Climatic and Anthropogenic Impacts on Environmental Conditions and Phytoplankton Community in the Gulf of Trieste (Northern Adriatic Sea)
Abstract
1. Introduction
2. Materials and Methods
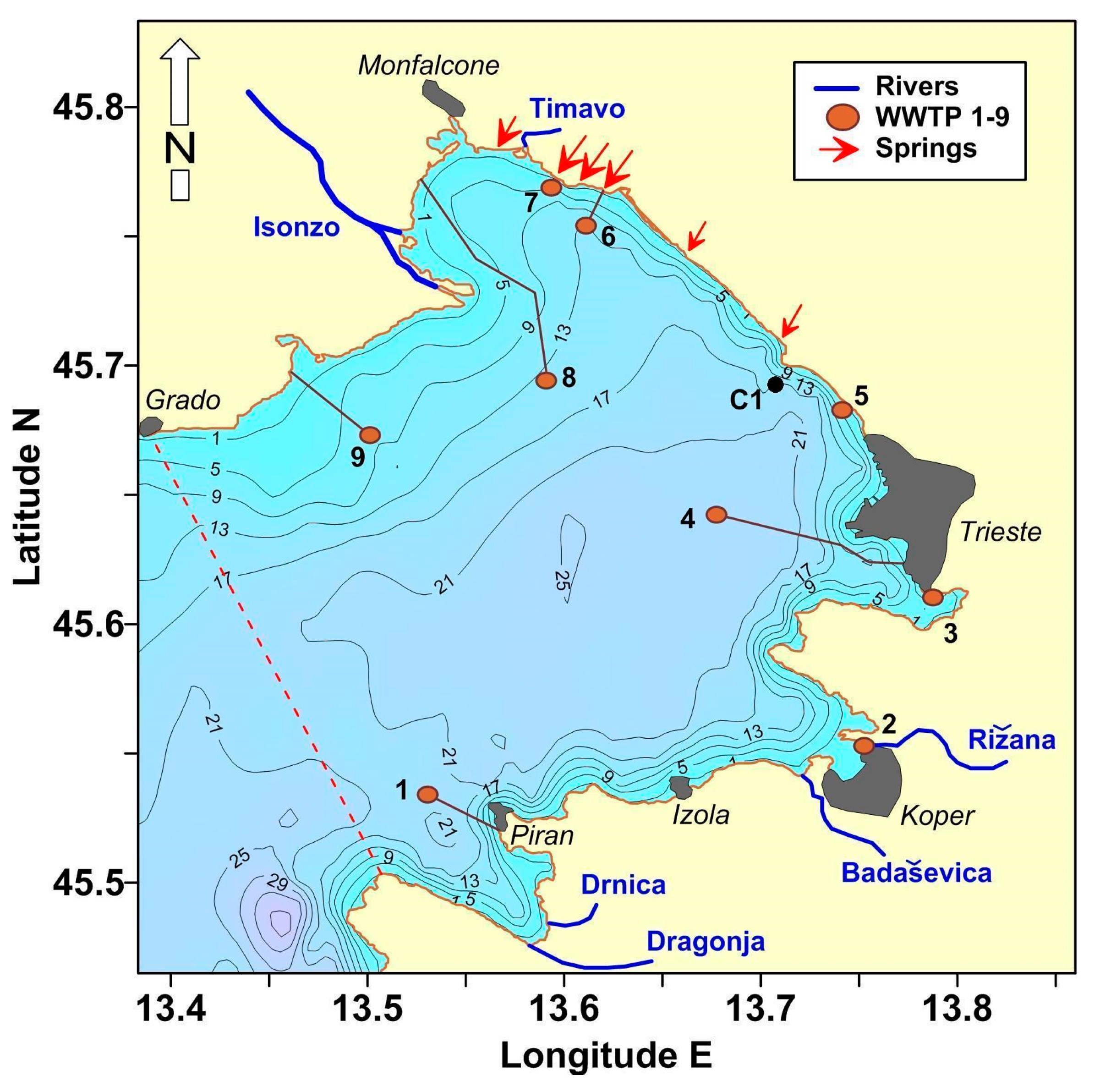
2.1. Study Site
2.2. Coastal Water Monitoring
2.3. Acquisition and Processing of Environmental Data
3. Results
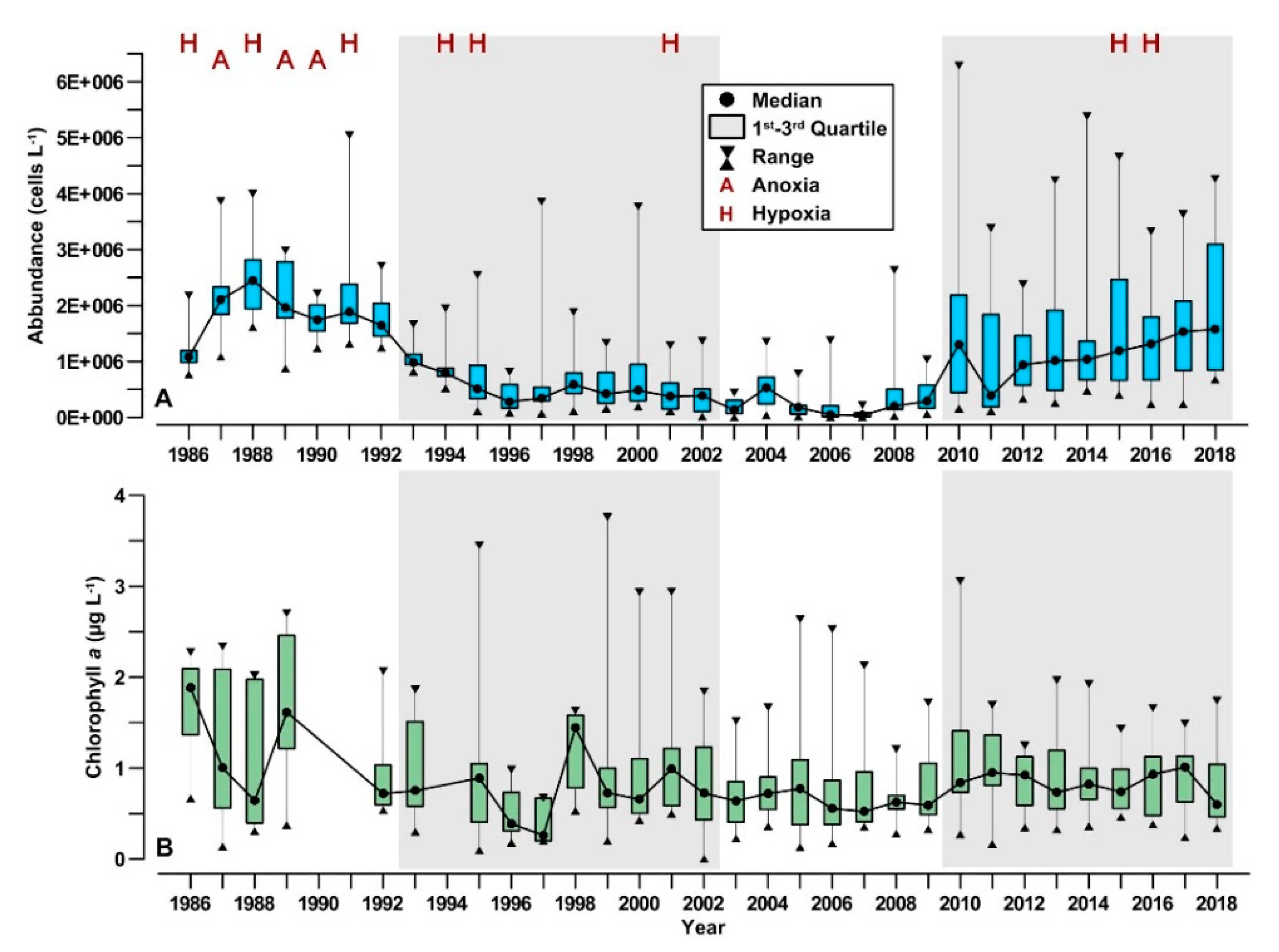
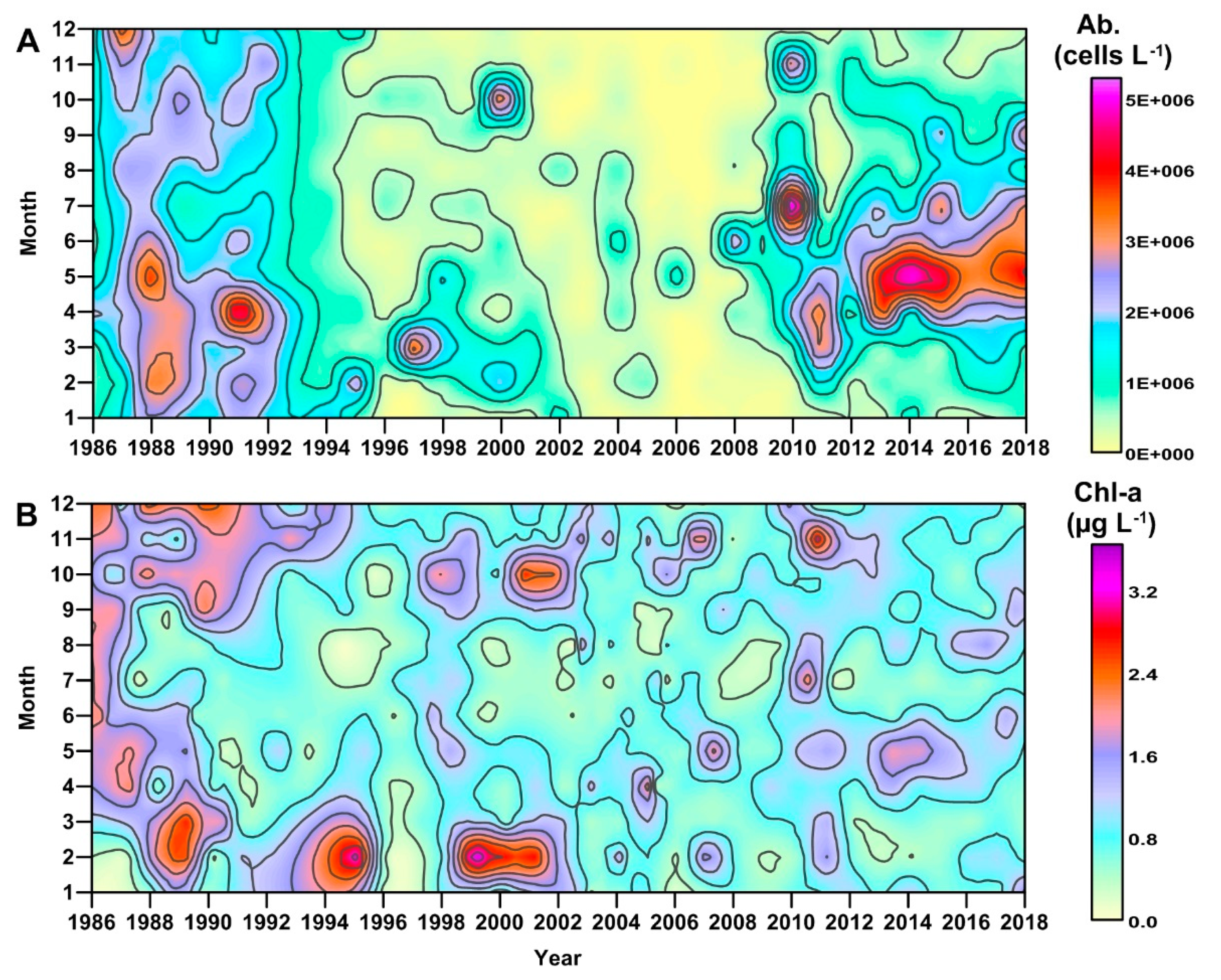
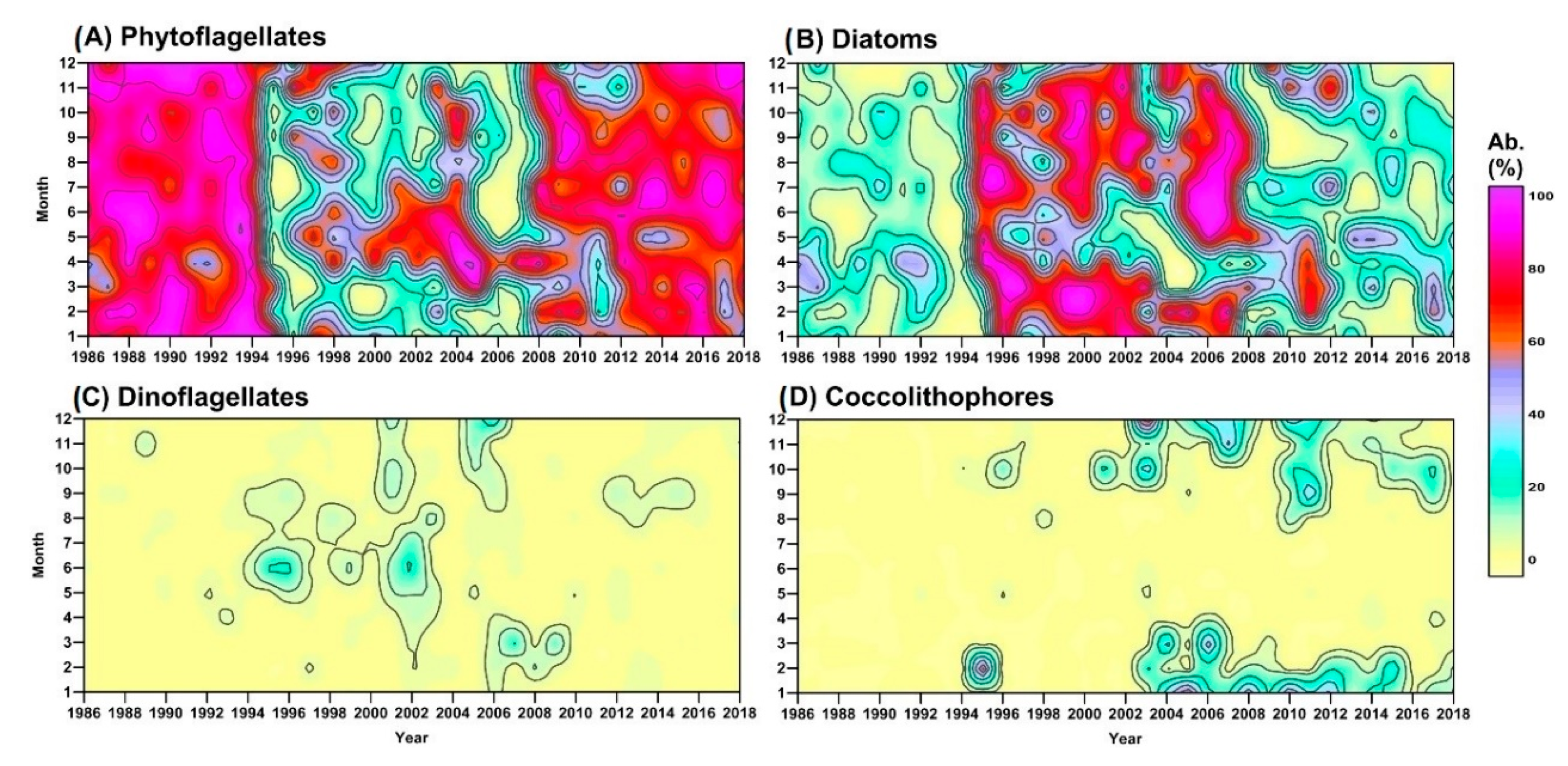
3.1. Long-Term Evolution of Phytoplankton Community
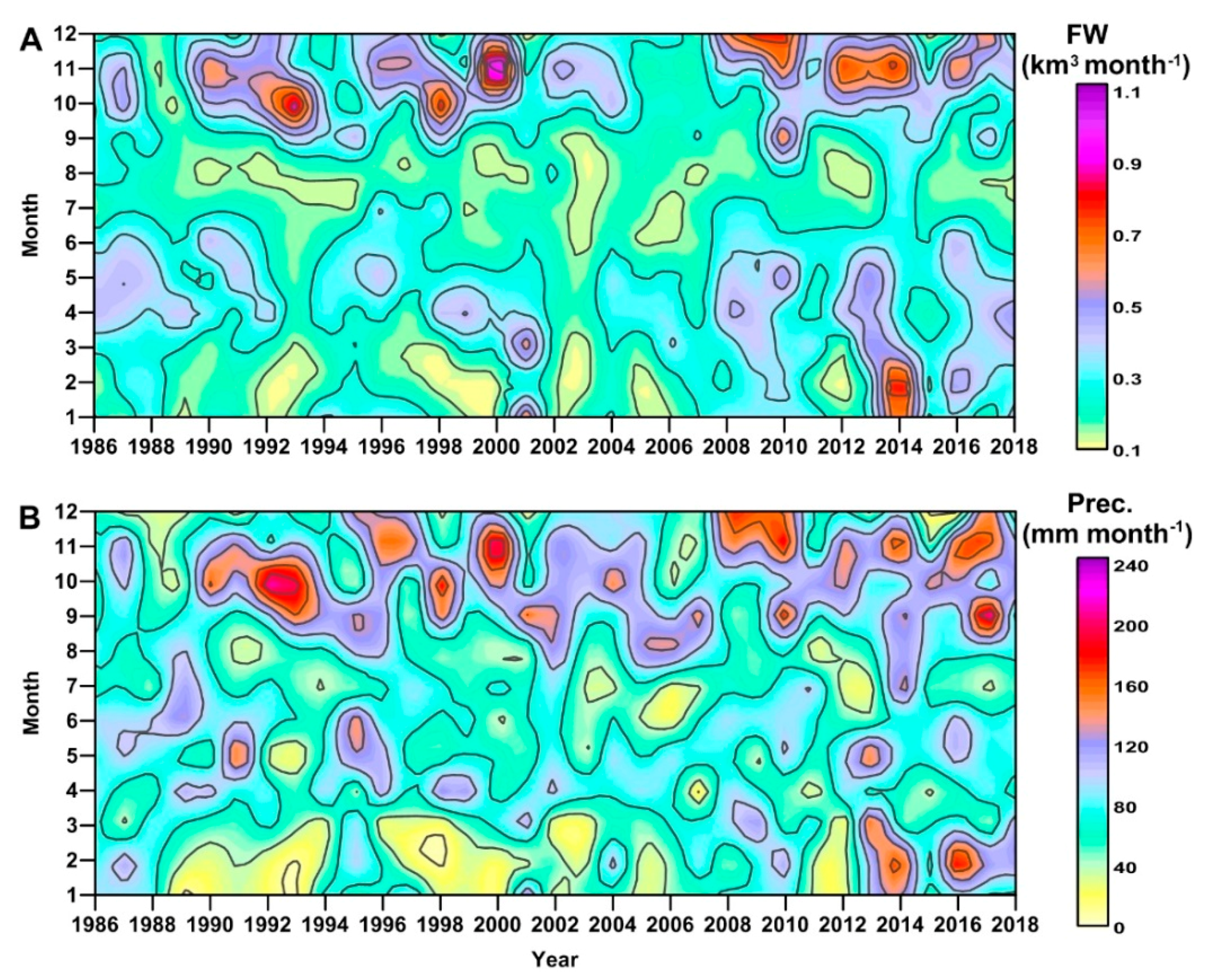
3.2. River Discharge and Precipitation in the GoT
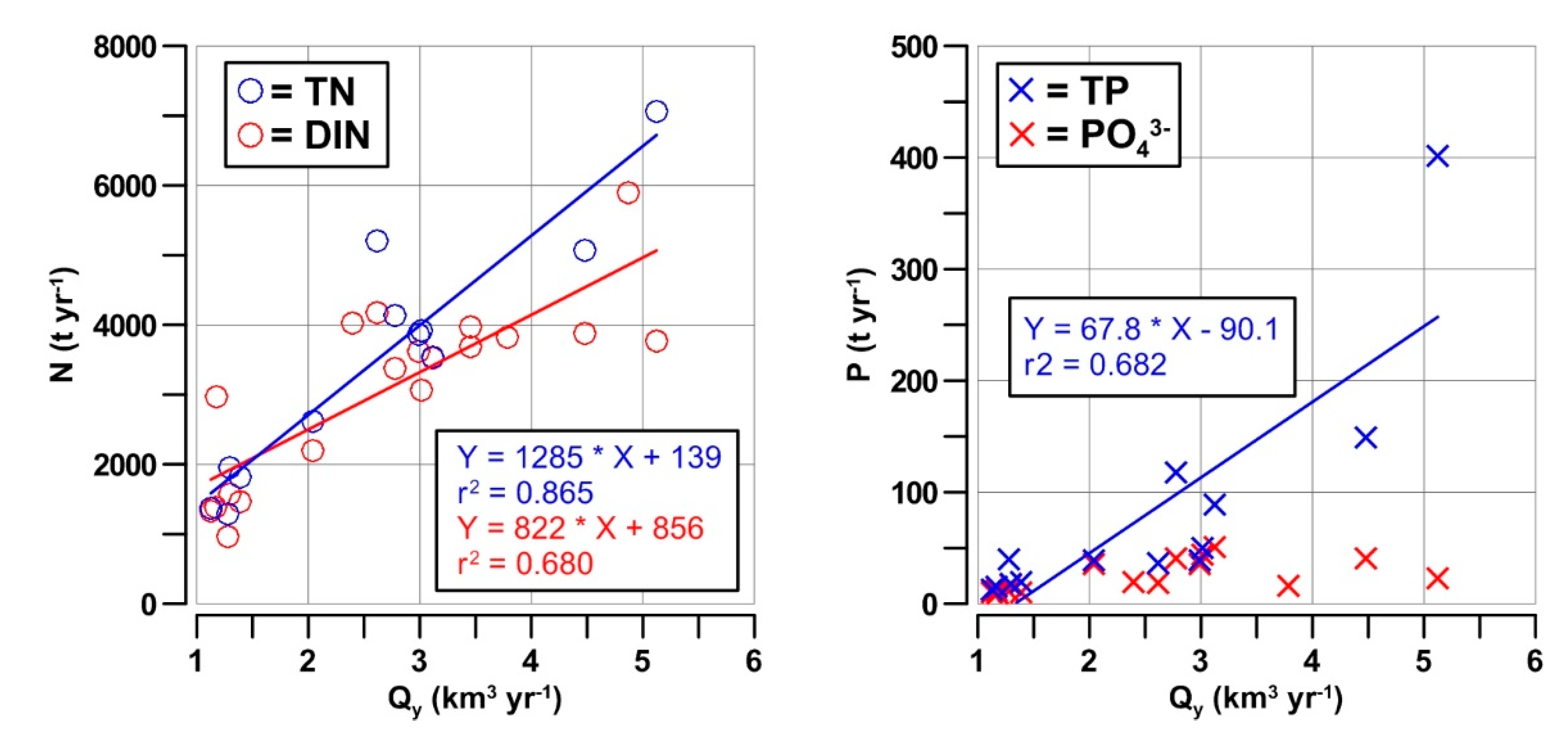
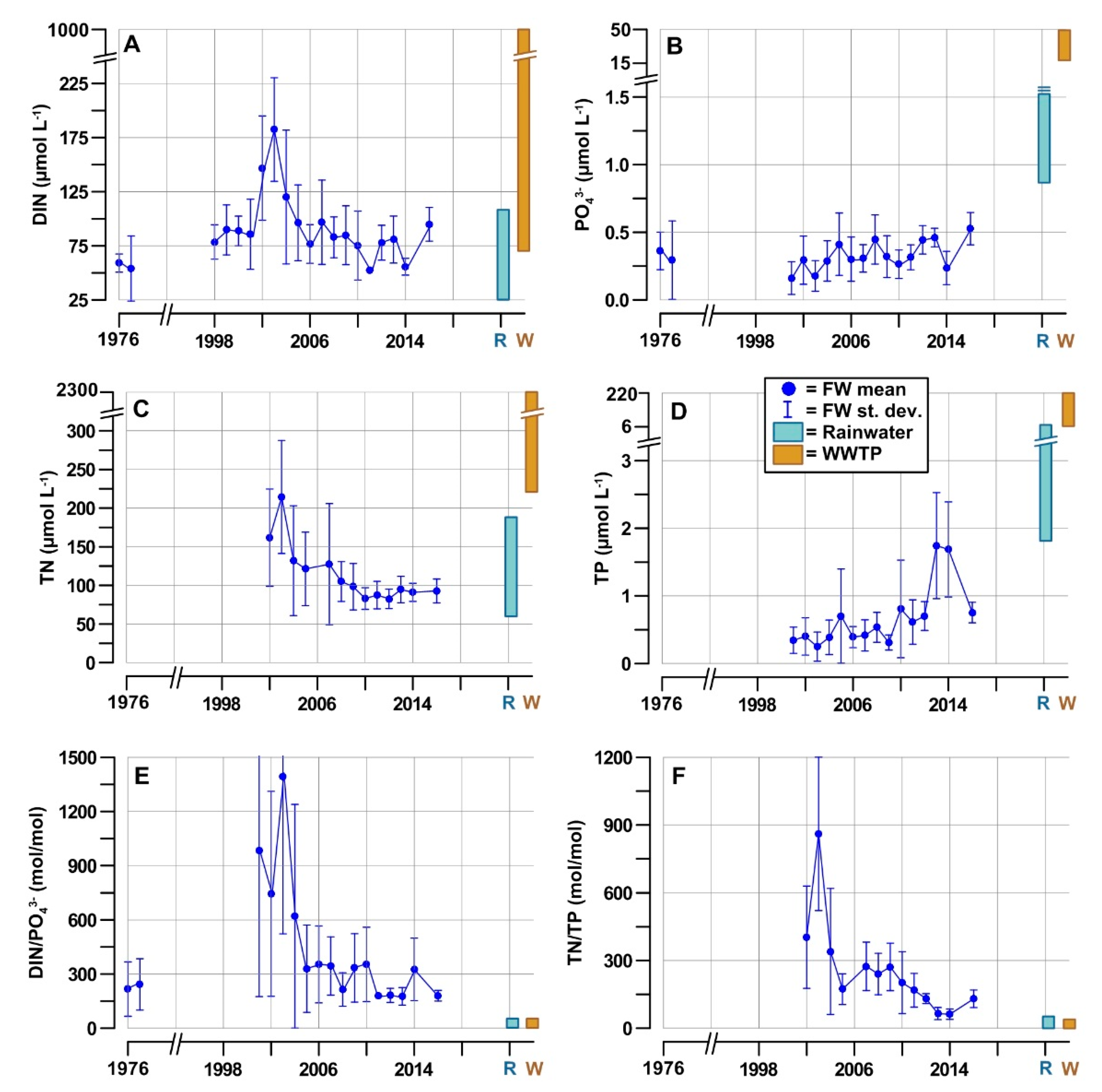
3.3. Nutrient Inputs in the GoT
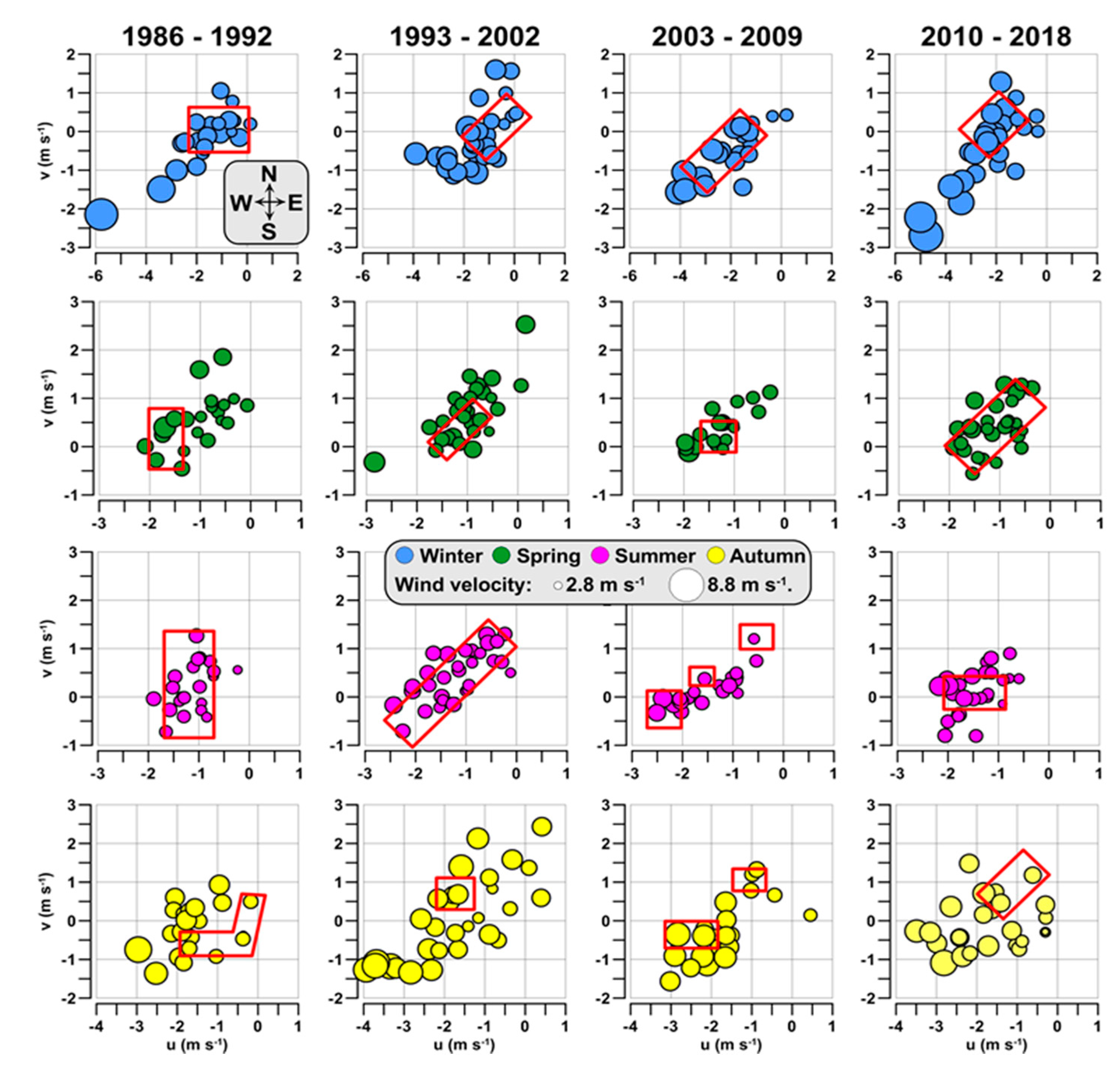
3.4. Hydrological Conditions and Climatic Circulation
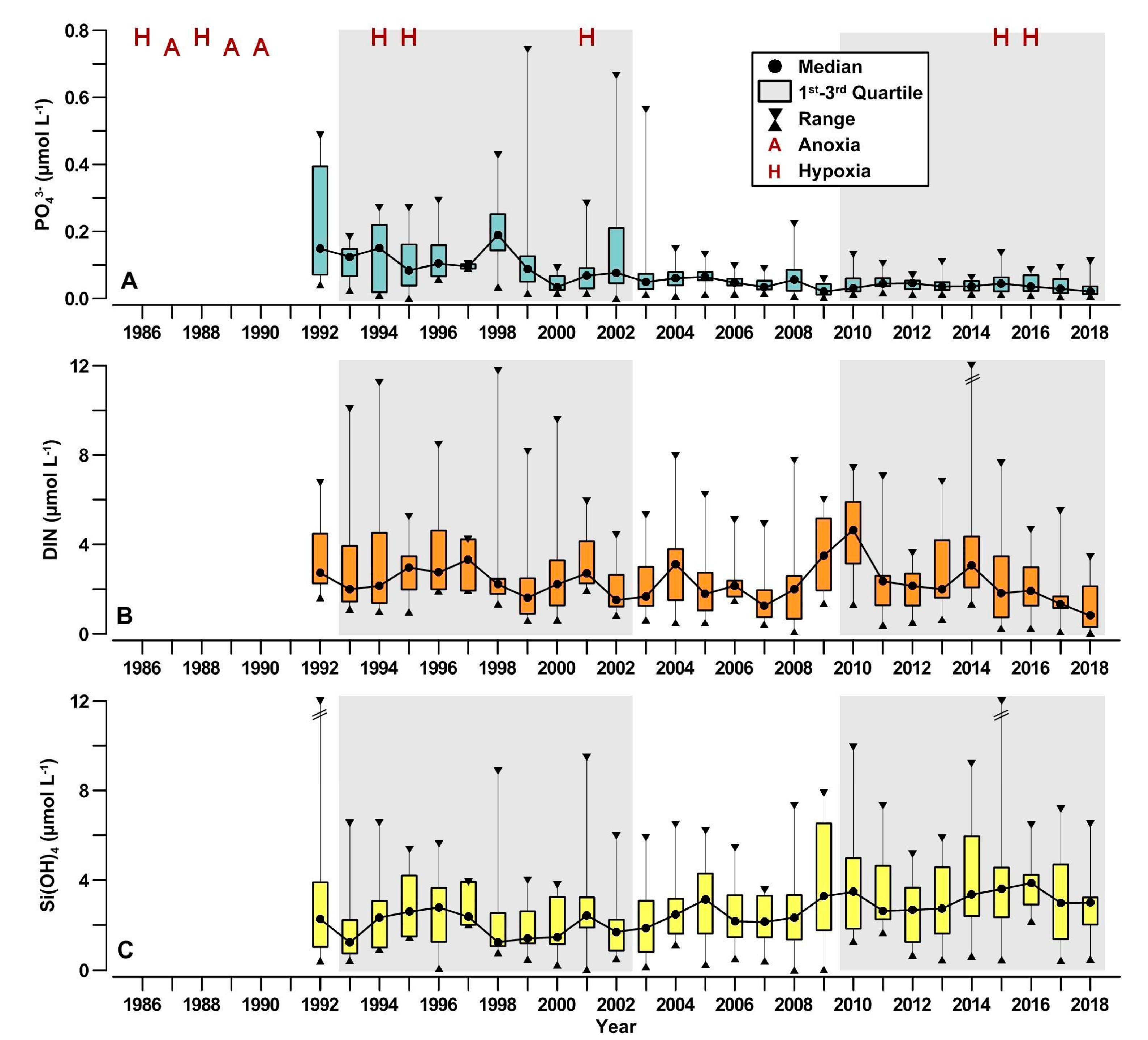
3.5. Nutrient Availability in the Coastal Waters
4. Discussion
4.1. Freshwater Influence
4.2. Climatic Effects
4.3. Effects of Natural and Anthropogenic Nutrient Inputs
4.4. Nutrient Availability in the Coastal Waters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Year | NO3− µmol L−1 | NO2− µmol L−1 | NH4+ µmol L−1 | DIN µmol L−1 | PO43− µmol L−1 | Si(OH)4 µmol L−1 | TN µmol L−1 | TP µmol L−1 | DIN/PO43− mol/mol | TN/TP mol/mol |
|---|---|---|---|---|---|---|---|---|---|---|
| 1976 | 57.58 | 0.35 | 1.09 | 59.01 | 0.36 | 36.20 | - | - | 217 | - |
| 1977 | 52.38 | 0.23 | 1.36 | 53.97 | 0.29 | 40.51 | - | - | 243 | - |
| 1998 | 73.34 | 0.65 | 4.57 | 78.57 | - | - | - | - | - | - |
| 1999 | 69.07 | 0.41 | 20.23 | 89.71 | - | - | - | - | - | - |
| 2000 | 83.36 | 0.46 | 4.90 | 88.72 | - | - | - | - | - | - |
| 2001 | 81.98 | 0.46 | 1.11 | 85.65 | 0.16 | - | - | 0.34 | 983 | - |
| 2002 | 143.62 | 0.32 | 1.62 | 146.86 | 0.29 | - | 161.80 | 0.40 | 744 | 404 |
| 2003 | 178.78 | 0.39 | 2.08 | 182.44 | 0.18 | - | 214.36 | 0.25 | 1394 | 861 |
| 2004 | 115.48 | 0.29 | 3.21 | 120.05 | 0.29 | - | 131.90 | 0.39 | 620 | 340 |
| 2005 | 95.13 | 0.35 | 1.88 | 96.23 | 0.41 | - | 121.37 | 0.70 | 330 | 173 |
| 2006 | 73.42 | 0.41 | 1.39 | 76.68 | 0.30 | - | - | 0.39 | 354 | - |
| 2007 | 93.64 | 0.24 | 2.86 | 96.74 | 0.31 | - | 127.35 | 0.42 | 345 | 274 |
| 2008 | 79.60 | 0.38 | 2.86 | 82.84 | 0.45 | - | 104.95 | 0.54 | 214 | 240 |
| 2009 | 81.39 | 0.30 | 3.03 | 84.72 | 0.32 | - | 98.28 | 0.31 | 334 | 272 |
| 2010 | 71.27 | 0.25 | 3.69 | 75.21 | 0.26 | - | 82.96 | 0.81 | 353 | 202 |
| 2011 | 54.44 | 0.22 | 1.78 | 52.33 | 0.31 | - | 87.46 | 0.61 | 180 | 168 |
| 2012 | 71.19 | 0.24 | 2.24 | 77.84 | 0.44 | - | 82.61 | 0.70 | 182 | 131 |
| 2013 | 78.83 | 0.25 | 1.85 | 80.76 | 0.46 | - | 94.60 | 1.74 | 176 | 65 |
| 2014 | 53.63 | 0.28 | 1.94 | 55.67 | 0.24 | - | 91.03 | 1.69 | 326 | 62 |
| 2016 | 90.91 | 1.09 | 2.83 | 94.82 | 0.53 | - | 92.81 | 0.75 | 180 | 130 |
| Site, Station | Years | TN µmol L−1 | TP µmol L−1 | NO3− µmol L−1 | NH4+ µmol L−1 | DIN µmol L−1 | PO43− µmol L−1 | Si(OH)4 µmol L−1 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Gulf of Trieste | 1978–1979 | 41.7 | - | 21.0 | 20.4 | 41.6 * | 0.7 | - | [60] |
| Rijeka, St. 1 | 1984–1991 | - | - | 38.0 | - | - | - | - | [61] |
