Abstract
Microplastics are an emerging environmental pollutant, whose global ubiquity is becoming increasingly evident. Conventional wastewater treatment does not completely remove them, and there are growing concerns about microplastics in source water and post-treatment drinking water. Microplastics have been reported to alter the development, physiology, and behavior of various aquatic organisms; however, limited knowledge exists on their effect on natural phytoplankton communities. Many studies also use uniformly spherical plastic beads, while most scrub particles in consumer products and secondary microplastics in the environment have various shapes and sizes. We tested the effects of two types of microplastics, 50 µm polystyrene (PS) calibration beads and polylactic acid (PLA) plastic body wash scrub particles, and one type of plant-derived body wash scrub particle on a natural phytoplankton assemblage through a 7-day incubation experiment in a temperate, mesotrophic lake. The calibration beads and the plant-derived particles generally did not alter the taxonomic composition of the phytoplankton in the mesocosms, while the PLA body wash microplastics eliminated cryptophytes (p < 0.001) and increased chrysophytes (p = 0.041). Our findings demonstrate differential effects of irregularly shaped PLA body wash microplastics vs. PS calibration beads on lake phytoplankters and empirically support potential bottom-up alteration of the aquatic food web by secondary microplastics.
1. Introduction
Microplastics (MPs), or plastic pieces that are typically <5 mm, have come to be widely recognized as an emerging pollutant in recent years. While the earliest major scientific report of aquatic microplastic pollution dates back to 1971 [1], MPs did not draw much attention as a research topic until the 21st century. Since then, we have gained substantial knowledge on the extent of the global MP pollution and its impact on the development, physiology, and behavior of various marine and freshwater organisms [2,3,4,5,6,7]. We have also learned that conventional wastewater treatment plants do not completely remove MPs and can be point sources of aquatic MPs [8,9,10,11]. Simultaneously, MPs in source water and processed or bottled drinking water have been a growing concern for water utilities and the general public [12]. Controlled lab studies are elucidating the molecular pathways of organismal responses to aquatic MPs, including nanoplastics (NPs) [13,14,15]. Data are severely limited, however, on how MPs affect natural phytoplankton communities instead of lab monocultures where a single, well-studied species from a culture collection is grown in defined growth media at constant temperature and regular light/dark cycles. Such studies have revealed physiological effects of MPs on phytoplankton cells and their gene expression using certain enzymes and reactive oxygen species as responses [16]. The influences of MPs at the population and community levels, however, are yet to be studied to the same extent, although remarkable advances are being made for biofilm communities [17,18]. Many of these MP studies used uniformly spherical and fluorescent plastic beads, while many scrub particles in consumer products [16,19,20,21] and MPs found in environmental samples [22,23] have various shapes and sizes. Non-spherical MPs, including those resulting from fragmentation of macroscopic plastic debris, are expected to be the majority of MPs encountered by aquatic organisms. Yet, non-spherical MPs are rarely used in MP bioassays because analytic-grade MP beads are readily available and facilitate straight-forward study design and data interpretation.
We aimed to investigate whether analytic-grade MP beads are a reasonable surrogate to other types of anthropogenic microparticles that may be released in household wastewater and interact with lake phytoplankton. A novel, 7-day summertime in situ incubation experiment was conducted in a temperate, mesotrophic lake where different types of spherical and non-spherical microparticles were added to mesocosms containing natural lake phytoplankton. We first hypothesized that MPs have quantitative and/or qualitative effect(s) on the lake phytoplankton, while plant-derived particles have no such effect. Plant-derived scrub particles from a body wash were included as one of the experimental treatments. They are often chosen by personal care product manufacturers as alternatives to plastic microbeads, which have been banned or are being voluntarily phased out of production by manufacturers [24,25]. We also hypothesized that planktonic cyanobacterial colonies become smaller and more numerous with MPs. In previous years, cyanobacteria such as Aphanizomenon, Microcystis, Dolichospermum, and Planktothrix were regularly observed in summer at the study site, where they only formed very localized and/or diffuse “blooms”. The same cyanobacterial taxa, however, often caused harmful blooms in other local lakes, and MPs may have an effect on colony formation and maintenance in these taxa, which can cause harmful blooms in eutrophic lakes.
2. Materials and Methods
The overall design of our in situ incubation experiment was based on [26], utilizing 15.2 cm × 15.2 cm Bitran® polyethylene bags (Thermo Fisher Scientific, Waltham, MA, USA) as mesocosms, deployed in floating racks at a near-shore lake surface (Figure 1). We collected ~20 L and 2 L of surface lake water containing phytoplankton from mesotrophic Otsego Lake, New York, USA, on 6 and 13 July 2016, respectively. The pre-incubation sample (20 L) was filtered through a 118 µm Nitex® (Nitex, Singapore) mesh to exclude most large grazers before treatment. Three types of microparticles constituted treatments in this experiment: 50 µm diameter polystyrene calibration beads (DC) (Dri-Cal® Particle Size Standards Beads, DC-50, Thermo Fisher Scientific, Waltham, MA, USA), plant-derived scrub particles from Softsoap® (Softsoap, Chaska, MN, USA) Coconut Scrub body wash (CS), and plastic scrub particles from Dial® (Dial, Scottsdale, AZ, USA) Power Scrub body wash (DP). Figure 2 shows images of representative microparticles used. CS and DP (Supplementary Materials) were harvested in a lab from June to August 2013 through repeated rounds of dilution with deionized water and centrifugation. They were subsequently dried and stored at room temperature. The detailed harvesting method and size distributions for CS and DP are described in [20]. CS burned and DP melted, respectively, when exposed to heat similar to the hot needle test used in [27], meaning that DP was a plastic and CS was not. CS was most likely an apricot kernel powder, as it was the only scrubbing agent listed on the bottle. Both DC and DP sank when mixed into deionized water. Fourier transform infrared (FTIR) analysis by a Frontier FT-IR/NIR spectrometer with a built-in standard reference library (PerkinElmer, USA) was performed on CS and DP for further characterization. Full FTIR spectra were compared to reference spectra by Open Specy [28] with default preprocessing parameters (smoothing polynomial = 3, baseline correction polynomial = 8) and a spectral range of 650 to 4000 cm−1. Each treatment was applied at 67 mg L−1 (~30 mg particles per 450 mL pre-incubation ambient lake water per mesocosm) with 4 replicates per treatment. Control mesocosms did not receive any microparticle addition but otherwise were prepared in the same manner as the treatment mesocosms. With the control group, a total of 16 mesocosms were incubated for 7 days. A 7-day incubation was selected as it was long enough for the phytoplankton population to build up in the mesocosms, yet no signs of CO2 limitation nor a shift from planktonic to attached growth were observed.

Figure 1.
(a) Experimental mesocosms. (b) Deployment site.
