Kinetics and Mechanistic Studies of Photochemical and Oxidative Stability of Galaxolide
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Analytical Procedures
2.2.1. Absorbance (A) Measurements
2.2.2. Chromatographic Analysis
2.3. Irradiation Procedures
2.3.1. Direct Photolysis
2.3.2. H2O2-Assisted Photodegradation Process
2.4. Separation of the Products of Photodecomposition of HHCB from an Aqueous Matrix
2.5. Computational Methodology
3. Results and Discussion
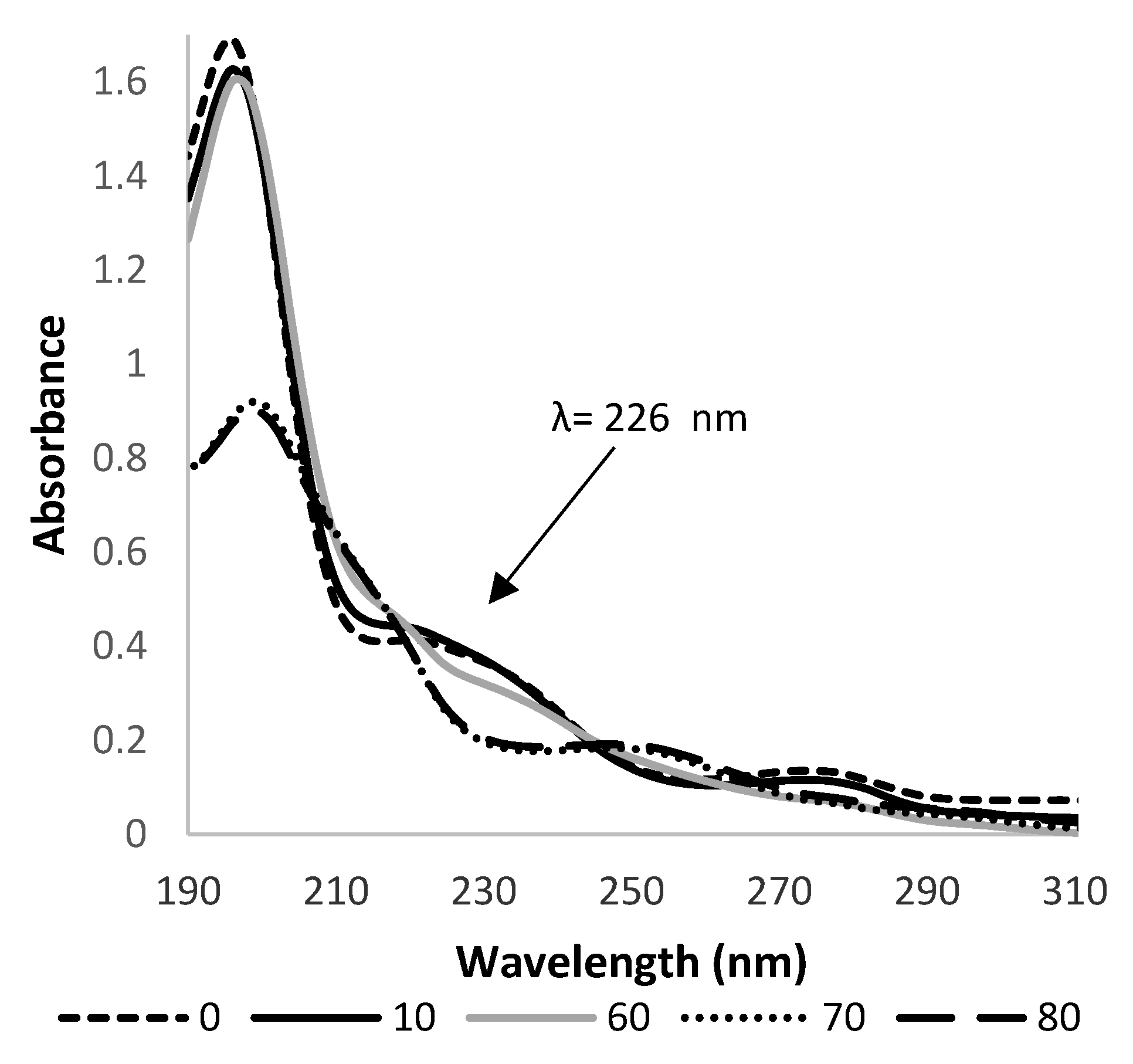
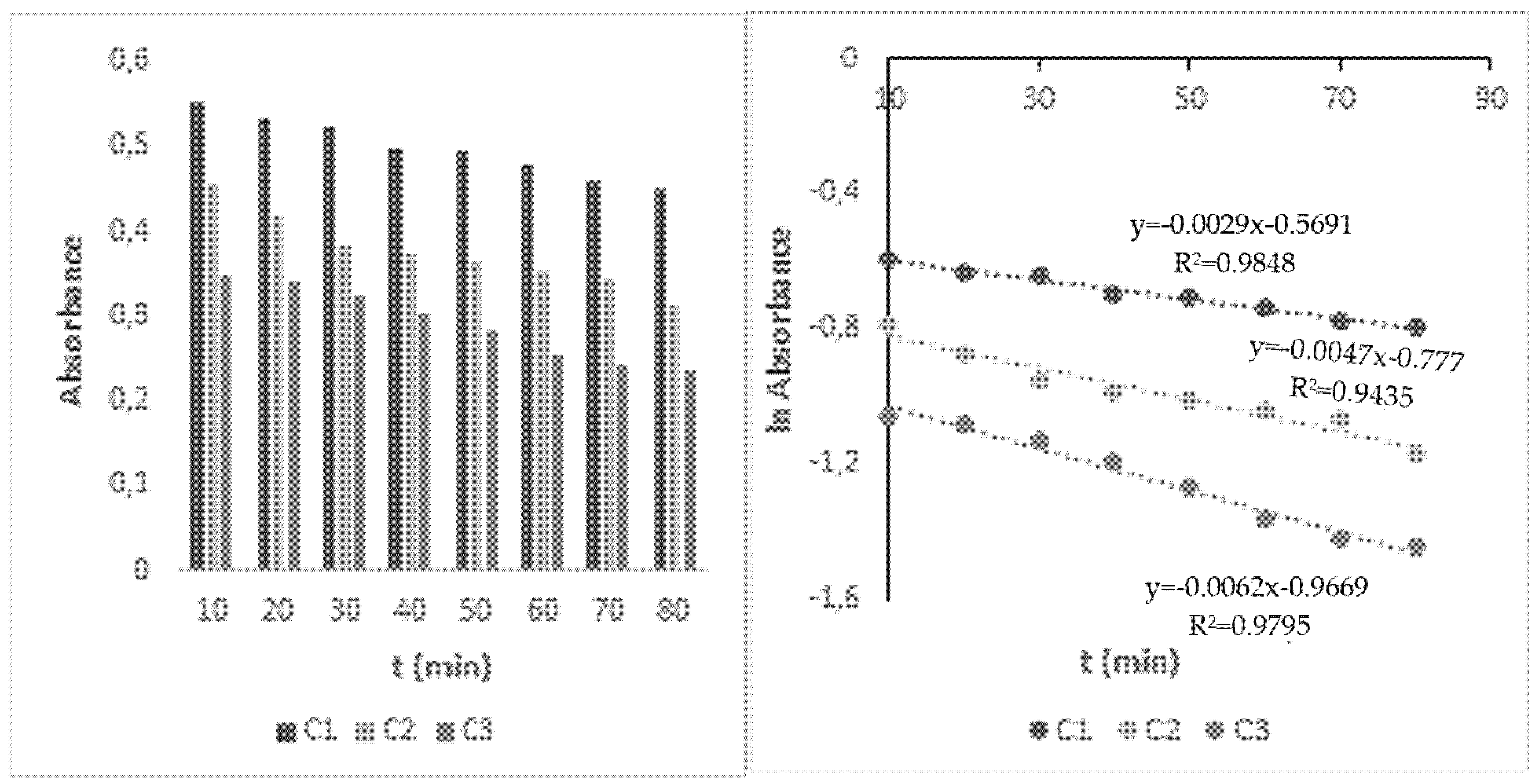
3.1. Kinetics of HHCB Direct Photolysis under Influence of Light
3.2. Kinetics of HHCB Decomposition under Influence of UV/H2O2
3.3. Photolysis of HHCB in Environmental Condition
3.4. Identification of Transformation Photoproducts of HHCB
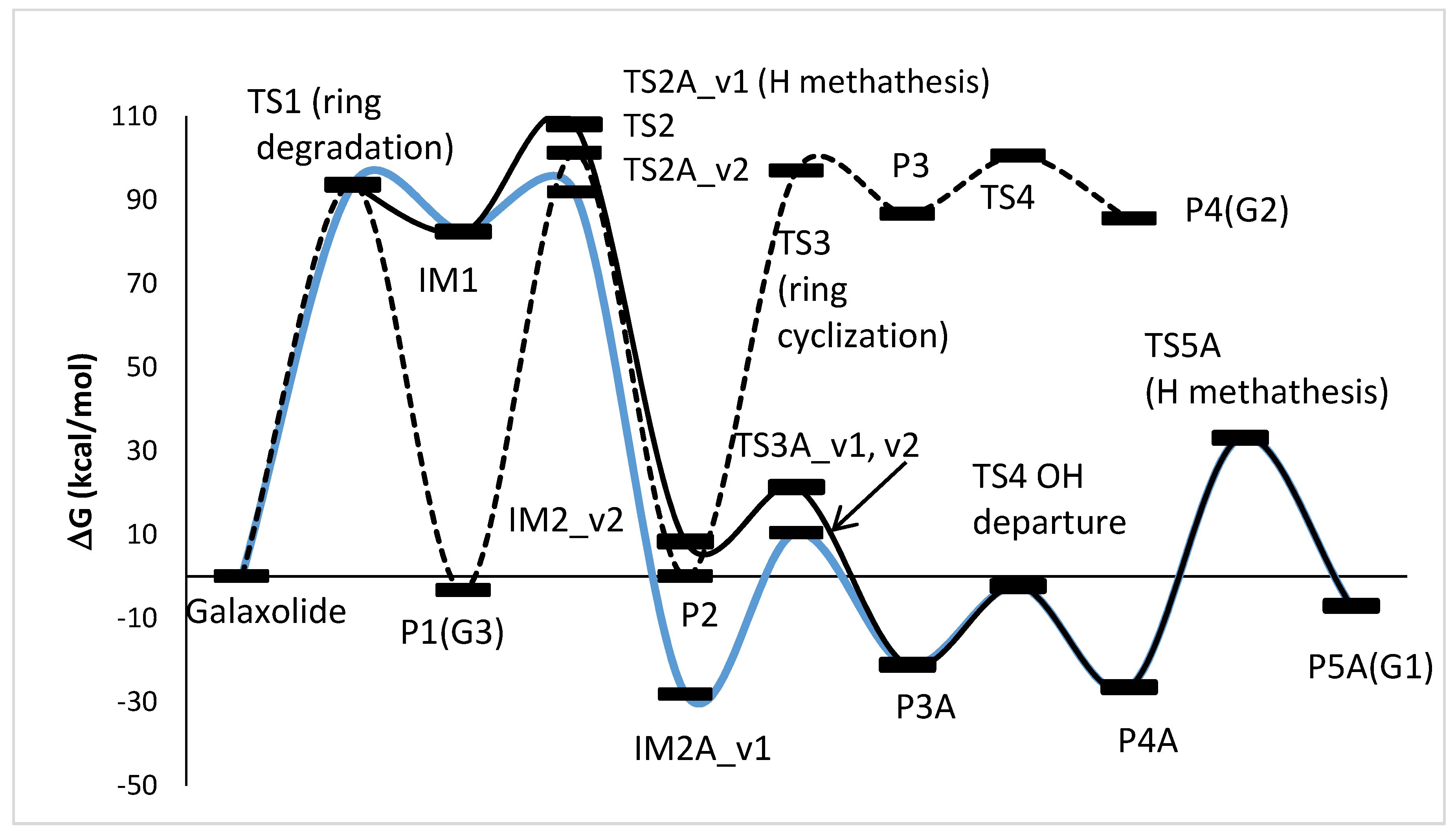
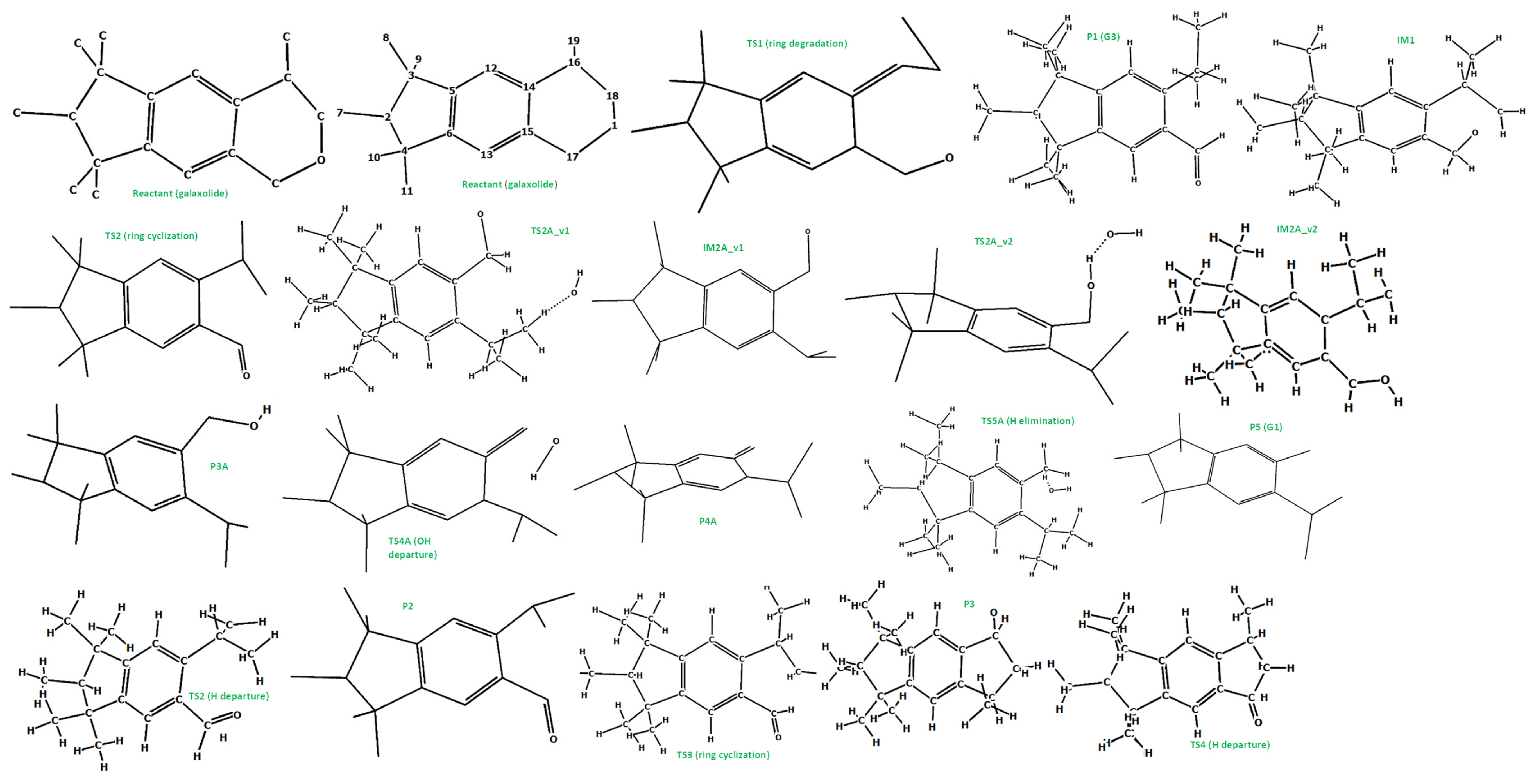
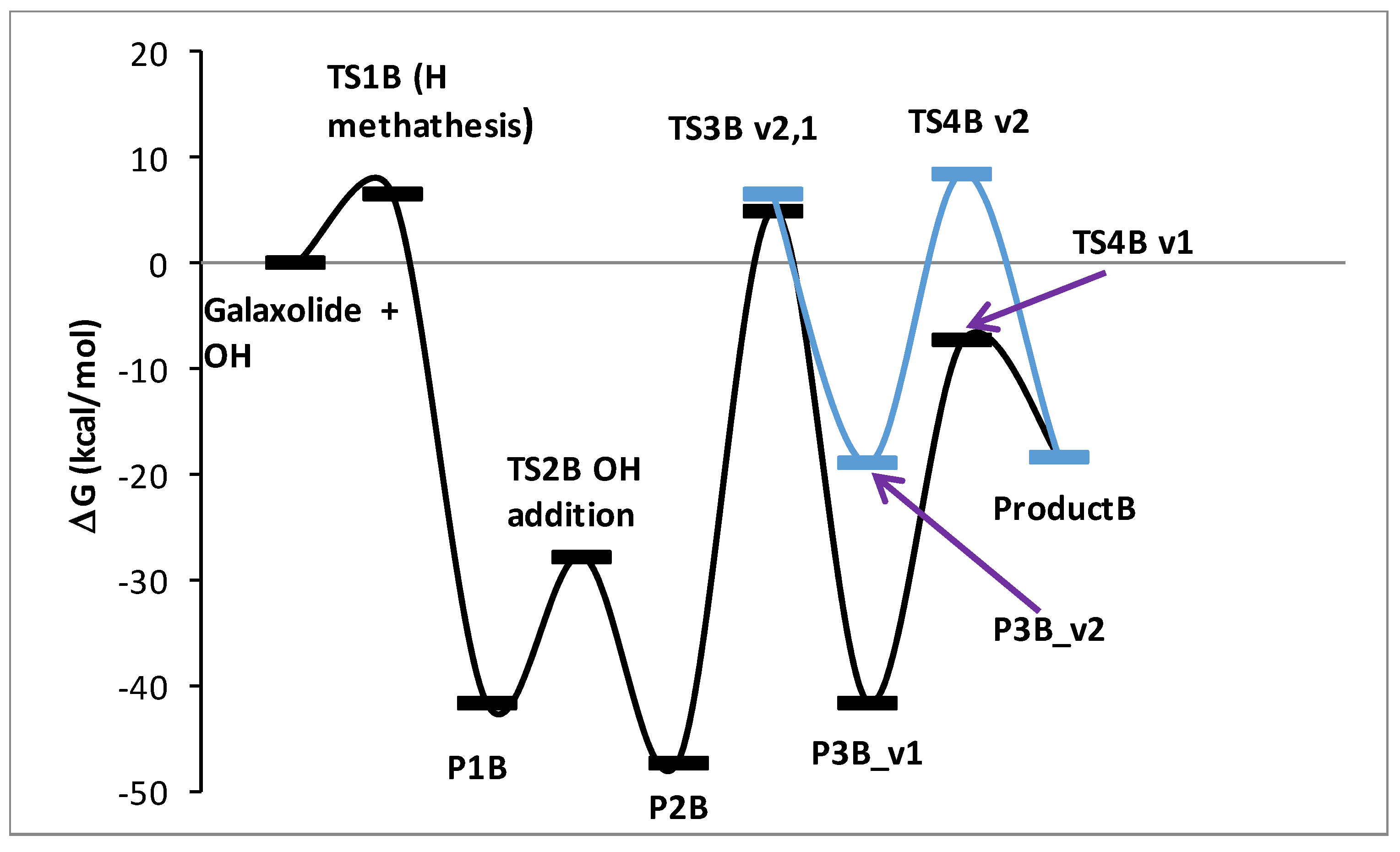
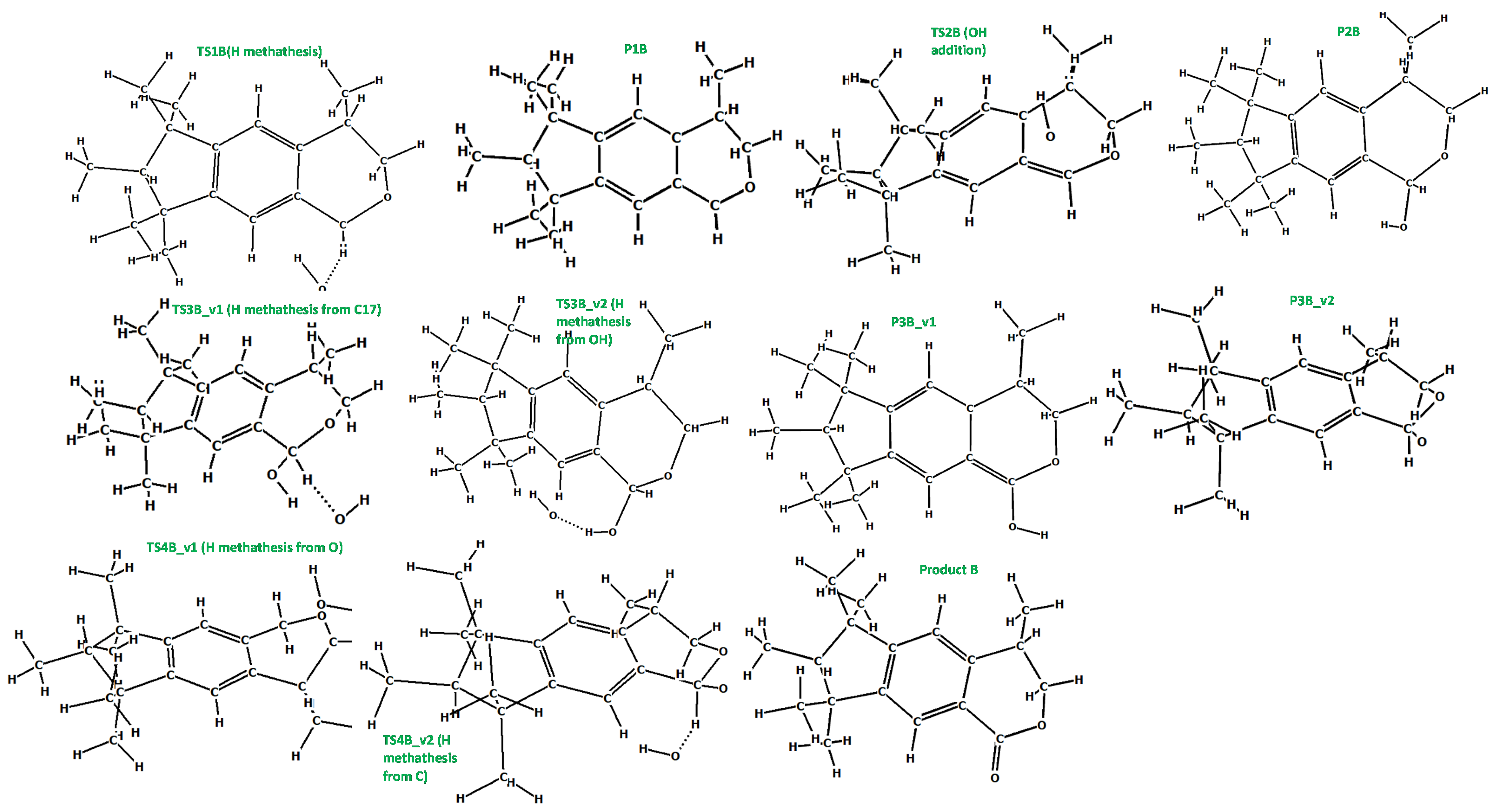
3.5. A DFT Mechanistic Study of the Galaxolide Decay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiner, J.L.; Kannan, K. A survey of polycyclic musks in selected household commodities from the United States. Chemosphere 2005, 62, 867–873. [Google Scholar] [CrossRef] [PubMed]
- European Union Risk Assessment Report. In 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-Hexamethylcyclopenta-γ-2-Benzopyran, (1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-Hexamethylin-Deno[5,6-C]Pyran-Hhcb); 1222-05-5; European Chemicals Bureau: Ispra, Italy, 2008.
- Bester, K. Polycyclic musks in the Ruhr catchment area–transport discharges of waste water, and transformation of HHCB, AHTN and HHCB-lactone. J. Environ. Monit. 2005, 7, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bester, K. Analysis of musk fragrances in environmental samples. J. Chromatogr. A 2009, 1216, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.M.; Hornbuckle, K.C. Synthetic musk fragrances in Lake Michigan. Environ. Sci. Technol. 2004, 28, 367–372. [Google Scholar] [CrossRef]