| Kvarner Bay, St. 2 | 1988–1991 | - | - | 38.0 | - | - | - | - | [61] |
| Lovran, St. 3 | 1988–1991 | - | - | 41.0 | - | - | - | - | [61] |
| Kvarner Bay, St. 4 | 1988–1991 | - | - | 34.0 | - | - | - | - | [61] |
| Gulf of Trieste | 1993 | 117 (66–185) | 3.8 (1.7–5.7) | 49.9 (11–77) | 23.3 (4.5–36) | 73.2 (16–113) | 3.0 (0.9–4.8) | 3.0 (1.1–6.0) | [27] |
| Padez Basin, | 2006–2007 | - | - | 42.8 | 28.6 | 71.4 | - | - | [62] |
| Kvarner Bay, St. 4 | 1996–2005 | - | - | 23.7 | 46.5 | 70.2 | - | - | [63] |
| Pula, St. 18 | 1981–2006 | - | - | 92.1 | 62.1 | 154.2 | - | - | [64] |
| Pazin, St. 15 | 1981–2006 | - | - | 37.8 | 29.3 | 67.1 | - | - | [64] |
| Zavizan, St. 31 | 1981–2006 | - | - | 31.4 | 31.4 | 62.8 | - | - | [64] |
| Rijeka | 2001–2010 | - | - | 37.1 | 29.3 | 66.4 | - | - | [65] |
| Komiza | 2001–2010 | - | - | 30.0 | 18.6 | 48.5 | - | - | [65] |
| Rijeka, St.1 | 1990–1991 | - | - | 49.0 | 79.0 | 128.0 | - | - | [66] |
| Rijeka, St.2 | 1990–1991 | - | - | 77.0 | 151.0 | 228.0 | - | - | [66] |
| WWTP | IE | Flow m3 s−1 | TN µmol L−1 | DIN µmol L−1 | TP µmol L−1 | PO43− µmol L−1 | TN/TP mol/mol | DIN/PO43− mol/mol |
|---|---|---|---|---|---|---|---|---|
| 1 Piran | 33,000 | 0.08 | 678 (260–1960) | - | 80 (16–219) | - | 8.5 | - |
| 2 Koper + Izola | 84,500 | 0.17 | 339 (228–1128) | 148 (71–668) | 43 (6–99) | - | 7.8 | - |
| 3 Trieste (Zaule) | 67,000 | 0.27 | - | - | - | - | - | - |
| 4 Trieste (Servola) | 220,000 | 1.39 | 1299 (571–2284) | 828 (471–1024) | 52 (26–184) | 36 (17–52) | 25 | 23 |
| 5 Trieste (Barcola) | 7500 | 0.09 | - | - | - | - | - | - |
| 6 Sistiana | 3500 | 0.01 | - | - | - | - | - | - |
| 7 Duino | 750 | 0.001 | - | - | - | - | - | - |
| 8 Staranzano + Monfalcone | 62,500 | 0.2 | 659 (71–1356) | 553 (75–1195) | 46 (1–143) | - | 14 | - |
| 9 Grado | 11,000–80,000 * | 0.18 | 1112 (271-3034) | 947 (166-2898) | 44 (1–151) | - | 25 | - |
| TOTAL | 489,750–558,750 * | 4.01 | - | - | - | - | - | - |
| Area | Years | NH4+ mmol N m−2 d−1 | NO3− mmol N m−2 d−1 | PO43− mmol P m−2 d−1 | Si(OH)4 mmol Si m−2 d−1 | Reference |
|---|---|---|---|---|---|---|
| Central GoT | 1995–1996 | 0.8 ± 07 + | 0.17 ± 0.73 + | 0.03 ± 0.05 + | 2.6 ± 2.3 + | [54] |
| Central GoT | 1996 | 0.94 ° (0.73 *) | −0.08 ° | 0.03° (0.07 *) | - | [42] |
| Southern GoT | 1996 | - | −0.14 ° | - | - | [42] |
| Southern GoT | Mar. 1996 | 0.28 ° (1.14 *) | - | 0.01 ° (0.06 *) | - | [67] |
| Southern GoT | Sep. 1996 | 0.52 ° (2.28 *) | - | 0.01 ° (0.06 *) | - | [67] |
| Parameter | Isonzo Flow | Precipitation | Wind Velocity | TSW | Total Abundance | Chlorophyll a | Phyto-Flagellates | Diatoms | Dino-Flagellates | Cocco-Lithophores | DIN | PO43− | Si(OH)4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isonzo flow (km3 month−1) | 1.000 | 0.655 | 0.306 | −0.107 | 0.112 | 0.166 | 0.156 | −0.013 | 0.086 | 0.203 | 0.376 | 0.001 | 0.213 |
| Precipitation (mm month−1) | 0.655 | 1.000 | 0.222 | 0.179 | −0.046 | 0.066 | 0.001 | −0.056 | 0.082 | 0.145 | 0.214 | 0.054 | 0.184 |
| Wind velocity (m s−1) | 0.306 | 0.222 | 1.000 | −0.521 | −0.192 | −0.021 | −0.176 | −0.150 | −0.300 | 0.135 | 0.270 | 0.063 | 0.160 |