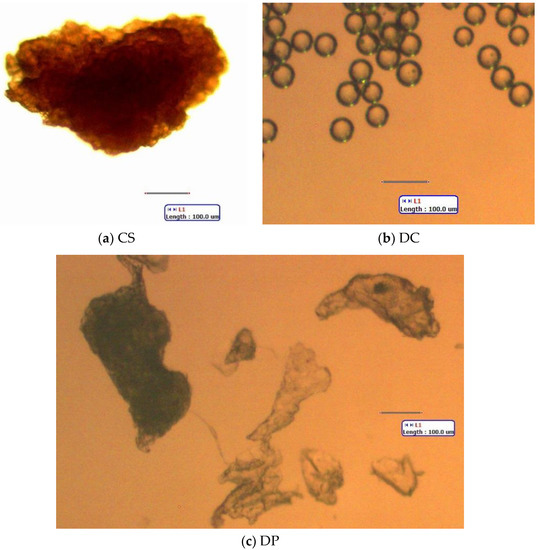
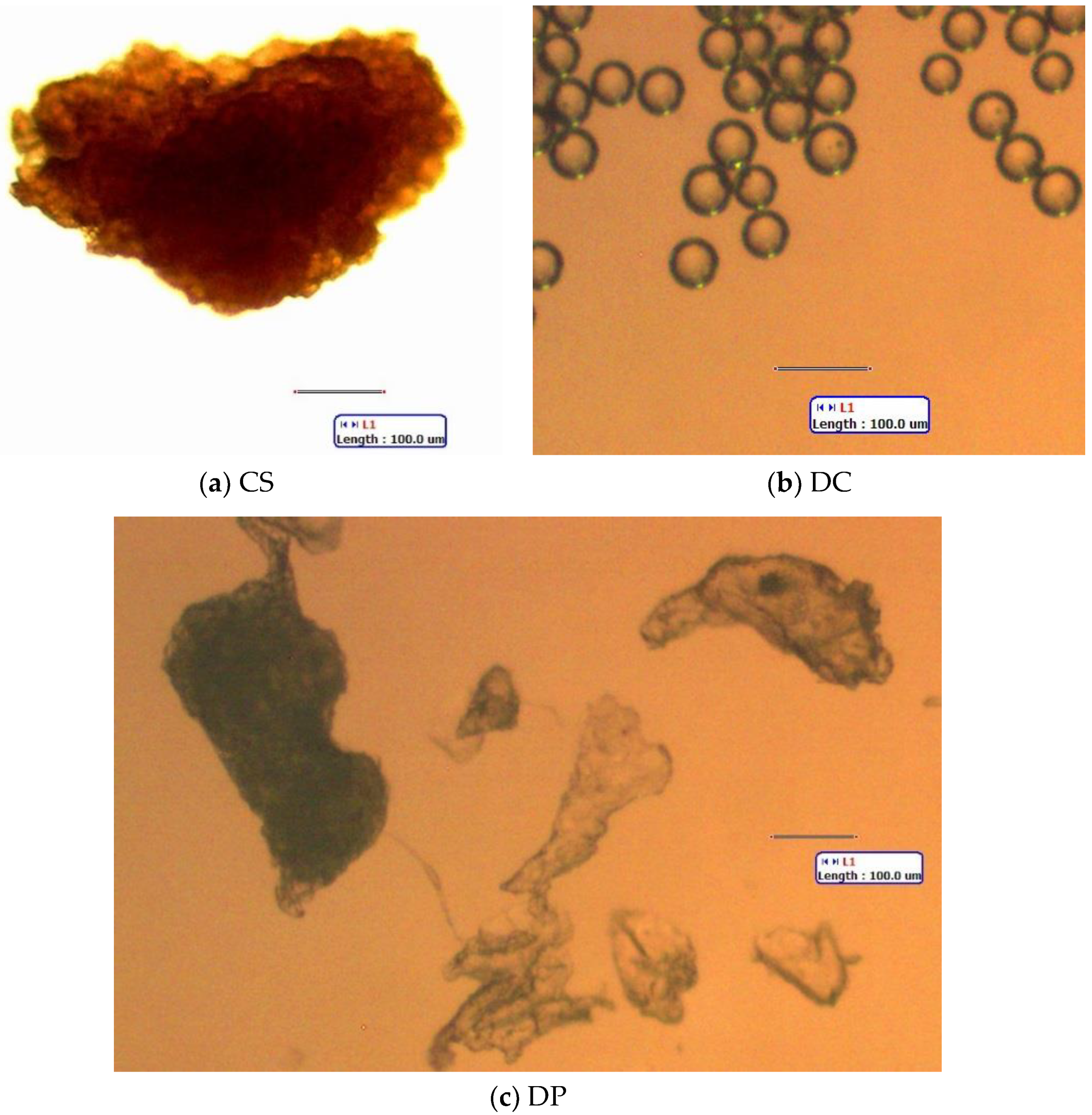
Figure 2.
Representative microparticles added to lake phytoplankton. (a) CS (apricot kernel powder from a “coconut scrub” body wash). (b) DC (Dri-Cal polystyrene (PS) beads, 50 µm). (c) DP (polylactic acid fragments from a body wash). All scale bars: 100 µm. Standard light microscopy.
Chlorophyll a (chl. a) was analyzed by filtration onto Whatman GF/F filters (nominal pore size: 0.7 µm) (GE Healthcare, Chicago, IL, USA), followed by extraction by acetone and fluorometry on a TD-700 laboratory fluorometer (Turner Designs, Sunnyvale, CA, USA) [29,30]. Sample volumes that were filtered onto the GF/F filters were 150 mL for each experimental replicate and 500 mL for the pre- and post-incubation ambient lake water samples (all were pre-filtered through a 118 µm mesh). All chl. a filters were prepared as soon as possible, within an hour of ambient lake water collection and within two hours of mesocosm takedown, and then kept frozen until the acetone extraction step. Phytoplankton samples from the ambient lake water and experimental mesocosms were preserved with Lugol’s iodine and stored at 4 °C. They were settled in 10 mL Utermöhl chambers overnight before identification and quantification under an inverted light microscope (Axiovert, Zeiss, Dublin, CA, USA). At 400X, the first 100 identifiable cells (i.e., >5 µm in the largest dimension) were counted along a transect across the bottom of the Utermöhl chamber. Examination of 5 to 17 fields of view were typically required to reach 100 cells. Phytoplankters were first identified to the genus level whenever possible and then grouped into major taxonomic groups. We examined the following variables as responses to the treatments: species richness (total number of taxa per replicate), Shannon–Wiener (SW) diversity index [31], and taxon-specific cell counts. Statistical analyses were performed on the open-source statistical software R [32] and RStudio [33] with packages multcomp [34], multcompview [35], tidyverse [36], and ggplot2 [37]. All response variables except chl. a were square-root (sqrt) transformed. Their normality was visually inspected in Q-Q plots, and normality of residuals in ANOVA models was confirmed by Shapiro–Wilk test. Assumption of equal variance among treatments was justified by the results of Bartlett’s K-squared test.
