Abstract
The baseline quality of pre-drilling shallow groundwater is essential for the evaluation of potential environmental impacts of shale gas development. The Xishui region in the northern Guizhou Province of Southwest China has the potential for shale gas development but there is a lack of commercial production. As for the future environmental concerns in this undeveloped area, this study presented the hydrochemical and isotopic characteristics of shallow groundwater and its dissolved gas before shale gas development and determined the sensitive monitoring indicators. Results showed that shallow groundwater with an average pH of 7.73 had low total dissolved solids (TDS) ranging between 102 and 397 mg/L, with the main water chemistry types of HCO3-Ca and HCO3-Ca·Mg. The quality of most groundwater samples satisfied the drinking water standards of China. The mass concentration of dissolved methane in groundwater was below the detection limit (<0.01 mg/L), suggesting the low baseline value of hydrocarbon. The shallow groundwater was mainly recharged by local precipitation based on water isotopes. Water chemistry was modified by the dominant dissolution of carbonate rocks and partial dissolution of clastic rocks, as indicated by δ13C-DIC, 87Sr/86Sr, and δ11B. Evidence from carbon isotopes of dissolved methane and CO2 (δ13C-CH4 and δ13C-CO2) and noble gas isotopes (3He/4He and 4He/20Ne) demonstrated that the biogenic methane mainly originated from acetate fermentation and the dissolved noble gas was a result of the dissolution of air. Based on the geochemical and isotopic differences between shallow groundwater and flowback and produced water (including shale gas) from the Weiyuan and Fuling shale gas fields as well as shale gas from Xishui, this study has provided the sensitive monitoring indicators and methods for identifying potential pollution of regional shallow groundwater related to shale gas development in the future.
1. Introduction
Due to advances in horizontal drilling and hydraulic fracturing, the rapid increase of shale gas production in North America has affected the global energy pattern [1,2]. China has developed shale gas exploration since 2012 and has the largest technically recoverable shale gas resources worldwide, which are estimated to include 21.8 trillion m3 of natural gas [3,4]. The black shale from the Lower Silurian Longmaxi Formation is the main shale gas reservoir in South China, accounting for 20% of national shale gas resources [5]. In the Sichuan Basin, Southwest China, several large gas fields have been developed with the Longmaxi Formation as reservoir formation, including Fuling, Weiyuan, and Changning [6]. In the Guizhou Province of Southwest China, the Longmaxi Formation is mainly located in Northern Guizhou and its shale gas reserves can be evaluated to 1.83 × 1012 m3, which has the potential for large commercial shale gas development [7,8,9].
However, large-scale hydraulic fracturing (HF) may cause potential risks to the geological environment [10,11,12]. One of the widely concerned environmental issues related to shale gas development is the groundwater pollution problems [13,14,15]. Groundwater pollution caused by shale gas production is mainly divided into stray gas (natural gas) pollution and wastewater pollution through different pollution pathways, such as wastewater leakage from impounding reservoirs, fluid leakage associated with casing and cementing defects during hydrocarbon extraction, and migration of deep reservoir fluids to shallow groundwater through induced faults [16,17,18]. Some earlier studies indicated that dissolved CH4 of shallow groundwater in Pennsylvania increased as it moved closer to shale gas wells [13,19]. This is most likely due to the small sample size, which was from a planned sampling campaign in a known contamination area [20]. A few follow-up studies using a larger dataset and a geospatial analysis tool suggested that such an impact was negligible except for a few hotspots [20,21]. In Texas, specifically in Parker-Hood counties, a detailed noble gas study indicated that the stray gas in shallow groundwater was most likely from the Strawn Group rather than the Barnett Shale [22,23]. Furthermore, lacking pre-drilling baseline data of shallow groundwater quality makes these findings of stray gas contamination controversial [24,25]. Recent research has compared the post-drilling and pre-drilling data and found that high concentrations of methane and dissolved solids of shallow groundwater were not related to the shale gas development, but to natural processes in most areas [25,26,27]. Thus, establishing geochemical baseline datasets of shallow groundwater systems before shale gas production is necessary, which can not only provide direct evidence when contamination occurs, but can also reduce controversies of shallow groundwater pollution after hydraulic fracturing [28,29,30]. Currently, a few sites, such as Pennsylvania, USA [31], Colorado, USA [32], Quebec, Canada [33], North Yorkshire, UK [34], and Chongqing, China [35,36], have developed baseline investigation of groundwater before drilling shale gas wells.
This study aims to present a case for the comprehensive evaluation of shallow groundwater quality before shale gas production. The study area is located in the Xishui region of Northern Guizhou, where there are plans for shale gas development, but where there is not yet commercial production. Previous studies in the USA and in the Qaidam Basin in North China show that hydrocarbon isotopes of methane can be used to identify the stray gas contamination of shallow groundwater [13,19,25], while hydrochemistry and isotopes of boron, lithium, and strontium are accessible and sensitive to recognizing the shallow groundwater pollution from flowback and produced water (FPW) [37,38,39,40,41]. Thus, this study investigated the systematic geochemical baseline values of groundwater and dissolved gas from 21 springs supplying local drinking water, by using multiple hydrochemical and isotopic compositions, including major elements, trace elements, and environmental isotopes (2H, 18O, 13C, 87Sr/86Sr, 11B, 3He). Comparing shallow groundwater (including dissolved gas) of Xishui with the FPW (including shale gas) from the Weiyuan gas field (FPW-W) [42], the Fuling gas field (FPW-F) in Sichuan Basin [43], and a shale gas sample from Xishui, this study also concludes that sensitive geochemical indicators and integrated methods of groundwater pollution identification should be included in a routine monitoring program.
2. Study Area and Sampling
The study area is located in the Xishui area (25°06’35″–28°50′15″ N and 105°50′20″–106°44′30″ E), a northern region of Guizhou Province, Southwest China (Figure 1). It is typical of a subtropical humid monsoon climate with an average annual temperature of 13.1 °C. The annual average precipitation is 1138 mm (max 1160 mm; min 777 mm), of which approximately 79% occurs in May–October. Multiple rivers developing in the area belong to the Wujiang river system, including the Chishui River and the Xishui River. The study area is a mountainous landform with rare arable land. The land use/land cover (LULC) in Xishui is mainly forest (62%) and farmland (31%) [44].
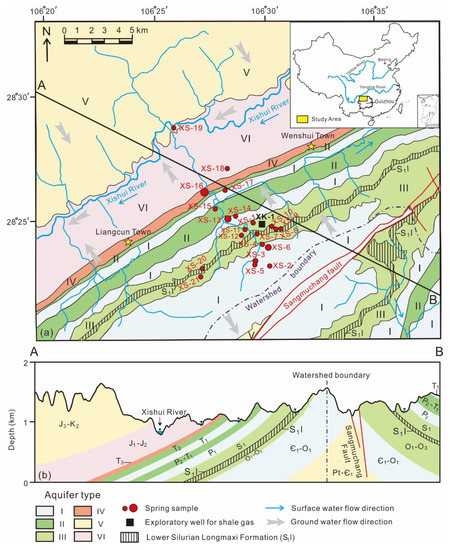
Figure 1.
Study area. (a) The planimetric map with the distribution of aquifers; (b) the section map of aquifers. The introduction of aquifer types can be found in the text. The bigger dots indicate the spring water samples with higher discharges, while the Lower Silurian Longmaxi Formation (S1l) is the target stratum for shale gas exploration.
Tectonically, the study area belongs to the transitional zone between the northern part of the Central Guizhou Uplift and the southeastern region of the Sichuan Basin [45]. The sampling area is located in the northwestern limb of the Sangmuchang anticline, while the axial direction is 40–50° north by east [46]. The oldest stratum exposed at the core of the anticline is the Proterozoic dolomite rock. Those strata from the core to northwestern (from old to new) comprise the Cambrian dolomite, Ordovician shale and limestone, Lower Silurian shale, Permian and Triassic limestone, and Jurassic and Upper Cretaceous mudstone and sandstone [47]. As the major shale gas reservoir in the study area, the black shale of the Lower Silurian Longmaxi Formation is deposited in the deep water continental sedimentary environment, the same as those in the Sichuan Basin [45,48]. It is characterized by large thickness (50–200 m), sapropelic kerogen, and a moderate burial depth (generally less than 1–2 km) [49]. As for its organic chemical characteristics, it is high in organic matter content (w(TOC) of 0.5–3.92%), gas content (saturated adsorption gas content of 1.52–2.77 m3/t), and thermal maturity, while it is low in porosity and permeability [50,51,52], indicating the advantages of shale gas accumulation and hydraulic fracturing.
Shallow groundwater aquifers in the study area can be divided into six types according to the lithology, water-bearing media, water yield property based on lithology, and water abundance (Figure 1) [53]: (I) Fracture-cave water mainly occurring in the limestone and dolomite of Lower Cambrian to Lower Ordovician (Є1–O1), Lower Permian (P1), and Lower Triassic (T1), with the discharge of springs of 10–100 L/s. (II) Fracture-cave water commonly presenting in the limestone with little mudstone of Upper Permian to Lower Triassic (P2–T1) and Middle Triassic (T2), with the discharge of springs of 1–10 L/s. (III) Cave-fracture water generally occurring in the shale with little limestone of Lower to Upper Ordovician (O1–O3) and Lower Silurian (S1) (including Longmaxi Formation), with the discharge of springs of 0.1–10 L/s. (IV) Pore-fracture water only flowing in the sandstone of Upper Triassic (T3), with a relatively rich water yield of 1000–5000 m3/d for a well with the drawdown value of 40 m. (V) One kind of fracture water existing in the shale of Proterozoic to Lower Cambrian (Pt–Є1) and the sandstone of Upper Jurassic to Upper Cretaceous (J3–K2), with a groundwater runoff modulus of 1.0–2.0 L/(s·km2). (VI) Other fracture water distributing in the mudstone of Lower to Middle Jurassic (J1–J2), with an extremely low groundwater runoff modulus of less than 1.0 L/(s·km2). Because of the various lithology and the strong anisotropy of shallow aquifers, shallow groundwater in the study area may occur heterogeneously with mixing sources of geochemical components [54]. In Figure 1, the major direction of groundwater flow in the study area is from the watershed boundary to the Xishui River (the largest river in study area). Fracture groundwater in the study area is usually recharged by local rainfall through infiltration and represents the average chemical and isotopic compositions of rainfall in the past several months to several years based on the local hydrogeological conditions [53]. Therefore, the seasonal variation has been homogenized. Shallow groundwater drainages mainly occur in springs and recharges into river water [54].
As the Xishui region is one of the most favorable exploration zones of shale gas in northern Guizhou [9,49], several exploratory wells have been developed for evaluating potential shale gas resources of the Longmaxi Formation, including XK-1 (shown in Figure 1). However, the study area is mainly an underdeveloped area without conventional and unconventional oil and gas development as well as other industrial activities. Therefore, it is an ideal site to access the baseline quality of local shallow groundwater.
3. Experiment and Analyses
Because there was almost no well in the study area, the 21 shallow groundwater samples were all collected from springs (outcrops of groundwater) in June 2018, some of which were used to provide drinking water for local people. The sites of the springs were distributed in different types of aquifers with various lithology (like silicate and carbonate rocks) and diverse ages (Cambrian to Cretaceous) (Figure 1). Despite complex lithology and different ages of aquifers in the study area, we concluded all samples to represent the holistic shallow groundwater and provide general baseline quality of the local shallow groundwater system. A shale gas sample (SG-1) was collected from the scientific shallow shale gas well (XK-3) in March 2019.
