, 189–227. [Google Scholar] [CrossRef]
- Brown, J.L.; Choe, J.C. Behavioral Ecology and Sociobiology. In Encyclopedia of Animal Behavior; Choe, J.C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 103–108. ISBN 9780128132517. [Google Scholar]
- Marin, I.N.; Palatov, D.M. Lifestyle Switching and Refugee Availability Are the Main Factors in the Evolution and Distribution of the Genus Synurella Wrześniowski, 1877 (Amphipoda: Crangonyctidae). Arthropoda Sel. 2022, 31, 393–448. [Google Scholar] [CrossRef]
- Kilada, R.; Driscoll, J.G. Age Determination in Crustaceans: A Review. Hydrobiologia 2017, 799, 21–36. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Sun, C.-L.; Chen, Y.; Yeh, S.-Z. Modelling the Growth of Crustacean Species. Rev. Fish Biol. Fish. 2012, 22, 157–187. [Google Scholar] [CrossRef]
- O’Donovan, V.; Tully, O. Lipofuscin (Age Pigment) as an Index of Crustacean Age: Correlation with Age, Temperature and Body Size in Cultured Juvenile Homarus gammarus L. J. Exp. Mar. Biol. Ecol. 1996, 207, 1–14. [Google Scholar] [CrossRef]
- Bluhm, B.A.; Brey, T.; Klages, M.; Arntz, W.E. Occurrence of the Autofluorescent Pigment, Lipofuscin, in Polar Crustaceans and Its Potential as an Age Marker. Polar Biol. 2001, 24, 642–649. [Google Scholar] [CrossRef]
- Kilada, R.; Sainte-Marie, B.; Rochette, R.; Davis, N.; Vanier, C.; Campana, S. Direct Determination of Age in Shrimps, Crabs, and Lobsters. Can. J. Fish. Aquat. Sci. 2012, 69, 1728–1733. [Google Scholar] [CrossRef]
- Walters, E.A.; Crowley, C.E.; Gandy, R.L.; Behringer, D.C. A Reflex Action Mortality Predictor (RAMP) for Commercially Fished Blue Crab Callinectes sapidus in Florida. Fish. Res. 2022, 247, 106188. [Google Scholar] [CrossRef]
- Wahle, R.A.; Tully, O.; O’Donovan, V. Lipofuscin as an Indicator of Age in Crustaceans: Analysis of the Pigment in the American Lobster Homarus americanus. Mar. Ecol. Prog. Ser. 1996, 138, 117–123. [Google Scholar] [CrossRef]
- Bluhm, B.A.; Brey, T.; Klages, M. The Autofluorescent Age Pigment Lipofuscin: Key to Age, Growth and Productivity of the Antarctic Amphipod Waldeckia obesa (Chevreux, 1905). J. Exp. Mar. Biol. Ecol. 2001, 258, 215–235. [Google Scholar] [CrossRef]
- Belchier, M.; Edsman, L.; Sheehy, M.R.J.; Shelton, P.M.J. Estimating Age and Growth in Long-Lived Temperate Freshwater Crayfish Using Lipofuscin. Freshw. Biol. 1998, 39, 439–446. [Google Scholar] [CrossRef]
- Sheehy, M.R.J.; Bannister, R.C.A.; Wickins, J.F.; Shelton, P.M.J. New Perspectives on the Growth and Longevity of the European Lobster (Homarus gammarus). Can. J. Fish. Aquat. Sci. 1999, 56, 1904–1915. [Google Scholar] [CrossRef]
- Glenn, D.; Pakes, M.J.; Caldwell, R.L. Fluorescence in Arthropoda Informs Ecological Studies in Anchialine Crustaceans, Remipedia, and Atyidae. J. Crustac. Biol. 2013, 33, 620–626. [Google Scholar] [CrossRef]
- Enin, U.I.; Lowenberg, U.; Kunzel, T. Population Dynamics of the Estuarine Prawn (Nematopalaemon hastatus Aurivillius 1898) off the Southeast Coast of Nigeria. Fish. Res. 1996, 26, 17–35. [Google Scholar] [CrossRef]
- Etim, L.; Sankare, Y. Growth and Mortality, Recruitment and Yield of the Fresh-Water Shrimp, Macrobrachium völlenhovenii, Herklots 1851 (Crustacea, Palaemonidae) in the Fahe Reservoir, Côte d’Ivoire, West Africa. Fish. Res. 1998, 38, 211–223. [Google Scholar] [CrossRef]
- Jayawardane, P.A.A.T.; McLusky, D.S.; Tytler, P. Estimation of Population Parameters and Stock Assessment of Penaeus indicus (H. Milne Edwards) in the Western Coastal Waters of Sri Lanka. Asian Fish Sci. 2002, 15, 155–166. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Hylen, A.; Østvedt, O.-J.; Alvsvaag, J.; Iles, T.C. Growth, Maturation, and Longevity of Maturation Cohorts of Norwegian Spring-Spawning Herring. ICES J. Mar. Sci. 2004, 61, 165–175. [Google Scholar] [CrossRef]
- Bintoro, G.; Setyohadi, D.; Lelono, T.D.; Maharani, F. Biology and Population Dynamics Analysis of Fringescale Sardine (Sardinella fimbriata) in Bali Strait Waters, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 391, 012024. [Google Scholar] [CrossRef]
- Amponsah, S.K.K.; Asiedu, B.; Failler, P. Population Parameters of Oreochromis niloticus (L) from a Semi-Open Lagoon (Sakumo II), Ghana and Its Implications on Management. Egypt. J. Aquat. Biol. Fish. 2020, 24, 195–207. [Google Scholar] [CrossRef]
- Castillo-Jordán, C.; Cubillos, L.A.; Navarro, E. Inter-Cohort Growth Rate Changes of Common Sardine (Strangomera bentincki) and Their Relationship with Environmental Conditions off Central Southern Chile. Fish. Res. 2010, 105, 228–236. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A Quantitative Theory of Organic Growth (Inquiries on Growth Laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Amin, S.M.N.; Arshad, A.; Siraj, S.S.; Sidik, B.J. Population Structure, Growth, Mortality and Yield per Recruit of Segestid Shrimp, Acetes japonicus (Decapoda: Sergestidae) from the Coastal Waters of Malacca, Peninsular Malaysia. Indian J. Mar. Sci. 2009, 38, 57–68. [Google Scholar]