3. Results
3.1. FTIR Analysis of Body Wash Particles
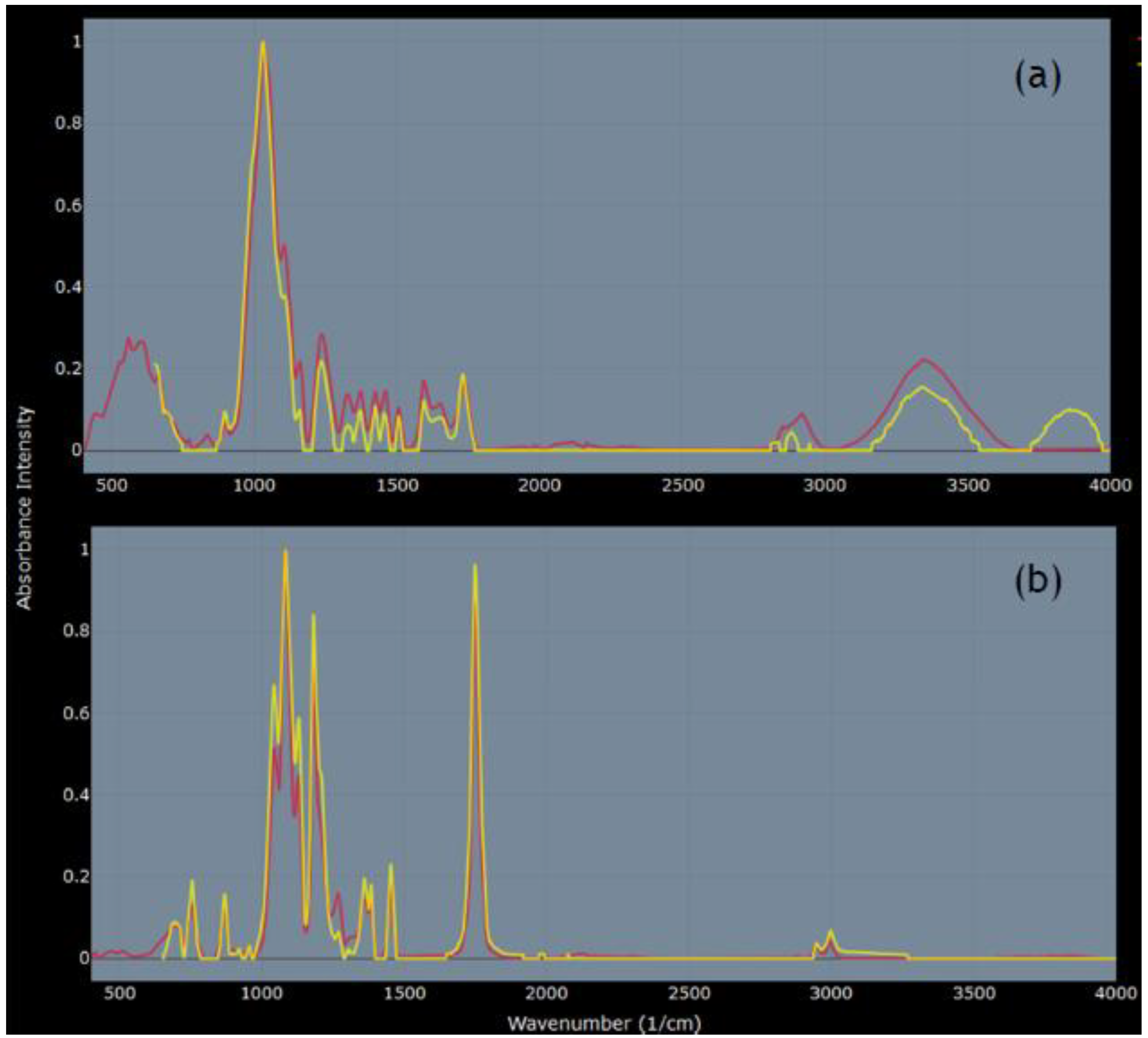
FTIR analysis confirmed that CS was a plant material; all top 10 matches (r ≥ 0.93) on Open Specy [28] were plant materials, with the top three (fiber poplar down, fiber kapok, and fiber jute) sharing the best match at r = 0.96 (spectra source: [38]) (Figure 3a). The top matches for DP were two spectra for polylactic acid (PLA) (r = 0.99 and 0.98) and fiber polylactide (0.98) (spectra source: [38]) (Figure 3b).
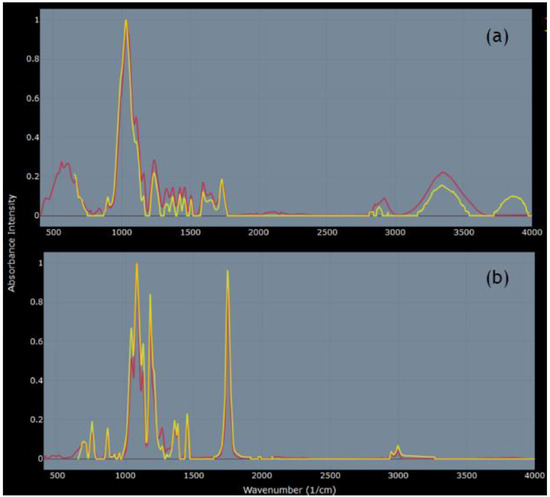
Figure 3.
Comparisons of FTIR spectra on Open Specy [28]. (a) Yellow line: CS (plant-derived microparticles); red line: fiber poplar down [38], r = 0.96. (b) Yellow line: DP (body wash microplastic (MP)); red line: polylactic acid (PLA) [38], r = 0.99.
3.2. Chl. a and Diversity Indices
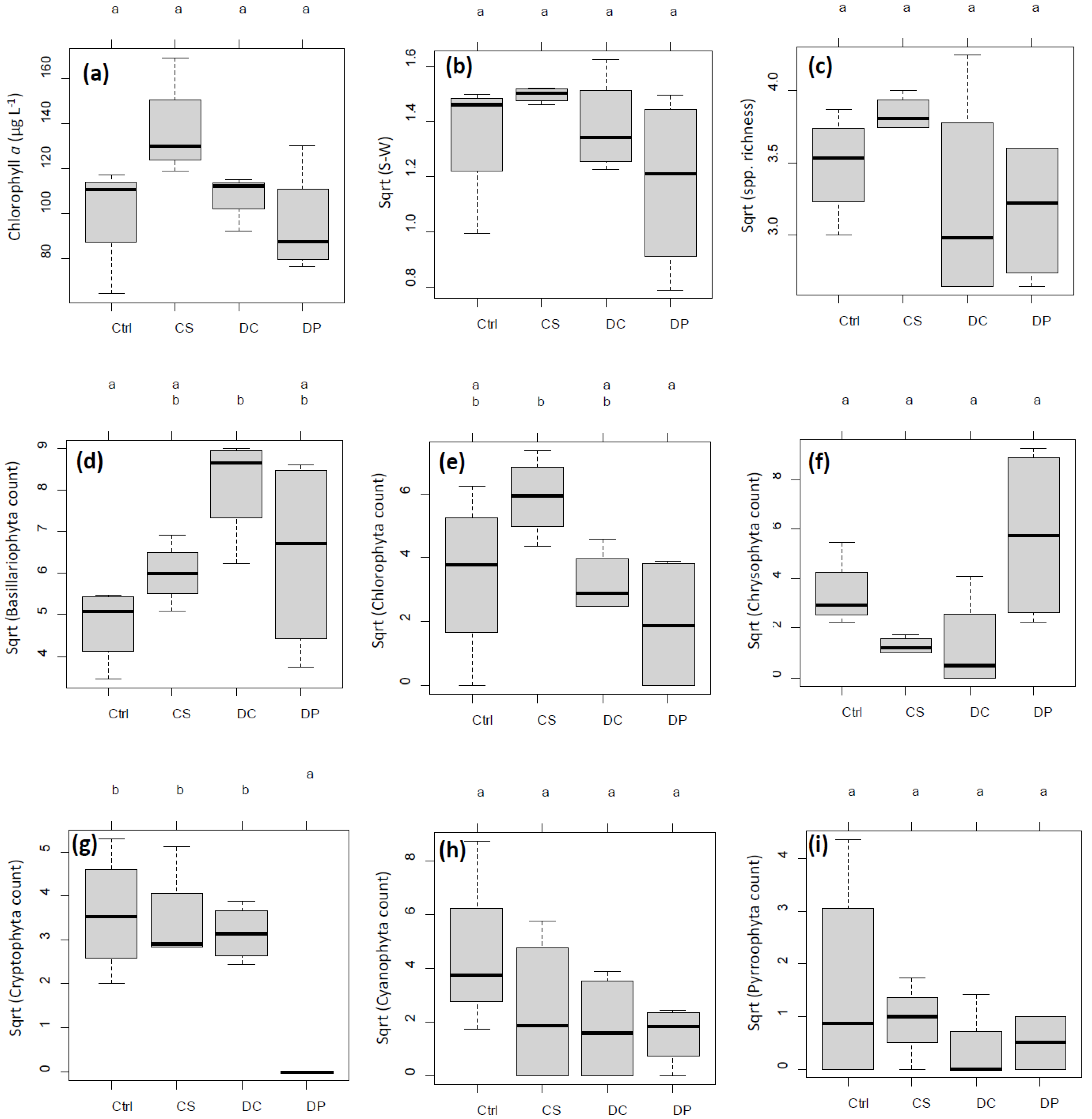
Chl. a concentrations in untreated pre- and post-incubation ambient lake water were 8.6 µg L−1 and 8.1 µg L−1, respectively. After the 7-day incubation, mean chl. a concentrations in the mesocosms were 100.9, 137.1, 106.6, and 95.4 µg L−1 for control, CS, DC, and DP, respectively, with no significant difference across treatments (Figure 4a). Similarly, the mean SW index was 1.88, 2.24, 1.96, and 1.46; the mean species richness was 12.2, 14.8, 10.8, and 10.2, respectively. No significant difference was detected among treatments in SW index or species richness (Figure 4b,c).