Field parameters of water samples, including temperature, pH, oxidation-reduction potential (ORP) and electrical conductivity (EC), were measured in situ by a portable multi-parameter meter (HQ40D, Hach). The titration of HCO3/CO3 was accomplished in the field using a 16900 Digital Titrator (Hach) with 0.8 mol/L sulfuric acid for titration and phenolphthalein and methyl orange as indicators. After the filtration through 0.45 μm membrane in the field, all samples were sealed in different capacities of polyethylene bottles according to the sample volumes required by different chemical tests.
Major ions and water stable isotopes (δ18O and δ2H) were analyzed in the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGG-CAS). Major cations (K+, Na+, Ca2+, and Mg2+) and anions (F−, Cl−, NO3− and SO42−) were measured by an Ion Chromatography (Dionex ICS1100) with a detection limit of 0.05 mg/L. After water samples were filtered by 0.22 μm membrane as sample pretreatment in the laboratory, δ18O and δ2H of samples were measured by a laser absorption water isotope analyzer (Picarro L1102–i) with detection precisions of ±0.1 and ±0.5‰, respectively. Trace elements were determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) of NexION 300D in the Beijing Research Institute of Uranium Geology (BRIUG). For the concentrations of Hg and As, an atomic fluorescence spectrometer (ASF2202) of BRIUG was used for the measurement, with analytical limits of 0.6 and 0.1 μg/L, respectively. The content of Br- was tested in BRIUG using a Gas Chromatography Analyzer (Panna A90), with a detection limit of 0.02 mg/L. For the measurement of the content of CH4 in groundwater, each water sample was sealed in two 40-mL brown glass bottles added with hydrochloric acid as an antimicrobial agent and the samples were analyzed by Gas Chromatograph Mass Spectrometer (GC/MS) in Shanghai SEP Analytical Service Co., Ltd., with a detection limit of 0.01 mg/L.
The carbon isotope of dissolved inorganic carbon (δ13C-DIC) in groundwater was determined by Isotope Ratio Mass Spectrometers (IRMS) of MAT253 in the Laboratory for Stable Isotope Geochemistry, IGG-CAS. The detection accuracy is ±0.1‰ (standard value of VPDB). For the measurement of boron isotope (δ11B), water samples were condensed to the required testing concentration and they were measured by Thermal Ionization Mass Spectrometry (TIMS) in the Qinghai Institute of Salt Lakes, CAS. The measurement precision is better than 0.4‰. The detection limit of δ11B is generally 10–20 μg/L. The ratio of strontium isotope (87Sr/86Sr) was analyzed by TIMS (Phoenix) in BRIUG. The 87Sr/86Sr of the standard sample (NBS 987) from the National Institute of Standards and Technology (NIST) is 0.710248 ± 0.000008. The uncertainty of the measurement of 87Sr/86Sr is ±0.000008. represents the normalization of the 87Sr/86Sr ratio of a water sample, by using the strontium isotope value of modern seawater (0.709169) [38]:
The samples of dissolved gas were collected from 12 selected springs through a conventional negative pressure method. A dissolved gas sample was injected into a 50 mL glass bottle with pristine water using the method of dissolved gas collection [55] and the bottle was further stored in a 500 mL brown plastic jar full of the pristine water. The tests for gas components (volumetric percentage) and the isotopes of carbon and noble gas were accomplished in the Key Laboratory of Petroleum Resources Research, CAS. The gas compositions of gas samples were tested by IRMS (MAT 271). The relative standard deviation was ±5% and the detection limit was 0.0001%. The carbon isotopes of methane (δ13C-CH4) and carbon dioxide (δ13C-CO2) were measured by IRMS (Thermo-Fisher Scientific Delta Plus XP) with an error range of ±0.2‰. The isotope values of noble gas (3He/4He and 4He/20Ne) as well as the volumetric percentage of He were analyzed by a noble gas isotope mass spectrometer (Noblesse, Nu instruments). The measurement precision is ±3%. The standard gas of AIRLZ2007, representing Ra, is from the air on Gaolan Mountain, West China, of which the value of 3He/4He is 1.39 × 10−6. The results of 3He/4He can be expressed as R/Ra:
R/Ra = (3He/4He)sample/(3He/4He)standard gas
The full results are provided in Tables 1 and 2, including samples of groundwater, dissolved gas, and shale gas. The geochemical data of FPW-W and FPW-F come from Ni et al. (2018) [42] and Huang et al. (2020) [43], respectively. Besides, the isotopic and geochemical data of shale gas in the Weiyuan gas field are from Cao (2017) [56]. Though the Longmaxi Formation in the study area is undeveloped for shale gas, it is reasonable to compare natural shallow groundwater (including dissolved gas) with the FPW (including shale gas) from two gas fields extracting shale gas from the Longmaxi Formation, for concluding the major differences and establishing methods for identifying pollution from FPW and shale gas.
4. Results and Discussion
4.1. Baseline Values of Shallow Groundwater
4.1.1. Hydrochemistry
TDS values varied between 102 and 397 mg/L, with an average value of was 237 mg/L. The hydrochemical types of shallow groundwater were mainly HCO3-Ca and HCO3-Ca·Mg with low contents of Cl and Na (averages of 2.0 and 2.6 mg/L, respectively), except that only two samples (XS-17, XS-18) belonged to HCO3·SO4-Ca due to the high percentages of milligram equivalent of SO4 (26.6 and 25.9 meq%) (Figure 2). The cations of shallow groundwater were dominated by Ca and Mg, with the average percentages of milligram equivalent of 76 and 21 meq%, respectively while the anions primarily consisted of HCO3 (average of 80 meq%) with a minor amount of SO4 (average of 16 meq%). The concentrations of both potassium and fluorine were low in groundwater, with ranges of 0.1–2.8 mg/L and 0.1–0.3 mg/L, respectively. In the karst area of Guizhou province, shallow aquifers affected by the dissolution of carbonate rock usually develop the HCO3-Ca and HCO3-Ca·Mg water types and low TDS of groundwater, indicating that the dissolution of dolomite and limestone may contribute to the compositions of major ions of local shallow groundwater [57,58,59].
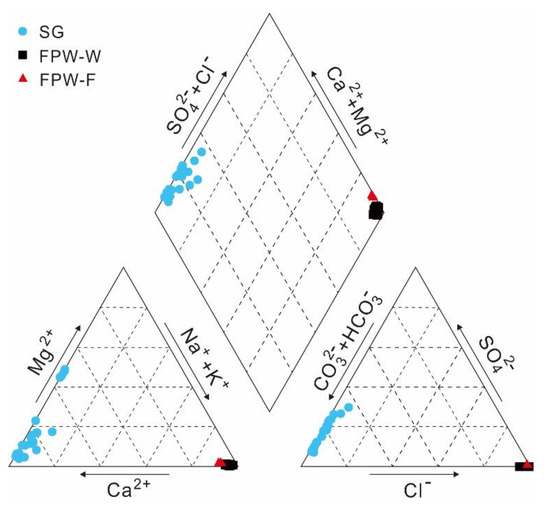
Figure 2.
The Piper diagram of shallow groundwater (SG) of the Xishui region, flowback and produced water from Weiyuan (FPW-W) and Fuling (FPW-F).
Compared with the undetected content of sulfate in FPW [42,43], local shallow groundwater samples had a relatively high content of SO4, ranging between 13 and 77 mg/L, with an average value of 34 mg/L, but far lower than the drinking water standard of China (GB5749-2006) of 250 mg/L. The main sources of sulfate in groundwater can be from human activity, precipitation, oxidation of sulfides and dissolution of sulfate-rich evaporite minerals (i.e., gypsum, anhydrite) [60,61]. The residents live dispersedly in the study area and the sampling spring outcrops were far away from the residence, suggesting that it had little possibility of groundwater pollution from the sewage water. The saturation indexes (SI) of gypsums and anhydrite of shallow groundwater, calculated by PHREEQC 3.0 with the database of llnl.dat [62], were all lower than −1.5. The low values of SI may be related to a quick circulation rate, strong renewability, and a relatively low content of evaporite minerals in the local karst area, implying that the dissolution of sulfate minerals may have little impact on local groundwater. The average milligram equivalent ratio of (SO4)/(SO4 + HCO3 + CO3 + Cl + NO3) of groundwater was 16%, which was lower than values of the area influenced by oxidation of sulfides in Southwest China (24–29%) [63], indicating that most local groundwater may be neglectfully affected by oxidation of sulfides. The sulfate content of rain in nine cities evenly distributed in Southwest China had an average value of 10.6 mg/L [64], while the enrichment factor (evapotranspiration from rain to spring) had the range of 2–5 based on some sites with similar hydrogeological conditions in Southwest China [65,66], suggesting that the spring water with no loss or other source during recharge should have the range of SO4 concentrations between 21 and 53 mg/L. The theoretical range covered the SO4 values of most local springs, but two samples of XS-17 (74 mg/L) and XS–19 (77 mg/L) significantly exceeded the range. These two samples also had high ratios of (SO4)/(SO4 + HCO3 + CO3 + Cl + NO3) of 22% and 23%, respectively, close to the range of 24–29%, suggesting that the oxidation of sulfides may affect two samples. Thus, the sulfate of most samples was mainly derived from local precipitation, while two samples with high sulfate concentrations may be additionally affected by the oxidation of sulfide deposits.
The concentrations of NO3 in groundwater varied between <0.05 and 10.5 mg/L, with an average value of 4.0 mg/L, except two samples (XS-15, XS-17) with high values of 63.9 and 28.4 mg/L, respectively. The NO3 concentrations of most water samples were below the threshold limit of 44 mg/L for the drinking water standard in China, except for one sample (XS-15). A few corn fields were in the vicinity of the sampling sites of XS-15, and XS-17, and the high content of nitrate may be related to agricultural pollution such as nitrogen fertilizer.
Bromide concentrations of most water samples were below the detection limit of 0.02 mg/L and only five samples had quite low contents, ranging between 0.02 and 1.00 mg/L, with an average value of 0.25 mg/L. In China, there is no standard limit of methane for both drinking water (GB5749-2006) and groundwater quality (GB/T 14848-2017). In America, the defined action level of methane for hazard mitigation (10–28 mg/L), recommended by the US Office of the Interior, can be considered as a standard limit of methane for natural groundwater [67]. The methane contents of all groundwater samples were less than 0.01 mg/L and were far lower than the action level, implying that the CH4 contents of shallow groundwater in the region were low background values. If the stray gas from shale gas wells leaks into shallow groundwater, it may cause the increasing mass concentrations of CH4 of the polluted groundwater.
The concentrations of all measured trace elements in each sample were below the threshold limits of the drinking water standard (GB5749-2006). The contents of Hg and As were undetected, while other typical toxicity indexes like Cd, Cr, Pb, and Sb were all lower than the threshold limits. Compared with other trace elements, Sr, Ba, Zn, and Cu had much higher averages and wider ranges of variation. For example, the concentrations of Sr, Ba, Zn, and Cu were 18–1949, 5.1–236, 0.36–163, and 0.05–88 μg/L, with averages of 444, 53, 13, and 6.9 μg/L.
