Accounting for the Three-Dimensional Distribution of Escherichia coli Concentrations in Pond Water in Simulations of the Microbial Quality of Water Withdrawn for Irrigation
Abstract
1. Introduction
2. Methodology
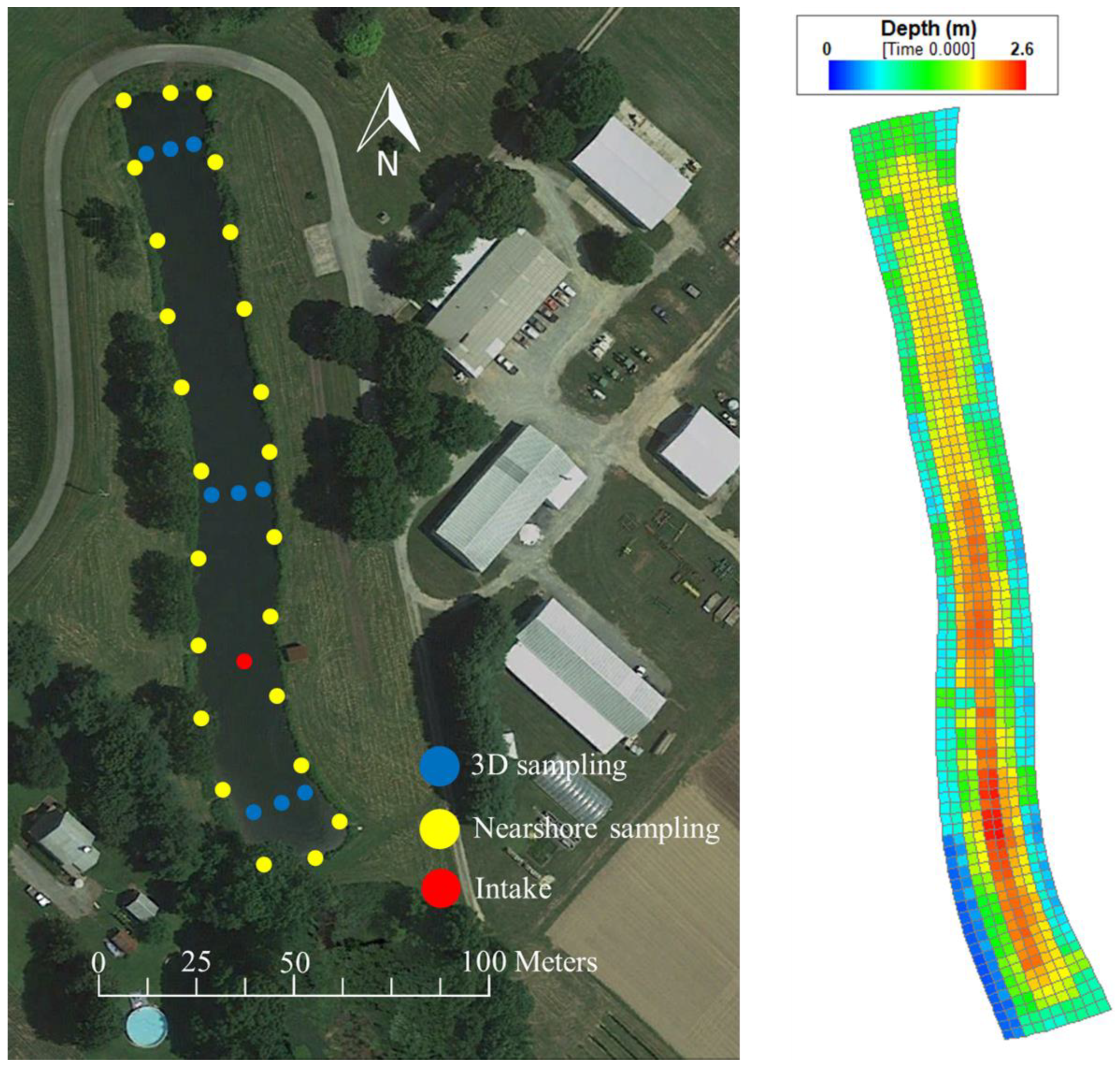
2.1. Study Area
2.2. Data Acquisition
3. Model and Simulation Setup
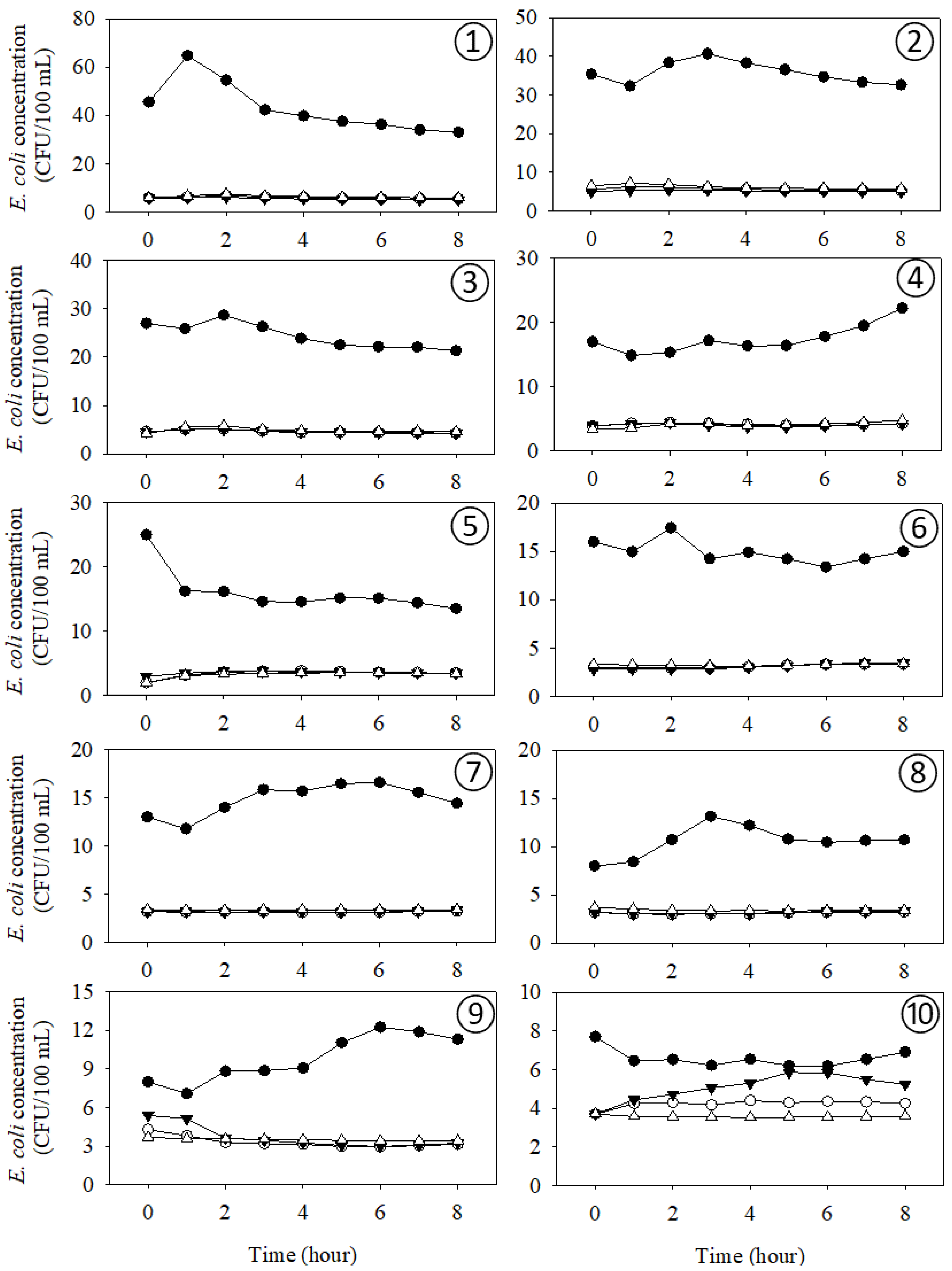
4. Results
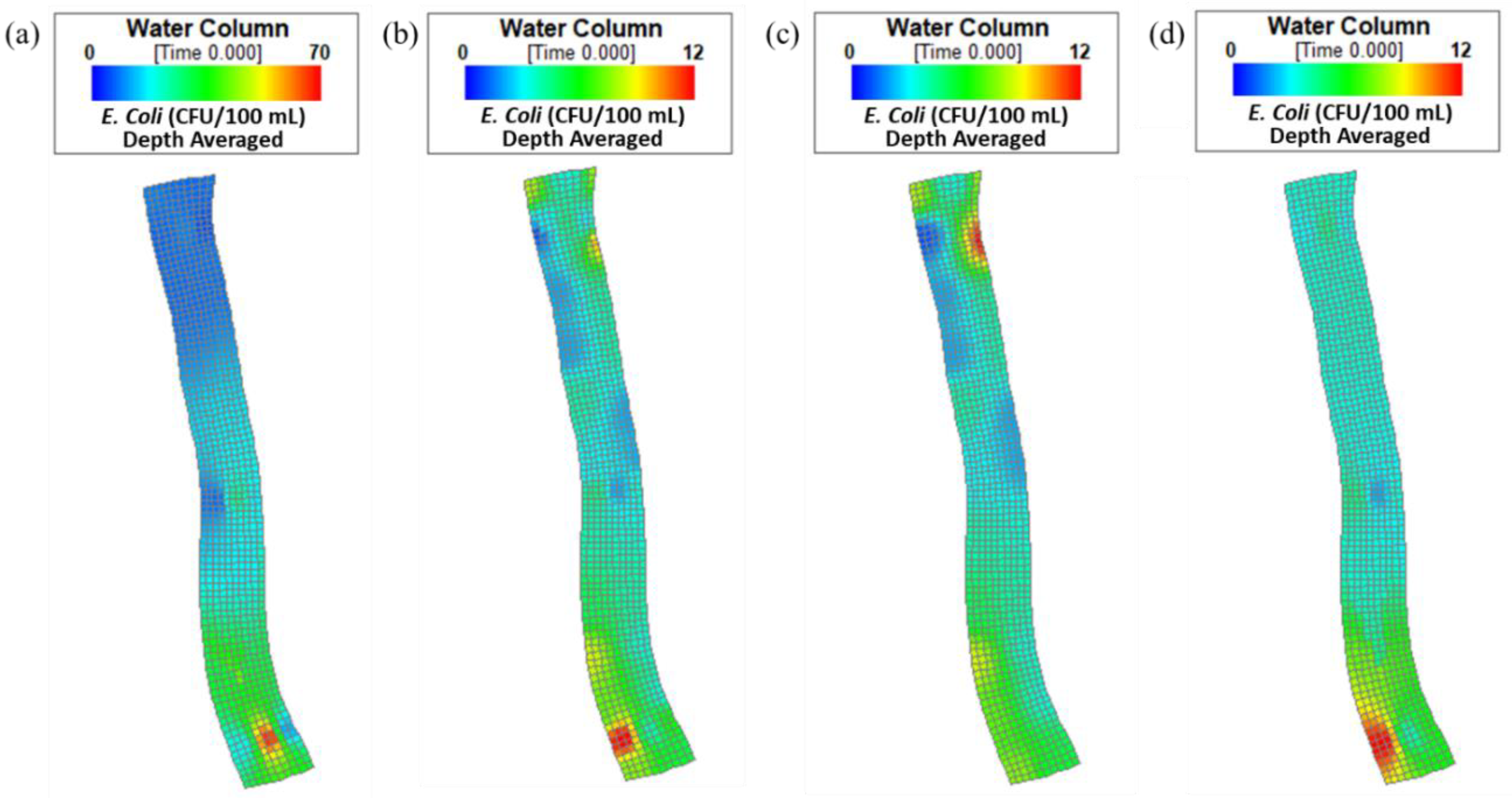
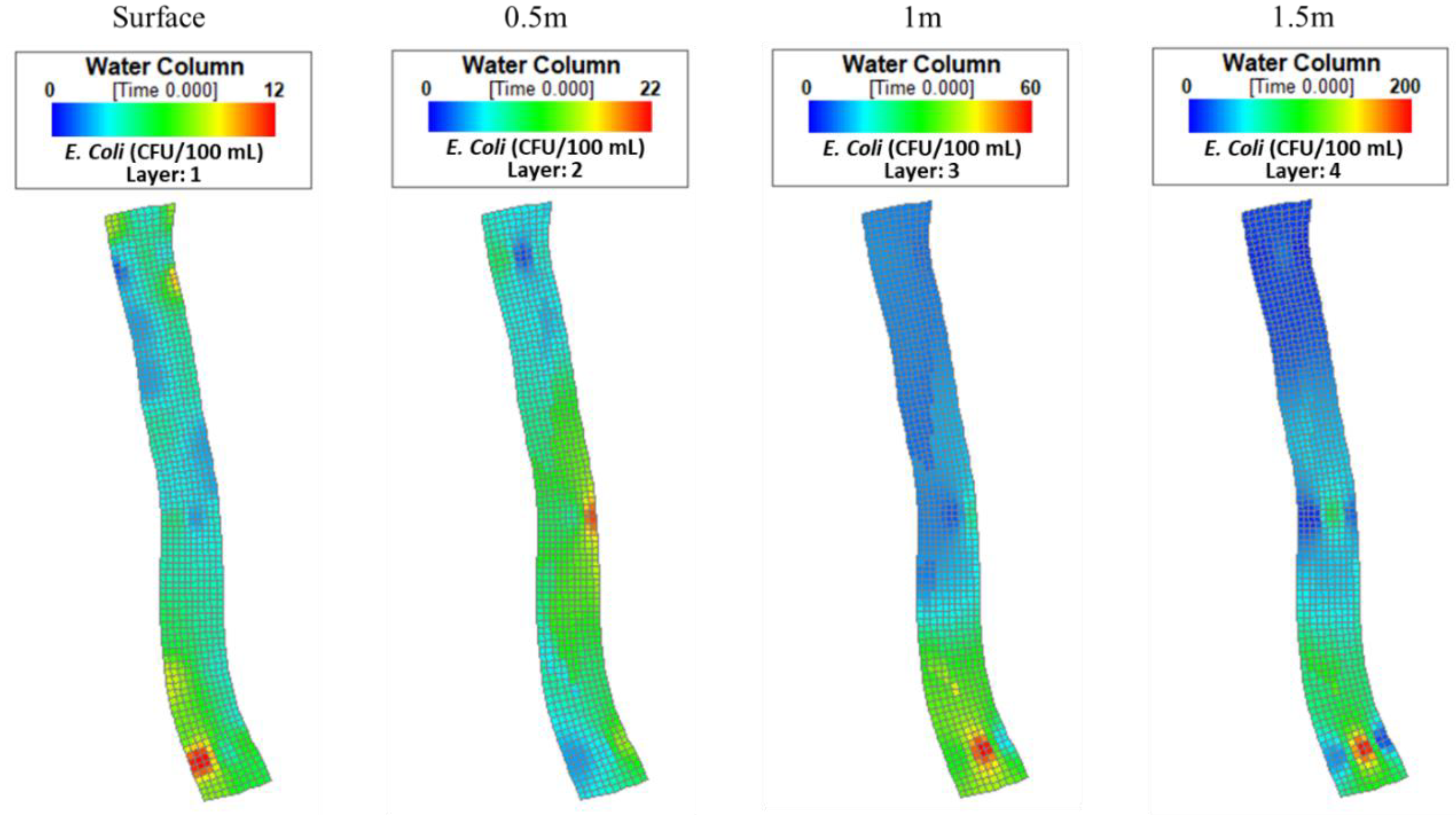
4.1. Spatial Variability of E. coli Concentrations
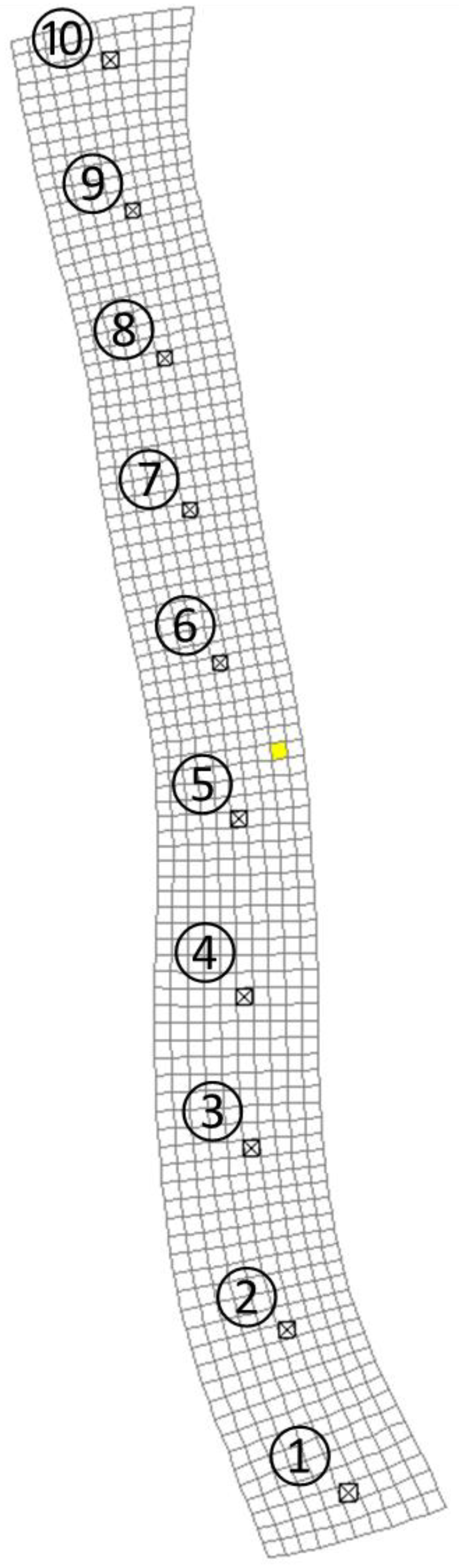
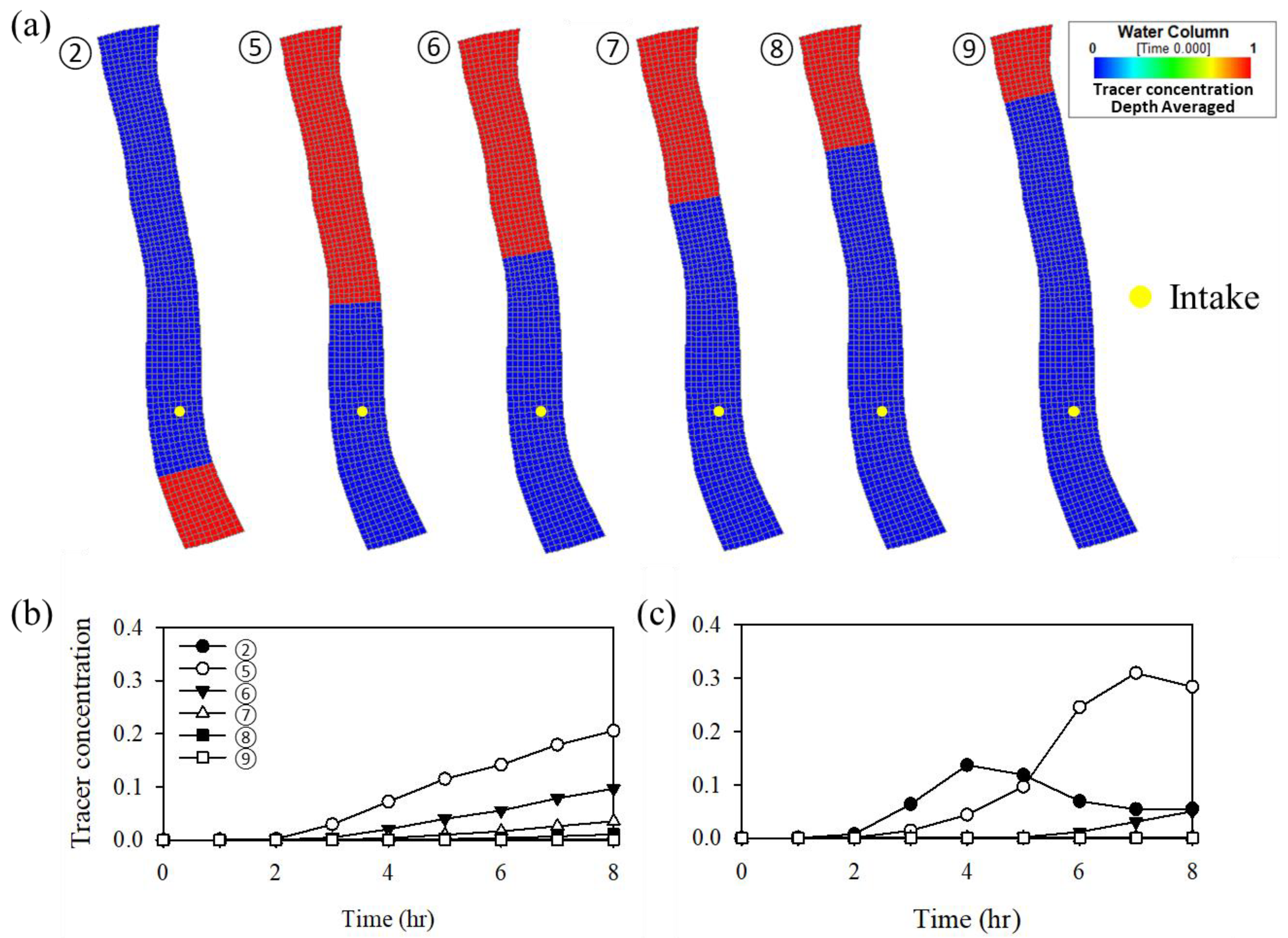
4.2. Simulations with Different Intake Locations
4.3. Assessment of the Zone of Influence for the Intake
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO’s First Ever Global Estimates of Foodborne Diseases Find Children under 5 Account for Almost One Third of Deaths; World Health Organization: Geneva, Switzerland, 2015; Volume 37, pp. 109–110. [Google Scholar]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.; Shelton, D.R.; McLain, J.E.; Patel, J.; Mandrell, R.E. Irrigation waters as a source of pathogenic microorganisms in produce: A review. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2011; Volume 113, pp. 75–141. [Google Scholar]