- Arculeo, M.; Vitale, S.; Cannizaro, L.; Lo Brutto, S. Growth Parameters and Population Structure of Aristeus antennatus (Decapoda, Penaeidae) in the South Tyrrhenian Sea (Southern Coast of Italy). Crustaceana 2011, 84, 1099–1109. [Google Scholar] [CrossRef]
- Gómez, J.; Defeo, O. Life History of the Sandhopper Pseudorchestoidea brasiliensis (Amphipoda) in Sandy Beaches with Contrasting Morphodynamics. Mar. Ecol. Prog. Ser. 1999, 182, 209–220. [Google Scholar] [CrossRef]
- Bacela, K.; Konopacka, A.; Grabowski, M. Reproductive Biology of Dikerogammarus haemobaphes: An Invasive Gammarid (Crustacea: Amphipoda) Colonizing Running Waters in Central Europe. Biol. Invasions 2009, 11, 2055–2066. [Google Scholar] [CrossRef]
- Cházaro-Olvera, S.; García-Delgado, X.; Winfield, I.; Ortiz, M. A Population Study of the Amphipod Nototropis minikoi (Gammaridea, Atylidae) in the Sian Ka’an Biosphere Reserve, Quintana Roo, Mexico. Crustaceana 2017, 90, 337–348. [Google Scholar] [CrossRef]
- Correia, D.; Banha, F.; Gama, M.; Anastácio, P.M. Population Dynamics and Expansion of Crangonyx pseudogracilis, a Potentially Invasive Amphipod. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 8. [Google Scholar] [CrossRef]
- Bouchard, L.; Winkler, G. Life Cycle, Growth and Reproduction of Neomysis americana in the St. Lawrence Estuarine Transition Zone. J. Plankton. Res. 2018, 40, 693–707. [Google Scholar] [CrossRef]
- Fonseca, D.B.; Veloso, V.G.; Cardoso, R.S. Growth, Mortality, and Reproduction of Excirolana braziliensis Richardson, 1912 (Isopoda, Cirolanidae) on the Prainha Beach, Rio De Janeiro, Brazil. Crustaceana 2000, 73, 535–545. [Google Scholar] [CrossRef]
- Caetano, C.H.S.; Cardoso, R.S.; Veloso, V.G.; Silva, E.S. Population Biology and Secondary Production of Excirolana braziliensis (Isopoda: Cirolanidae) in Two Sandy Beaches of Southeastern Brazil. J. Coast. Res. 2006, 22, 825–835. [Google Scholar] [CrossRef]
- Defeo, O.; Gomez, J.; Lercari, D. Testing the Swash Exclusion Hypothesis in Sandy Beach Populations: The Mole Crab Emerita brasiliensis in Uruguay. Mar. Ecol. Prog. Ser. 2001, 212, 159–170. [Google Scholar] [CrossRef]
- Cardoso, R.S.; Veloso, V.G.; Caetano, C.H.S. Life History of Emerita brasiliensis (Decapoda: Hippidae) on Two Beaches with Different Morphodynamic Characteristics. J. Coast. Res. 2003, 35, 392–401. [Google Scholar]
- Fidalgo, M.L.; Santos, P.; Ferreira, C.; Silva, A. Population Structure and Dynamics of the Freshwater Shrimp (Millet, 1831) in the Lower River Minho (NW Portugal). Crustaceana 2015, 88, 657–673. [Google Scholar] [CrossRef]
- Taddei, F.G.; Reis, S.D.S.; David, F.S.; Da Silva, T.E.; Fransozo, V.; Fransozo, A. Population Structure, Mortality, and Recruitment of Macrobrachium amazonicum (Heller, 1862) (Caridea: Palaemonidae) in the Eastern Amazon Region, Brazil. J. Crustac. Biol. 2017, 37, 131–141. [Google Scholar] [CrossRef]
- Lolas, A.; Vafidis, D. Population Dynamics, Fishery, and Exploitation Status of Norway Lobster (Nephrops norvegicus) in Eastern Mediterranean. Water 2021, 13, 289. [Google Scholar] [CrossRef]
- Grabowski, M.; Bącela-Spychalska, K.; Pešić, V. Reproductive Traits and Conservation Needs of the Endemic Gammarid Laurogammarus scutarensis (Schäferna, 1922) from the Skadar Lake System, Balkan Peninsula. Limnologica 2014, 47, 44–51. [Google Scholar] [CrossRef]
- Glazier, D.S.; Butler, E.M.; Lombardi, S.A.; Deptola, T.J.; Reese, A.J.; Satterthwaite, E. V Ecological Effects on Metabolic Scaling: Amphipod Responses to Fish Predators in Freshwater Springs. Ecol. Monogr. 2011, 81, 599–618. [Google Scholar] [CrossRef]
- Prata, P.F.S.; Pires, D.M.; Fonseca, D.B.; Dumont, L.F.C. Occurrence and Quantification of the Autofluorescent Pigment Neurolipofuscin in the Brains of Red Shrimp Pleoticus muelleri (Bate, 1888) (Decapoda: Solenoceridae). Panam. J. Aquat. Sci. 2017, 12, 108–116. [Google Scholar]
- Redžović, Z.; Erk, M.; Gottstein, S.; Cindrić, M. Energy Status of Stygophilous Amphipod Synurella ambulans as a Promising Biomarker of Environmental Stress in the Hyporheic Zone. Water 2023, 15, 3083. [Google Scholar] [CrossRef]
- Komatina, D.; Grošelj, S. Transboundary Water cooperation for Sustainable Development of the Sava Basin. In The Sava River; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; Volume 31, pp. 1–25. [Google Scholar]
- ISRBC (International Sava River Basin Commission) Sava River Basin Analysis Report; Zagreb, 2009. Available online: https://www.savacommission.org/documents-and-publications/water-management-1957/sava-river-basin-analysis-report/10360 (accessed on 18 October 2022).