According to the data of FPW-W and FPW-F, the hydrochemical characteristics were distinctly different from those of shallow groundwater, shown in Figure 2 and Figure 3. The hydrochemical types of FPW-W and FPW-F were Cl-Na, with high TDS (averages of 18,568 and 62,075 mg/L) and salinity (averages of Cl contents of 10,998 and 38,756 mg/L) (Figure 2). Unlike the extremely low content of Na in shallow groundwater, the averages of concentrations of Na in FPW-W and FPW-F were 3648 and 11,286 times higher than those of shallow groundwater, as the result of a mixing of high-saline formation water entrapped in Longmaxi formation (Figure 3a). Besides, when HCO3 is only derived from the dissolution of carbonate minerals (calcite and dolomite) and/or anorthite reacting with the aqueous soil CO2 in groundwater, the milligram equivalent ratio of (Ca + Mg)/(HCO3) should be 1:1 [68,69]. In Figure 3b, shallow groundwater samples mostly fell along the 1:1 line with the average equivalent ratio of 1.2, suggesting that the concentrations of dissolved inorganic carbon (DIC) were related to the carbonate dissolution. In contrast with the high correlation coefficient of groundwater (r = 0.91), both FPW-W and FPW-F showed no correlation between (Ca + Mg) and (HCO3). Compared with shallow groundwater (Br/Cl: 0.009 to 0.18), FPW-W and FPW-F had the higher concentrations of Br (21–179 mg/L, 139–153 mg/L) with lower molar ratios of Br/Cl (1.7 × 10−3 to 3.9 × 10−3, 6.5 × 10−4 to 6.9 × 10−4). FPW-W and FPW-F from the Sichuan Basin were also abundant in some trace elements due to the origin of evaporated seawater [42,43]. Ba, Sr, Rb, B, and Li in both FPW-W and FPW-F were significantly higher than that of shallow groundwater by at least two orders of magnitude, suggesting that Ba, Sr, Rb, B, and Li may rise in groundwater mixed with FPW (Figure 3d–f).
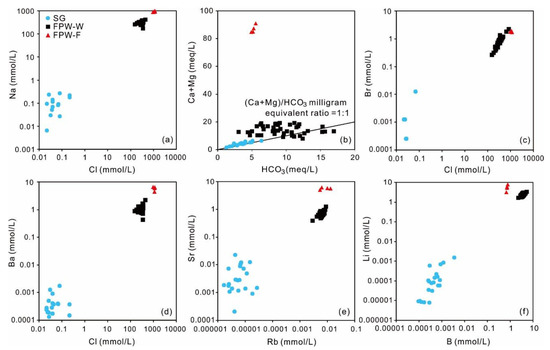
Figure 3.
(a) Relationship between Na and Cl; (b) relationship between Ca + Mg and HCO3; (c) relationship between Br and Cl; (d) relationship between Ba and Cl; (e) relationship between Sr and Rb; (f) relationship between Li and B.
4.1.2. Isotopes of Oxygen, Hydrogen, Carbon, Strontium, and Boron
δ18O and δ2H
The stable isotopes of oxygen (18O) and hydrogen (2H) can effectively trace the origin and hydrological processes of groundwater [70]. The values of δ18O-H2O and δ2H-H2O of shallow groundwater in the study area had ranges of −8.2 to −6.4‰, and −47.7 to −36.8‰, respectively (Table 1). The global meteoric water line (GMWL) [71] and the local meteoric water line (LMWL) are usually combined to reveal the origin of groundwater. The LMWL is composed of isotopic data of δ18O and δ2H obtained from the Zunyi station of the GNIP database [72]. The data from GNIP consist of monthly average values of stable isotopes from 1986 to 1991, so the LMWL is based on five-year data reflecting the overall situation of the area.

Table 1.
Chemical and isotopic composition of shallow groundwater samples of the study area.
For the shallow groundwater, most samples fell along the LMWL, suggesting that shallow groundwater mainly originated from the local precipitation (Figure 4). However, most groundwater samples were located slightly above the LWML with a relatively high weighted average d-excess of 15.8‰ compared with an average annual d-excess of 11.8‰, implying that the source of precipitation may have more fractions of terrestrial recycling vapor when recharging the groundwater [73]. A similar phenomenon also occurred in Chishui City, which is adjacent to Zunyi City [74]. Besides, the δ18O and δ2H of precipitation will become depleted with the rise of altitude and the decline of temperature, such as the depletion of δ18O will be between −0.15 and −0.5‰ per 100 m rise of altitude [70]. The samples from the outcrops of springs above the altitude of 1000 m had more depleted values of δ18O (an average value of −7.4‰) than those below 1000 m (an average value of −6.8‰). For example, the samples of XS-2, 3, and 5 with most depleted isotopic values (average δ18O value of −8.2‰) were collected from outcrops located in Cambrian strata with higher altitudes, while the samples of XS-11, 13, and 17 with most enriched values (an average δ18O value of −6.4‰) were from the outcrops of springs with lower altitudes (Figure 4). Thus, the altitude effect may cause the differences of δ18O and δ2H between various groundwater samples.
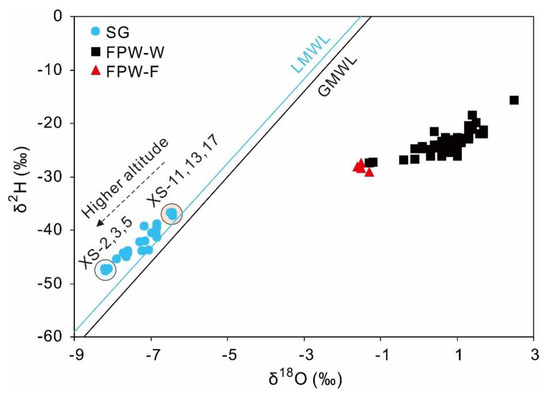
Figure 4.
The plot of δ18O-H2O and δ2H-H2O of shallow groundwater from Xishui, FPW-W, and FPW-F. GMWL (global meteoric water line): δ2H = 8δ18O + 10 [71]; LMWL (local meteoric water line) for Zunyi: δ2H = 7.89δ18O + 11.95 [72].
Distinguished from shallow groundwater, the stable isotopes of FPW-W were much heavier, with δ18O and δ2H ranging from −1.3 to +2.5‰, and from −27.4 to −15.6‰, respectively (Figure 4). It was the result of the blending consisting of injected fracturing water with low values of δ18O and δ2H, and formation water derived from evaporated seawater with high δ18O and δ2H [42]. The values of δ18O (−1.6 to −1.3‰) and δ2H (−29.1 to −27.4‰) of FPW-F were slightly below the range of FPW-W, but they were significantly different from shallow groundwater [43].
δ13C-DIC
The content of dissolved inorganic carbon (DIC) and the variation of δ13C reflect the specific geochemical processes and the biogeochemical cycle of carbon [75,76]. The sources of DIC in groundwater mainly consist of dissolved CO2 from soil and atmosphere, oxidation of organic matter, and water–rock interaction. In this study, the pH of groundwater was from 7.12 to 8.29. When the values of pH are between 6.4 and 8.3, and the DIC in water is mainly composed of bicarbonate (HCO3−) [77,78]. Thus, HCO3− was the major species of DIC in groundwater.
The concentrations of HCO3− were in the range of 78 and 390 mg/L, with an average value of 214 mg/L. As for the partial pressure of carbon dioxide (PCO2), the PCO2 in groundwater were all higher than the PCO2 of the atmosphere (10−3.5), with a range from 10−3.11 to 10−1.51, indicating that other sources of DIC had important impacts on groundwater. Except for sample XS-21 that had the negative value of SI-calcite (−0.82), most values of SI-calcite in groundwater were larger than 0, ranging from 0.11 to 0.88, suggesting that shallow groundwater mostly had the over-saturated level of calcite. Meanwhile, the values of δ13C of DIC in groundwater varied between −13.8 and −7.8‰ with an average value of −12.0‰ without an obvious correlation with concentrations of HCO3 (r = 0.33) (Figure 5). The δ13C-DIC values of groundwater samples overlapped the results of groundwater in Zunyi City (−14.4 to −6.3‰) [79]. According to the positive values of SI-calcite, the ratios of (Ca + Mg)/(HCO3) close to 1:1, high PCO2 within the range of soil CO2 (10−3–10−1), and high 87Sr/86Sr of some samples shown in Figure 6b, the values of δ13C-DIC in local groundwater may be affected by the dissolution of carbonate rocks, the exchange of carbon isotopes between DIC (HCO3) in water, and soil CO2 in an open groundwater system, and the input of silicate weathering (e.g., albite, anorthite).
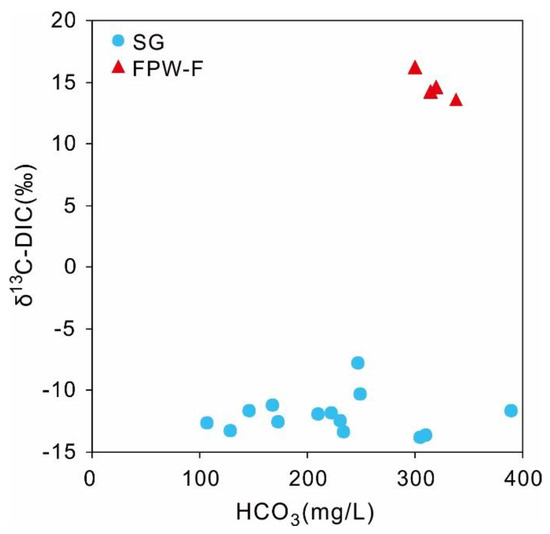
Figure 5.
The diagram of δ13C-DIC vs. HCO3 for shallow groundwater and FPW-F.
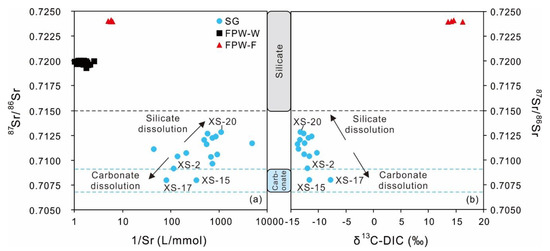
Figure 6.
The diagrams of 87Sr/86Sr vs. 1/Sr (a) and 87Sr/86Sr vs. δ13C-DIC (b) for shallow groundwater, FPW-W, and FPW-F. The 87Sr/86Sr values from the weathering of silicate rocks were higher than 0.7150 [84,85,86]. The ratios of 87Sr/86Sr of carbonate rocks were in a range between 0.7068 and 0.7091 [83].
Compared with the depleted δ13C-DIC of groundwater, the FPW from Fuling gas field had more enriched values of δ13C-DIC varying between +13.6 and +16.2‰ with HCO3 contents of 300–338 mg/L (Figure 5). The positive values of FPW may be related to the methanogenesis process causing the large isotopic fractionation between CH4 and CO2 (and hence DIC), but not the normal processes of both rainfall-infiltration and mineral dissolution generally controlling the δ13C values of shallow groundwater in karst zones [43].
87Sr/86Sr
Due to negligible isotope mass fractionation and various compositions in rocks and minerals, strontium isotopes are usually used for tracing hydrochemical processes in different geological settings, such as in the sourcing and transporting of materials, migration and transformation of pollutants, mixing patterns of water systems, weathering, and water–rock interaction [57,80,81]. In most cases, carbonate rocks and evaporates have higher concentrations of strontium and lower 87Sr/86Sr compared with silicate rocks with lower contents of strontium and higher 87Sr/86Sr [82]. The carbonate rocks in Xishui are mainly marine carbonates. The 87Sr/86Sr ratios of marine carbonates throughout the Phanerozoic were approximately within a range between 0.7068 and 0.7091 [83]. Meanwhile, the results of some studies show that Sr derived from the weathering of silicate rocks commonly had a ratio of 87Sr/86Sr higher than 0.7150 [84,85,86]. The concentrations of Sr in shallow groundwater ranged between 0.02 and 1.97 mg/L, with an average value of 0.44 mg/L. The ratios of 87Sr/86Sr of groundwater varied from 0.70797 to 0.71283 and had an average value of 0.71083, while had a range of −16.9 to 51.6 with an average value of 23.5. In Figure 6a, most of the water samples were in the transition between carbonate and silicate with three samples in the scope of carbonate. Besides, the groundwater affected by silicate weathering commonly had higher 87Sr/86Sr ratios and lower δ13C-DIC values than the groundwater controlled by carbonate weathering [87]. In Figure 6b, the springs in the range of transition had a δ13C-DIC average of −12.4‰, while the others in the range of carbonate had a δ13C-DIC average value of −10.5‰. Thus, silicate weathering partially affected the compositions of carbon and strontium isotopes for shallow groundwater. The relatively high ratios of 87Sr/86Sr also existed in the groundwater of urban areas in Zunyi, with a range of 0.70729 to 0.71727, reflecting the input of silicate weathering [88].