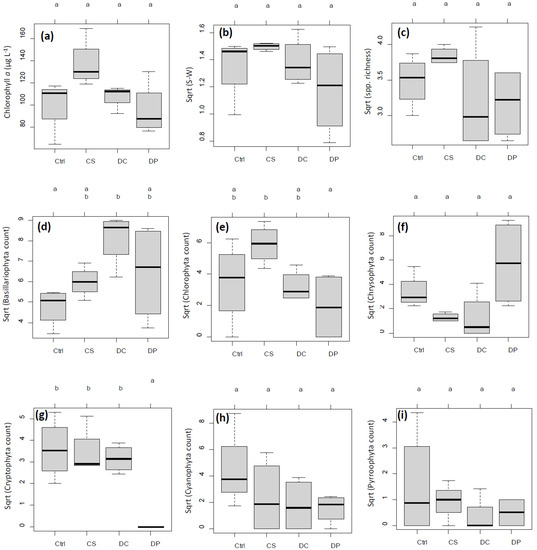
Figure 4.
Boxplots of major experimental responses after a 7-day incubation by treatment groups. n = 4 each for control (Ctrl), plant particles (CS), calibration beads (DC), and body wash MPs (DP), except chlorophyll a (chl. a) for DC (n = 3 due to sample loss). All responses except chl. a were square-root (sqrt) transformed to better meet model assumptions. Letters a and b on top of each panel indicate groupings by the results of multiple pairwise comparisons among the treatment groups by Tukey’s honestly significant difference (HSD) test at α = 0.05. (a) chl. a (µg L−1), (b) Shannon–Wiener (SW) index, (c) species richness, (d) Bacillariophyta count, (e) Chlorophyta count, (f) Chrysophyta count, (g) Cryptophyta count, (h) Cyanophyta count, and (i) Pyrrophyta count.
3.3. Phytoplankton Composition
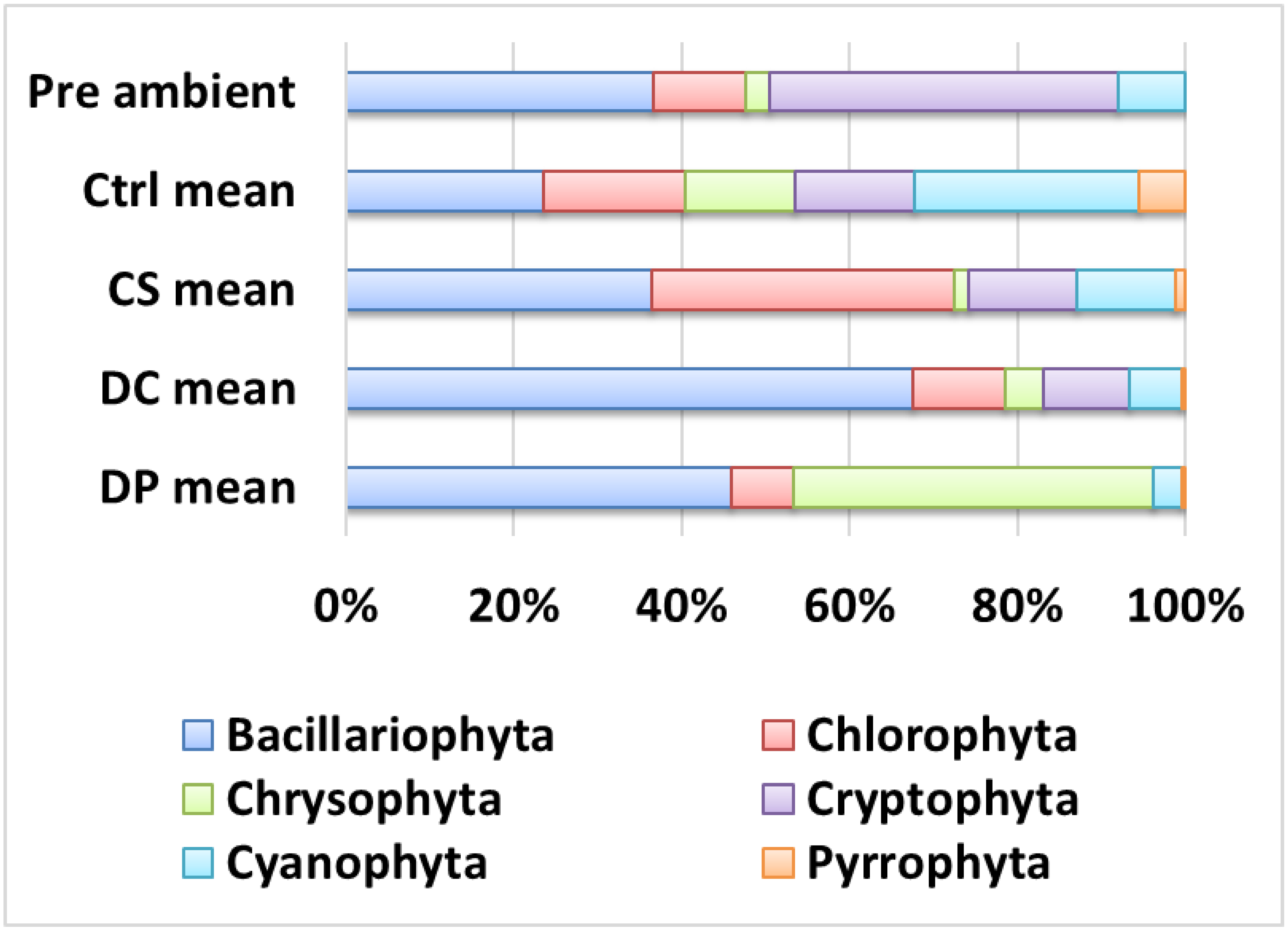
The pre-incubation phytoplankton community was represented by five major taxonomic groups: Cryptophyta (including Synurophyta) (42.0% by cell count), Bacillariophyta (diatoms) (37%), Chlorophyta (11.0%), and Cyanophyta (8.0%) (Figure 5). Pyrrophyta (dinoflagellates) were not counted in the pre-ambient sample but were detected in post-incubation samples at low frequencies (means: 5.5, 1.3, 0.5, and 0.5% for control, CS, DC, and DP, respectively). Cyanophytes were predominantly small colonies of picocyanobacteria such as Aphanocapsa and Gloeocapsa.