- Peng, F.-J.; Ying, G.-G.; Pan, C.G.; Seleck, H.; Salvino, D.; Van den Brink, P.J. Bioaccumulation and biotransformation of triclosan and galaxolide in the freshwater oligocaete Limnodrilus hoffmeisteri in a water/sediment microcosm. Environ. Sci Technol. 2018, 52, 8390–8398. [Google Scholar] [CrossRef] [Green Version]
- Havranek, I.; Coutris, C.; Norli, H.R.; River, P.-A.; Joner, E.J. Uptake and elimination kinetics of the biocide triclosan and the synthetic musks galaxolide and tonalide in the earthworm Dendrobaena veneta when exposed to sewage sludge. Environ. Toxicol. Chem. 2017, 36, 2068–2073. [Google Scholar] [CrossRef]
- Cunha, S.C.; Trabalon, L.; Jacobs, S.; Castro, M.; Fernandez-Tejedor, M.; Granby, K.; Verbeke, W.; Kwadijk, C.; Ferrari, F.; Robbens, J.; et al. UV-filters and musk fragrances in seafood commercialized in Europe Union: Occurrence, risk and exposure assessment. Environ. Res. 2018, 161, 399–408. [Google Scholar] [CrossRef]
- Macherius, A.; Eggen, T.; Lorenz, W.G.; Reemtsma, T.; Winkler, U.; Moeder, M. Uptake of galaxolide, tonalide, and triclosan by carrot, barley, and meadow fescue plants. J. Agric. Food Chem. 2012, 60, 7785–7791. [Google Scholar] [CrossRef]
- Rivier, P.-A.; Havranek, I.; Courtis, C.; Norli, H.R. Joner, E.J. Transfer of organic pollutants from sewage sludge to earthworms and barley under field conditions. Chemosphere 2019, 222, 954–960. [Google Scholar] [CrossRef]
- Sanyal, S.; Amrani, F.; Dallongeviille, A.; Banerjee, S.; Blanchard, O.; Deguen, S.; Costet, N.; Zmirou-Navier, D.; Annesi-Maesano, I. Estimating indoor galaxolide concentrations using predictive models based on objective assessments and data about dwelling characteristics. Inhal. Toxicol. 2017, 29, 611–619. [Google Scholar] [CrossRef]