Compared with local groundwater, the FPW-W and FPW-F had higher contents of Sr (34–109 mg/L, 447–542 mg/L) and larger ratios of 87Sr/86Sr (0.71926–0.72001, 0.72397–0.72413), related to the mineral dissolution of bulk host shale (0.740246–0.740248) [42,43].
δ11B
The relatively large mass difference between boron isotopes (10B and 11B) leads to various isotopic values of δ11B in natural water (e.g., seawater, river, groundwater), ranging between −16 and +59‰ [89]. Among different waters, groundwater has a wide range of δ11B, between −15.9 and +32.4‰ [90]. The boron in unpolluted groundwater may be derived from water–rock interaction, atmospheric input (e.g., meteoric cyclic salts), or mixing with water of different origin [91,92]. The variability of δ11B in natural groundwater is the result of the large differences among the natural B sources and partitioning reactions during adsorption/desorption [93]. According to data from different sites in different countries [43,94,95,96,97], the δ11B of fresh groundwater (TDS < 1 g/L without anthropogenic pollution) had a wide range of δ11B, between −14 and +27‰ with B contents of 7.8–670 μg/L (Figure 7). Apart from one sample (XS-19) with a high concentration of B (39.3 μg/L), the other samples had relatively low concentrations of boron varying between 1.1 and 12.7 μg/L, with an average value of 5.0 μg/L, overlapping the range of fresh groundwater. Due to the excessively low contents of B in shallow groundwater, the δ11B values of most samples were undetectable, except for the samples of XS-12 (+6.4‰) and XS-19 (−7.8‰). In Figure 7, both XS-12 and XS-19 were in the range of global fresh groundwater, implying that the B contents and δ11B of local groundwater belonged to the natural background values of fresh groundwater. The depleted δ11B value and relatively high B concentration of XS-19 was likely related to water–rock interaction, such as adsorption/desorption between water and aquifer sediment [98], leaching of host rock [93], and dissolution of clay minerals [94].
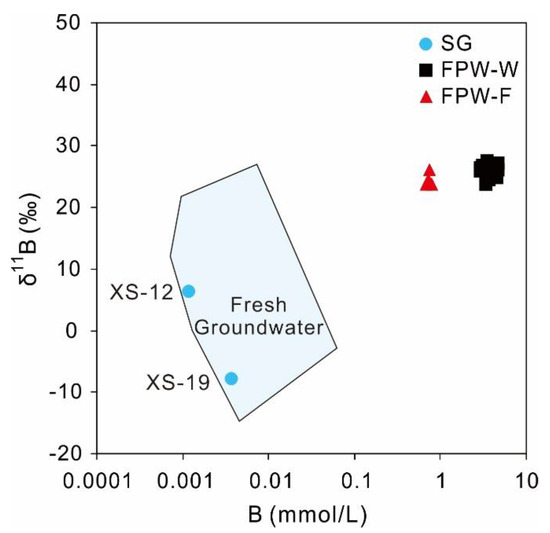
Figure 7.
The plot of δ11B versus B for shallow groundwater, FPW-W, and FPW-F. Data of fresh groundwater come from different researches of Southern Britain [94], Central Europe [95], Pennsylvania, USA [96], Tibet, China [97], Chongqing, China [43].
Although the δ11B data of groundwater in this study were limited and groundwater had a relatively wide range of δ11B, shallow groundwater had significantly lower B concentrations and δ11B, in contrast with FPW from Weiyuan (B: 2.4–56.2 mg/L; δ11B: +23.8 to +27.6‰) [42], Fuling (B: 7.3–8.4 mg/L; δ11B: +23.9 to +26.1‰) [43], the Marcellus shale (B: 5.2–62.6 mg/L; δ11B: +25.5 to +32.3‰) [39], and the Fayetteville shale (B: 2.4–21.1 mg/L; δ11B: +26.4 to +33.2‰) [99]. But the molar ratios of B/Cl for FPW-W (6.5 × 10−3 to 21 × 10−3) and FPW-F (6.5 × 10−4 to 6.9 × 10−4) were within or below the range of local shallow groundwater (1.3 × 10−3 to 44 × 10−3). Due to the significant differences of δ11B and B contents, it is possible to use boron concentrations and isotopes to identify the groundwater pollution related to FPW.
4.2. Origin of Dissolved Gas
4.2.1. Compositions of Dissolved Gas
The compositions of dissolved gas samples are shown in Table 2. In 12 gas samples, the volumetric percentages of N2 had a narrow range between 72.57 and 85.32% with an average value of 77.65% while the percentages of O2 varied between 10.69 and 25.45% with an average value of 20.01%. The composition of gas samples were similar to those of natural air with the percentages of N2 and O2 of around 78% and 21%, respectively, implying that the dissolved gas in spring water may be related to the air. The contents of CO2 and Ar were lower than 2.5%, with averages of 0.90 and 1.38%, respectively. The percentages of CH4 were in a range between 0.0025% and 0.3754%, showing the low content of hydrocarbons. The proportions of H2S were lower than the detection limit, except for the sample XS-9 with a value of 0.0009%. Only three samples (XS-8, 16, 17) had the percentages of He (0.0006, 0.0009, and 0.0007%). The values of H2 were below the measurement limit. The acidic gases (e.g., HCl and SO2) were absent in the gas samples. As for the shale gas sample (SG-1), the volumetric percentages of CH4, N2, O2, CO2, Ar, and He were 97.40%, 1.88%, 0.15%, 0.015%, 0.02%, and 0.0538%, respectively. The percentages of H2S and H2 were lower than the measurement limit.

Table 2.
Volume fraction, isotopic composition of dissolved gas samples of shallow groundwater and a shale gas sample (SG-1).
4.2.2. Carbon and Helium Isotopes
The geofluid system may have different sources of dissolved methane, including biogenic, thermogenic, and abiotic methane. Understanding the origin of methane in shallow groundwater is important, which can be used to identify the sources of potential methane pollution in groundwater, like stray methane from shale gas wells [11,25,100]. The carbon isotope is a useful tracer for determining the origin of dissolved methane in the water system and for tracing the stray gas pollution [13,19]. The impact of the degassing sampling method on the isotopic composition of methane can be negligible because the low isotope fractionation between CH4 (dissolved) and CH4 (gas) (1.00033–1.0005) leads to little difference between the δ13C-CH4 (dissolved) and δ13C-CH4 (gas) (0.3–0.5‰) [101,102].
The δ13C values of biogenic methane are generally lower than −50‰, while the δ13C-CH4 of thermogenic and abiotic origin can be commonly higher than −50‰ [70]. Besides, the abiotic methane derived from the modern seafloor hydrothermal vents has enriched δ13C values, ranging between −26 and −9‰ [103,104]. Based on the unique isotopic compositions of different kinds of methane, the origin of methane in geological systems can be determined. The δ13C-CH4 value of a shale gas sample from Xishui was −31.9‰, suggesting the thermogenic origin of methane. The shale gas from the Weiyuan gas field had high values of δ13C-CH4 varying between −37.3 and −34.3‰, indicating the typical thermogenic origin of methane without abiotic methane (Figure 8) [56]. Unlike the methane from shale gas, the δ13C-CH4 values of shallow groundwater were lower, with a narrow range between −53.1 and −50.5‰, indicating the biogenic origin of dissolved methane.
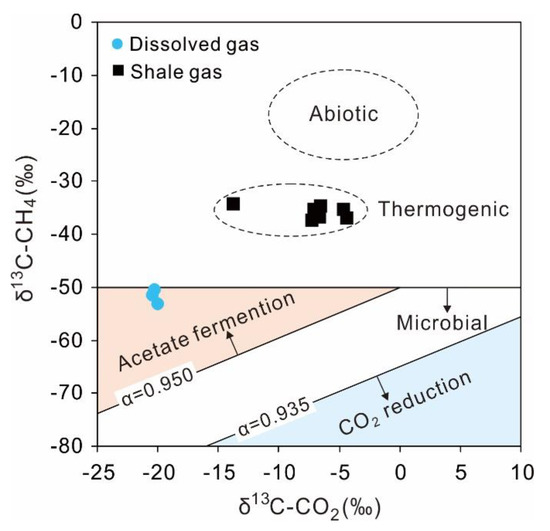
Figure 8.
The plot of δ13C-CH4 versus δ13C-CO2 with the dissolved gas of shallow groundwater in Xishui and shale gas from Weiyuan. The abiotic range is from Ueno et al. (2006) [104] The lines of α = 0.935 and α = 0.950 stand for the boundary line of CO2 reduction and acetate fermentation, respectively [70].
The pathways of biological methanogenesis normally includes acetate fermentation and CO2 reduction. Two pathways can occur solely or jointly in freshwater systems with the effect of mixed bacteria decomposing organic materials and producing CH4 [70]. The fractionation of 13C between coexisting CO2 and CH4 (α13CCH4-CO2) is an effective tool for identifying two pathways:
α13CCH4-CO2 = (δ13CCH4 + 1000)/(δ13CCO2 + 1000)
The fractionation factor of CO2 reduction producing CH4 is generally less than 0.935, while that of acetate fermentation is usually higher than about 0.950 [105,106]. The α13CCH4-CO2 values of shallow groundwater were all higher than 0.950 in a narrow range from 0.966 to 0.969 (Figure 8), demonstrating that acetate fermentation was the main origin of dissolved methane for local groundwater. In the reaction of acetate fermentation (2CH2O → CH3COOH → CH4+CO2, CH2O stands for complex organic materials), the produced CH4 and CO2 will have δ13C values of −61 and +9‰, assuming δ13C for organic matter is about −26‰ and the enrichment factor between CH4 and CO2 is 70‰. However, the values of δ13C-CO2 of shallow groundwater were more depleted, ranging between −21.9 and −20.0‰, close to soil CO2 (−23‰ for C3 vegetation). The PCO2 of groundwater (10−3.11 to 10−1.51) was close to the PCO2 of soil (10−3 to 10−1), showing that the local groundwater may be in an open groundwater system. Therefore, the depleted values of δ13C-CO2 were probably related to both finite acetate fermentation with limited CH4 and the open groundwater system dominated by soil CO2.
Due to the inert chemical feature, noble gases are widely used to trace the origins of gas and fluid. As the different isotopic compositions of helium in atmosphere, mantle, and crust with little content of 20Ne in mantle and crust, the ratios of 3He/4He and 4He/20Ne can effectively identify the sources of gases (including fluids) and evaluate the influence of atmosphere in gases or fluids, such as thermal fluid and natural gas [107,108,109].
The 3He/4He ratio in the atmosphere (Ra) is around 1.39 × 10−6 [109,110], while 4He/20Ne of the atmosphere can be 0.318 [107]. The 3He/4He ratios in other geological systems are usually expressed by R. The R/Ra ratio in the mantle, commonly represented by mid-oceanic ridge basalts (MORB), has an average value of 8.75 [111]. The typical R/Ra ratio of the crust is approximately 0.025 [112]. According to the different isotopic values of noble gas in the air, mantle and crust, the plot of R/Ra versus 4He/20Ne can be applied for tracing the origin of gas, with the three end-members including air (R/Ra = 1, 4He/20Ne = 0.318), mantle (R/Ra = 8.75, 4He/20Ne = 100,000), and crust (R/Ra = 0.025, 4He/20Ne = 100,000) (Figure 9). Although the 4He/20Ne ratio of 100,000 is the assumed value for mantle and crust, the extremely high 4He/20Ne can occur in the natural gas from several gas fields, such as the Hugoton-Panhandle gas field in the USA (up to 46,000) [113] and gas wells in Polish and Ukrainian Carpathians (up to 41,200) [114].