| TSW (C) | −0.107 | 0.179 | −0.521 | 1.000 | −0.023 | −0.063 | 0.025 | 0.040 | 0.287 | 0.015 | −0.265 | −0.009 | −0.052 |
| Total abundance (cell L−1) | 0.112 | −0.046 | −0.192 | −0.023 | 1.000 | 0.377 | 0.868 | 0.552 | 0.490 | −0.007 | −0.142 | −0.139 | −0.145 |
| Chlorophyll a (µg L−1) | 0.166 | 0.066 | −0.021 | −0.063 | 0.377 | 1.000 | 0.250 | 0.339 | 0.226 | 0.093 | 0.053 | −0.034 | 0.028 |
| Phytoflagellates (cell L−1) | 0.156 | −0.001 | −0.176 | 0.025 | 0.868 | 0.250 | 1.000 | 0.185 | 0.474 | 0.013 | −0.075 | −0.110 | 0.075 |
| Diatoms (cell L−1) | −0.013 | −0.056 | −0.150 | 0.040 | 0.552 | 0.339 | 0.185 | 1.000 | 0.312 | −0.009 | −0.230 | −0.124 | −0.375 |
| Dinoflagellates (cell L−1) | 0.086 | 0.082 | −0.300 | 0.287 | 0.490 | 0.226 | 0.474 | 0.312 | 1.000 | 0.193 | −0.145 | −0.097 | −0.043 |
| Coccolithophores (cell L−1) | 0.203 | 0.145 | 0.135 | 0.015 | −0.007 | 0.093 | 0.013 | −0.009 | 0.193 | 1.000 | 0.066 | −0.111 | 0.276 |
| DIN (µmol L−1) | 0.376 | 0.214 | 0.270 | −0.265 | −0.142 | 0.053 | −0.075 | −0.230 | −0.145 | 0.066 | 1.000 | 0.328 | 0.516 |
| PO43− (µmol L−1) | 0.001 | 0.054 | 0.063 | −0.009 | −0.139 | −0.034 | −0.110 | −0.124 | −0.097 | −0.111 | 0.328 | 1.000 | 0.132 |
| Si(OH)4 (µmol L−1) | 0.213 | 0.184 | 0.160 | −0.052 | −0.145 | 0.028 | 0.075 | −0.375 | −0.043 | 0.276 | 0.516 | 0.132 | 1.000 |
References
- Rabalais, N.N.; Turner, R.E.; Dıaz, R.J.; Justic, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Comley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Nillen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Hall, N.S.; Paerl, H.W.; Peierls, B.L.; Whipple, A.C.; Rossignol, K.L. Effects of climatic variability on phytoplankton community structure and bloom development in the eutrophic, microtidal, New River Estuary, North Carolina, USA. Estuar. Coast. Shelf Sci. 2013, 117, 70–82. [Google Scholar] [CrossRef]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013. [Google Scholar] [CrossRef]
- Matos, J.B.; de Oliveira, S.M.O.; Pereira, L.C.C.; da Costa, R.M. Structure and temporal variation of the phytoplankton of a macrotidal beach from the Amazon coastal zone. An. Acad. Bras. Ciênc 2016, 88, 1325–1339. [Google Scholar] [CrossRef]
- Mutshinda, C.M.; Finkel, Z.V.; Widdicombe, C.E.; Irwin, A.J. Bayesian inference to partition determinants of community dynamics from observational time series. Community Ecol. 2019, 20, 238–251. [Google Scholar] [CrossRef]
- Kovats, R.S.; Valentini, R.; Bouwer, L.M.; Georgopoulou, E.; Jacob, D.; Martin, E.; Rounsevell, M.; Soussana, J.-F. Europe. Contribution of Working Group II to the Fifth Assessment Report of IPCC. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1267–1326. ISBN 978-1-107-05816-3. [Google Scholar]
- Chowdhury, M.; Hardikar, R.; Kesavan, H.C.; Thomas, J.; Mitra, A.; Rokade, M.A.; Naidu, V.S.; Sukumaran, S. Nutrient stoichiometry and freshwater flow in shaping of phytoplankton population in a tropical monsoonal estuary (Kundalika Estuary). Estuar. Coast. Shelf Sci. 2017, 198, 73–91. [Google Scholar] [CrossRef]
- Basu, S.; Mackey, K.R.M. Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef]