- Illies, J. Limnofauna Europaea. A Checklist of the Animals Inhabiting European Inland Waters, with an Account of Their Distribution and Ecology, 2nd ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1978. [Google Scholar]
- Nakić, Z.; Ružičić, S.; Posavec, K.; Mileusnić, M.; Parlov, J.; Bačani, A.; Durn, G. Conceptual Model for Groundwater Status and Risk Assessment—Case Study of the Zagreb Aquifer System. Geol. Croat. 2013, 66, 55–77. [Google Scholar] [CrossRef]
- Schwarz, U. Hydropower Projects in Protected Areas in the Balkan Region; RiverWatch & EuroNatur: Vienna, Austria; Radolfzell, Germany, 2015; pp. 1–34. [Google Scholar]
- RiverWatch—Hydroelectric Power Plant Projects on the Sava River. Available online: https://riverwatch.eu/en/balkanrivers/map (accessed on 19 October 2022).
- Meštrov, M. Faunističko-Ekološka i Biocenološka Istraživanja Podzemnih Voda Savske Nizine. Period. Biol. 1960, 13, 73–108. (In Croatian) [Google Scholar]
- Meštrov, M.; Stilinović, B.; Habdija, I.; Lattinger, R.; Maloseja, Ž.; Kerovec, M.; Čičin-Šain, L. The Ecological Characteristics of Intertstitial Underground Waters in Relation to the Water of the River Sava. Acta Biol. 1983, 48, 5–33. (In Croatian) [Google Scholar]
- Bou, C.; Rouch, R. Un Nouveau Champ de Recherches Sur La Faune Aquatique Souterraine. Comptes Rendus L’Academie Sci. 1967, 265, 369–370. [Google Scholar]
- Karaman, G.S. Anfipodi Delle Acque Dolci Italiane; Calderini: Bologna, Italiy, 1993; Volume XXXI. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: Washington, DC, USA, 1985; pp. 1–1268. [Google Scholar]
- Deutsches Institut für Normung. Deutsche Einheitsverfahren Zur Wasser-, Abwasserund Schlammuntersuchung, 16th ed.; Verlag Chemie: Weinheim, Germany, 1986; Volume II. [Google Scholar]
- Dafner, E.V.; Wangersky, P.J. A Brief Overview of Modern Directions in Marine DOC Studies. Part I.—Methodological Aspects. J. Environ. Monit. 2002, 4, 48–54. [Google Scholar] [CrossRef]
- Dautović, J.; Vojvodić, V.; Tepić, N.; Ćosović, B.; Ciglenečki, I. Dissolved Organic Carbon as Potential Indicator of Global Change: A Long-Term Investigation in the Northern Adriatic. Sci. Total Environ. 2017, 587–588, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Dautović, J.; Strmečki, S.; Pestorić, B.; Vojvodić, V.; Plavšić, M.; Krivokapić, S.; Ćosović, B. Organic Matter in the Karstic Enclosed Bay (Boka Kotorska Bay, South Adriatic Sea). Influence of Freshwater Input. Fresenius Environ. Bull. 2012, 21, 995–1006. [Google Scholar]
- Gayanilo, F.C., Jr.; Sparre, P.; Pauly, P. FAO-ICLARM Stock Assessment Tools II (FISAT II). Revised Version. User’s Guide; FAO Computerized Information Series (Fisheries): Rome, Italy, 2005; Volume 8. [Google Scholar]
- Bhattacharya, C.G. A Simple Method of Resolution of a Distribution into Gaussian Components. Biometrics 1967, 23, 115–135. [Google Scholar] [CrossRef]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO Fisheries Technical Paper; Food & Agriculture Organization: Rome, Italy, 1983; Volume 234, pp. 1–52. [Google Scholar]
- Defeo, O.; Arreguín-Sánchez, F.; Sánchez, J. Growth Study of the Yellow Clam Mesodesma mactroides: A Comparative Analysis of Three Length-Based Methods. Sci. Mar. 1992, 56, 53–59. [Google Scholar]
- Pauly, D. On the Interrelationships between Natural Mortality, Growth Parameters, and Mean Environmental Temperature in 175 Fish Stocks. J. Cons. Int. Pour L’exploration Mer 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Pauly, D. Length-Converted Catch Curves and the Seasonal Growth of Fishes. Fishbyte 1990, 8, 24–29. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer Version 6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5). In Microcomputer Power; Canoco: Ithaca, NY, USA, 2002. [Google Scholar]