- Oliveira, M.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Presence and survival of Escherichia coli O157: H7 on lettuce leaves and in soil treated with contaminated compost and irrigation water. Int. J. Food Microbiol. 2012, 156, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Akinde, S.B.; Sunday, A.A.; Adeyemi, F.M.; Fakayode, I.B.; Oluwajide, O.O.; Adebunmi, A.A.; Oloke, J.K.; Adebooye, C.O. Microbes in Irrigation Water and Fresh Vegetables: Potential Pathogenic Bacteria Assessment and Implications for Food Safety. Appl. Biosaf. 2016, 21, 89–97. [Google Scholar] [CrossRef]
- Steele, M.; Mahdi, A.; Odumeru, J. Microbial assessment of irrigation water used for production of fruit and vegetables in Ontario, Canada. J. Food Prot. 2005, 68, 1388–1392. [Google Scholar] [CrossRef]
- Boehm, A.B.; Sassoubre, L.M. Enterococci as indicators of environmental fecal contamination. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Chandrasekaran, R.; Hamilton, M.J.; Wang, P.; Staley, C.; Matteson, S.; Birr, A.; Sadowsky, M.J. Geographic isolation of Escherichia coli genotypes in sediments and water of the Seven Mile Creek—A constructed riverine watershed. Sci. Total Environ. 2015, 538, 78–85. [Google Scholar] [CrossRef]