- Eschke, H.-D. Synthetic musks in different water matrices. In The Handbook of Environmental Chemistry; Rimkus, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3, pp. 17–28. [Google Scholar]
- Balk, F.; Ford, R.A. Environmental risk assessment for the polycyclic musks AHTN and HHCB in the EU: I. Fate and Exposure Assessment. Toxicol. Lett. 1999, 111, 57–79. [Google Scholar] [CrossRef]
- Rusconi, M.; Brenna, E.; Polesello, S. Can the ratio galaxolide-lactone: Galaxolide be a good tracer of wastewater in freshwaters? Integr. Environ. Assess. Manag. 2017, 13, 214–216. [Google Scholar] [CrossRef]
- Ilyas, H.; van Hullebush, E.D. Performance comparison of different constructed wetlands for the removal of personal care products. Environ. Res. Public Health 2020, 17, 3091. [Google Scholar]
- Bester, K. Retention characteristics and balance assessment for two polycyclic musk fragrances (HHCB and AHTN) in a typical German sewage treatment plant. Chemosphere 2020, 57, 863–870. [Google Scholar] [CrossRef]
- Simonich, S.L.; Federle, T.W.; Eckhoff, W.S.; Rottiers, A.; Webb, S.; Sabaliunas, D.; de Wolf, W. Removal of fragrance materials during U.S. and European wastewater treatment. Environ. Sci. Technol. 2020, 36, 2839–2847. [Google Scholar] [CrossRef]
- Tasselli, S.; Guzzella, L. Polycyclic musk fragrances (PMFs) in wastewater and activated sludge: Analytical protocol and application to a real case study. Environ. Sci. Pollut. Res. 2020, 27, 30977–30986. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, J.A.E.; Trought, K.; Mitchell, C.; Northcott, G.; Tremblay, L.A. Assessment of endocrine disruption and oxidative potential of bisphenol A, triclosan, nonylophenol, diethylhexyl phthalate, galaxolide, and carbamazepine, common contaminants of municipal biosolids. Toxicol. Vitr. 2018, 48, 342–349. [Google Scholar] [CrossRef]
