Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Chemicals and Analysis
2.2. Biosorbent Material Collection and Preparation
2.3. Chromium Adsorption Using Batch System
2.4. Modeling and Statistical Analysis
2.5. Analyses of Rice Husk Using FTIR Spectroscopy
3. Results and Discussion
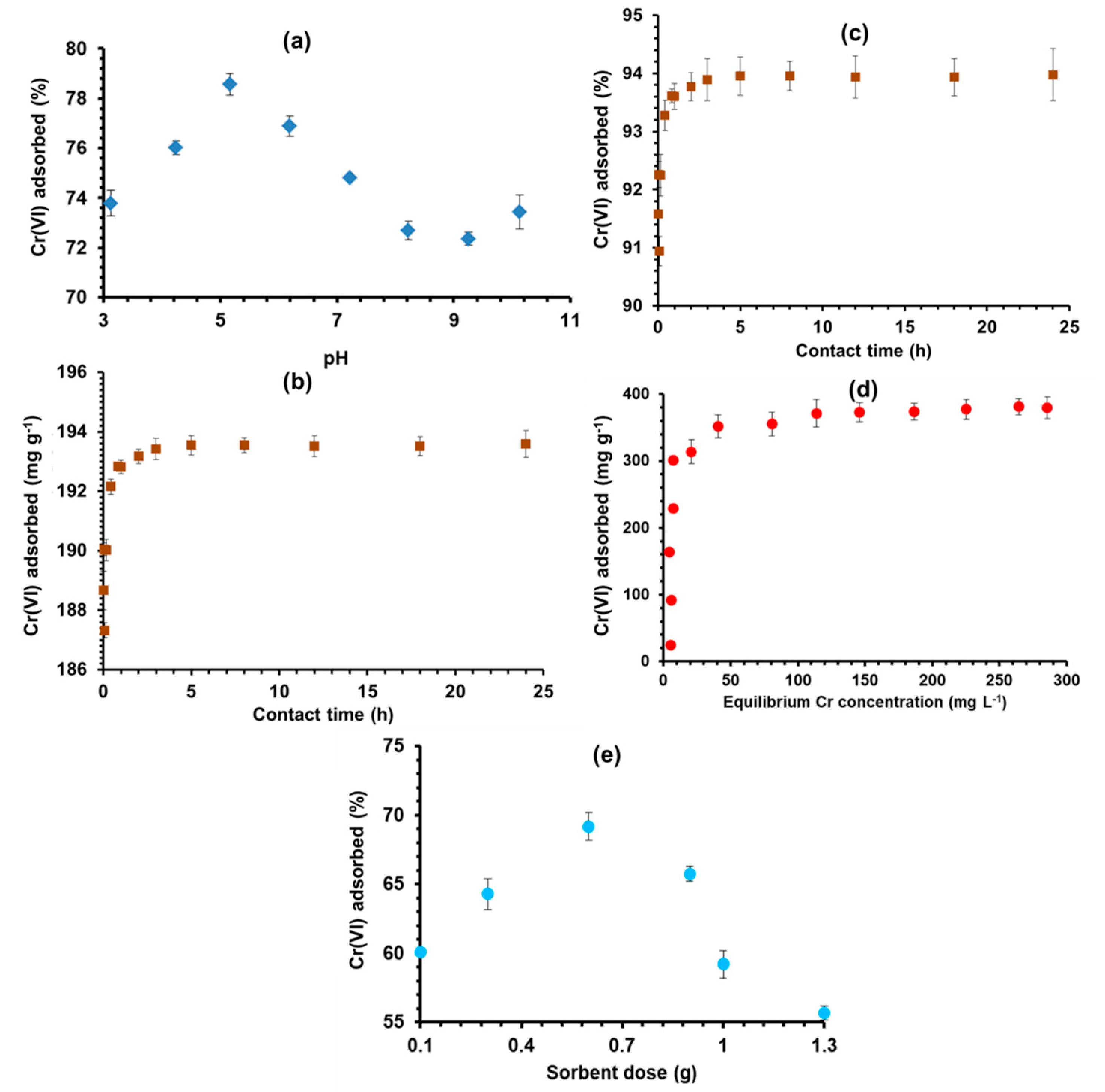
3.1. Influence of Varying pH Levels
3.2. Contact Time
3.3. Initial Chromium Concentration
3.4. Sorbent Dosage
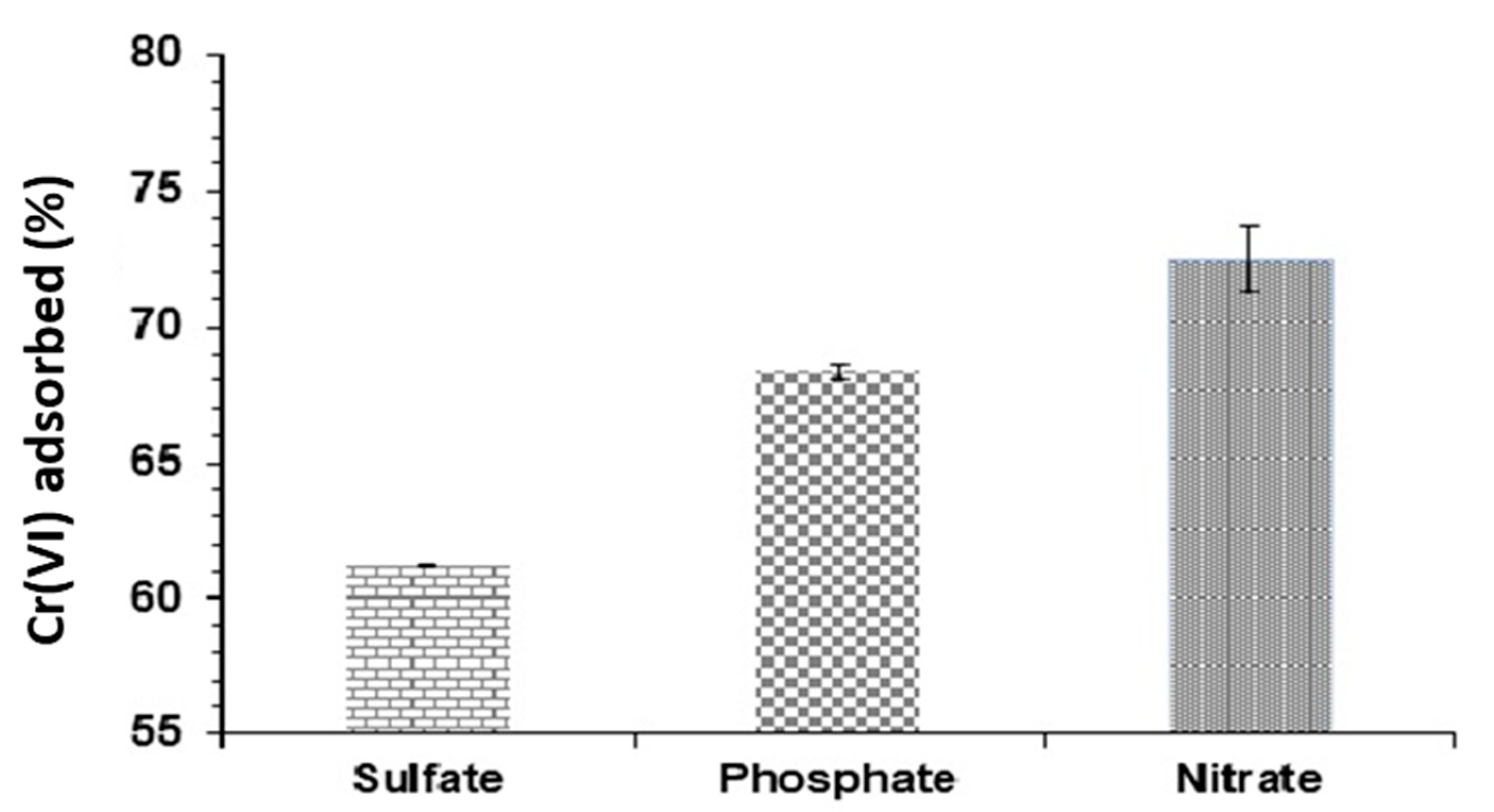
3.5. Co-occurring Ions
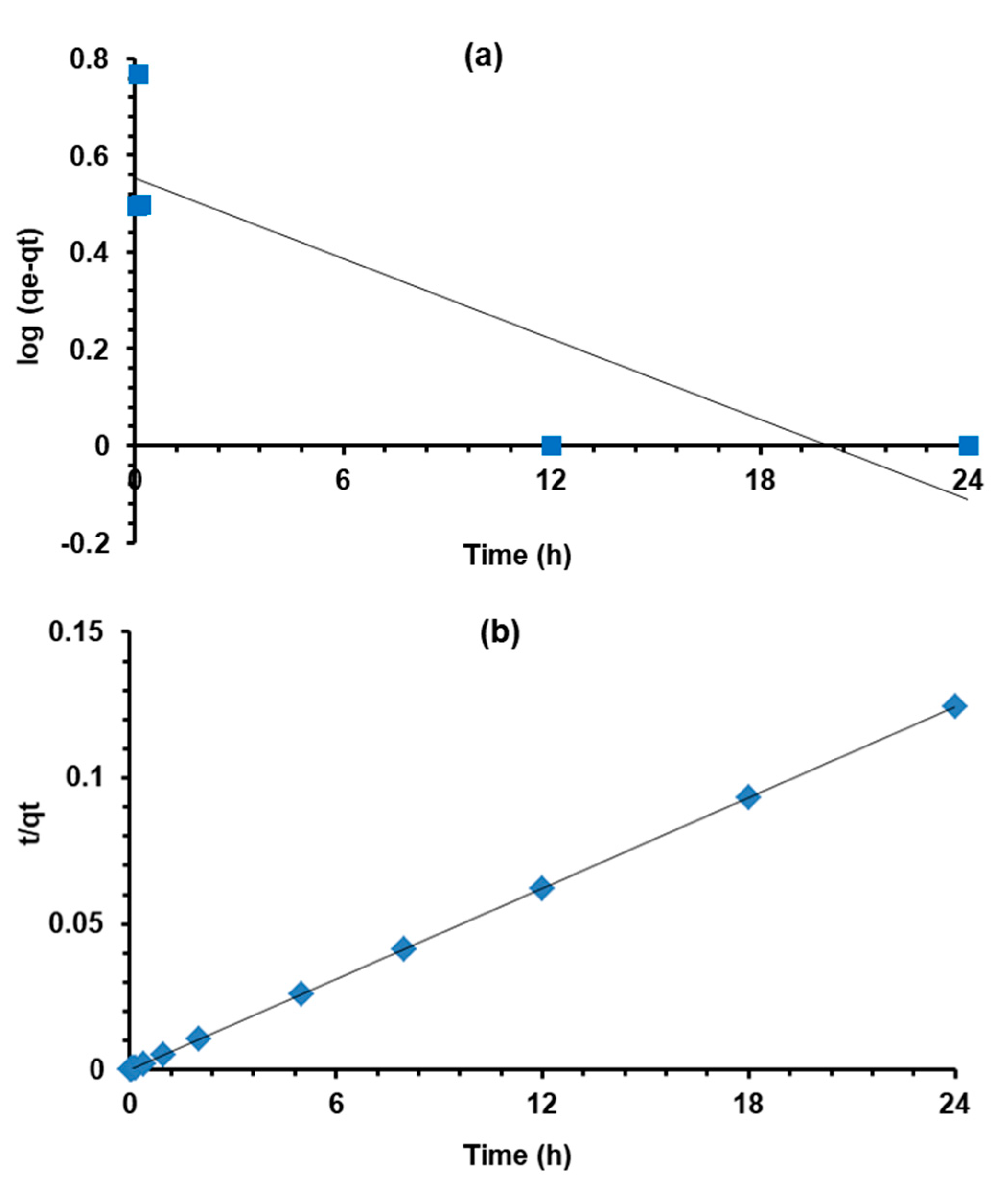
3.6. Sorption Kinetic Modeling
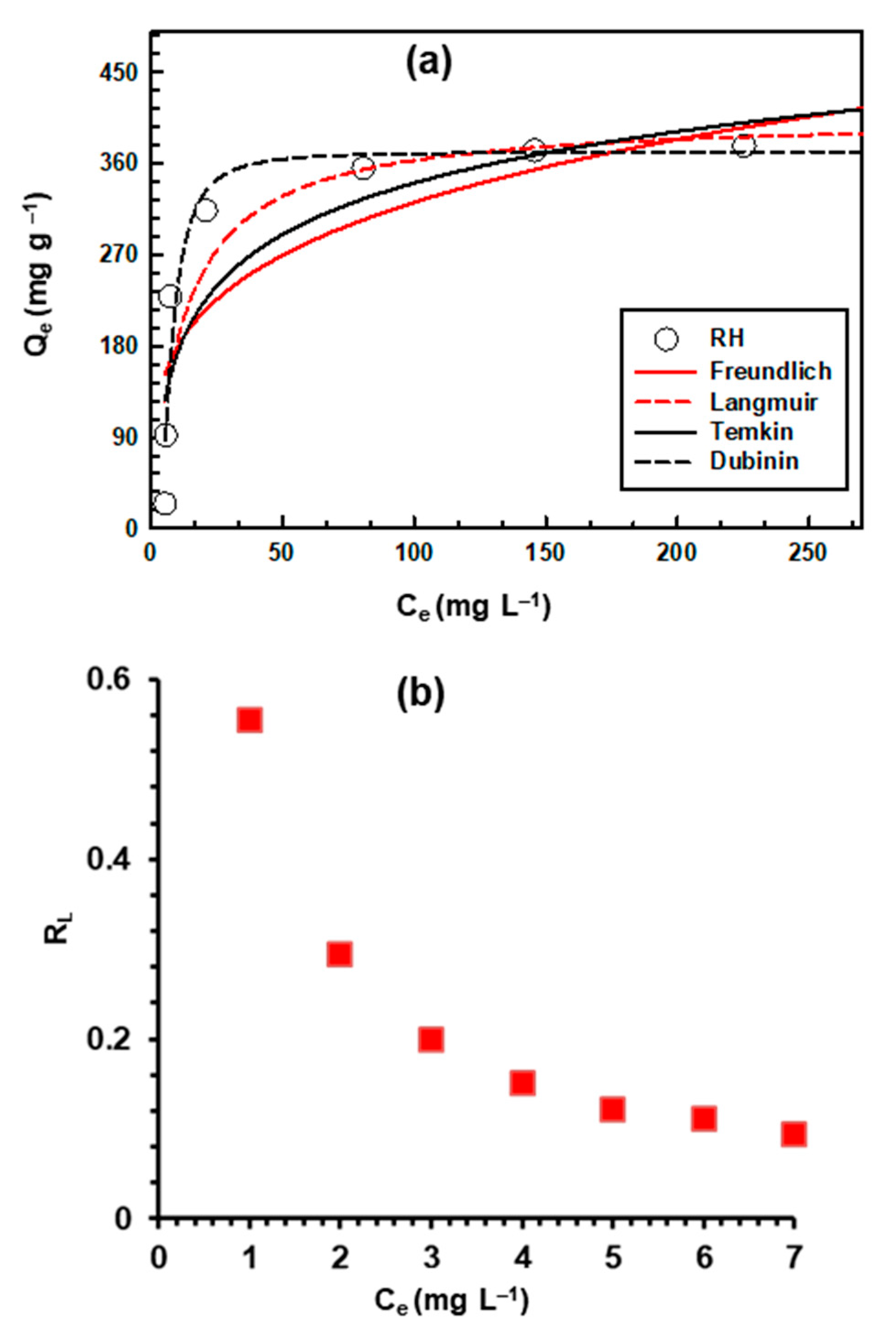
3.7. Sorption Isotherm Modeling
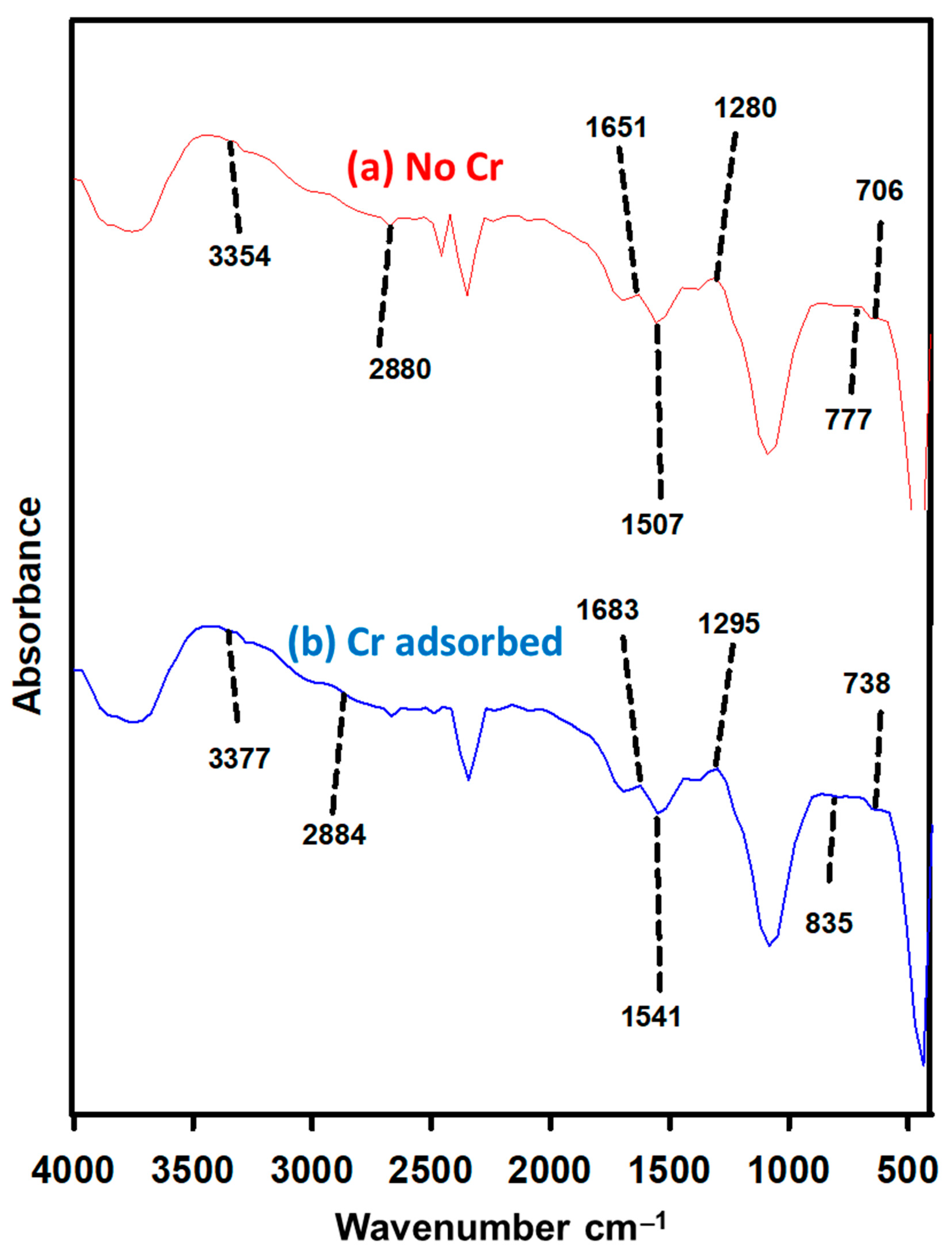
3.8. Cr (VI) Biosorption Mechanism through FTIR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T.V. Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram (Cicer arientinum). Electr. J. Biotechnol. 2005, 8, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Dhanakumar, S.; Solaraj, G.; Mohanraj, R.; Pattabhi, S. Removal of Cr (VI) from aqueous solution by adsorption using cooked tea dust. Ind. J. Sci. Technol. 2007, 1, 1–6. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Naghipour, D.; Vaezi, F.; Nazmara, S. Teawaste as an adsorbent for heavy metal removal from industrial wastewaters. Am. J. Appl. Sci. 2005, 2, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, S.; Kannadasan, T.; Velmurugan, S.; Muthu, S.; Vinoth Kumar, P. Biosorption of chromium (VI) from industrial effluent using neem leaf adsorbent. Res. J. Chem. Sci. 2013, 3, 48–53. [Google Scholar]