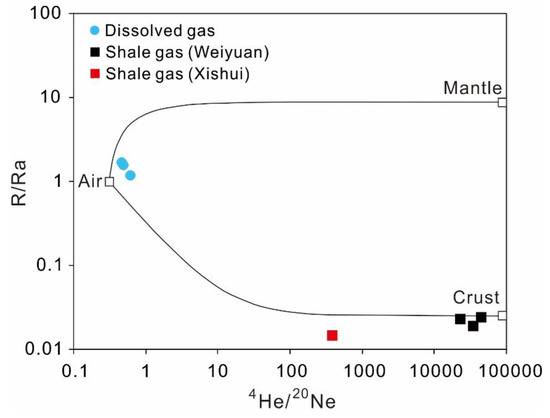
Figure 9.
Diagram of R/Ra versus 4He/20Ne with dissolved gas in shallow groundwater and shale gas from Weiyuan and Xishui.
In Figure 9, the dissolved gas of shallow groundwater was close to the range of air, with the R/Ra ratios ranging from 1.19 to 1.68 and the 4He/20Ne ratios varying between 0.46 and 0.61, indicating that the dissolved noble gas was mainly derived from dissolution of air. For the shale gas from the Weiyuan gas field, the R/Ra ratios varied between 0.019 and 0.025, with high 4He/20Ne ratios of 22,570 to 44,505, conforming to the typical characteristic of crustal natural gas enriched in radiogenic 4He [56]. Compared with the shale gas from Weiyuan, the shale gas from Xishui had lower ratios of R/Ra (0.015) and 4He/20Ne (383). Because of the large differences between shale gas and dissolved gas of groundwater in terms of isotopic compositions of both noble gas and CH4, the isotopes of noble gas and CH4 may help to recognize potential shale gas pollution occurring in local shallow groundwater during the shale gas exploitation.
4.3. Sensitive Indicators for Monitoring Groundwater
4.3.1. Sensitive Indicators
Due to the complex dissolved organic and inorganic components, wastewater generated from shale gas wells may cause potential or controversial environmental impacts on surface water, groundwater, and stream sediment [11]. Thus, the sensitive monitoring indicators are essential to monitor post-drilling groundwater quality and trace potential groundwater pollution caused by shale gas development. Most of the (semi) volatile organic compounds, pesticides and polychlorinated biphenyls (PCBs) are detected sporadically with low concentrations in FPW and therefore, the analysis of them may be unnecessary [115]. Based on the details of Section 4.1, FPW can be used as an end-member for identifying sensitive indicators. When FPW has significantly different geochemical and isotopic compositions compared with shallow groundwater, sensitive indicators can be gained through end-member comparison analysis [38,39,43]. Due to the lack of the shale gas wells in the Xishui region where the Longmaxi Formation is the future target stratum for shale gas, FPW-W and FPW-F are considered as hypothetic end-members for identifying indicators, assuming the compositions of ions and isotopes of the future FPW in Xishui will be similar to either FPW-W or FPW-F.
For the natural groundwater without contamination, the value of a specific ion and/or its isotopic composition has a random variation in a range of (average value ± 3σ) with a probability of 99.7% (Pauta criterion), where σ is the standard deviation. According to the determination method from Huang et al. (2020) [43], if shallow groundwater (SG) is mixed with X% of FPW, the specific ion and/or its isotopic composition in the polluted groundwater will change, where Δ represents the variation. For ions,
Δion = ((X/100)CFPW + (1 − X/100)CSG) − CSG)
For isotopes (e.g., δ13C, δ2H, δ18O, δ11B, ),
where C is the average concentration for specific ions of FPW-W or FPW-F and shallow groundwater (SG) and R is the isotopic value. Under the condition of |Δ| = 3σ, X% can be regarded as a threshold value of mixing rate from FPW-W and FPW-F causing statistical changes in ions or/and isotopes. The threshold values are put in order from smallest to largest and the first several indexes can be chosen as sensitive indicators, although sensitive indicators may vary from different sites with variable hydrogeological conditions.
Δisotope = ((X/100)CFPWRFPW + (1 − X/100)CSGRSG)/((X/100)CFPW + (1 − X/100)CSG) − RSG
Although the concentrations of most inorganic components (e.g., Na, Cl) in FPW increase with flowback time, we choose the averages of these components to represent major geochemical characteristics of the FPW. The results (Figure 10, Table 3) show that less than 0.1% of FPW-W would lead to significant changes of Li (0.05%), Cl (0.05%), δ11B (0.06%), B (0.06%), Na (0.08%) for shallow groundwater while less than 0.1% of FPW-F would cause statistical rises of Li (0.02%), Cl (0.02%), Na (0.02%), Ba (0.02%), and (0.05%) for shallow groundwater. Besides, a mineral may precipitate from supersaturated conditions during the mixing of different waters. By using a mix of the geochemical model PHREEQC [62], if shallow groundwater blends with either 0.12% of FPW-W or 0.02% of FPW-F (threshold values of Ba), the saturation indexes (SI) of barite (BaSO4) in both mixtures are 0.44, suggesting possible precipitation of Ba. However, the saturation indexes merely represent the tendency of hydrochemical processes (subsaturation means dissolution and supersaturation suggests precipitation) and only a few cases demonstrate the control of solutes by equilibrium with minerals [78,116]. The other ions (e.g., Ca, Mg, Br) and stable isotopes (δ18O and δ2H) are less sensitive to identify FPW with threshold values higher than 0.5%, such as at least 53% of FPW-W mixed with shallow groundwater causing statistical increases of δ18O and δ2H (shown in Table 3).
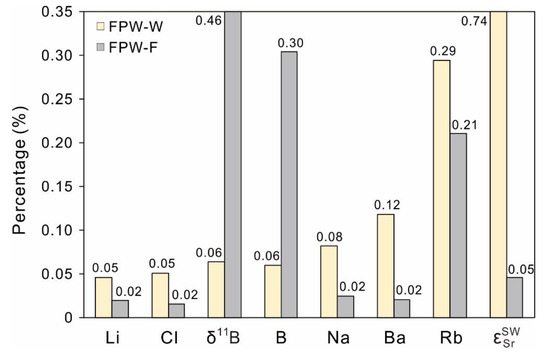
Figure 10.
The sensitive monitoring indicators (ions and isotopes) for identifying either FPW-W or FPW-F mingling with shallow groundwater in Xishui. The percentages are the threshold values of FPW mixed with the shallow groundwater, causing statistical changes of specific indicators (|Δ|) that are equal to the natural variation of shallow groundwater (3σ).

Table 3.
The threshold values of specific ions and their isotopes during the mixing process between shallow groundwater from Xishui and FPW-W or FPW-F. X1 represents the threshold value for identifying the mixing of FPW-W, while X2 stands for threshold values for recognizing the mixing of FPW-F. GB5479-2006 stands for the drinking water standard of China.
For collecting more indicators with enough sensibility to monitor the quality of shallow groundwater, those tracers of both FPW-W and FPW-F with threshold values below 0.1% can be considered as sensitive monitoring indicators. This means that a small portion of FPW releasing to shallow groundwater in Xishui can be likely recognized by using indicators of Na, Li, Cl, Ba, B, δ11B, and , which ought to be analyzed as a priority for tracing potential contamination of the future FPW in Xishui. For example, assuming B and Sr behave conservatively, a mixture consisting of 0.1% FPW-F and 99.9% shallow groundwater would lead to obvious increases of 13.7‰ for δ11B and 98.3 for , as well as significant rises in concentrations of Sr (112%), B (118%), Rb (115%), Na (828%), Ba (1463%), Cl (1910%), and Li (2416%) (using Mix of geochemical model PHREEQC).
Although the spring water samples were collected in the wet season (June 2018), the little temporary variability of geochemical and isotopic compositions of springs as groundwater in the study area has limited impact on the changes of threshold values of specific indicators. Springs in the study area represents the average chemical and isotopic compositions of rainfall in the past several months to several years [53] and the seasonal variation has been homogenized. According to the geochemical and isotopic data of spring water collected in Jiaoshiba, Chongqing with similar hydrogeological conditions [43], the major ions (Na, Cl), trace elements (B, Li,) and isotopes (δ13C-DIC) of springs had no significant variation between the dry season and the wet season. Besides, the geochemical (Na, Cl, B, Li, Ba, Sr) and isotopic compositions (87Sr/86Sr, δ13C-DIC) of a spring (unpublished data), collected in Xishui region in March, 2019 (dry season), were in the range of spring water samples (wet season) in this study. Assuming that the contents of major ions and trace elements of 21 spring water samples in the dry season would be abnormally three times higher than that in wet season (87Sr/86Sr and δ11B are assumed to be stable), the threshold values of sensitive indicators used for identifying the mixing of FPW-W and shallow groundwater (including the dry and the wet season) would remain low, such as Na (0.21%), Cl (0.13%), B (0.14%), Li (0.11%), and δ11B (0.13%). Although the δ11B of most spring water samples are undetectable due to extremely low boron concentrations, the contaminated groundwater mixed with FPW would be likely qualified to be measured for δ11B. For example, when 0.06% of FPW-W (threshold value) mingles with 99.94% of shallow groundwater, the boron concentration of polluted groundwater would be 30.6 μg/L, which can be totally qualified to the δ11B measurement in the laboratory. Meanwhile, a spring, polluted by FPW in Jiaoshiba, had a high boron concentration of 60.3–69.0 μg/L and could be measured for δ11B [43]. Therefore, if the pollution of FPW occurs, the polluted spring will be likely eligible to the δ11B detection and the δ11B can be used to verify the contamination of FPW.
The sensitive indictors should be included in the routine monitoring program and used in priority for tracing potential contamination of FPW during the shale gas development. As for the future shale gas development in the Xishui region, the routine monitoring parameters of shallow groundwater should contain the concentrations of Na, Li, Cl, Ba, and B, while and δ11B can be further used to confirm potential contamination of PFW, assuming that the pollution trend and/or abnormal values occur. Meanwhile, when a small portion of FPW is mixed with shallow groundwater, some ions can reach or exceed the limit values of the drinking water standard, causing groundwater quality to deteriorate. For example, when the portion of FPW-F is 0.08%, the concentration of Ba in contaminated groundwater can reach the drinking water standard in China (700 μg/L), which means that 1 m3 of FPW-F can pollute 1250 m3 of natural shallow groundwater. Therefore, the hazard of FPW should be highly concerning.
4.3.2. Method for Identifying Pollution
Compared with the complex species of degradable organic components, it may be relatively accessible to trace the groundwater pollution related to shale gas development using stray gas and dissolved inorganic constituents. In this study, the methods could be composed of two parts for tracing pollution of inorganic components and stray gas.