- Parolini, M.; Magni, S.; Traversi, I.; Villa, S.; Finizio, A.; Binelli, A. Environmentally relevant concentrations of galaxolide (HHCB) and tonalide (AHTN) induced oxidative and genetic damage in Dreissena polymorpha. J. Hazard. Mater. 2015, 285, 1–10. [Google Scholar] [CrossRef]
- Ehiguese, F.O.; Alam Md, R.; Pinado-herrera, M.; Araujo, C.V.M.; Martin-Diaz, M.L. Potential of environmental concentrations of the musks galaxolide and tonalide to induce oxidative stress and genotoxicity in the marine environment. Mar. Environ. Res. 2020, 160, 105019–105027. [Google Scholar] [CrossRef]
- Ding, T.; Li, W.; Cai, M.; Jia, X.; Yang, M.; Yang, B.; Li, J. Algal toxicity, accumulation and metabolic pathway of galaxolide. J. Hazard. Mater. 2020, 384, 121360–121366. [Google Scholar] [CrossRef]
- Martin, C.; Moeder, M.; Daniel, X.; Krauss, G.; Schlosser, D. Biotransformation of the polycyclic musks HHCB and AHTN and metabolite formation by fungi occurring in freshwater environments. Environ. Sci. Technol. 2007, 41, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.; Cruz, A.; Santos, L.; Alvares, A. Human dermal exposure to galaxolide from personal care products. Int. J. Cosmet. Sci. 2013, 35, 299–309. [Google Scholar] [CrossRef]
- Kannan, K.; Reiner, J.L.; Yun, S.H.; Perrotta, E.E.; Tao, L.; Johnson-Restrepo, B.; Rodan, B.D. Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States. Chemosphere 2005, 61, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, H.; Li, J.; Wu, Y.; Shao, B. Occurrence of synthetic musks in human breast milk samples from twelve provinces in China. Food Addit. Contam. A 2016, 33, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Moribe, M.; Inoue, K.; Someya, T.; Ryuda, T.; Ichiba, M.; Miyamata, T.; Kunisue, T.; In, H.; Marud, K.; et al. Synthetic musk fragrances in human breast milk and adipose tissue from Japan. Environ. Res. Asia 2009, 247–252. [Google Scholar]