- Monnot, A.D.; Christian, W.V.; Paustenbach, D.J.; Finley, B.L. Correlation of blood Cr(III) and adverse health effects: Application of PBPK modeling to determine non-toxic blood concentrations. Crit. Rev. Toxicol. 2014, 44, 618–637. [Google Scholar] [CrossRef]
- Levankumar, L.; Uthukumaran, V.; Gobinath, M. Batch adsorption and kinetics of chromium(VI) removal from aqueous solutions by Ocimum americanum L. seed pods. J. Hazard. Mater. 2009, 161, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.N.; Pandey, N.; Thomas, H. Adsorption of Cr (VI) from aqueous solutions on tea waste and coconut husk. Indian J. Chem. Technol. 2012, 19, 111–117. [Google Scholar]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surfaces B Biointerfaces 2008, 63, 116–121. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Hamdy, W.; Kassem, A.; Abdel Hady, A. Comparison of different rice straw based adsorbents for chromium removal from aqueous solutions. Desalin. Water Treat. 2016, 57, 6991–6999. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 1–38. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S.; et al. Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Ahmed, I.; Attar, S.J.; Parande, M.G. Removal of Hexavalent Chromium (Cr (VI)) from Industrial Wastewater by Using Biomass Adsorbent (Rice Husk Carbone). Int. J. Adv. Eng. Stud. 2012, 1, 92–94. [Google Scholar]