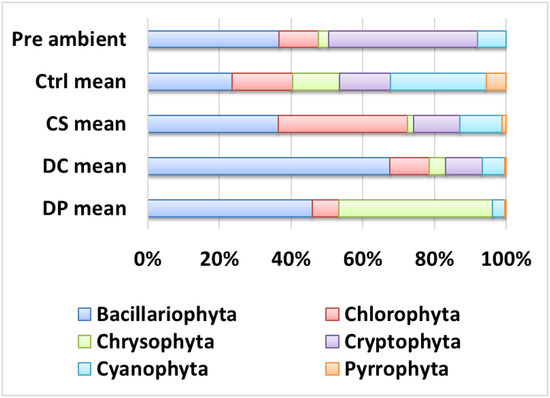
Figure 5.
Mean taxonomic composition of the phytoplankton in the untreated pre-incubation lake water (“Pre ambient”) and in the experimental mesocosms after 7 days of in situ incubation. Ctrl: control; CS: plant particles; DC: calibration beads; DP: body wash MPs.
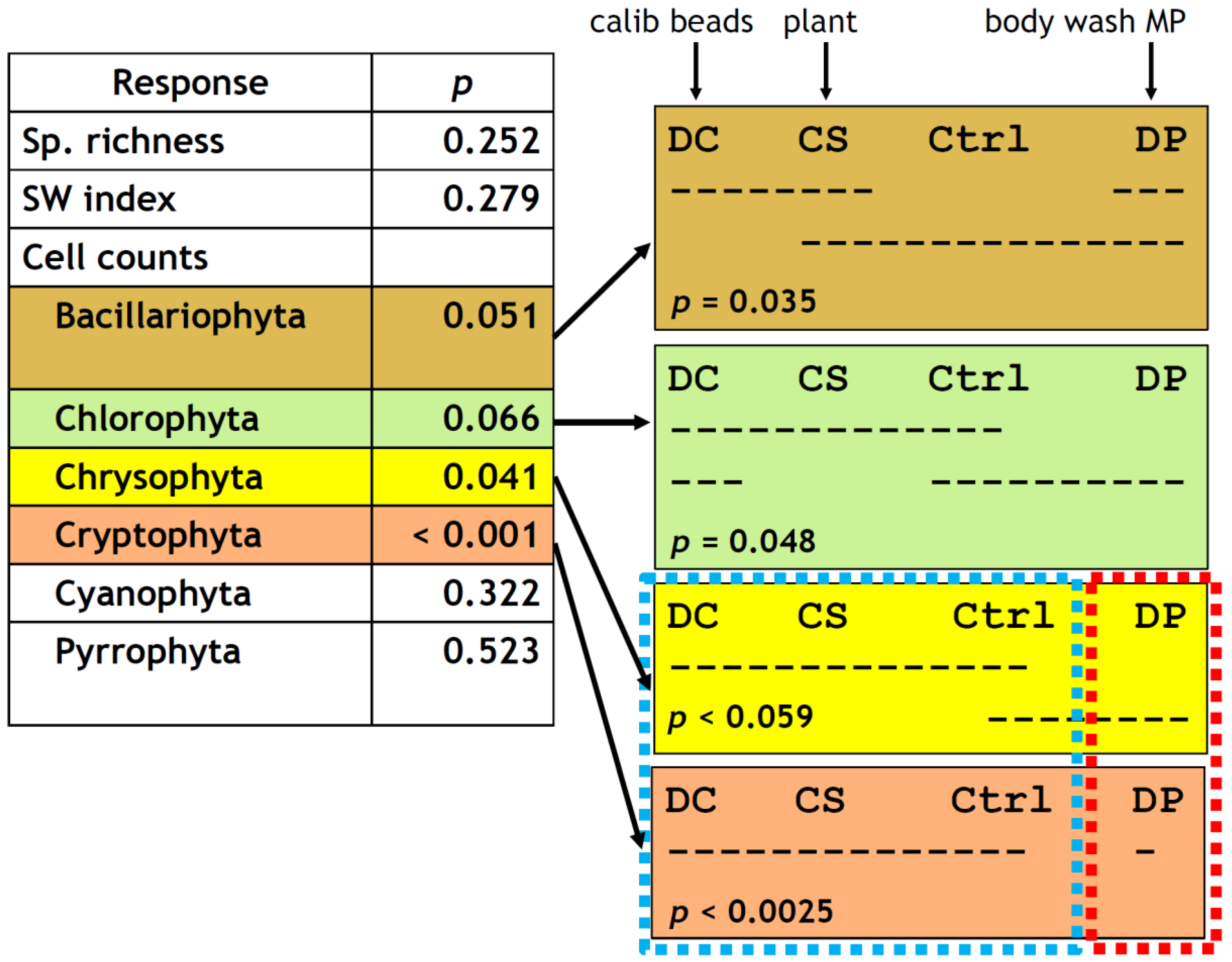
Consistent with the chl. a results above, there was no noticeable difference in general abundance of identifiable phytoplankton cells among treatments after the 7-day incubation. Cell counts by major taxonomic groups, however, showed a treatment effect, most evidently for cryptophytes (one-way ANOVA, p < 0.001), followed by chrysophytes (p = 0.041) and marginally by bacillariophytes (p = 0.051) and chlorophytes (p = 0.066) (Figure 6). DP had such a strong inhibitory effect on cryptophytes that it was totally separated from the other three treatment groups (Tukey’s honestly significant difference (HSD), p < 0.0025, Figure 4g and Figure 6). DP was also marginally distinct from DC and CS for chrysophytes (p < 0.059, Figure 6) but had an overlap with the control. This weak trend was recognized but not indicated as statistically significant in Figure 4f at α = 0.05.
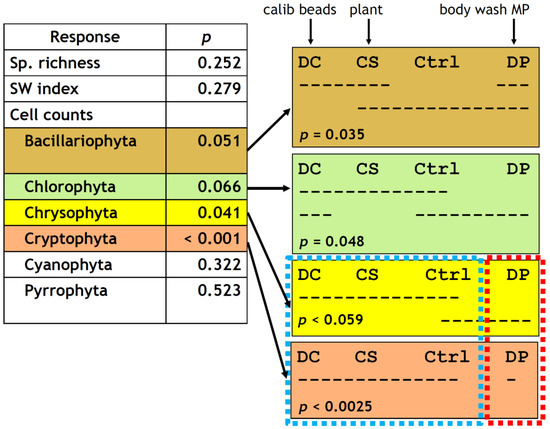
Figure 6.