- US FDA. 2018. Available online: https://www.fda.gov/food/foodborneillnesscontaminants/peopleatrisk/ucm352830.htm (accessed on 4 May 2020).
- Dieter, C.A.; Caldwell, R.R.; Harris, M.A.; Ivahnenko, T.I.; Lovelace, J.K.; Barber, N.L.; Lisney, K.S. Estimated use of water in the United States in 2015: U.S. Geol. Surv. Circ. 2018, 1441, 65. [Google Scholar]
- Jones, L.A.; Worobo, R.W.; Smart, C.D. Plant-pathogenic oomycetes, Escherichia coli strains, and Salmonella spp. frequently found in surface water used for irrigation of fruit and vegetable crops in New York State. Appl. Environ. Microbiol. 2014, 80, 4814–4820. [Google Scholar] [CrossRef]
- Antaki, E.M.; Vellidis, G.; Harris, C.; Aminabadi, P.; Levy, K.; Jay-Russell, M.T. Low concentration of Salmonella enterica and generic Escherichia coli in farm ponds and irrigation distribution systems used for mixed produce production in Southern Georgia. Foodborne Pathog. Dis. 2016, 13, 551–558. [Google Scholar] [CrossRef]
- Gu, G.; Luo, Z.; Cevallos-Cevallos, J.M.; Adams, P.; Vellidis, G.; Wright, A.; van Bruggen, A.H. Factors affecting the occurrence of Escherichia coli O157 contamination in irrigation ponds on produce farms in the Suwannee River Watershed. Can. J. Microbiol. 2012, 59, 175–182. [Google Scholar] [CrossRef]