For identifying the dissolved inorganic components of FPW, the pre-drilling shallow groundwater and FPW after drilling could be chosen as two end-members. Based on sensitive monitoring indicators discussed in Section 4.3.1, the concentrations of Na, Li, Cl, B, and Ba can be used to identify potential contamination trends of post-drilling shallow groundwater in the Xishui region, while isotopes of δ11B and can further demonstrate whether FPW is the pollution source of groundwater. Elemental ratios of FPW are also different from that of shallow groundwater. For example, FPW-W and FPW-F had lower concentration ratios of Br/Cl (3.8 × 10−3–8.7 × 10−3, 3.4 × 10−3–4.1 × 10−3) and higher values of Sr/Ca (0.18–0.45, 0.32–0.35), compared with shallow groundwater with high Br/Cl (0.02–0.40) and low Sr/Ca (4.2 × 10−4–0.02). When 0.1% of FPW-F is mixed with 99.9% of groundwater, Br/Cl of contaminated groundwater would sharply decrease to 9.6 × 10−3, while Sr/Ca would significantly increase to 1.4 × 10−2, in contrast with the high Br/Cl (average of 0.14) and low Sr/Ca (average of 6.8 × 10−3) of shallow groundwater. Therefore, specific elemental ratios should also be considered as sensitive indexes for tracing contamination of FPW. When the pollution of shallow groundwater occurs, the mixing ratio of FPW in the polluted groundwater can be appropriately quantified, assuming Cl, B, and Sr behave conservatively. According to the method from Huang et al. (2020) [43], assuming the polluted shallow groundwater (PSG) is a simple mixture of natural shallow groundwater (SG) and FPW without water–rock reactions, precipitation, or other hydrochemical processes, the mixing ratio (X%) of FPW in polluted groundwater could be approximately obtained based on the solute and isotopes mass balances. For specific ions (i.e., Cl), the X is given by:
Xion = 100(CPSG − CSG)/(CFPW − CSG)
For isotopes of conservative ions (i.e., δ11B and ), the X is given by:
where C is the average concentration for a specific ion of PSG, SG, and FPW, and R is the isotopic value of a specific ion. Although the contents of some ions (e.g., Na, Cl) in FPW increase with flowback time, the concentrations of these tracers for FPW at the early flowback stage are still distinctive to the shallow groundwater. For example, the Cl content of FPW-W can be up to 11,167 mg/L in the first day after hydraulic fracturing. Therefore, the averages of tracers are chosen to roughly represent the overall FPW. With the calculation of different sensitive indicators, an appropriate range of mixing ratios of FPW can be obtained, which can quickly and evidently assess shallow groundwater contamination caused by FPW.
Xisotope = 100(CPSGRPSG − CSGRSG)/(CFPWRFPW − CSGRSG)
As methane is the main component in shale gas (over 90% in Weiyuan shale gas), the stray methane is the major stray gas contamination. The δ13C of biogenic methane generally developing in shallow groundwater was significantly different to that of thermogenic methane in shale gas (Figure 8). It is, therefore, a logical deduction that if thermogenic methane of shale gas leaks into the shallow groundwater with limited biogenic methane, the δ13C of mixing methane in polluted groundwater is likely to shift, which may be affected by oxidation or diffusion. Besides, the ratios of R/Ra and 4He/20Ne of dissolved gas in Xishui were significantly different from that of shale gas (Figure 9). Thus, with the dissolved gas of pre-drilling shallow groundwater and shale gas as two end-members, a deterministic model containing δ13C-CH4, R/Ra, and 4He/20Ne can be used to trace potential stray shale gas leaking into shallow groundwater in the Xishui region. In the zone of shallow groundwater in the Xishui region, δ13C-CH4 of biogenic origin is from −50 to −80‰) [70], while R/Ra and 4He/20Ne vary between the air (R/Ra = 1, 4He/20Ne = 0.318) and the highest values of dissolved noble gas in Xishui (R/Ra = 1.68, 4He/20Ne = 0.61). According to the data of shale gas from three gas fields in the Sichuan Basin (Fuling, Weiyuan, Changning) [56,117] and Xishui, the zone of shale gas can be restricted to the ranges of δ13C-CH4 (−37.3 to −26.7‰), R/Ra (0.007 to 0.028), and 4He/20Ne (383 to 44,505). If shale gas strays into a shallow groundwater system in Xishui during the shale gas development, the dissolved methane and noble gas in contaminated groundwater may be in the transition range between two end-members (Figure 11). Though this method needs to be further verified, it may provide a new tool to trace the stray gas contamination related to shale gas development. Besides, other gas indexes, such as δ13C-C2H6, δ2H-CH4, and 36Ar/40Ar, should be added to the future baseline investigation of shallow groundwater and further provide more robust results about shale gas pollution, due to their different compositions between natural dissolved gas and shale gas [19,100].
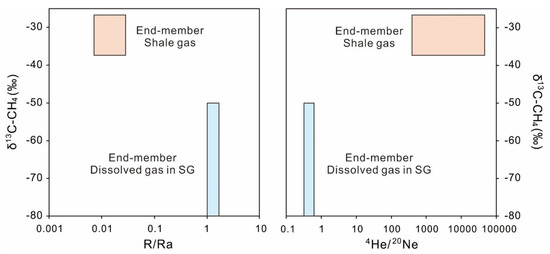
Figure 11.
The plots of δ13C-CH4 versus R/Ra, δ13C-CH4 versus 4He/20Ne with dissolved gas and shale gas as two end-members. The red zone is restricted to the data of shale gas from three gas fields in Sichuan Basin [56,117] and Xishui, while the blue zone represents the range of dissolved gas in local shallow groundwater. If shale gas strays into the dissolved gas, the δ13C-CH4, R/Ra, and 4He/20Ne of contaminated groundwater may lie between two zones.
5. Conclusions
This study provides baseline water quality before shale gas exploitation in the Xishui shale gas play. The geochemical and isotopic compositions for FPW are significantly different from shallow groundwater. Therefore, sensitive tracers can be obtained from end-member analysis. Taking shallow groundwater of Xishui and FPW from Weiyuan and Fuling as two end-members, some sensitive tracers (Na, B, Cl, Li, Ba, δ11B, and ) should be analyzed as a priority during monitoring of groundwater quality in the study area. Besides, the indexes of δ13C-CH4, R/Ra, and 4He/20Ne can be used to identify the mixing of stray shale gas in the polluted groundwater. With these sensitive inorganic indicators and gas indexes, we can not only effectively monitor the change of the post-drilling groundwater quality, but also confirm and quantify the potential or possible groundwater pollution related to FPW and shale gas in future shale gas development.
Author Contributions
Conceptualization, T.H. and Z.L.; methodology, T.H. and Z.L.; formal analysis, Z.L., Y.L.(Yin Long), F.Z. and Y.L.(Yiman Li); investigation, B.M., J.T. and Z.L.; writing—original draft preparation, Z.L.; writing—review and editing, T.H.; supervision, T.H. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant 41877207), the “Strategic Priority Research Program (B)” of the Chinese Academy of Sciences (Grant XDB10030603), IAEA Project (RAS7035), the Youth Innovation Promotion Association CAS (2018087) and a CAS Scholarship (201825).
Acknowledgments
The authors thank Shuo Yang and Min Lyu for their help during fieldwork. The authors also wish to express their appreciation to the editor and three anonymous reviewers whose detailed comments were very helpful in improving the clarity and focus of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Chen, J.; Li, J.; Yang, Z.; Sun, Q.; Lu, J.; Zhang, G. Natural gas in China: Development trend and strategic forecast. Nat. Gas. Ind. 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Shale Gas Planning. Available online: http://zfxxgk.nea.gov.cn/auto86/201609/t20160930_2306.htm (accessed on 15 September 2019).
- Zou, C.; Dong, D.; Wang, Y.; Li, X.; Huang, J.; Wang, S.; Guan, Q.; Zhang, C.; Wang, H.; Liu, H.; et al. Shale gas in China: Characteristics, challenges and prospects (II). Pet. Explor. Dev. 2016, 43, 166–178. [Google Scholar] [CrossRef]
- China Geological Survey. The Shale Gas. Resources in China; China Geological Survey: Beijing, China, 2015; pp. 1–23.
- Wang, R.; Hu, Z.; Long, S.; Liu, G.; Zhao, J.; Dong, L.; Du, W.; Wang, P.; Yin, S. Differential characteristics of the Upper Ordovician–Lower Silurian Wufeng–Longmaxi shale reservoir and its implications for exploration and development of shale gas in/around the Sichuan Basin. Acta Geol. Sin. Engl. 2019, 93, 520–535. [Google Scholar] [CrossRef]
- Yang, R.; Chen, W.; Zhou, R. Characteristics of organic-rich shale and exploration area of shale gas in Guizhou Province. Nat. Gas. Geosci. 2012, 23, 340–347. [Google Scholar]
- Zhang, B.; Yao, L.; Luo, S.; Zhao, Y.; Sun, W. Current status and outlook of shale gas exploration and development in Guizhou Province. Nat. Gas. Technol. Econ. 2016, 10, 57–59. [Google Scholar]
- Zhang, B.; Han, Z.; Zhang, W.; Si, F. Research on shale gas of Wufeng–Longmaxi Formation, Guizhou Province. Nat. Gas. Technol. Econ. 2017, 11, 19–23. [Google Scholar]
- Vengosh, A.; Warner, N.; Jackson, R.; Darrah, T. The effects of shale gas exploration and hydraulic fracturing on the quality of water resources in the United States. Procedia Earth Planet. Sci. 2013, 7, 863–866. [Google Scholar] [CrossRef]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef]
- Vengosh, A.; Kondash, A.; Harkness, J.; Lauer, N.; Warner, N.; Darrah, T.H. The geochemistry of hydraulic fracturing fluids. Procedia Earth Planet. Sci. 2017, 17, 21–24. [Google Scholar] [CrossRef]
- Osborn, S.G.; Vengosh, A.; Warner, N.R.; Jackson, R.B. Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc. Natl. Acad. Sci. USA 2011, 108, 8172–8176. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, G.A.; Krkosek, W.; Anderson, L.; McBean, E.; Mohseni, M.; Bazri, M.; Mauro, I. Impacts of hydraulic fracturing on water quality: A review of literature, regulatory frameworks and an analysis of information gaps. Env. Rev. 2016, 24, 122–131. [Google Scholar] [CrossRef]
- Wilson, M.P.; Worrall, F.; Davies, R.J.; Hart, A. Identifying groundwater compartmentalisation for hydraulic fracturing risk assessments. Environ. Sci. Proc. Impacts 2019, 21, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.W.; Ingraffea, A.; Engelder, T. Should fracking stop? Nature 2011, 477, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of shale gas development on regional water quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, T. Environmental impacts of hydraulic fracturing in shale gas development in the United States. Pet. Explor. Dev. 2015, 42, 801–807. [Google Scholar] [CrossRef]
- Jackson, R.B.; Vengosh, A.; Darrah, T.H.; Warner, N.R.; Down, A.; Poreda, R.J.; Osborn, S.G.; Zhao, K.; Karr, J.D. Increased stray gas abundance in a subset of drinking water wells near Marcellus shale gas extraction. Proc. Natl. Acad. Sci. USA 2013, 110, 11250–11255. [Google Scholar] [CrossRef]