- Correia, P.; Cruz, A.; Santos, L.; Alvares, A. Risk of children’s dermal exposure to galaxolide through personal care products. Cosmetics 2015, 2, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Bagasra, O.; Golkar, Z.; Garcia, M.; Rice, L.N.; Pace, D.G. Role of perfumes in pathogenesis of autism. Med. Hypothesis 2013, 80, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Prado, L.; Lourido, M.; Lores, M.; Llompart, M.; Garcia-Jares, C.; Cela, R. Study of the photoinduced degradation of polycyclic musk compounds by solid-phase microextraction and gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1186–1192. [Google Scholar] [CrossRef]
- Liu, X.; Chena, Z.; Wanga, L.; Wub, Y.; Garoma, T. Degradation of polycyclic musk HHCB in water by O3, UV, and UV/O3. J. Photochem. Photobiol. A 2012, 230, 1–9. [Google Scholar] [CrossRef]
- Santiago-Morales, J.; Gomez, M.J.; Herrera, S.; Fernandez-Alba, A.R.; Garcia-Calveo, E.; Rosal, E. Oxidative and photochemical processes for the removal of galaxolide and tonalide from wastewater. Water Res. 2012, 46, 4435–4447. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nanaboina, V.; Chen, F.; Korshin, G.V. Removal of polycyclic synthetic musks and antineoplastic drugs in ozonated wastewater: Quantification based on the data of differential spectroscopy. J. Hazard. Mater. 2016, 304, 242–250. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.A.; Medana, C.; Islam, M.A.; Raso, E.; Panagiotou, K.; Albanis, T. Efficiency of TiO2 photocatalytic degradation of HHCB (1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta[γ]-2-benzopyran) in natural aqueous solutions by nested experimental design and mechanism of degradation. Appl. Catal. B Environ. 2010, 99, 314–320. [Google Scholar] [CrossRef]
- Lopez, S.H.; Hernando, D.; Gómez, M.J.; Santiago-Morales, J.; Rosal, R.; Fernández-Alba, A.R. Investigation of Galaxolide Degradation Products Generated under Oxidative and Irradiation Processes by Liquid chromatography/hybrid Quadrupole Time-of-Flight Mass Spectrometry and Comprehensive Two-Dimensional Gas chromatography/Time-of-Flight Mass Spect. Rapid Commun. Mass Spectrom. 