- Lee, D.; Tertuliano, M.; Vellidis, G.; Harris, C.; Grossman, M.K.; Rajeev, S.; Levy, K. Evaluation of Grower-Friendly, Science-Based Sampling Approaches for the Detection of Salmonella in Ponds Used for Irrigation of Fresh Produce. Foodborne Pathog. Dis. 2018, 15, 627–636. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Vazquez, K.M.; Topalcengiz, Z.; Muñoz-Carpena, R.; Danyluk, M.D. Evaluating the US Food Safety Modernization Act Produce Safety Rule standard for microbial quality of agricultural water for growing produce. J. Food Prot. 2017, 80, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Truitt, L.N.; Vazquez, K.M.; Pfuntner, R.C.; Rideout, S.L.; Havelaar, A.H.; Strawn, L.K. Microbial quality of agricultural water used in produce preharvest production on the Eastern Shore of Virginia. J. Food Prot. 2018, 81, 1661–1672. [Google Scholar] [CrossRef]
- Partyka, M.L.; Bond, R.F.; Chase, J.A.; Kiger, L.; Atwill, E.R. Multistate evaluation of microbial water and sediment quality from agricultural recovery basins. J. Environ. Qual. 2016, 45, 657–665. [Google Scholar] [CrossRef] [PubMed]