Summary of one-way ANOVA (left) and grouping by Tukey’s HSD test (right) on the taxonomic responses of the phytoplankton to the treatments. n = 4 for all four treatment groups except for chl. a for DC (n = 3 due to sample loss). Dashed horizontal lines in the right panel indicate Tukey’s HSD groupings; for example, for Bacillariophyta (top right box), DC, CS, and DP formed one group and CS, Ctrl (control), and DP formed another group. While an overlap existed between the two groups, DC and Ctrl never belonged to the same group and therefore had statistically different responses in this case (p = 0.035 for the DC–Ctrl contrast). Similarly, CS–DP contrast for Chlorophyta was significant (p = 0.048), DP–DC and DP–CS contrasts had p < 0.059, and DP–DC, DP–CS, and DP–Ctrl contrasts had p < 0.0025.
4. Discussion
Our first hypothesis that MPs, but not plant-derived particles, affect the lake phytoplankton was partially supported. Microparticle addition to the lake phytoplankton did not lead to any statistically significant increase or decrease in chl. a in the control nor any of the three microparticle treatments (one-way ANOVA and Tukey’s HSD at α = 0.05), which was interpreted as no quantitative effect on overall phytoplankton biomass. Responses of each taxonomic group to different types of microparticles, however, showed previously unreported qualitative effects: Cryptophytes were eliminated (Figure 4g and Figure 5) and chrysophyte counts increased (Figure 4f and Figure 5) in response to the addition of irregularly shaped and sized MP fragments recovered from a body wash (DP). Plant-derived scrub particles (CS) had no effect (α = 0.05) on cell counts of any of the taxonomic groups included in this study (Figure 4, Figure 5 and Figure 6), supporting the idea that plant-derived microparticles are a more environmentally sustainable alternative to MPs as scrubbing agents in personal care products. Yet, the effects of the perfectly spherical calibration beads (DC) on the phytoplankton were either nondetectable or indistinguishable from those of CS in this study. Other studies have reported negative effects of analytic calibration beads on aquatic primary producers [39,40,41]. If these experiments were repeated with MPs that are more representative of secondary MPs found in the environment, more pronounced and/or different results may be observed. Such studies are going to be critical as we seek to predict long-term effects of secondary MPs in our aquatic ecosystems and to mitigate them at strategic points in the modern hydrologic cycles such as wastewater treatment plants. Our second hypothesis that planktonic cyanobacterial colonies become smaller and more numerous with MPs was not testable as the cyanobacteria observed in this study were predominantly small colonies of picocyanobacteria. With certain NPs being reported to increase microcystin production in cyanobacteria [42], repeating this experiment with a more eutrophic source water and in late summer to early fall when the water temperature is higher would be interesting and may potentially have an applied implication to management of harmful cyanobacterial blooms, an increasing concern for water utilities with respect to cyanotoxins as well as taste and odor compounds [43].
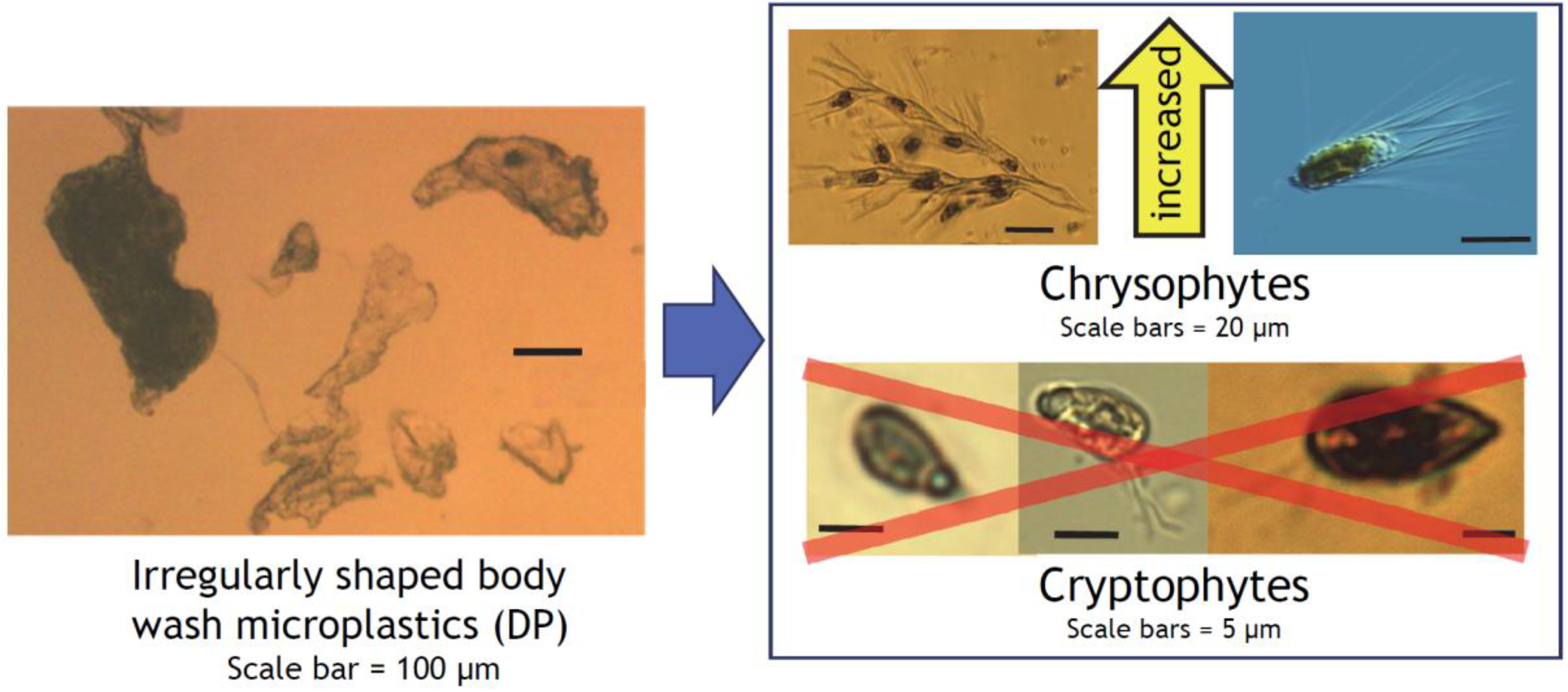
The differential effects of the calibration beads (DC) vs. irregularly shaped body wash MP particles (DP) on cryptophytes and chrysophytes were totally unexpected, as they were both “sinking” MPs (i.e., not made of “floating” high- or low-density polyethylene or polypropylene), and their size difference was within one order of magnitude. DP had an extremely close (r = 0.99) match with PLA, a family of plant-based biodegradable plastics widely used in medicine as suture threads, bone fixation hardware, and drug delivery devices and also noted for its brittleness and slow degradation [44]. PLAs derived from corn-based starch are also widely used in biodegradable/compostable single-use clamshell food containers [45] and tableware [46]. Chroomonas and Rhodomonas are flagellated, “naked” unicellular algae, and small cryptophytes resembling them (definitive species identification is not possible with light microscopy) comprised the majority of our cryptophytes (Figure 7), the group eliminated by DP (Figure 4g and Figure 5). Cryptophytes have an external covering called periplast, which is composed of a plasma membrane and plate(s) embedded underneath [47,48,49]. This would be expected to offer some extra support, but this reinforcement appears limited [50] and easily compromised by trypsin digestion [47]. Cryptophytes therefore do not have physical protection for their plasma membranes against degradation products from MPs, including proteolytic metabolites from surface biofilm. Trypsin has been detected in coastal surface seawater [51], and proteolytic enzymes in general have been associated with both live and dead bacteria in lakes [52].