2013, 27, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Zhang, Q. Theoretical and kinetic properties of OH radical-initiated oxidation of galaxolide in the atmosphere. J. Phys. Chem. A 2018, 122, 9151–9159. [Google Scholar] [CrossRef]
- Wang, X.-H.; Lin, A.Y.-C. Is the phototransformation of pharmaceuticals a natural purification process that decreases ecological and human health risks? Environ. Pollut. 2014, 186, 203–215. [Google Scholar] [CrossRef]
- Kulthong, K.; Srisung, S.; Boonpavanitchakul, K.; Kangwansupamonkon, W.; Maniratanachote, R. Research Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part. Fibre Toxicol. 2010, 1, 7–8. [Google Scholar]
- Vallecillos, L.; Borrull, F.; Pocurull, E. Recent approaches for the determination of synthetic musk fragrances in environmental samples. Trends Anal. Chem. 2015, 72, 80–92. [Google Scholar] [CrossRef]
- Panagiotou, A.N.; Sakkas, V.A.; Albanis, T.A. Application of chemometric assisted dispersive liquid–liquid microextraction to the determination of personal care products in natural waters. Anal. Chim. Acta 2009, 649, 135–140. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. (Eds.) Gaussian 16; Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chemcraft-Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 7 April 2021).
- Barone, W.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Phys. Chem. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Karpińska, J.; Sokół, A.; Kołdys, J.; Ratkiewicz, A. Studies on the Kinetics of Doxazosin Degradation in Simulated Environmental Conditions and Selected Advanced Oxidation Processes. Water 2019, 11, 1001. [Google Scholar] [CrossRef] [Green Version]