- Park, D.; Yun, Y.S.; Ahn, C.K.; Park, J.M. Kinetics of the reduction of hexavalent chromium with the brown seaweed Ecklonia biomass. Chemosphere 2007, 66, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.S.; Atoyebi, O.M.; Adegoke, K.A.; Fehintola, E.O.; Ojo, A.O. Removal of toxicant chromium (VI) from aqueous solution using different adsorbents. J. Chem. Soc. Pak. 2015, 37, 190. [Google Scholar]
- Vempati, R.K.; Musthyala, S.C.; Mollah, M.Y.A.; Cocke, D.L. Surface analyses of pyrolysed rice husk using scanning force microscopy. Fuel 1995, 74, 1722–1725. [Google Scholar] [CrossRef]
- Gao, W.; Li, H.; Karnowo; Song, B.; Zhang, S. Integrated Leaching and Thermochemical Technologies for Producing High-Value Products from Rice Husk: Leaching of Rice Husk with the Aqueous Phases of Bioliquids. Energies 2020, 13, 6033. [Google Scholar] [CrossRef]
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of Rice Husk Ash-Based Geopolymers Coating Composite for Enhancement in Flexural Properties and Microstructure Using Response Surface Methodology. Coatings 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M. Tea Waste as a Potential Biowaste for Removal of Hexavalent Chromium from Wastewater: Equilibrium and kinetic studies. Arab. J. Geosci. 2018, 11, 573. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Nawaz, R.; Hussain, F.; Raza, M.; Ali, S.; Rizwan, M.; Ahmad, S. Human health implications, risk assessment and remediation of As-contaminated water: A critical review. Sci. Total Environ. 2017, 601, 756–769. [Google Scholar] [CrossRef]