- McEgan, R.; Mootian, G.; Goodridge, L.D.; Schaffner, D.W.; Danyluk, M.D. Predicting Salmonella populations from biological, chemical, and physical indicators in Florida surface waters. Appl. Environ. Microbiol. 2013, 79, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Pahl, D.M.; Telias, A.; Newell, M.; Ottesen, A.R.; Walsh, C.S. Comparing source of agricultural contact water and the presence of fecal indicator organisms on the surface of ‘Juliet’grape tomatoes. J. Food Prot. 2013, 76, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.; Kierzewski, R.; Stocker, M.; Sellner, K.; Mulbry, W.; Lee, H.; Kim, M. Temporal stability of Escherichia coli concentrations in waters of two irrigation ponds in Maryland. Appl. Environ. Microbiol. 2018, 84, e01876-17. [Google Scholar]
- Dias, D.F.C.; Passos, R.G.; Von Sperling, M. A review of bacterial indicator disinfection mechanisms in waste stabilisation ponds. Rev. Environ. Sci. Bio/Technol. 2017, 16, 517–539. [Google Scholar] [CrossRef]
- Dias, D.F.; von Sperling, M. Vertical profiling and modelling of Escherichia coli decay in a shallow maturation pond operating in a tropical climate. Environ. Technol. 2018, 39, 759–769. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Lemckert, C.; Roiko, A.; Stratton, H. On the hydrodynamics and treatment efficiency of waste stabilisation ponds: From a literature review to a strategic evaluation framework. J. Clean. Prod. 2018, 183, 495–514. [Google Scholar] [CrossRef]