- Ter Braak, C.J.F. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Poltermann, M. Growth, Production and Productivity of the Arctic Sympagic Amphipod Gammarus wilkitzkii. Mar. Ecol. Prog. Ser. 2000, 193, 109–116. [Google Scholar] [CrossRef]
- Taylor, S.J.; Webb, D.W. Subterranean Amphipoda (Crustacea) of Illinois’ Salem Plateau: Spatial and Temporal Components of Microdistribution; University of Illinois at Urbana-Champaign Library: Urbana, IL, USA, 2000. [Google Scholar]
- Nair, K.K.C.; Anger, K. Life Cycle of Corophium insidiosum (Crustacea, Amphipoda) in Laboratory Culture. Helgoländer Wiss. Meeresunters. 1979, 32, 279–294. [Google Scholar] [CrossRef]
- Cunha, M.R.; Sorbe, J.C.; Moreira, M.H. The Amphipod Corophium multisetosum (Corophiidae) in Ria de Aveiro (NW Portugal). I. Life History and Aspects of Reproductive Biology. Mar. Biol. 2000, 137, 637–650. [Google Scholar] [CrossRef]
- Hakala, I. Distribution, Population Dynamics and Production of Mysis relicta (Lovén) in Southern Finland. Ann. Zool. Fennici. 1978, 15, 243–258. [Google Scholar]
- Mauchline, J. The Biology of Mysids and Euphausiids; Blaxter, J.H.S., Russel, S.F.S., Yonge, S.M., Eds.; Academic Press: London, UK, 1980; Volume 18. [Google Scholar]
- Lima, J.d.F.; Da Silva, L.M.A.; Da Silva, T.C.; Garcia, J.d.S.; Pereira, I.d.S.; Amaral, K.D.S. Reproductive Aspects of Macrobrachium amazonicum (Decapoda: Palaemonidae) in the State of Amapá, Amazon River Mouth. Acta Amazon 2014, 44, 245–254. [Google Scholar] [CrossRef]
- Veloso, V.G.; Cardoso, R.S. Population Biology of the Mole Crab Emerita brasiliensis (Decapoda: Hippidae) at Fora Beach, Brazil. J. Crustac. Biol. 1999, 19, 147–153. [Google Scholar] [CrossRef]
- Watts, M.M.; Pascoe, D.; Carroll, K. Population Responses of the Freshwater Amphipod Gammarus pulex (L.) to an Environmental Estrogen, 17α-Ethinylestradiol. Environ. Toxicol. Chem. 2002, 21, 445–450. [Google Scholar] [CrossRef]
- Maranhão, P.; Marques, J.C. The Influence of Temperature and Salinity on the Duration of Embryonic Development, Fecundity and Growth of the Amphipod Echinogammarus marinus Leach (Gammaridae). Acta Oecologica 2003, 24, 5–13. [Google Scholar] [CrossRef]
- Highsmith, R.C.; Coyle, K.O. Amphipod Life Histories: Community Structure, Impact of Temperature on Decoupled Growth and Maturation Rates, Productivity, and P:B Ratios. Am. Zool. 1991, 31, 861–873. [Google Scholar] [CrossRef]
- Dugan, J.E.; Hubbard, D.M.; Wenner, A.M. Geographic Variation in Life History of the Sand Crab, Emerita analoga (Stimpson) on the California Coast: Relationships to Environmental Variables. J. Exp. Mar. Biol. Ecol. 1994, 181, 255–278. [Google Scholar] [CrossRef]
- Lercari, D.; Defeo, O. Effects of Freshwater Discharge in Sandy Beach Populations: The Mole Crab Emerita brasiliensis in Uruguay. Estuar. Coast. Shelf Sci. 1999, 49, 457–468. [Google Scholar] [CrossRef]
- Defeo, O.; Brazeiro, A.; De Alava, A.; Riestra, G. Is Sandy Beach Macrofauna Only Physically Controlled? Role of Substrate and Competition in Isopods. Estuar. Coast. Shelf Sci. 1997, 45, 453–462. [Google Scholar] [CrossRef]
- Sprules, W.G. The Life Cycle of Crangonyx richmondensis laurentianus Bousfield (Crustacea: Amphipoda). Can. J. Zool. 1967, 45, 877–884. [Google Scholar] [CrossRef]
- Piscart, C.; Devin, S.; Beisel, J.N.; Moreteau, J.C. Growth-Related Life-History Traits of an Invasive Gammarid Species: Evaluation with a Laird-Gompertz Model. Can. J. Zool. 2003, 81, 2006–2014. [Google Scholar] [CrossRef]