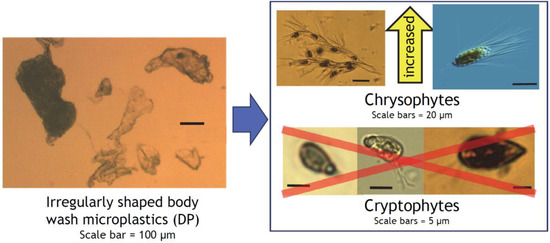
Figure 7.
Conceptual diagram of the differential effects of irregularly shaped body wash MPs on Chrysophytes and Cryptophytes, with images of representative taxa identified in this study. Images are not to scale. Inside the right box: Mallomonas sp. image (upper right) is licensed under CC BY-NC-SA 2.0 [53]; images of Dinobryon divergens (upper left), Chroomonas- (lower left and middle) and Rhodomonas-like (lower right) cryptophytes are from this and past studies from Otsego Lake phytoplankton.
In contrast, chrysophytes, mostly Dinobryon and Mallomonas in our samples, increased their occurrence with DP addition. These taxa have protective siliceous lorica or plates [48,54] that may afford them protection from MPs and potentially harmful chemicals leaching from or adsorbed to MPs. At the same time, DP did not affect cell counts of Bacillariophytes (diatoms), which are protected by siliceous frustules (valves) and mucilage [48,55] and considered resistant to abrasion and disturbance in stream periphyton [56]. The vast majority of diatoms observed in this study were non-colony forming, and MP effects on diatom colony sizes could not be evaluated. We thus postulate that a physically protective cellular outer covering is an important factor for phytoplankters to avoid negative effects from irregularly shaped and brittle aquatic MPs with large and uneven surface areas. The negative effects could include physical abrasion, electrostatic interaction, and chemical leaching from jagged and constantly eroding surfaces. Perfectly spherical MPs (DC), which are smaller in particle size and collectively have a large but smooth surface area, are more likely to roll along the surface of a cell than to damage it, which may explain them having no effect on cryptophyte counts in this study (Figure 4g). Plant-derived CS particles also tend to have rounded edges due to their cellular composition, as visible along the outline of the representative particle in Figure 2a. DP particles, on the other hand, have many projections and sharp edges that seemed to have resulted from exuding of melted plastic through a fine mesh-like structure or mechanical shredding or shaving of larger pieces of plastic. Sinking rates of diatoms have been reported to decrease with formation of diatom-microbead aggregates, while those of cryptophyte-microbead aggregates increased their sinking rate [39]. This can lead to more diatoms remaining in the photic zone while the flagellate and motile cryptophytes may experience higher mortality due to sinking into the aphotic zone. If the motile cryptophytes sense increased sinking, this may also manifest as a subtle decrease in fitness due to increased energy expenditure to swim against gravity in order to remain in the photic zone.
The findings from this study also point to the potential danger of extrapolating results from precision-manufactured calibration beads and nanoparticles to secondary MPs found in the environment. We fully acknowledge the fact that harvesting microparticles from consumer products can be extremely labor intensive and that obtaining the raw particles from a vendor is often difficult, as the exact entity of the scrub particles is not necessarily disclosed in the ingredients list. Vendors are also unlikely to respond to researcher inquiries in general. Whenever possible, however, comparison between calibration beads and scrub particles in consumer products that are expected in wastewater effluents or lab-generated secondary particles that mimic those in the environment (e.g., [57]) is advised in studies of biotic responses to MPs. Use of MPs in personal care products is either banned or being voluntarily phased out in the European Union, United Kingdom, and North America and assumed to be responsible for a relatively small portion of the overall aquatic MP pollution [24,25]; however, in this study the MPs harvested from a body wash served as a superior surrogate for secondary MPs found in source and wastewater than uniformly sized and shaped calibration beads (primary MPs). The body wash MPs better mimicked the products of fragmentation of larger pieces of plastic waste and were most likely still associated with chemical residue of the body wash. Such residue would contain chemicals commonly expected in household effluent and potentially adsorbed by aquatic MPs, e.g., surfactants, fragrances, colorants, moisturizers, stabilizers, and preservatives. Effects of MPs on aquatic organisms are expected to be driven by the physical (size and shape) and chemical characteristics of MPs themselves as well as externally associated chemicals from the surrounding water.
This study focused mainly on the effects of MPs that would be classified as fragments in environmental samples. Fibers are the most irregularly shaped MPs with a large surface-to-volume ratio and a miniscule mass, resulting in a very slow sinking rate. Fibers are therefore more likely to interact with living cells that they encounter in the environment than beads and fragments. With our increasing awareness of their true ubiquity [9,23,58], further investigation of the effects of MPs, including fibers on phytoplankton communities, and potential bottom-up effects on aquatic food webs are warranted.
5. Conclusions
This study focused on the interaction between a freshwater lake phytoplankton community and 50 µm polystyrene calibration beads, PLA-based MP from a body wash, and plant-derived scrub particles from another body wash through a 7-day, summertime, in situ incubation experiment in a mesotrophic, northern temperate lake. Taxonomic composition of the phytoplankton in the 450 mL mesocosms were not affected by the calibration beads and the plant-derived particles, while the body wash MPs eliminated cryptophytes (p < 0.001) and increased chrysophytes (p = 0.041). These differential effects of irregularly shaped body wash MPs on certain taxa of lake phytoplankters suggest potential bottom-up alteration of aquatic food webs by secondary MPs, including those considered biodegradable. Increased monitoring data and sample sharing between utilities operators and researchers will accelerate our understanding of how environmental MPs interact with freshwater biotas before they enter the ocean.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/12/9/2650/s1, Supplementary File 1: photographs of the product ingredient lists of CS and DP. Raw data tables for phytoplankton, water chemistry, and CS and DP FTIR spectra are available as a data package through the Environmental Data Iintiative (EDI) data portal: https://portal.edirepository.org/nis/mapbrowse?scope=edi&identifier=615. doi:10.6073/pasta/103799f7d2cf93e5a54582750ac28436.