- Dursun, F.; Tas, S. Variations in abundance and diversity of phytoplankton in the surface waters of the Golden Horn Estuary (Sea of Marmara). J. Mar. Biol. Assoc. UK 2019, 99, 279–290. [Google Scholar] [CrossRef]
- Trombetta, T.; Vidussi, F.; Mas, S.; Parin, D.; Simier, M.; Mostajir, B. Water temperature drives phytoplankton blooms in coastal waters. PLoS ONE 2019, 14, e0214933. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bolch, C.J.S.; Brand, T.D.; Narayanaswamy, B.E. The Relationship between Phytoplankton Distribution and Water Column Characteristics in North West European Shelf Sea Waters. PLoS ONE 2012, 7, e34098. [Google Scholar] [CrossRef] [PubMed]
- Malone, T.; Azzaro, M.; Bode, A.; Brown, E.; Duce, R.; Kamykowski, D.; Ho Kang, S.; Kedong, Y.; Thorndyke, M.; Wang, J.; et al. Chapter 6: Primary Production, Cycling of Nutrients, Surface Layer and Plankton. In Assessment of Major Ecosystem Services from the Marine Environment (Other than Provisioning Services); United Nations, Ed.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Grizzetti, B.; Bouraoui, F. Assessment of Nitrogen and Phosphorus Environmental Pressure at European Scale; EUR 22526 EN; European Commission, DG Joint Research Centre, Institute for Environment and Sustainability, Publications Office of the European Union: Luxembourg, 2006; p. 66. ISBN 92-79-03739-0. [Google Scholar]
- Cozzi, S.; Ibáñez, C.; Lazar, L.; Raimbault, P.; Giani, M. Flow Regime and Nutrient Loading Trends from the largest South European Watersheds: Implications for the productivity of Mediterranean and Black Sea’s coastal areas. Water 2019, 11, 1. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Fonda Umani, S. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Volf, G.; Atanasova, N.; Skerjanec, M.; Ozanic, N. Hybrid modeling approach for the northern Adriatic watershed management. Sci. Total Environ. 2018, 635353–635363. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Allen, E.B.; Branquinho, C.; Cruz, C.; Dias, T.; Fenn, M.E.; Manrique, E.M.; Pérez-Corona, E.; Sheppard, L.J.; Stock, W.D. Nitrogen deposition effects on Mediterranean-type ecosystems: An ecological assessment. Environ. Pollut. 2011, 159, 2265–2279. [Google Scholar] [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Goberville, E.; Beaugrand, G.; Sautour, B.; Tréguer, P.; SOMLIT Team. Climate-driven changes in coastal marine systems of western Europe. Mar. Ecol. Prog. Ser. 2010, 408, 129–147. [Google Scholar] [CrossRef]
- Lipizer, M.; De Vittor, C.; Falconi, C.; Comici, C.; Tamberlich, F.; Giani, M. Effects of intense meteorological and biological forcing factors on biogeochemical properties of coastal waters (Gulf of Trieste, Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2012, 115, 40–50. [Google Scholar] [CrossRef]
- Manna, V.; Fabbro, C.; Cerino, F.; Bazzaro, M.; Del Negro, P.; Celussi, M. Effect of an extreme cold event on the metabolism of planktonic microbes in the northernmost basin of the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2019, 225, 106252. [Google Scholar] [CrossRef]