- Žganec, K.; Đurić, P.; Gottstein, S. Life History Traits of the Endangered Endemic Amphipod Echinogammarus cari (Crustacea, Gammaridae) from the Dinaric Karst. Int. Rev. Hydrobiol. 2011, 96, 686–708. [Google Scholar] [CrossRef]
- Tuck, I.D.; Chapman, C.J.; Atkinson, R.J.A. Population Biology of the Norway Lobster, Nephrops norvegicus (L.) in the Firth of Clyde, Scotland—I: Growth and Density. ICES J. Mar. Sci. 1997, 54, 125–135. [Google Scholar] [CrossRef]
- Maynou, F.; Sardà, F. Nephrops norvegicus Population and Morphometrical Characteristics in Relation to Substrate Heterogeneity. Fish. Res. 1997, 30, 139–149. [Google Scholar] [CrossRef]
- Vogt, G. Ageing and Longevity in the Decapoda (Crustacea): A Review. Zool. Anz. 2012, 251, 1–25. [Google Scholar] [CrossRef]
- Biorede—Corophium multisetosum. Available online: http://www.biorede.pt/index4.htm (accessed on 24 April 2020).
- Hervant, F.; Mathieu, J.; Messana, G. Locomotory, Ventilatory and Metabolic Responses of the Subterranean Stenasellus virei (Crustacea, Isopoda) to Severe Hypoxia and Subsequent Recovery. Comptes Rendus L’académie Sci. Paris Sci. Vie 1997, 320, 139–148. [Google Scholar] [CrossRef]
- Spicer, J.I. Is the Reduced Metabolism of Hypogean Amphipods Solely a Result of Food Limitation? Hydrobiologia 1998, 377, 201–204. [Google Scholar] [CrossRef]
- Werner, I.; Auel, H.; Garrity, C.; Hagen, W. Pelagic Occurrence of the Sympagic Amphipod Gammarus wilkitzkii in Ice-Free Waters of the Greenland Sea—Dead End or Part of Life-Cycle? Polar Biol. 1999, 22, 56–60. [Google Scholar] [CrossRef]
- Schwamborn, R. How Reliable Are the Powell–Wetherall Plot Method and the Maximum-Length Approach? Implications for Length-Based Studies of Growth and Mortality. Rev. Fish Biol. Fish. 2018, 28, 587–605. [Google Scholar] [CrossRef]
- Mathews, C.P.; Samuel, M. The Relationship between Maximum and Asymptotic Length in Fishes. Fishbyte 1990, 8, 14–16. [Google Scholar]
- Castiglioni, D.D.S.; Ozga, A.V.; Rodrigues, S.G.; Bueno, A.A.D.P. Population Dynamics of a Freshwater Amphipod from South America (Crustacea, Amphipoda, Hyalellidae). Nauplius 2016, 24, e2016028. [Google Scholar] [CrossRef]
- Pilgrim, W.; Burt, M.D.B. Effect of Acute PH Depression on the Survival of the Freshwater Amphipod Hyalella azteca at Variable Temperatures: Field and Laboratory Studies. Hydrobiologia 1993, 254, 91–98. [Google Scholar] [CrossRef]
- Henry, K.S.; Danielopol, D.L. Oxygen Dependent Habitat Selection in Surface and Hyporheic Environments by Gammarus roeseli Gervais (Crustacea, Amphipoda): Experimental Evidence. Hydrobiologia 1998, 390, 51–60. [Google Scholar] [CrossRef]
- Brey, T.; Gage, J.D. Interactions of Growth and Mortality in Benthic Invertebrate Populations: Empirical Evidence for a Mortality-Growth Continuum. Arch. Fish. Mar. Res. 1997, 54, 45–59. [Google Scholar]
- Pennafirme, S.; Soares-Gomes, A. Population Dynamics and Secondary Production of a Key Benthic Tanaidacean, Monokalliapseudes schubarti (Mañé-Garzón, 1949) (Tanaidacea, Kalliapseudidae), from a Tropical Coastal Lagoon in Southeastern Brazil. Crustaceana 2017, 90, 1483–1499. [Google Scholar] [CrossRef]
- Cardoso, R.S.; Veloso, V.G. Population Biology and Secondary Production of the Sandhopper Pseudorchestoidea brasiliensis (Amphipoda: Talitridae) at Prainha Beach, Brazil. Mar. Ecol. Prog. Ser. 1996, 142, 111–119. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Austad, S.N. Why Do We Age? Nature 2000, 408, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Arntz, W.E.; Brey, T.; Gallardo, V.A. Antarctic Zoobenthos. Oceanogr. Mar. Biol. Annu. Rev. 1994, 32, 241–304. [Google Scholar]