- Maïga, Y.; Wethe, J.; Denyigba, K.; Ouattara, A.S. The impact of pond depth and environmental conditions on sunlight inactivation of Escherichia coli and enterococci in wastewater in a warm climate. Can. J. Microbiol. 2009, 55, 1364–1374. [Google Scholar] [CrossRef]
- Sah, L.; Rousseau, D.P.; Hooijmans, C.M.; Lens, P.N. 3D model for a secondary facultative pond. Ecol. Model. 2011, 222, 1592–1603. [Google Scholar] [CrossRef]
- Sah, L.; Rousseau, D.P.; Hooijmans, C.M. Numerical modelling of waste stabilization ponds: Where do we stand? Water Air Soil Pollut. 2012, 223, 3155–3171. [Google Scholar] [CrossRef]
- Khan, S.; Melville, B.W.; Shamseldin, A.Y.; Fischer, C. Investigation of flow patterns in storm water retention ponds using CFD. J. Environ. Eng. 2013, 139, 61–69. [Google Scholar]
- Hamrick, J.M. A Three-Dimensional Environmental Fluid Dynamics Computer Code: Theoretical and Computational Aspects. Special Report in Applied Marine Science and Ocean Engineering; Virginia Institute of Marine Science, William & Mary: Gloucester Point, VA, USA, 1992. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Liu, Y.; He, B.; Zhu, X.; Zou, R.; Zhu, Y. Three-dimensional hydrodynamic and water quality model for TMDL development of Lake Fuxian, China. J. Environ. Sci. 2012, 24, 1355–1363. [Google Scholar] [CrossRef]
- Seo, D.; Kim, M.; Ahn, J.H. Prediction of chlorophyll-a changes due to weir constructions in the Nakdong River using EFDC-WASP modelling. Environ. Eng. Res. 2012, 17, 95–102. [Google Scholar] [CrossRef]
- Wool, T.A.; Davie, S.R.; Rodriguez, H.N. Development of three-dimensional hydrodynamic and water quality models to support total maximum daily load decision process for the Neuse River Estuary, North Carolina. J. Water Resour. Plan. Manag. 2003, 129, 295–306. [Google Scholar] [CrossRef]
- He, G.; Fang, H.; Bai, S.; Liu, X.; Chen, M.; Bai, J. Application of a three-dimensional eutrophication model for the Beijing Guanting Reservoir, China. Ecol. Model. 2011, 222, 1491–1501. [Google Scholar] [CrossRef]
- Luo, X.; Li, X. Using the EFDC model to evaluate the risks of eutrophication in an urban constructed pond from different water supply strategies. Ecol. Model. 2018, 372, 1–11. [Google Scholar] [CrossRef]
- EFDC_Explorer 8 User Guide. 2020. Available online: https://eemodelingsystem.atlassian.net/wiki/spaces/EEREF/overview (accessed on 7 June 2020).