- Sadef, Y.; Poulsen, T.G.; Bester, K. Modeling organic micro pollutant degradation kinetics during sewage sludge composting. Waste Manag. 2014, 34, 2007–2013. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Zhou, Q.; Zhou, Q.; Zhang, Y.; Zhu, L. Polycyclic Musks in the Environment: A Review of Their Concentrations and Distribution, Ecological Effects and Behavior, Current Concerns and Future Prospects. Crit. Rev. Environ. Sci. Technol. 2021, 51, 323–377. [Google Scholar] [CrossRef]
- Gao, S.; Tian, B.; Zeng, X.; Yu, Z. Enantiomeric Analysis of Polycyclic Musks AHTN and HHCB and HHCB-Lactone in Sewage Sludge by Gas chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Biselli, S.; Gatermann, R.; Kallenborn, R.; Sydnes, L.K.; Hühnerfuss, H. Biotic and abiotic transformation pathways of synthetic musks in the aquatic environment. In Synthetic Musk Fragrances in the Environment. The Handbook of Environmental Chemistry; Hutzinger, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 189–211. [Google Scholar]
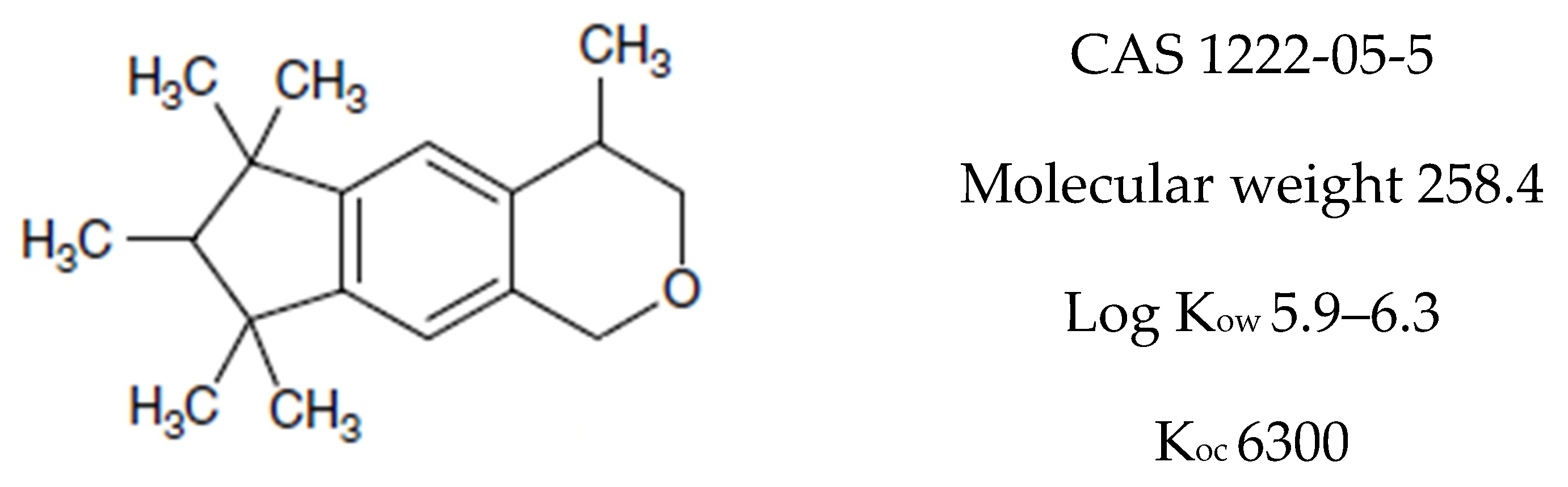
| Validation Parameter | Value/Equation |
|---|---|
| Linear range (mol L−1) | 4 × 10−9–9 × 10−6 |
| Slope | 8.18 × 1011 |
| Intercept | 41712 |
| Correlation coefficient (r) | 0.9996 |
| LOD mol L−1 | 3.63 × 10−10 |
| LOQ mol L−1 | 1.10 × 10−9 |
| Precision (n = 4), R.S.D. % | 2.08 |
| Reproducibility R.S.D. % | 0.68 |
| Studied Process | pH | The Concentration of H2O2 mol L−1 | Molar Ratio H2O2/HHCB | k min−1 × 10−3 | t1/2 min | % of Degradation after 80 min |
|---|---|---|---|---|---|---|
| Irradiation time: 80 min | ||||||
| Vis (E 750 W/m2) | 5.8 | - | - | 3.5 | 198 | 21.3 |
| 8.7 | - | - | 4.3 | 161 | 25.9 | |
| Vis (E 375 W/m2) | 5.8 | - | - | 3.45 | 201 | 23.6 |
| UV | 5.8 | - | - | 1.3 | 533 | 12.1 |
| 8.7 | - | - | 1.4 | 495 | 12.2 | |
| Vis/H2O2 (E 750 W/m2) | 5.8 | 10−3 | 20 | 3.7 | 187 | 21.4 |
| 10−2 | 200 | 3.7 | 187 | 22.3 | ||
| 8.7 | 10−2 | 200 | 2.9 | 239 | 18.4 | |
| 10−3 | 20 | 6.2 | 112 | 68.0 | ||
| 5 × 10−4 | 10 | 4.7 | 147 | 28.6 | ||
| UV/H2O2 | 5.8 | 10−3 | 20 | 2.1 | 330 | 19.1 |