- Sudo, H. Effect of Temperature on Growth, Sexual Maturity and Reproduction of Acanthomysis robusta (Crustacea: Mysidacea) Reared in the Laboratory. Mar. Biol. 2003, 143, 1095–1107. [Google Scholar] [CrossRef]
- Leber, K.M. Seasonality of Macroinvertebrates on a Temperate, High Wave Energy Sandy Beach. Bull. Mar. Sci. 1982, 32, 86–98. [Google Scholar]
- Dou, Q.; Du, X.; Cong, Y.; Wang, L.; Zhao, C.; Song, D.; Liu, H.; Huo, T. Influence of Environmental Variables on Macroinvertebrate Community Structure in Lianhuan Lake. Ecol. Evol. 2022, 12, e8553. [Google Scholar] [CrossRef] [PubMed]
- Polančec, V. Statistički Ljetopis Grada Zagreba; Polančec, V., Šiško, D., Nevistić, S., Pongrac, I., Bešlić, Ž., Šaravanja, R., Krndelj, N., Eds.; Grad Zagreb, Gradski ured za strategijsko planiranje i razvoj Grada: Zagreb, Croatia, 2017. [Google Scholar]
- Hydrometeorological Service SFRY (Hidrometeorološka služba SFRJ). Atlas Klime SFRJ, Tablični Podaci; Hidrometeorološka služba: Beograd, Serbia, 1969. [Google Scholar]
- Zaninović, K.; Gajić-Čapka, M.; Perčec Tadić, M.; Vučetić, M.; Milković, J.; Bajić, A.; Cindrić, K.; Cvitan, L.; Katušin, Z.; Kaučić, D.; et al. Climate Atlas of Croatia 1961–1990, 1971–2000. (Klimatski Atlas Hrvatske); Zaninović, K., Gajić-Čapka, M., Milković, J., Perčec Tadić, M., Vučetić, M., Eds.; Meteorological and Hydrological Service of Croatia: Zagreb, Croatia, 2008; ISBN 978-953-7526-01-6. [Google Scholar]
- De Alava, A.; Defeo, O. Distributional Pattern and Population Dynamics of Exciroluna armata (Isopoda: Cirolanidae) in a Uruguayan Sandy Beach. Estuar. Coast. Shelf Sci. 1991, 33, 433–444. [Google Scholar] [CrossRef]
- Yannicelli, B.; Palacios, R.; Giménez, L. Activity Rhythms of Two Cirolanid Isopods from an Exposed Microtidal Sandy Beach in Uruguay. Mar. Biol. 2001, 138, 187–197. [Google Scholar] [CrossRef]
- Alam, M.K.; Negishi, J.N.; Pongsivapai, P.; Yamashita, S.; Nakagawa, T. Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone. Water 2021, 13, 1340. [Google Scholar] [CrossRef]
- Stubbington, R.; Wood, P.J.; Reid, I. Spatial Variability in the Hyporheic Zone Refugium of Temporary Streams. Aquat. Sci. 2011, 73, 499–511. [Google Scholar] [CrossRef]
- Grant, A.; Morgan, P.J.; Olive, P.J.W. Use Made in Marine Ecology of Methods for Estimating Demographic Parameters from Size/Frequency Data. Mar. Biol. 1987, 95, 201–208. [Google Scholar] [CrossRef]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Assessing Sources of Uncertainty in Length-Based Estimates of Body Growth in Populations of Fishes and Macroinvertebrates with Bootstrapped ELEFAN. Ecol. Modell. 2019, 393, 37–51. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Growth in Crustacea—Twenty Years On. Hydrobiologia 2001, 449, 111–122. [Google Scholar] [CrossRef]
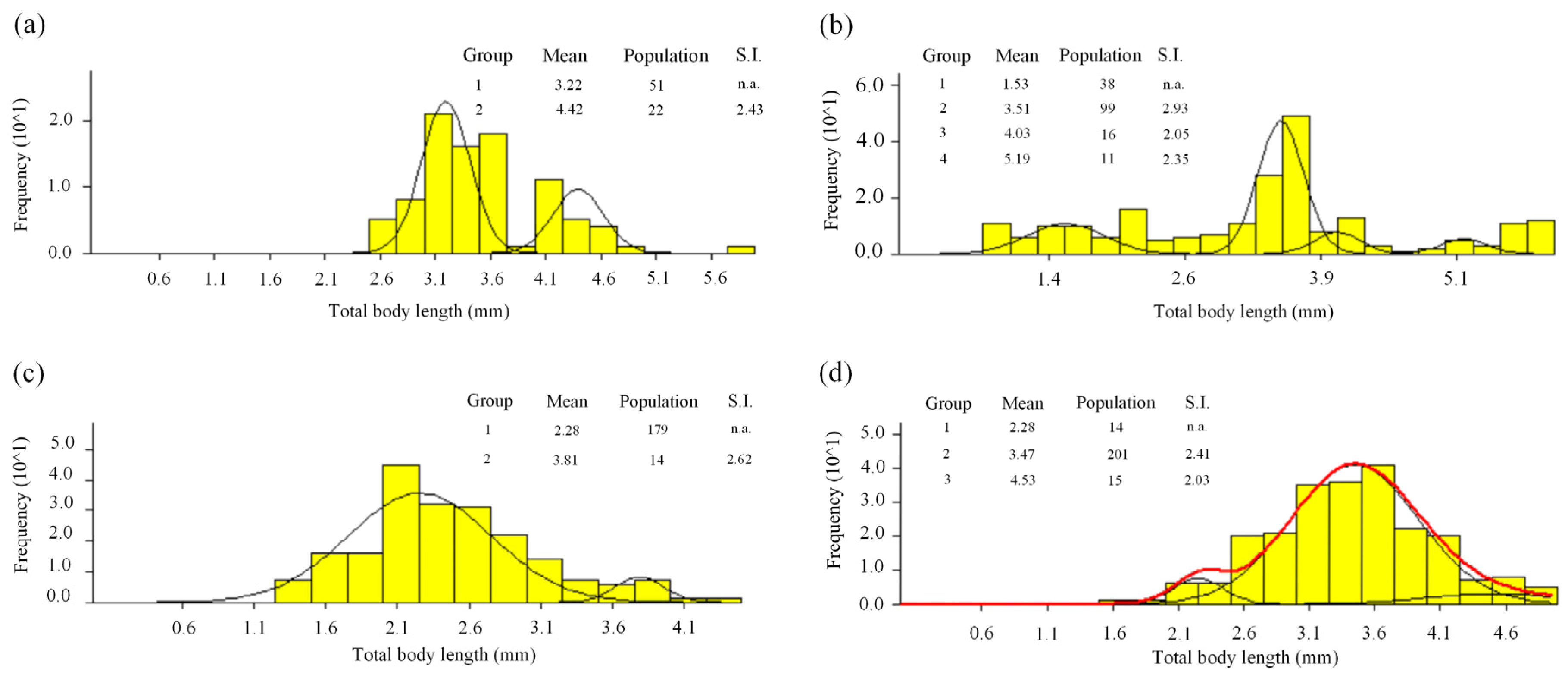
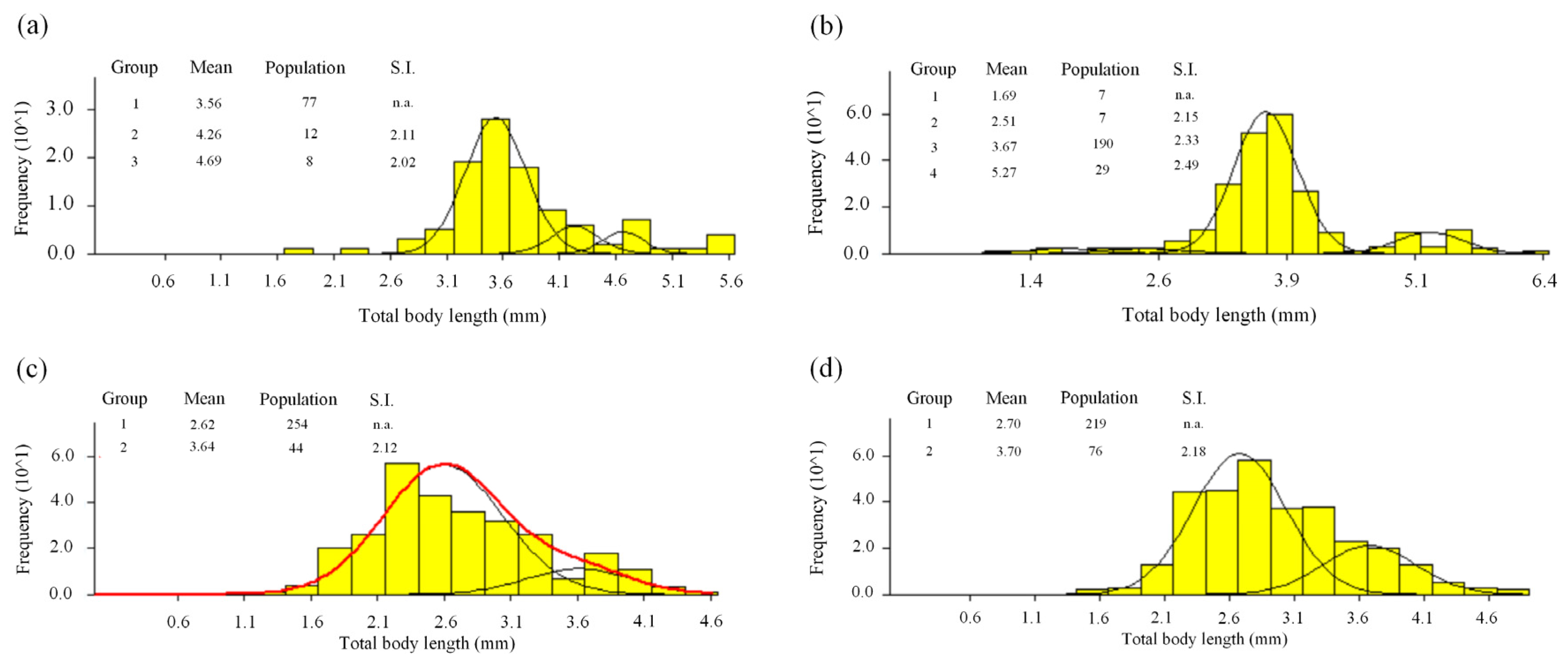


| Total Body Length (mm) | Sex Ratio (F:M) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Females | Males | ||||||||
| n | Min–Max | Mean | ±SD | n | Min–Max | Mean | ±SD | ||
| Winter | 26 | 3.30–6.00 | 4.39 | 0.50 | 65 | 2.65–4.15 | 3.38 | 0.32 | 0.40:1 * |
| Spring | 40 | 2.80–6.00 | 5.16 | 1.04 | 118 | 2.40–4.50 | 3.70 | 0.38 | 0.34:1 * |
| Summer | 15 | 2.80–4.10 | 3.26 | 0.49 | 131 | 1.75–4.50 | 2.78 | 0.55 | 0.11:1 * |
| Autumn | 104 | 2.80–5.10 | 3.97 | 0.54 | 117 | 2.30–4.00 | 3.28 | 0.40 | 0.89:1 |
| Total | 185 | 2.80–6.00 | 4.20 | 0.79 | 431 | 1.75–4.50 | 3.29 | 0.38 | 0.43:1 * |
| Total Body Length (mm) | Sex Ratio (F:M) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Females | Males | ||||||||
| n | Min–Max | Mean | ±SD | n | Min–Max | Mean | ±SD | ||
| Winter | 20 | 4.13–5.78 | 4.92 | 0.50 | 83 | 3.00–5.00 | 3.77 | 0.34 | 0.24:1 * |