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Stocker, M.D.; Pachepsky, Y.A.; Hill, R.L.; Sellner, K.G.; Macarisin, D.; Staver, K.W. Intraseasonal variation of E. coli and environmental covariates in two irrigation ponds in Maryland, USA. Sci. Total Environ. 2019, 670, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Maraccini, P.A.; Mattioli, M.C.M.; Sassoubre, L.M.; Cao, Y.; Griffith, J.F.; Ervin, J.S.; Van De Werfhorst, L.C.; Boehm, A.B. Solar inactivation of enterococci and Escherichia coli in natural waters: Effects of water absorbance and depth. Environ. Sci. Technol. 2016, 50, 5068–5076. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.E.; Allen, C.D.; Charlton, M.N. Dissolved organic matter and ultraviolet radiation penetration in the Laurentian Great Lakes and tributary waters. J. Great Lakes Res. 2004, 30, 367–380. [Google Scholar] [CrossRef]
- Smith, P.; Carroll, C.; Wilkins, B.; Johnson, P.; Gabhainn, S.N.; Smith, L.P. The effect of wind speed and direction on the distribution of sewage-associated bacteria. Lett. Appl. Microbiol. 1999, 28, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Wang, L.; Chen, Y. Analysis of agricultural pollution by flood flow impact on water quality in a reservoir using a three-dimensional water quality model. J. Hydroinform. 2012, 15, 1061–1072. [Google Scholar] [CrossRef]
- Hatvani, I.G.; Kirschner, A.K.; Farnleitner, A.H.; Tanos, P.; Herzig, A. Hotspots and main drivers of fecal pollution in Neusiedler See, a large shallow lake in Central Europe. Environ. Sci. Pollut. Res. 2018, 25, 28884–28898. [Google Scholar] [CrossRef]
- Sokolova, E.; Pettersson, T.J.; Bergstedt, O.; Hermansson, M. Hydrodynamic modelling of the microbial water quality in a drinking water source as input for risk reduction management. J. Hydrol. 2013, 497, 15–23. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocker, M.D.; Jeon, D.J.; Sokolova, E.; Lee, H.; Kim, M.S.; Pachepsky, Y.A. Accounting for the Three-Dimensional Distribution of Escherichia coli Concentrations in Pond Water in Simulations of the Microbial Quality of Water Withdrawn for Irrigation. Water 2020, 12, 1708. https://doi.org/10.3390/w12061708
Stocker MD, Jeon DJ, Sokolova E, Lee H, Kim MS, Pachepsky YA. Accounting for the Three-Dimensional Distribution of Escherichia coli Concentrations in Pond Water in Simulations of the Microbial Quality of Water Withdrawn for Irrigation. Water. 2020; 12(6):1708. https://doi.org/10.3390/w12061708
Chicago/Turabian StyleStocker, Matthew D., Dong Jin Jeon, Ekaterina Sokolova, Hoonsoo Lee, Moon S. Kim, and Yakov A. Pachepsky. 2020. "Accounting for the Three-Dimensional Distribution of Escherichia coli Concentrations in Pond Water in Simulations of the Microbial Quality of Water Withdrawn for Irrigation" Water 12, no. 6: 1708. https://doi.org/10.3390/w12061708
APA StyleStocker, M. D., Jeon, D. J., Sokolova, E., Lee, H., Kim, M. S., & Pachepsky, Y. A. (2020). Accounting for the Three-Dimensional Distribution of Escherichia coli Concentrations in Pond Water in Simulations of the Microbial Quality of Water Withdrawn for Irrigation. Water, 12(6), 1708. https://doi.org/10.3390/w12061708