- Lloyd-Hughes, B.; Saunder, M.A. A drought climatology for Europe. Int. J. Clim. 2002, 22, 1571–1592. [Google Scholar] [CrossRef]
- Cabrini, M.; Fornasaro, D.; Cossarini, G.; Lipizer, M.; Virgilio, D. Phytoplankton temporal changes in a coastal northern Adriatic site during the last 25 years. Estuar. Coast. Shelf Sci. 2012, 115, 113–124. [Google Scholar] [CrossRef]
- Malej, A.; Mozetič, P.; Malačič, V.; Turk, V. Response of summer phytoplankton to episodic meteorological events (Gulf of Trieste, Adriatic Sea). PSZN Marine Ecol. 1997, 18, 273–288. [Google Scholar] [CrossRef]
- Scroccaro, I.; Ostoich, M.; Umgiesser, G.; De Pascalis, F.; Colugnati, L.; Mattassi, G.; Vazzoler, M.; Cuomo, M. Submarine wastewater discharges: Dispersion modelling in the Northern Adriatic Sea. Environ. Sci. Pollut. Res. 2010, 17, 844–855. [Google Scholar] [CrossRef]
- Cozzi, S.; Falconi, C.; Comici, C.; Čermelj, B.; Kovac, N.; Turk, V.; Giani, M. Recent evolution of river discharges in the Gulf of Trieste and their potential response to climate changes and anthropogenic pressure. Estuar. Coast. Shelf Sci. 2012, 115, 14–24. [Google Scholar] [CrossRef]
- Turk, V.; Mozetič, P.; Malej, A. Overview of eutrophication-related events and other irregular episodes in Slovenian sea (gulf of Trieste, Adriatic Sea). Ann. Ser. Hits. Nat. 2007, 17, 11–23. [Google Scholar]
- Kralj, M.; Lipizer, M.; Čermelj, B.; Celio, M.; Fabbro, C.; Brunetti, F.; Francé, J.; Mozetič, P.; Giani, M. Hypoxia and dissolved oxygen trends in the northeastern Adriatic Sea (Gulf of Trieste). Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2019, 164, 74–88. [Google Scholar] [CrossRef]
- Malačič, V.; Celio, M.; Cermelj, B.; Bussani, A.; Comici, C. Interannual evolution of seasonal thermohaline properties in the Gulf of Trieste (northern Adriatic) 1991–2003. J. Geophys. Res. 2006, 111, C08009. [Google Scholar] [CrossRef]
- Malačič, V.; Petelin, B. Climatic circulation in the Gulf of Trieste (northern Adriatic). J. Geophys. Res. 2009, 114, C07002. [Google Scholar] [CrossRef]
- Furlani, S.; Ninfo, A.; Zavagno, E.; Paganini, P.; Zini, L.; Biolchi, S.; Antonioli, F.; Coren, F.; Cucchi, F. Submerged notches in Istria and the Gulf of Trieste: Results from the Geoswim project. Quat. Int. 2014, 332, 37–47. [Google Scholar] [CrossRef]
- Zini, L.; Calligaris, C.; Zavagno, E. Classical Karst hydrodynamics: A shared aquifer within Italy and Slovenia. In Evolving Water Resources Systems: Understanding, Predicting and Managing Water–Society Interactions; Proceedings of ICWRS2014; IAHS Publication 364: Bologna, Italy, 2014; pp. 499–504. [Google Scholar]
- Malej, A.; Mozetič, P.; Malačič, V.; Terzić, S.; Ahel, M. Phytoplankton responses to freshwater inputs in a small semi-enclosed gulf (Gulf of Trieste, Adriatic Sea). Mar. Ecol. Progr. Ser. 1995, 120, 111–121. [Google Scholar] [CrossRef]
- Cantoni, C.; Cozzi, S.; Pecchiar, I.; Cabrini, M.; Mozetič, P.; Catalano, G.; Fonda Umani, S. Short-term variability of primary production and inorganic nitrogen uptake related to the environmental conditions in a shallow coastal area (Gulf of Trieste, N Adriatic Sea). Oceanol. Acta 2003, 26, 565–575. [Google Scholar] [CrossRef]