| Spring | 29 | 4.20–6.38 | 5.43 | 0.41 | 195 | 2.75–4.80 | 3.84 | 0.35 | 0.15:1 * |
| Summer | 85 | 2.10–4.50 | 3.03 | 0.59 | 179 | 1.80–4.62 | 2.94 | 0.63 | 0.47:1 * |
| Autumn | 62 | 2.75–4.90 | 3.83 | 0.54 | 239 | 1.80–4.40 | 2.99 | 0.48 | 0.26:1 * |
| Total | 196 | 2.10–6.38 | 4.30 | 1.08 | 696 | 1.80–5.00 | 3.39 | 0.49 | 0.28:1 * |
| Jarun | Medsave | |||||
|---|---|---|---|---|---|---|
| Growth Parameters | Females (n = 185) | Males (n = 431) | Combined Sex (n = 616) | Females (n = 196) | Males (n = 696) | Combined Sex (n = 892) |
| Growth coefficient (K) (year−1) | 0.92 | 0.59 | 1.50 | 0.34 | 1.20 | 0.50 |
| Hypothetical age at zero size (t0) (year) | −0.57 | −0.36 | −0.75 | −0.14 | −0.64 | −0.31 |
| Asymptotic length (L∞) (mm) | 6.30 | 5.40 | 4.40 | 7.20 | 4.00 | 6.60 |
| Longevity (tmax) (year) | 3.26 | 5.08 | 2.00 | 8.82 | 2.50 | 6.00 |
| Growth index φ′ | 1.56 | 1.23 | 1.46 | 1.25 | 1.28 | 1.34 |
| Species | Sex | Mean Size (mm) | L∞ (mm) | K (year−1) | t0 (year) | M or Z (year−1) | Sampling Period | Location | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Gammarus wilkitzkii ** | Females | 19.5–49.7 | 64.6 | 0.48 | Summer 1994 | Franz Josef Land, Arctic | [120] | ||
| Males | 62.3 (max) (min–max) | 69.2 | 0.47 | ||||||
| Nototropis minikoi ** | Females | 5.12 ± 0.59 | June 2015 | Campechen Lagoon, Quintana Roo, Caribbean Sea, Mexico | [82] | ||||
| Males | 4.04 ± 0.42 | ||||||||
| Sex combined | 0.94–6.34 (min–max) | 6.66 | 0.67 | 0.18 | / | ||||
| Pseudoichestoidea brasiliensis ** | Females | 7.3–12.2 (min–max) | May 1996–December 1997 | Arachani beach, Uruguay | [80] | ||||
| Males | |||||||||
| Sex combined | 8.74 3–12.2 (min–max) | 11.64 | 1.61 | −0.175 | 2.35 (M) | ||||
| Females | 7.8–12.1 (min–max) | Barra del Chuy beach, Uruguay | |||||||
| Males | |||||||||
| Sex combined | 8.92 3–12.2 (min–max) | 11.27 | 1.88 | −0.175 | 2.47 (M) | ||||
| Synurella ambulans * | Females | 4.23 ± 0.87 | 6.75 + | 0.63 | −0.36 | 0.86 (M) | Winter 2018–autumn 2019 | Sava River, Croatia | Present study |
| Males | 3.26 ± 0.56 | 4.70 + | 0.90 | −0.50 | 2.70 (M) | ||||
| Sex combined | 3.28 ± 0.96 | 5.50 + | 1.00 | −0.53 | 1.25 (M) | ||||
| Waldeckia obesa ** | Females | 5–31 (min–max) sex combined | 7.47 | 0.50 | (0.27/year) (Z) | January–March 1998 | eastern Weddell Sea, Antarctic | [66] | |
| Males | 6.92 | 0.60 | (0.43/year) (Z) |
| Jarun | Medsave | |||||
|---|---|---|---|---|---|---|
| Parameters | Females | Males | Combined Sex | Females | Males | Combined Sex |
| Total mortality (Z, year−1) | 3.13 | 4.60 | 3.48 | 1.77 | 1.92 | 2.10 |
| Natural mortality (M, year−1) | 1.96 | 1.53 | 2.98 | 0.99 | 2.65 | 1.30 |
| M/Z (%) | 62.62 | 33.26 | 85.63 | 55.93 | 138.02 | 61.90 |
| M/K | 2.13 | 2.59 | 1.99 | 2.91 | 2.21 | 2.60 |
| Z/K | 3.40 | 7.80 | 2.32 | 5.21 | 1.60 | 4.20 |
| Annual mean temp. (°C) | 14.99 | 15.10 | ||||
| Study Site | Jarun | Medsave | ||||||
|---|---|---|---|---|---|---|---|---|
| Season | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn |
| Position (m) | 0.22 ± 0.73 −0.30–3.00 | 0.83 ± 1.35 0.00–3.00 | 0.14 ± 0.07 0.00–0.20 | 1.86 ± 2.05 0.10–5.50 | 1.34 ± 2.29 0.30–8.00 | 0.16 ± 0.69 −0.30–2.00 | 0.00 ± 0.00 0.00–0.00 | 0.10 ± 0.00 0.10–0.10 |
| Depth (cm) | 65.28 ± 4.23 57–70 | 56.88 ± 4.32 50–65 | 51.50 ± 2.07 49–55 | 56.38 ± 8.75 45–65 | 65.43 ± 1.34 62–67 | 54.00 ± 1.15 52–56 | 51.25 ± 0.96 50–52 | 48.67 ± 3.21 45–51 |
| WT (°C) | 12.97 ± 0.77 12.30–13.80 | 12.66 ± 0.06 12.55–12.75 | 16.35 ± 0.16 16.00–16.50 | 16.56 ± 0.76 16.10–18.40 | 10.84 ± 0.94 9.50–11.50 | 12.14 ± 0.04 12.10–12.20 | 23.30 ± 0.00 23.30.−23.30 | 14.50 ± 0.00 14.50–14.50 |