| 10−2 | 200 | 1.9 | 365 | 11.8 | ||
| 8.7 | 10−3 | 20 | 4.4 | 157.5 | 31.4 | |
| 10−2 | 200 | 17.3 | 40 | 87.0 | ||
| Studied Process | Used Irradiation | k (min−1) × 10−2 | t1/2 (min) | % of Degradation after | |
|---|---|---|---|---|---|
| 40 min | 120 min | ||||
| Direct photolysis of laboratory solution | Vis (E 750 W/m2) UV | 4.93 | 14 | 93.6 | 99.9 |
| 2.92 | 24 | 58.9 | 99.8 | ||
| Direct photolysis in presence of surface water matrix | Vis (E750 W/m2) | 3.57 | 19 | 74.5 | 98.9 |
| Direct photolysis in presence of artificial sweat matrix | 3.16 | 22 | 91.9 | 97.6 | |
| Name | Structure | Retention Index (IR) | Retention Time (tr min) | Quantification Ion (m/z, %) | Identification Ions (m/z, %) |
|---|---|---|---|---|---|
| HHCB | 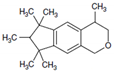 | 1872 | 13.44 | 243 (100) | 258 (30), 213 (36) |
| HHCB-lactone main peak | 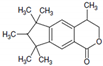 | 2206 | 16.60 | 257 (100) | 272 (14), 197 (12) |
| HHCB-lactone secondary peak |  | 2247 | 16.95 | 257 (100) | 272 (14), 197 (12) |
| Product transformation | NN–unidentified compound | 2161 | 16.20 | 243 (100) | - |
| Product transformation | NN–unidentified compound | 2287 | 17.20 | 273 (100) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, A.; Ratkiewicz, A.; Tomaszewska, I.; Karpinska, J. Kinetics and Mechanistic Studies of Photochemical and Oxidative Stability of Galaxolide. Water 2021, 13, 1813. https://doi.org/10.3390/w13131813
Sokol A, Ratkiewicz A, Tomaszewska I, Karpinska J. Kinetics and Mechanistic Studies of Photochemical and Oxidative Stability of Galaxolide. Water. 2021; 13(13):1813. https://doi.org/10.3390/w13131813
Chicago/Turabian StyleSokol, Aneta, Artur Ratkiewicz, Iwona Tomaszewska, and Joanna Karpinska. 2021. "Kinetics and Mechanistic Studies of Photochemical and Oxidative Stability of Galaxolide" Water 13, no. 13: 1813. https://doi.org/10.3390/w13131813