- Siegel, D.I.; Azzolina, N.A.; Smith, B.J.; Perry, A.E.; Bothun, R.L. Methane concentrations in water wells unrelated to proximity to existing oil and gas wells in northeastern Pennsylvania. Environ. Sci. Technol. 2015, 49, 4106–4112. [Google Scholar] [CrossRef]
- Wen, T.; Niu, X.; Gonzales, M.; Zheng, G.; Li, Z.; Brantley, S.L. Big groundwater data sets reveal possible rare contamination amid otherwise improved water quality for some analytes in a region of Marcellus shale development. Environ. Sci. Technol. 2018, 52, 7149–7159. [Google Scholar] [CrossRef]
- Wen, T.; Castro, M.C.; Nicot, J.P.; Hall, C.M.; Larson, T.; Mickler, P.; Darvari, R. Methane sources and migration mechanisms in shallow groundwaters in Parker and Hood Counties, Texas—A heavy noble gas analysis. Environ. Sci. Technol. 2016, 50, 12012–12021. [Google Scholar] [CrossRef]
- Wen, T.; Castro, M.C.; Nicot, J.P.; Hall, C.M.; Pinti, D.L.; Mickler, P.; Darvari, R.; Larson, T. Characterizing the noble gas isotopic composition of the Barnett Shale and Strawn Group and constraining the source of stray gas in the Trinity Aquifer, north-central Texas. Environ. Sci. Technol. 2017, 51, 6533–6541. [Google Scholar] [CrossRef] [PubMed]
- Rahm, B.G.; Riha, S.J. Evolving shale gas management: Water resource risks, impacts, and lessons learned. Environ. Sci. Proc. Impacts 2014, 16, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Harkness, J.S.; Darrah, T.H.; Warner, N.R.; Whyte, C.J.; Moore, M.T.; Millot, R.; Kloppmann, W.; Jackson, R.B.; Vengosh, A. The geochemistry of naturally occurring methane and saline groundwater in an area of unconventional shale gas development. Geochim. Cosmochim. Acta 2017, 208, 302–334. [Google Scholar] [CrossRef]
- Barth-Naftilan, E.; Sohng, J.; Saiers, J.E. Methane in groundwater before, during, and after hydraulic fracturing of the Marcellus Shale. Proc. Natl. Acad. Sci. USA 2018, 115, 6970–6975. [Google Scholar] [CrossRef] [PubMed]
- Botner, E.C.; Townsend-Small, A.; Nash, D.B.; Xu, X.; Schimmelmann, A.; Miller, J.H. Monitoring concentration and isotopic composition of methane in groundwater in the Utica Shale hydraulic fracturing region of Ohio. Env. Monit. Assess. 2018, 190, 322. [Google Scholar] [CrossRef]
- Jackson, R.E.; Gorody, A.W.; Mayer, B.; Roy, J.W.; Ryan, M.C.; Van Stempvoort, D.R. Groundwater protection and unconventional gas extraction: The critical need for field-based hydrogeological research. Ground Water 2013, 51, 488–510. [Google Scholar] [CrossRef]
- Siegel, D.I.; Smith, B.; Perry, E.; Bothun, R.; Hollingsworth, M. Pre-drilling water-quality data of groundwater prior to shale gas drilling in the Appalachian Basin: Analysis of the Chesapeake Energy Corporation dataset. Appl. Geochem. 2015, 63, 37–57. [Google Scholar] [CrossRef]
- Montcoudiol, N.; Banks, D.; Isherwood, C.; Gunning, A.; Burnside, N. Baseline groundwater monitoring for shale gas extraction: Definition of baseline conditions and recommendations from a real site (Wysin, Northern Poland). Acta Geophys. 2019, 67, 365–384. [Google Scholar] [CrossRef]
- Brantley, S.L.; Vidic, R.D.; Brasier, K.; Yoxtheimer, D.; Pollak, J.; Wilderman, C.; Wen, T. Engaging over data on fracking and water quality. Science 2018, 359, 395–397. [Google Scholar] [CrossRef]
- Son, J.H.; Hanif, A.; Dhanasekar, A.; Carlson, K.H. Colorado Water Watch: Real-time groundwater monitoring for possible contamination from oil and gas activities. Env. Monit. Assess. 2018, 190, 138. [Google Scholar] [CrossRef]
- Bordeleau, G.; Rivard, C.; Lavoie, D.; Lefebvre, R.; Malet, X.; Ladeveze, P. Geochemistry of groundwater in the Saint-Edouard area, Quebec, Canada, and its influence on the distribution of methane in shallow aquifers. Appl. Geochem. 2018, 89, 92–108. [Google Scholar] [CrossRef]
- Smedley, P.L.; Ward, R.S.; Bearcock, J.M.; Bowes, M.J. Establishing the baseline in groundwater chemistry in connection with shale-gas exploration: Vale of Pickering, UK. Procedia Earth Planet. Sci. 2017, 17, 678–681. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Pang, Z.; Wang, Y.; Jin, C. Geochemical characteristics of shallow groundwater in Jiaoshiba shale gas production area: Implications for environmental concerns. Water 2016, 8, 552. [Google Scholar] [CrossRef]
- Huang, T.; Pang, Z.; Tian, J.; Li, Y.; Yang, S.; Luo, L. Methane content and isotopic composition of shallow groundwater: Implications for environmental monitoring related to shale gas exploitation. J. Radioanal. Nucl. Chem. 2017, 312, 577–585. [Google Scholar] [CrossRef]
- Li, H.; Son, J.-H.; Carlson, K.H. Concurrence of aqueous and gas phase contamination of groundwater in the Wattenberg oil and gas field of northern Colorado. Water Res. 2016, 88, 458–466. [Google Scholar] [CrossRef]
- Chapman, E.C.; Capo, R.C.; Stewart, B.W.; Kirby, C.S.; Hammack, R.W.; Schroeder, K.T.; Edenborn, H.M. Geochemical and strontium isotope characterization of produced waters from Marcellus Shale natural gas extraction. Environ. Sci. Technol. 2012, 46, 3545–3553. [Google Scholar] [CrossRef]
- Warner, N.R.; Darrah, T.H.; Jackson, R.B.; Millot, R.; Kloppmann, W.; Vengosh, A. New tracers identify hydraulic fracturing fluids and accidental releases from oil and gas operations. Environ. Sci. Technol. 2014, 48, 12552–12560. [Google Scholar] [CrossRef]
- Phan, T.T.; Capo, R.C.; Stewart, B.W.; Macpherson, G.L.; Rowan, E.L.; Hammack, R.W. Factors controlling Li concentration and isotopic composition in formation waters and host rocks of Marcellus Shale, Appalachian Basin. Chem. Geol. 2016, 420, 162–179. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, H.; Chen, Z.; Li, X.; Zhu, P.; Cui, X. Hydrogeochemical and isotopic indicators of hydraulic fracturing flowback fluids in shallow groundwater and stream water, derived from Dameigou shale gas extraction in the Northern Qaidam Basin. Environ. Sci. Technol. 2017, 51, 5889–5898. [Google Scholar] [CrossRef]
- Ni, Y.; Zou, C.; Cui, H.; Li, J.; Lauer, N.E.; Harkness, J.S.; Kondash, A.J.; Coyte, R.M.; Dwyer, G.S.; Liu, D.; et al. The origin of flowback and produced waters from Sichuan Basin, China. Environ. Sci. Technol. 2018, 52, 14519–14527. [Google Scholar] [CrossRef]
- Huang, T.; Pang, Z.; Li, Z.; Li, Y.; Hao, Y. A framework to determine sensitive inorganic monitoring indicators for tracing groundwater contamination by produced formation water from shale gas development in the Fuling Gasfield, SW China. J. Hydrol. 2020, 581, 124403. [Google Scholar] [CrossRef]
- Liu, F.; Xie, S.; Xie, P.; Zhang, W. Analysis on characteristics of landscape pattern of land use in Xishui County of Guizhou Province. Tianjin Agr. Sci. 2015, 21, 84–88. [Google Scholar]
- Ma, W.; Liu, S.; Huang, W.; Zhang, C.; Xu, G.; Yuan, H. Characteristics of Silurian Paleo-oil reservoirs and their significance for petroleum exploration on the southeast margin of Sichuan Basin. Oil Gas. Geol. 2012, 33, 432–441. [Google Scholar]
- Li, S.J.; Zhou, Y.; Xiao, K.H.; Wo, Y.J.; Wang, X.W.; Liu, Q.Y. Characteristics of Silurian destroyed oil reservoir in Houtan section of Xishui area in southeastern margin of Sichuan Basin. Acta Pet. Sin. 2009, 30, 849–855. [Google Scholar]
- Guizhou Geological Bureau. The Geology Map of Tongzi in Guizhou (1:200,000); China Geology Map Press: Beijing, China, 1978.
- Su, W.; Li, Z.; Ettensohn, F.; Johnson, M.E.; Huff, W.; Wang, W.; Ma, C.; Li, L.; Zhang, L.; Zhao, H. Distribution of black shale in the Wufeng–Longmaxi Formations (Ordovician-Silurian), South China: Major controlling factors and implications. Earth Sci. J. China Univ. Geosci. 2007, 32, 819–827. [Google Scholar]
- Guo, S.; Guo, J.; Liu, C.; Zhang, L.; Guo, X.; Xiao, P. Shale gas accumulation potential of Lower Silurian Longmaxi formation in northern Guizhou. J. Cent. South. Univ. Sci. Technol. 2016, 47, 1973–1980. [Google Scholar]
- Wang, L.; Fu, Y.; Li, J.; Sima, L.; Wu, Q.; Jin, W.; Wang, T. Mineral and pore structure characteristics of gas shale in Longmaxi formation: A case study of Jiaoshiba gas field in the southern Sichuan Basin, China. Arab. J. Geosci. 2016, 9, 733. [Google Scholar] [CrossRef]
- Long, S.; Peng, Y.; Liu, H.; Zhao, C.; Zhao, J.; Yu, L.; Sun, C.; Tang, X. Micro-characteristics of the shale in the first member of Silurian Longmaxi Formation in southeastern Sichuan Basin, China. J. Nanosci. Nanotechnol. 2017, 17, 6662–6669. [Google Scholar] [CrossRef]
- Sui, H.; Gao, W.; Hu, R. A new evaluation method for the fracability of a shale reservoir based on the structural properties. Geofluids 2019, 2019, 2079458. [Google Scholar] [CrossRef]
- PLA’s 00932 Unit. The Integration Hydrogeology Map of Tongzi in Guizhou (1:200,000); China Geology Map Press: Beijing, China, 1978.
- Huang, Q.; Kang, Z.; Qin, X.; Yu, J.; Su, C. Distribution characteristics of Sr2+, Sr/Mg, Sr/Ca and its applications in karst water system of Xishui County. Geol. Sci. Technol. Inf. 2011, 30, 98–103. [Google Scholar]
- Luo, L.; Pang, Z.; Luo, J.; Li, Y.; Kong, Y.; Pang, J.; Wang, Y. Noble gas isotopes to determine the depth of the geothermal fluid circulation. Chin. J. Geol. 2014, 49, 888–898. [Google Scholar]
- Cao, C. Gas Geochemistry and Implication during Shale Gas Production from Longmaxi Formatiion in Sichuan Basin, China. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Lang, Y.; Liu, C.; Zhao, Z.; Li, S.; Han, G. Geochemistry of surface and ground water in Guiyang, China: Water/rock interaction and pollution in a karst hydrological system. Appl. Geochem. 2006, 21, 887–903. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Wu, P.; Zhang, R.; Zhang, C.; Sun, J. Hydrogeochemical characteristics and associated mechanism based on groundwater dating in a karstic basin, Guizhou Province, China. Environ. Earth Sci. 2015, 73, 67–76. [Google Scholar] [CrossRef]
- Wu, P.; Tang, C.; Zhu, L.; Liu, C.; Cha, X.; Tao, X. Hydrogeochemical characteristics of surface water and groundwater in the karst basin, southwest China. Hydrol. Process. 2009, 23, 2012–2022. [Google Scholar] [CrossRef]
- Hosono, T.; Delinom, R.; Nakano, T.; Kagabu, M.; Shimada, J. Evolution model of δ34S and δ18O in dissolved sulfate in volcanic fan aquifers from recharge to coastal zone and through the Jakarta urban area, Indonesia. Sci. Total Environ. 2011, 409, 2541–2554. [Google Scholar] [CrossRef]
- Samborska, K.; Halas, S.; Bottrell, S.H. Sources and impact of sulphate on groundwaters of Triassic carbonate aquifers, Upper Silesia, Poland. J. Hydrol. 2013, 486, 136–150. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-reaction, One-dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1999; pp. 1–312.