- Ucun, H.; Bayhan, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of chromium (VI) from aqueous solution by cone biomass of Pinus sylvestris. Bioresour. Technol. 2002, 85, 155–158. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Naushad, M.; Ali, R. Kinetic, equilibrium isotherm and thermodynamic studies of Cr (VI) adsorption onto low-cost adsorbent developed from peanut shell activated with phosphoric acid. Environ. Sci. Pollut. Res. 2013, 20, 3351–3365. [Google Scholar] [CrossRef] [PubMed]
- Singanan, M. Removal of lead (II) and cadmium (II) ions from wastewater using activated biocarbon. Sci. Asia 2011, 37, 115–119. [Google Scholar] [CrossRef]
- Sattar, M.S.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Niazi, N.K.; Jilani, A. Comparative efficiency of peanut shell and peanut shell biochar for removal of arsenic from water. Environ Sci Pollut Res. 2019, 26, 18624–18635. [Google Scholar] [CrossRef] [PubMed]
- Tajernia, H.; Ebadi, T.; Nasernejad, B.; Ghafori, M. Arsenic removal from water by sugarcane bagasse: An application of response surface methodology (RSM). Water Air Soil Pollut. 2014, 225, 2028. [Google Scholar] [CrossRef]
- Okafor, P.C.; Okon, P.U.; Daniel, E.F.; Ebenso, E.E. Adsorption capacity of coconut (Cocos nucifera L.) shell for lead, copper, cadmium and arsenic from aqueous solutions. Int. Electrochem. Sci. 2012, 7, 12354–12369. [Google Scholar]
- Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Danish, M.; Alghamdi, M.M. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes 2019, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Razmovski, R.; Šćiban, M. Biosorption of Cr (VI) and Cu (II) by waste tea fungal biomass. Ecol. Eng. 2008, 34, 179–186. [Google Scholar] [CrossRef]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farooqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic (V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremediation 2016, 18, 442–449. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A. Advanced biosorbents materials for removal of chromium from water and wastewaters. Environ. Prog. Sustain. Energy 2011, 30, 284–293. [Google Scholar] [CrossRef]
- Pehlivan, E.; Tran, H.T.; Ouedraogo, W.K.I.; Schmidt, C.; Zachmann, D.; Bahadir, M. Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As(V) from aqueous solutions. Food Chem. 2013, 138, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, R.; Manzoor, Q.; Iqbal, M.; Nisar, J. Biosorption of Pb (II) onto immobilized and native Mangifera indica waste biomass. J. Ind. Eng. Chem. 2015, 35, 185–195. [Google Scholar] [CrossRef]
- Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Shakoor, M.B.; Jilani, A.; Anjum, R. High sorption efficiency for As (III) and As (V) from aqueous solutions using novel almond shell biochar. Chemosphere 2020, 243, 125330. [Google Scholar] [CrossRef] [PubMed]
- Owalude, S.O.; Tella, A.C. Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Vinodhini, V.; Das, N. Mechanism of Cr (VI) biosorption by neem sawdust. Am.-Eurasian J. Sci. Res. 2009, 4, 324–329. [Google Scholar]
- Khazaei, I.; Aliabadi, M.; Hamed, M.H. Use of agricultural waste for removal of Cr(VI) from aqueous solution. Iran. J. Chem. Eng. 2011, 8, 11–23. [Google Scholar]
- Singha, B.; Naiya, T.K.; Kumar, B.A.; Das, S.K. Cr (VI) ions removal from aqueous solutions using natural adsorbents–FTIR studies. J. Environ. Prot. 2011, 2, 729. [Google Scholar] [CrossRef]
- Yi, Y.; Lv, J.; Liu, Y.; Wu, G. Synthesis and application of modified Litchi peel for removal of hexavalent chromium from aqueous solutions. J. Mol. Liquids 2017, 225, 28–33. [Google Scholar] [CrossRef]
- Peng, S.H.; Wang, R.; Yang, L.Z.; He, L.; He, X.; Liu, X. Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotoxicol. Environ. Saf. 2018, 165, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Meijide, J.; Tavares, T.; Pazos, M.; Sanromán, M.A. Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalent chromium. Proc. Saf. Environ. Prot. 2016, 101, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Vu, X.H.; Nguyen, L.H.; Van, H.T.; Nguyen, D.V.; Nguyen, T.H.; Nguyen, Q.T.; Ha, L.T. Adsorption of Chromium (VI) onto Freshwater snail shell-derived biosorbent from aqueous solutions: Equilibrium, kinetics, and thermodynamics. J. Chem. 2019. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Bhatnagar, A.; Ehrampoush, M.H.; Amrollahi, M.; Jamshidi, B.; Dehvari, M.; Taghavi, M. Biosorption of hexavalent chromium from aqueous solution onto pomegranate seeds: Kinetic modeling studies. Int. J. Environ. Sci. Technol. 2017, 14, 331–340. [Google Scholar] [CrossRef]
- Hu, H.; Gao, Y.; Wang, T.; Sun, L.; Zhang, Y.F.; Li, H. Removal of hexavalent chromium, an analogue of pertechnetate, from aqueous solution using bamboo (Acidosasa edulis) shoot shell. J. Radioanal. Nucl. Chem. 2019, 321, 427–437. [Google Scholar] [CrossRef]
- Aman, A.; Ahmed, D.; Asad, N.; Masih, R.; Abd ur Rahman, H.M. Rose biomass as a potential biosorbent to remove chromium, mercury and zinc from contaminated waters. Int. J. Environ. Stud. 2018, 75, 774–787. [Google Scholar] [CrossRef]
- Xie, Y.; Li, H.; Wang, X.; Ng, I.S.; Lu, Y.; Jing, K. Kinetic simulating of Cr (VI) removal by the waste Chlorella vulgaris biomass. J. Taiwan Inst. Chem. Eng. 2014, 45, 1773–1782. [Google Scholar] [CrossRef]
| Pseudo-First Order | Pseudo-Second Order |
|---|---|
| k1 (min−1) | k2 (g mg−1 min−1) |
| 0.02 | 0.005 |
| Isotherm Modelr | Parameters | Obtained Value |
|---|---|---|
| Langmuir | QL (mg g−1) | 33.68 |
| R2 | 0.84 | |
| KL (L g−1) | 0.08 | |
| Freundlich | QF (mg1−n g−1 Ln) | 99.5 |
| R2 | 0.70 | |
| n | 0.25 | |
| Temkin | b | 1.04 |
| R2 | 0.77 | |
| A | 73.28 | |
| Dubinin–Redushkevich | QD (mg g−1) | 371.73 |
| R2 | 0.93 | |
| E (kJ g−1) | 0.02 |