Author Contributions
Conceptualization, K.Y.; methodology, K.Y.; investigation, K.Y. and M.M.; formal analyses, M.M. and K.Y.; original draft preparation, K.Y.; review and editing, K.Y. and M.M.; funding acquisition, K.Y. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
K.Y. and M.M. thank the anonymous reviewers who provided constructive feedback and helped improve the manuscript, especially one reviewer who generously provided detailed guidance on FTIR data interpretation for MPs; Erica L. Majumder and Liyuan (Joanna) Hou for FTIR analysis of DP and CS samples at SUNY College of Environmental Sciences and Forestry; Courtney Widgahl-Perry (SUNY Fredonia) and other Northeast GLEON collaborators for the general design of the in situ incubation experiment; Emily Davidson Parry, Cody Hastings and Holly Waterfield for extracting microparticles from body wash products; 2016 BFS summer interns and BFS staff for general lab assistance; the Clark Foundation and Otsego County Conservation Association for financial support for the BFS Summer Internship Program, which funded M.M.; and SUNY Oneonta Faculty Research Grant and Biological Field Station Summer Faculty Research Stipend awarded to K.Y.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carpenter, E.J.; Smith, K.L.; Burke, J.A.; Schubert, W.K. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Sul, J.A.I.D.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. 2016, 56, 1720–1739. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics research—from sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar] [CrossRef]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R.C. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Hoellein, T.; McCormick, A.R.; Hittie, J.; London, M.G.; Scott, J.W.; Kelly, J.J. Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw. Sci. 2017, 36, 491–507. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7. [Google Scholar] [CrossRef]
- Smith, E.; Dziewatkoski, M.; Henrie, T.; Seidel, C.; Rosen, J. Microplastics: What Drinking Water Utilities Need to Know. J. Am. Water Work. Assoc. 2019, 111, 26–37. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.-P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of nano- and microplastics for freshwater ecosystems: A critical review. TrAC Trends Anal. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Yokota, K.; Waterfield, H.; Hastings, C.; Davidson, E.; Kwietniewski, E.; Wells, B. Finding the missing piece of the aquatic plastic pollution puzzle: Interaction between primary producers and microplastics. Limnol. Oceanogr. Lett. 2017, 2, 91–104. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M. Marine microplastic-associated biofilms—A review. Environ. Chem. 2015, 12, 551–562. [Google Scholar] [CrossRef]
- Miao, L.; Hou, J.; You, G.; Liu, Z.; Liu, S.; Li, T.; Mo, Y.; Guo, S.; Qu, H. Acute effects of nanoplastics and microplastics on periphytic biofilms depending on particle size, concentration and surface modification. Environ. Pollut. 2019, 255, 113300. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.; Hastings, C.; Waterfield, H.; Yokota, K. Development of Methods to Characterize & Extract Plastic Microparticles from Personal Cleansing Products; 47th Annual Report; Biological Field Station: Cooperstown, NY, USA, 2014; pp. 142–148. [Google Scholar]
- Kalčíková, G.; Alič, B.; Skalar, T.; Bundschuh, M.; Gotvajn, A. Žgajnar Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater. Chemosphere 2017, 188, 25–31. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Barrows, A.P.; Cathey, S.; Petersen, C. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kross, S.M.; Armstrong, J.B.; Bogan, M.T.; Darling, E.S.; Green, S.J.; Smyth, A.R.; Veríssimo, D. Scientific Evidence Supports a Ban on Microbeads. Environ. Sci. Technol. 2015, 49, 10759–10761. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Lewis, A.S.L.; Kim, B.S.; Edwards, H.L.; Wander, H.L.; Garfield, C.M.; Murphy, H.E.; Poulin, N.D.; Princiotta, S.D.; Rose, K.C.; Taylor, A.E.; et al. Prevalence of phytoplankton limitation by both nitrogen and phosphorus related to nutrient stoichiometry, land use, and primary producer biomass across the northeastern United States. Inland Waters 2020, 10, 42–50. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Cowger, W.; Gray, A.; Hapich, H.; Rochman, C.; Lynch, J.; Primpke, S.; Munno, K.; De Frond, H.; Herodotou, O. Open Specy. Available online: www.openspecy.org (accessed on 20 September 2020).
- Arar, E.J.; Collins, G.B. Method 445.0 in Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence; USEPA: Cincinnati, OH, USA, 1997; p. 22.
- Mehlrose, M.; Yokota, K. Evaluation of Chlorophyll a Extraction Techniques; State Universtiy of New York College at Oneonta Biological Field Station Annual Report; State Universtiy of New York College at Oneonta Biological Field Station: Cooperstown, NY, USA, 2016; pp. 66–75. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- R Studio Team. R Studio: Integrated Development for R; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Graves, S.; Piepho, H.-P.; Selzer, L. multcompView: Visualizations of Paired Comparisons. Available online: https://cran.r-project.org/web/packages/multcompView/index.html (accessed on 20 September 2020).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Primpke, S.; Wirth, M.; Lorenz, C.; Gerdts, G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal. Bioanal. Chem. 2018, 410, 5131–5141. [Google Scholar] [CrossRef]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Sjollema, S.B.; Redondo-Hasselerharm, P.; Leslie, H.; Kraak, M.H.S.; Vethaak, A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016, 170, 259–261. [Google Scholar] [CrossRef]
- Feng, L.-J.; Sun, X.-D.; Zhu, F.-P.; Feng, Y.; Duan, J.-L.; Xiao, F.; Li, X.-Y.; Shi, Y.; Wang, Q.; Sun, J.-W.; et al. Nanoplastics Promote Microcystin Synthesis and Release from Cyanobacterial Microcystis aeruginosa. Environ. Sci. Technol. 2020, 54, 3386–3394. [Google Scholar] [CrossRef]
- Smith, S.A. Beyond Toxins: A Source-to-Treatment Strategy for Harmful Algal Blooms. J. Am. Water Work. Assoc. 2019, 111, 14–22. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Madival, S.; Auras, R.; Singh, S.P.; Narayan, R. Assessment of the environmental profile of PLA, PET and PS clamshell containers using LCA methodology. J. Clean. Prod. 2009, 17, 1183–1194. [Google Scholar] [CrossRef]
- Fieschi, M.; Pretato, U. Role of compostable tableware in food service and waste management. A life cycle assessment study. Waste Manag. 2018, 73, 14–25. [Google Scholar] [CrossRef]
- Gantt, E. Micromorphology of the Periplast of Chroomonas sp. (Cryptophyceae)2. J. Phycol. 1971, 7, 177–184. [Google Scholar] [CrossRef]
- Sheath, R.G.; Wehr, J.D. Introduction to freshwater algae. In Freshwater Algae of North. America: Ecology and Classification; Academic Press: San Diego, CA, USA, 2003; pp. 1–9. ISBN 0-12-741550-5. [Google Scholar]
- Kugrens, P.; Lee, R.E. An Ultrastructural survey of Cryptomonad Periplasts Using Quick-Freezing Freeze Fracture Techniques. J. Phycol. 1987, 23, 365–376. [Google Scholar] [CrossRef]
- Siegelman, H.; Kycia, J. Algal biliproteins. In Handbook of Phycological Methods: Psychological Biochemistry Methods; Cambridge University Press: Cambridge, UK, 1978; pp. 71–79. [Google Scholar]
- Obayashi, Y.; Suzuki, S. Proteolytic enzymes in coastal surface seawater: Significant activity of endopeptidases and exopeptidases. Limnol. Oceanogr. 2005, 50, 722–726. [Google Scholar] [CrossRef]
- Kiersztyn, B.; Siuda, W.; Chróst, R.J. Persistence of bacterial proteolytic enzymes in lake ecosystems. FEMS Microbiol. Ecol. 2012, 80, 124–134. [Google Scholar] [CrossRef]
- Proyecto Agua Mallomonas, el cometa entre estrellas. S.O.S. Lago de Sanabria. Available online: https://www.flickr.com/photos/25898159@N07/49646121252. (accessed on 20 September 2020).
- Kristiansen, J.; Preisig, H.R. Phylum Chrysophyta (golden algae). In The Freshwater Algal Flora of the British Isles: An. Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2011; pp. 281–317. [Google Scholar]
- Lee, R.E. Phycology; Cambridge University Press (CUP): Cambridge, UK, 2008. [Google Scholar]
- Biggs, B.J.F.; Stevenson, R.J.; Lowe, R.L. A habitat matrix conceptual model for stream periphyton. Fundam. Appl. Limnol./Archiv für Hydrobiologie 1998, 143, 21–56. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Belda, M.T.; Pulido-Reyes, G.; Amariei, G.; Leganes, F.; Rosal, R.; Fernández-Piñas, F. Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environ. Sci. Nano 2019, 6, 1382–1392. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Spanjer, A.R.; Rosen, M.R.; Thom, T. Microplastics in Lake Mead National Recreation Area, USA: Occurrence and biological uptake. PLoS ONE 2020, 15, e0228896. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).