- Mozetič, P.; Francé, J.; Kogovsek, T.; Talaber, I.; Malej, A. Plankton trends and community changes in a coastal sea (northern Adriatic): Bottom-up vs. top-down control in relation to environmental drivers. Estuar. Coast. Shelf Sci. 2012, 115, 138–148. [Google Scholar] [CrossRef]
- Cozzi, S.; Reisenhofer, E.; Di Monte, L.; Cantoni, C.; Adami, G. Effect of environmental forcing on the fate of nutrients, dissolved organic matter and heavy metals released by a coastal wastewater pipeline. Chem. Ecol. 2008, 24, 87–107. [Google Scholar] [CrossRef]
- Mozetič, P.; Malačič, V.; Turk, V. A case study of sewage discharge in the shallow coastal area of the Northern Adriatic Sea (Gulf of Trieste). Mar. Ecol. 2008, 29, 483–494. [Google Scholar] [CrossRef]
- Cozzi, S.; Mistaro, A.; Sparnocchia, S.; Colugnati, L.; Bajt, O.; Toniatti, L. Anthropogenic loads and biogeochemical role of urea in the Gulf of Trieste. Sci. Total Environ. 2014, 493, 271–281. [Google Scholar] [CrossRef]
- Faganeli, J.; Ogrinc, N. Oxic–anoxic transition of benthic fluxes from the coastal marine environment (Gulf of Trieste, northern Adriatic Sea). Mar. Freshw. Res. 2009, 60, 700–711. [Google Scholar] [CrossRef]
- Testa, J.M.; Faganeli, J.; Giani, M.; Brush, M.J.; De Vittor, C.; Boynton, W.R.; Covelli, S.; Kemp, W.M.; Kovač, N.; Woodland, R. Advances in Our Understanding of Pelagic-Benthic Coupling. In Evolution and Comparative Analysis of Coastal Ecosystems; Malone, T., Malej, A., Faganeli, J., Eds.; AGU Wiley: Hoboken, NJ, USA. (In Press)
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1999; pp. 159–228. [Google Scholar] [CrossRef]
- Lorenzen, C.; Jeffrey, J. Determination of chlorophyll in sea water. UNESCO Tech. Pap. Mar. Sci. 1980, 35, 1–20. [Google Scholar]
- Throndsen, J. Preservation and storage. In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 2010; pp. 69–74. [Google Scholar]
- Zingone, A.; Totti, C.; Sarno, D.; Cabrini, M.; Caroppo, C.; Giacobbe, M.G.; Luglio, A.; Nuccio, C.; Socal, G. Fitoplancton: Metodiche di analisi quali-quantitativa. In Metodologie di Studio del Plancton Marino. Manuali e Linee Guida 56/2010; Socal, G., Buttino, I., Cabrini, M., Mangoni, O., Penna, A., Totti, C., Eds.; ISPRA SIBM: Roma, Italy, 2010; pp. 213–237. [Google Scholar]
- Chen, Z.; Grasby, S.E. Impact of decadal and century-scale oscillations on hydroclimate trend analyses. J. Hydrol. 2009, 365, 122–133. [Google Scholar] [CrossRef]
- Zhang, X.; Alexander, L.; Hegerl, G.C.; Jones, P.; Tank, A.K.; Peterson, T.C.; Trewin, B.; Zwiers, F.W. Indices for monitoring changes in extremes based on daily temperature and precipitation data. WIREs Clim. Chang. 2011, 2, 851–870. [Google Scholar] [CrossRef]
- Bregant, D.; Catalano, G. Chemical and Physical Conditions at Isonzo River Mouth (June 1976–December 1977); CNR-Istituto Talassografico of Trieste: Trieste, Italy, 1978; Volume 545, p. 12. [Google Scholar]
- Cozzi, S.; Giani, M. River water and nutrient discharges in the Northern Adriatic Sea: Current importance and long term changes. Cont. Shelf Res. 2011, 31, 1881–1893. [Google Scholar] [CrossRef]