- Liu, C.; Jiang, Y.; Tao, F.; Lang, Y.; Li, S. Chemical weathering of carbonate rocks by sulfuric acid and the carbon cycling in Southwest China. Geochimica 2008, 37, 404–414. [Google Scholar]
- Zhou, X.; Xu, Z.; Liu, W.; Wu, Y.; Zhao, T.; Jiang, H. Progress in the studies of precipitation chemistry in acid rain areas of Southwest China. Environ. Sci. 2017, 38, 4438–4446. [Google Scholar]
- Huang, T.; Fan, Y.; Long, Y.; Pang, Z. Quantitative calculation for the contribution of acid rain to carbonate weathering. J. Hydrol. 2019, 568, 360–371. [Google Scholar] [CrossRef]
- Huang, T.; Li, Z.; Ma, B.; Long, Y. Tracing the origin of groundwater nitrate in an area affected by acid rain using dual isotopic composition of nitrate. Geofluids 2019, 2019, 8964182. [Google Scholar] [CrossRef]
- Eltschlager, K.K.; Hawkins, J.W.; Ehler, W.C.; Baldassare, F. Technical Measures for the Investigation and Mitigation of Fugitive Methane Hazards in Areas of Coal Mining; U.S. Department of the Interior, Office of Surface Mining Reclamation and Enforcement: Pittsburgh, PA, USA, 2001; pp. 1–124.
- Liu, Z.; Yuan, D.; He, S. Stable carbon isotope geochemical and hydrochemical features in the system of carbonate–H2O–CO2 and their implications—Evidence from several typical karst area of China. Acta Geol. Sin. Engl. 1997, 71, 446–454. [Google Scholar]
- Huang, Q.; Qin, X.; Yang, Q.; Liu, P.; Zhang, J. Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate weathering using δ13CDIC and δ34S in karst area, northern China. Environ. Earth Sci. 2015, 75, 51. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 1–328. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Global Network of Isotopes in Precipitation (GNIP). Available online: http://www.iaea.org/water (accessed on 15 October 2019).
- Kong, Y.; Pang, Z.; Froehlich, K. Quantifying recycled moisture fraction in precipitation of an arid region using deuterium excess. Tellus B Chem. Phys. Meteorol. 2013, 65, 19251. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, H.; Zhan, L. Water contribution of fog drip to surface runoff in Chishui forest region during the dry season. Adv. Water Sci. 2016, 27, 377–384. [Google Scholar]
- Wigley, T.M.L.; Plummer, L.N.; Pearson, F.J. Mass transfer and carbon isotope evolution in natural water systems. Geochim. Cosmochim. Acta 1978, 42, 1117–1139. [Google Scholar] [CrossRef]
- Aucour, A.M.; Sheppard, S.M.F.; Guyomar, O.; Wattelet, J. Use of 13C to trace origin and cycling of inorganic carbon in the Rhône river system. Chem. Geol. 1999, 159, 87–105. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, Y.; Zhong, Y. Hydrogeochemistry; Geological Publishing House: Beijing, China, 1993; pp. 1–189. [Google Scholar]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; A.A. Balkema Publishers: Amsterdam, The Netherlands, 2005; pp. 1–634. [Google Scholar]
- Li, S.; Liu, C.; Lang, Y.; Tao, F.; Zhao, Z.; Zhou, Z. Stable carbon isotope biogeochemistry and anthropogenic impacts on karst ground water, Zunyi, Southwest China. Aquat. Geochem. 2008, 14, 211–221. [Google Scholar] [CrossRef]
- Douglas, G.; Gray, C.M.; Hart, B.; Beckett, R. A strontium isotopic investigation of the origin of suspended particulate matter (SPM) in the Murray-Darling River system, Australia. Geochim. Cosmochim. Acta 1995, 59, 3799–3815. [Google Scholar] [CrossRef]
- Blum, J.D.; Erel, Y.A. A silicate weathering mechanism linking increases in marine 87Sr/86Sr with global glaciation. Nature 1995, 373, 415–418. [Google Scholar] [CrossRef]
- Palmer, M.R.; Edmond, J.M. Controls over the strontium isotope composition of river water. Geochim. Cosmochim. Acta 1992, 56, 2099–2111. [Google Scholar] [CrossRef]
- Koepnick, R.B.; Denison, R.E.; Dahl, D.A. The Cenozoic seawater 87Sr/86Sr curve: Data review and implications for correlation of marine strata. Paleoceanography 1988, 3, 743–756. [Google Scholar] [CrossRef]
- Han, G.; Liu, C. Strontium isotope and major ion chemistry of the rainwaters from Guiyang, Guizhou Province, China. Sci. Total Environ. 2006, 364, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.J.; Jacobsen, S.B. The Nd and Sr isotopic systematics of river-water dissolved material: Implications for the sources of Nd and Sr in seawater. Chem. Geol. 1987, 66, 245–272. [Google Scholar] [CrossRef]
- Négrel, P.; Allègre, C.J.; Dupré, B.; Lewin, E. Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in river water: The Congo Basin case. Earth. Planet. Sci. Lett. 1993, 120, 59–76. [Google Scholar] [CrossRef]
- Currell, M.J.; Cartwright, I. Major-ion chemistry, δ13C and 87Sr/86Sr as indicators of hydrochemical evolution and sources of salinity in groundwater in the Yuncheng Basin, China. Hydrogeol. J. 2011, 19, 835–850. [Google Scholar] [CrossRef]
- Lang, Y.; Liu, C.; Han, G.; Zhao, Z.; Li, S. Characterization of water-rock interaction and pollution of karstic hydrological system: A study on water chemistry and Sr isotope of surface/ground water of the Guiyang area. Quat. Sci. 2005, 25, 655–662. [Google Scholar]
- Vengosh, A.; Heumann, K.G.; Juraske, S.; Kasher, R. Boron isotope application for tracing sources of contamination in groundwater. Environ. Sci. Technol. 1994, 28, 1968–1974. [Google Scholar] [CrossRef]
- Lang, Y.; Liu, C.; Zhao, Z. A review of studies on sources and migration of various contaminants in surface and ground waters by using boron and its isotopes. Earth Sci. Front. 2002, 9, 409–415. [Google Scholar]
- Davidson, G.R.; Bassett, R.L. Application of boron isotopes for identifying contaminants such as fly ash leachate in groundwater. Environ. Sci. Technol. 1993, 27, 172–176. [Google Scholar] [CrossRef]
- Bassett, R.L.; Buszka, P.M.; Davidson, G.R.; Chong-Diaz, D. Identification of groundwater solute sources using boron isotopic composition. Environ. Sci. Technol. 1995, 29, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.R. Geochemical and boron, oxygen and hydrogen isotopic constraints on the origin of salinity in groundwaters from the crystalline basement of the Alpine Foreland. Appl. Geochem. 2000, 15, 937–952. [Google Scholar] [CrossRef]
- Mather, J.D.; Porteous, N.C. The geochemistry of boron and its isotopes in groundwaters from marine and non-marine sandstone aquifers. Appl. Geochem. 2001, 16, 821–834. [Google Scholar] [CrossRef]
- Barth, S.R. Stable isotope geochemistry of sediment-hosted groundwater from a Late Paleozoic–Early Mesozoic section in central Europe. J. Hydrol. 2000, 235, 72–87. [Google Scholar] [CrossRef]
- Senior, L.A. A Reconnaissance Spatial and Temporal Baseline Assessment of Methane and Inorganic Constituents in Groundwater in Bedrock Aquifers, Pike County, Pennsylvania, 2012–13; U.S. Geological Survey: Reston, VA, USA, 2014; pp. 1–91.
- Yuan, J.; Guo, Q.; Wang, Y. Geochemical behaviors of boron and its isotopes in aqueous environment of the Yangbajing and Yangyi geothermal fields, Tibet, China. J. Geochem. Explor. 2014, 140, 11–12. [Google Scholar] [CrossRef]
- Pennisi, M.; Bianchini, G.; Muti, A.; Kloppmann, W.; Gonfiantini, R. Behaviour of boron and strontium isotopes in groundwater–aquifer interactions in the Cornia Plain (Tuscany, Italy). Appl. Geochem. 2006, 21, 1169–1183. [Google Scholar] [CrossRef]
- Warner, N.R.; Kresse, T.M.; Hays, P.D.; Down, A.; Karr, J.D.; Jackson, R.B.; Vengosh, A. Geochemical and isotopic variations in shallow groundwater in areas of the Fayetteville Shale development, north-central Arkansas. Appl. Geochem. 2013, 35, 207–220. [Google Scholar] [CrossRef]
- Darrah, T.H.; Vengosh, A.; Jackson, R.B.; Warner, N.R.; Poreda, R.J. Noble gases identify the mechanisms of fugitive gas contamination in drinking-water wells overlying the Marcellus and Barnett Shales. Proc. Natl. Acad. Sci. USA 2014, 111, 14076–14081. [Google Scholar] [CrossRef]
- Fuex, A.N. Experimental evidence against an appreciable isotopic fractionation of methane during migration. Phys. Chem. Earth 1980, 12, 725–732. [Google Scholar] [CrossRef]
- Bacsik, Z.; Lopes, J.N.C.; Gomes, M.F.C.; Jancso, G.; Mink, J.; Padua, A.A.H. Solubility isotope effects in aqueous solutions of methane. J. Chem. Phys. 2002, 116, 10816–10824. [Google Scholar] [CrossRef]
- Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Jean-Baptiste, P.; Holm, N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14′N, MAR). Chem. Geol. 2002, 191, 345–359. [Google Scholar] [CrossRef]
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Lansdown, J.M.; Quay, P.D.; King, S.L. CH4 production via CO2 reduction in a temperate bog: A source of 13C-depIeted CH4. Geochim. Cosmochim. Acta 1992, 56, 3493–3503. [Google Scholar] [CrossRef]
- Sano, Y.; Wakita, H. Geographical distribution of 3He/4He ratios in Japan: Implications for arc tectonics and incipient magmatism. J. Geophys. Res. Sol. Earth 1985, 90, 8729–8741. [Google Scholar] [CrossRef]
- Hilton, D.R.; Hammerschmidt, K.; Teufel, S.; Friedrichsen, H. Helium isotope characteristics of Andean geothermal fluids and lavas. Earth. Planet. Sci. Lett. 1993, 120, 265–282. [Google Scholar] [CrossRef]
- Ozima, M.; Podosek, F.A. Noble Gas Geochemistry, 2nd ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 1–286. [Google Scholar]
- Clarke, W.B.; Jenkins, W.J.; Top, Z. Determination of tritium by mass spectrometric measurement of 3He. Int. J. Appl. Radiat. Isot. 1976, 27, 515–522. [Google Scholar] [CrossRef]
- Graham, D.W. Noble gas isotope geochemistry of mid-ocean ridge and ocean island basalts: Characterization of mantle source reservoirs. Rev. Miner. Geochem. 2002, 47, 247–317. [Google Scholar] [CrossRef]
- Hoke, L.; Lamb, S.; Hilton, D.R.; Poreda, R.J. Southern limit of mantle-derived geothermal helium emissions in Tibet: Implications for lithospheric structure. Earth. Planet. Sci. Lett. 2000, 180, 297–308. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Lollar, B.S. Regional groundwater focusing of nitrogen and noble gases into the Hugoton-Panhandle giant gas field, USA. Geochim. Cosmochim. Acta 2002, 66, 2483–2497. [Google Scholar] [CrossRef]
- Kotarba, M.J.; Nagao, K. Composition and origin of natural gases accumulated in the Polish and Ukrainian parts of the Carpathian region: Gaseous hydrocarbons, noble gases, carbon dioxide and nitrogen. Chem. Geol. 2008, 255, 426–438. [Google Scholar] [CrossRef]
- Hayes, T. Sampling and Analysis of Water Streams Associated with the Development of Marcellus Shale Gas; Gas Technology Institute: Des Plaines, IL, USA, 2009; pp. 1–210. [Google Scholar]
- Drever, J.I. The Geochemistry of Natural Waters, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1988; pp. 1–437. [Google Scholar]
- Wang, Z.; Li, X.; Zhang, J.; Xu, H.; Wang, G.; Zhou, B.; Yang, J.; Wang, Y. Longmaxi formation shale gas geochemical features comparison between different blocks in Sichuan Basin. Coal Geol. China 2016, 28, 22–27. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).