| Biosorbent | Adsorption (mg g−1) | References |
|---|---|---|
| Litchi peel | 7.05 | Yi et al. [40] |
| Foxtail millet shell | 11.70 | Peng et al. [41] |
| Grapefruit peelings | 39.06 | Rosales et al. [42] |
| Freshwater snail shell | 8.85 | Vu et al. [43] |
| Pomegranate seeds | 3.32 | Ghaneian et al. [44] |
| Bamboo shoot shell | 28.72 | Hu et al. [45] |
| Rose biomass | 5.26 | Aman et al. [46] |
| Waste Chlorella vulgaris biomass | 43.3 | Xie et al. [47] |
| Rice husk | 351.92 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, U.; Shakoor, M.B.; Ali, S.; Ahmad, S.R.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation. Water 2021, 13, 263. https://doi.org/10.3390/w13030263
Khalil U, Shakoor MB, Ali S, Ahmad SR, Rizwan M, Alsahli AA, Alyemeni MN. Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation. Water. 2021; 13(3):263. https://doi.org/10.3390/w13030263
Chicago/Turabian StyleKhalil, Usman, Muhammad Bilal Shakoor, Shafaqat Ali, Sajid Rashid Ahmad, Muhammad Rizwan, Abdulaziz Abdullah Alsahli, and Mohammed Nasser Alyemeni. 2021. "Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation" Water 13, no. 3: 263. https://doi.org/10.3390/w13030263
APA StyleKhalil, U., Shakoor, M. B., Ali, S., Ahmad, S. R., Rizwan, M., Alsahli, A. A., & Alyemeni, M. N. (2021). Selective Removal of Hexavalent Chromium from Wastewater by Rice Husk: Kinetic, Isotherm and Spectroscopic Investigation. Water, 13(3), 263. https://doi.org/10.3390/w13030263