- Raicich, F.; Colucci, R.R. A near-surface sea temperature time series from Trieste, northern Adriatic Sea (1899–2015). Earth Syst. Sci. Data 2019, 11, 761–768. [Google Scholar] [CrossRef]
- Fonda Umani, S.; Beran, A.; Parlato, S.; Virgilio, D.; Zollet, T.; De Olazabal, A.; Lazzarini, B.; Cabrini, M. Noctiluca scintillans MACARTNEY in the Northern Adriatic Sea: Long–term dynamics, relationships with temperature and eutrophication, and role in the food web. J. Plankton Res. 2004, 26, 545–561. [Google Scholar] [CrossRef]
- Bertuzzi, A.; Faganelj, J.; Welker, C.; Brambati, A. Benthic fluxes of dissolved inorganic carbon, nutrients and oxygen in the Gulf of Trieste (Northern Adriatic). Water Air Soil Pollut. 1997, 99, 305–314. [Google Scholar] [CrossRef]
- Goffart, A.; Hecq, J.-H.; Legendre, L. Drivers of the winter-spring phytoplankton bloom in a pristine NW Mediterranean site, the Bay of Calvi (Corsica): A long-term study (1979–2011). Prog. Oceanogr. 2015, 137, 121–139. [Google Scholar] [CrossRef]
- Grilli, F.; Accoroni, S.; Acri, F.; Bernardi Aubry, F.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and interannual trends of oceanographic parameters over 40 years in the northern Adriatic Sea in relation to nutrient loadings from EMODnet Chemistry data portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Cerino, F.; Malinverno, E.; Fornasaro, D.; Kralj, M.; Cabrini, M. Coccolithophore diversity and dynamics at a coastal site in the Gulf of Trieste (northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2017, 196, 331–345. [Google Scholar] [CrossRef]
- Olivotti, R.; Faganeli, J.; Malej, A. Impact of “organic” pollutants on coastal waters, Gulf of Trieste. Water Sci. Technol. 1986, 18, 57–68. [Google Scholar] [CrossRef]
- Volf, G.; Atanasova, N.; Kompare, B.; Ožanić, N. Modeling nutrient loads to the northern Adriatic. J. Hydrol. 2013, 504182–504193. [Google Scholar] [CrossRef]
- Faganeli, J.; Tusnik, P. Carbon, nitrogen and phosphorus nutrients in the eastern part of the Gulf of Trieste (Northern Adriatic). Acta Adriat. 1983, 24, 25–41. [Google Scholar] [CrossRef]
- Alebić-Juretić, A. Precipitation chemistry within Kvarner Bay area, Northern Adriatic (Croatia), 1984-1991. Water Air Soil Pollut. 1994, 78, 343–357. [Google Scholar] [CrossRef]
- Rusjan, S.; Vidmar, A. The role of seasonal and hydrological conditions in regulating dissolved inorganic nitrogen budgets in a forested catchment in SW Slovenia. Sci. Total Environ. 2017, 575, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Alebić-Juretić, A. Airborne ammonia and ammonium within the Northern Adriatic area, Croatia. Environ. Pollut. 2008, 154, 439–447. [Google Scholar] [CrossRef]
- Špoler Čanić, K.; Vidič, S.; Bencetić Klaić, Z. Precipitation chemistry in Croatia during the period 1981-2006. J. Environ. Monit. 2009, 11, 839–851. [Google Scholar] [CrossRef]
- Ðjuričić, V.; Kosanović, C.; Škevin-Sović, J. Atmospheric contribution to eutrophication of the Adriatic Sea. WIT Trans. Ecol. Environ. 2012, 157, 377–386. [Google Scholar] [CrossRef]