| DO (mg O2 L−1) | 6.09 ± 0.94 5.07–6.91 | 5.72 ± 0.16 5.45–5.88 | 4.37 ± 0.10 4.20–4.50 | 6.45 ± 1.46 4.40–7.59 | 8.41 ± 1.17 7.64–10.18 | 5.73 ± 0.15 5.50–5.90 | 6.04 ± 0.14 5.90–6.20 | 8.94 ± 0.02 8.92–8.95 |
| Sat (%) | 44.63 ± 24.24 18.30–65.70 | 56.35 ± 1.38 54.60 ± 58.60 | 44.50 ± 1.41 43–47 | 65.90–14.43 45–77 | 77.24 ± 8.06 72.20–89.50 | 56.59 ± 1.21 54.90–58.60 | 6.04 ± 0.14 5.90–6.20 | 8.94 ± 0.02 8.92–8.95 |
| pH | 8.05 ± 0.07 7.97–8.11 | 7.45 ± 0.04 7.39–7.52 | 7.42 ± 0.05 7.30–7.46 | 7.80 ± 0.24 7.51–8.00 | 8.30 ± 0.09 8.24–8.44 | 8.33 ± 0.04 8.25–8.37 | 8.51 ± 0.08 8.40–8.58 | 7.96 ± 0.06 7.92–8.03 |
| Cond (µS cm−1) | 541 ± 27.61 517–571 | 578.75 ± 2.31 575–580 | 584.88 ± 1.55 583–587 | 576.25 ± 42.25 522–634 | 447.86 ± 39.57 388–488 | 420 ± 2.38 418–425 | 431 ± 1.29 430–433 | 439 ± 1.73 437–440 |
| Alk (mg CaCO3 L−1) | 261.67 ± 15.34 245–275 | 278.13 ± 3.72 270–280 | 212.50 ± 8.02 200–225 | 279.38 ± 10.92 265–290 | 242.86 ± 11.72 225–250 | 157.25 ± 0.26 157–157.50 | 201.75 ± 2.36 200–205 | 215 ± 25.98 200–245 |
| TWH (mg CaCO3 L−1) | 225.27 ± 7.28 218.94–233.18 | 277.68 ± 0.00 | 270.56 ± 0.00 | 235 ± 33.38 202.92–267.00 | 242.08 ± 0.00 | 243.86 ± 0.00 | 137.06 ± 0.00 | 267.00 ± 0.00 |
| P-PO43− (mg L−1) | 0.08 ± 0.02 0.06–0.10 | 0.24 ± 0.00 | 0.17 ± 0.00 | 0.16 ± 0.05 0.08–0.20 | 0.06 ± 0.00 | 0.10 ± 0.00 | 0.04 ± 0.00 | 0.10 ± 0.00 |
| N-NO2− (mg L−1) | 0.12 ± 0.02 0.10–0.13 | 0.18 ± 0.00 | 0.07 ± 0.00 | 0.14 ± 0.01 0.12–0.15 | 0.14 ± 0.00 | 0.06 ± 0.00 | 0.09 ± 0.00 | 0.16 ± 0.00 |
| N-NO3− (mg L−1) | 0.69 ± 0.10 0.60–0.80 | 1.96 ± 0.00 | 0.24 ± 0.00 | 1.53 ± 0.86 0.65–2.34 | 0.46 ± 0.00 | 2.31 ± 0.00 | 1.48 ± 0.00 | 2.72 ± 0.00 |
| COD (mg O2 L−1) | 1.76 ± 0.08 1.69–1.85 | 5.34 ± 0.00 | 2.55 ± 0.00 | 2.63 ± 0.97 1.69–3.54 | 1.89 ± 0.00 | 3.77 ± 0.00 | 5.07 ± 0.00 | 4.48 ± 0.00 |
| DOC (mg L−1) | 0.63 ± 0.02 0.60–0.65 | 0.82 ± 0.00 | 0.70 ± 0.00 | 0.90 ± 0.00 | 1.36 ± 0.00 | 1.37 ± 0.00 | 1.37 ± 0.00 | 0.94 ± 0.00 |
| POC (mg L−1) | 0.71 ± 0.15 0.55–0.84 | 0.59 ± 0.00 | 0.91 ± 0.00 | 2.63 ± 0.00 | 0.13 ± 0.00 | 0.37 ± 0.20 0.13–0.52 | 0.56 ± 0.05 0.52–0.62 | 0.69 ± 0.00 |
| TOC (%) | 2.32 ± 0.00 | 1.77 ± 0.00 | 1.92 ± 0.00 | 1.73 ± 0.00 | 1.07 ± 0.00 | 2.68 ± 0.00 | 2.97 ± 0.00 | 2.83 ± 0.00 |
| PC1 | PC2 | |
|---|---|---|
| Eigen values | 4.63 | 3.69 |
| % of variance | 27.2 | 21.7 |
| Position | −0.067 | 0.206 |
| Depth | −0.163 | 0.366 |
| WT | 0.164 | −0.26 |
| DO | −0.31 | 0.193 |
| Sat | −0.226 | 0.073 |
| pH | −0.432 | −0.097 |
| Cond | 0.406 | 0.191 |
| Alk | 0.156 | 0.404 |
| TWH | 0.209 | 0.114 |
| P-PO43− | 0.381 | 0.008 |
| N-NO2− | 0.077 | 0.313 |
| N-NO3− | 0.076 | −0.323 |
| COD | 0.136 | −0.31 |
| DOC | −0.332 | −0.159 |
| POC | 0.27 | 0 |
| TOC | 0.044 | −0.402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottstein, S.; Redžović, Z.; Erk, M.; Sertić Perić, M.; Dautović, J.; Cindrić, M. Life History Traits of the Stygophilous Amphipod Synurella ambulans in the Hyporheic Zone of the Lower Reaches of the Upper Sava River (Croatia). Water 2023, 15, 3188. https://doi.org/10.3390/w15183188
Gottstein S, Redžović Z, Erk M, Sertić Perić M, Dautović J, Cindrić M. Life History Traits of the Stygophilous Amphipod Synurella ambulans in the Hyporheic Zone of the Lower Reaches of the Upper Sava River (Croatia). Water. 2023; 15(18):3188. https://doi.org/10.3390/w15183188
Chicago/Turabian StyleGottstein, Sanja, Zuzana Redžović, Marijana Erk, Mirela Sertić Perić, Jelena Dautović, and Mario Cindrić. 2023. "Life History Traits of the Stygophilous Amphipod Synurella ambulans in the Hyporheic Zone of the Lower Reaches of the Upper Sava River (Croatia)" Water 15, no. 18: 3188. https://doi.org/10.3390/w15183188