- Alebić-Juretić, A.; Šojat, V. Chemical composition of rainwater collected at two sampling sites in the city of Rijeka. Arh. Hig. Rada Toksikol. 1998, 49, 245–250. [Google Scholar]
- Ogrinc, N.; Faganeli, J. Phosphorus regeneration and burial in near-shore marine sediments (the Gulf of Trieste, northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2006, 67, 579–588. [Google Scholar] [CrossRef]

| Source | Flow (km3 yr−1) | TN (t-N yr−1) | TP (t-P yr−1) |
|---|---|---|---|
| Isonzo River | 1.63–5.02 | 1285–7063 | 12–401 |
| Precipitation | 0.38–0.76 | 626–1239 | 45–89 |
| WWTP | 0.13 | 1290–1470 | 270–306 |
| Benthic fluxes | - | 2718° | 186° |
| Month | TSW Range (°C) | Slope (°C yr−1) | α |
|---|---|---|---|
| January | 6.1–12.3 | 0.05 | 0.05 |
| Febreary | 4.0–11.5 | 0.02 | >0.10 |
| March | 6.3–13.9 | 0.01 | >0.10 |
| April | 7.9–18.1 | 0.02 | >0.10 |
| May | 10.8–25.4 | 0.02 | >0.10 |
| June | 14.0–27.6 | 0.00 | >0.10 |
| July | 18.1–29.5 | 0.04 | 0.01 |
| August | 18.8–28.3 | 0.00 | >0.10 |
| September | 18.3–26.2 | 0.01 | >0.10 |
| October | 13.9–22.5 | 0.00 | >0.10 |
| November | 11.4–18.8 | 0.03 | >0.10 |
| December | 7.2–16.0 | 0.04 | 0.10 |
| Annual values | 4.0–29.5 | 0.02 | 0.05 |
| Month | DIN/PO43− | Si/PO43− | Si/DIN | |||
|---|---|---|---|---|---|---|
| Slope (yr−1) | α | Slope (yr−1) | α | Slope (yr−1) | α | |
| January | 2.1 | 0.05 | 3.4 | 0.001 | 0.01 | >0.10 |
| February | 2.4 | 0.10 | 2.9 | 0.01 | 0.02 | >0.10 |
| March | 2.4 | >0.10 | 3.5 | 0.05 | 0.02 | >0.10 |
| April | 0.7 | >0.10 | 4.4 | 0.01 | 0.10 | 0.001 |
| May | 1.5 | 0.10 | 3.9 | 0.01 | 0.04 | 0.10 |
| June | 0.4 | >0.10 | 4.9 | 0.001 | 0.10 | 0.001 |
| July | 1.3 | 0.10 | 4.3 | 0.05 | 0.11 | 0.05 |
| August | 1.7 | 0.10 | 3.8 | 0.01 | 0.09 | 0.05 |
| September | 0.2 | >0.10 | 2.9 | 0.05 | 0.09 | 0.001 |
| October | 0.7 | >0.10 | 3.3 | 0.05 | 0.06 | 0.05 |
| November | 1.8 | 0.01 | 4.0 | 0.001 | 0.05 | 0.01 |
| December | 2.1 | 0.01 | 3.3 | 0.001 | 0.02 | 0.05 |
| Annual value | 1.4 | 0.001 | 3.7 | 0.001 | 0.04 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzi, S.; Cabrini, M.; Kralj, M.; De Vittor, C.; Celio, M.; Giani, M. Climatic and Anthropogenic Impacts on Environmental Conditions and Phytoplankton Community in the Gulf of Trieste (Northern Adriatic Sea). Water 2020, 12, 2652. https://doi.org/10.3390/w12092652
Cozzi S, Cabrini M, Kralj M, De Vittor C, Celio M, Giani M. Climatic and Anthropogenic Impacts on Environmental Conditions and Phytoplankton Community in the Gulf of Trieste (Northern Adriatic Sea). Water. 2020; 12(9):2652. https://doi.org/10.3390/w12092652
Chicago/Turabian StyleCozzi, Stefano, Marina Cabrini, Martina Kralj, Cinzia De Vittor, Massimo Celio, and Michele Giani. 2020. "Climatic and Anthropogenic Impacts on Environmental Conditions and Phytoplankton Community in the Gulf of Trieste (Northern Adriatic Sea)" Water 12, no. 9: 2652. https://doi.org/10.3390/w12092652
APA StyleCozzi, S., Cabrini, M., Kralj, M., De Vittor, C., Celio, M., & Giani, M. (2020). Climatic and Anthropogenic Impacts on Environmental Conditions and Phytoplankton Community in the Gulf of Trieste (Northern Adriatic Sea). Water, 12(9), 2652. https://doi.org/10.3390/w12092652








