Structural Diversity in Early-Stage Biofilm Formation on Microplastics Depends on Environmental Medium and Polymer Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastic Particles
2.2. Incubation of Microplastic Particles in Environmental Media
2.3. Sample Preparation for Scanning Electron Microscopy
2.4. Classification of Observed Structural Diversity via SEM Images
2.5. ζ-Potential
3. Results
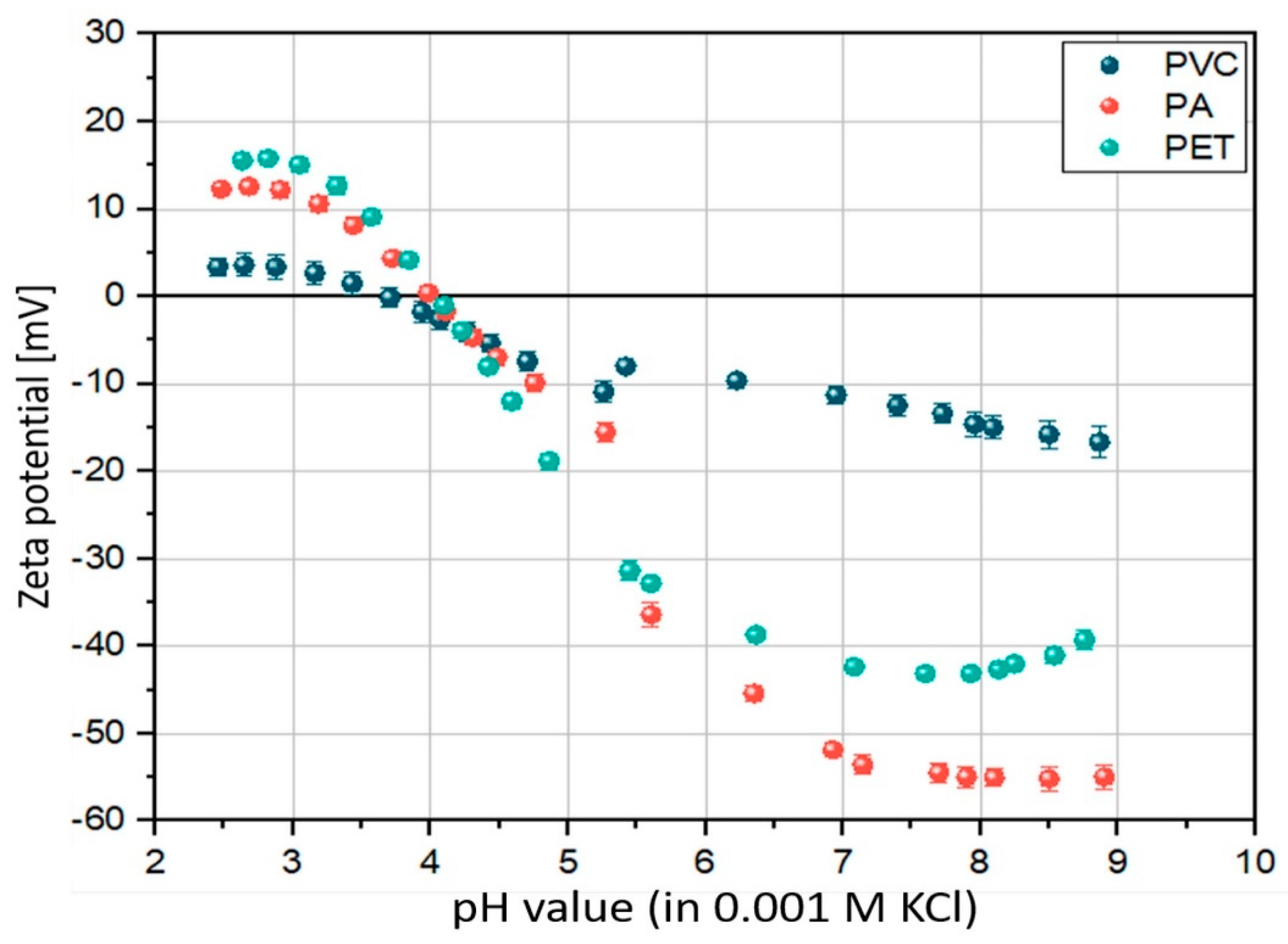
3.1. Surface Properties—ζ-Potential
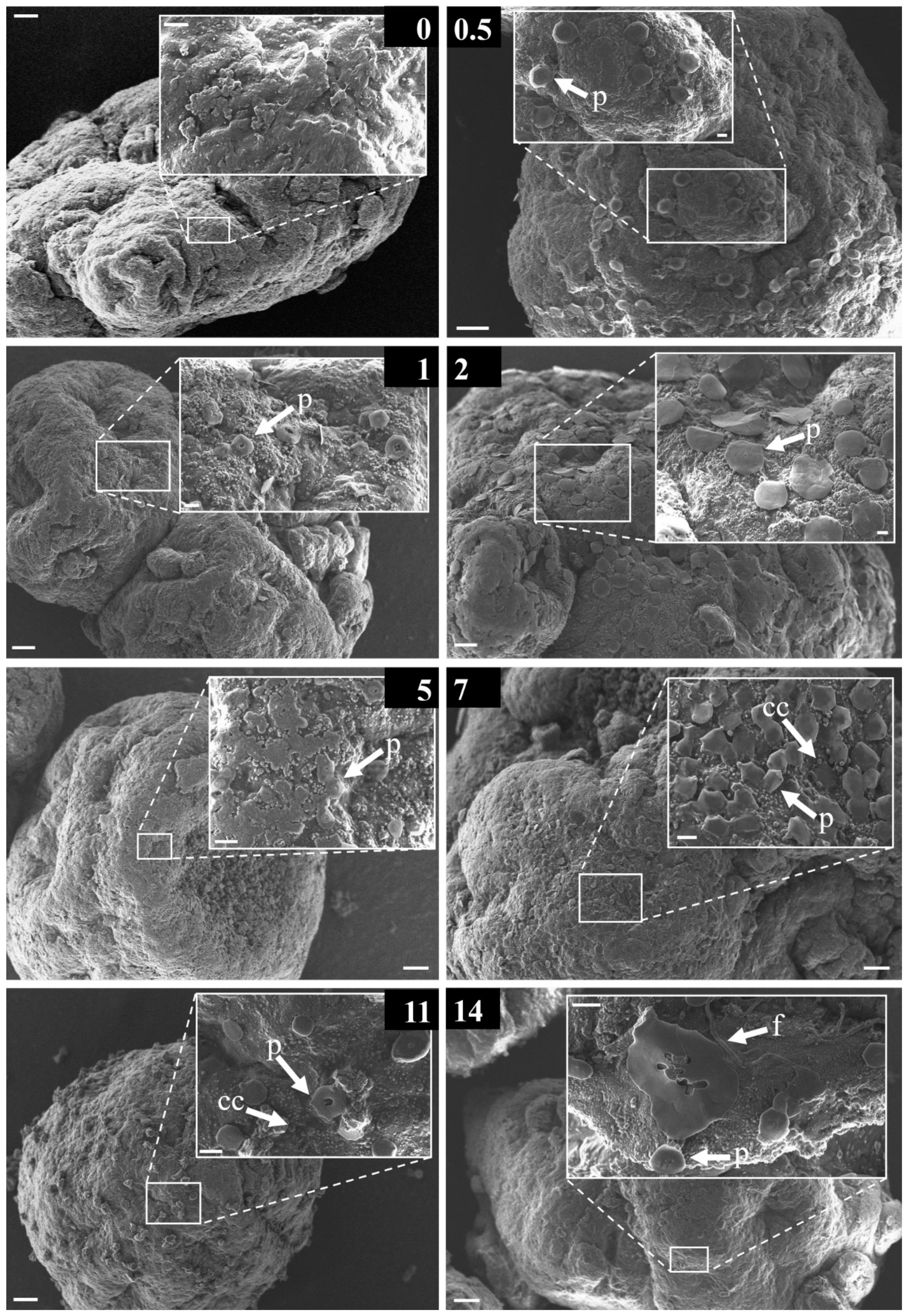
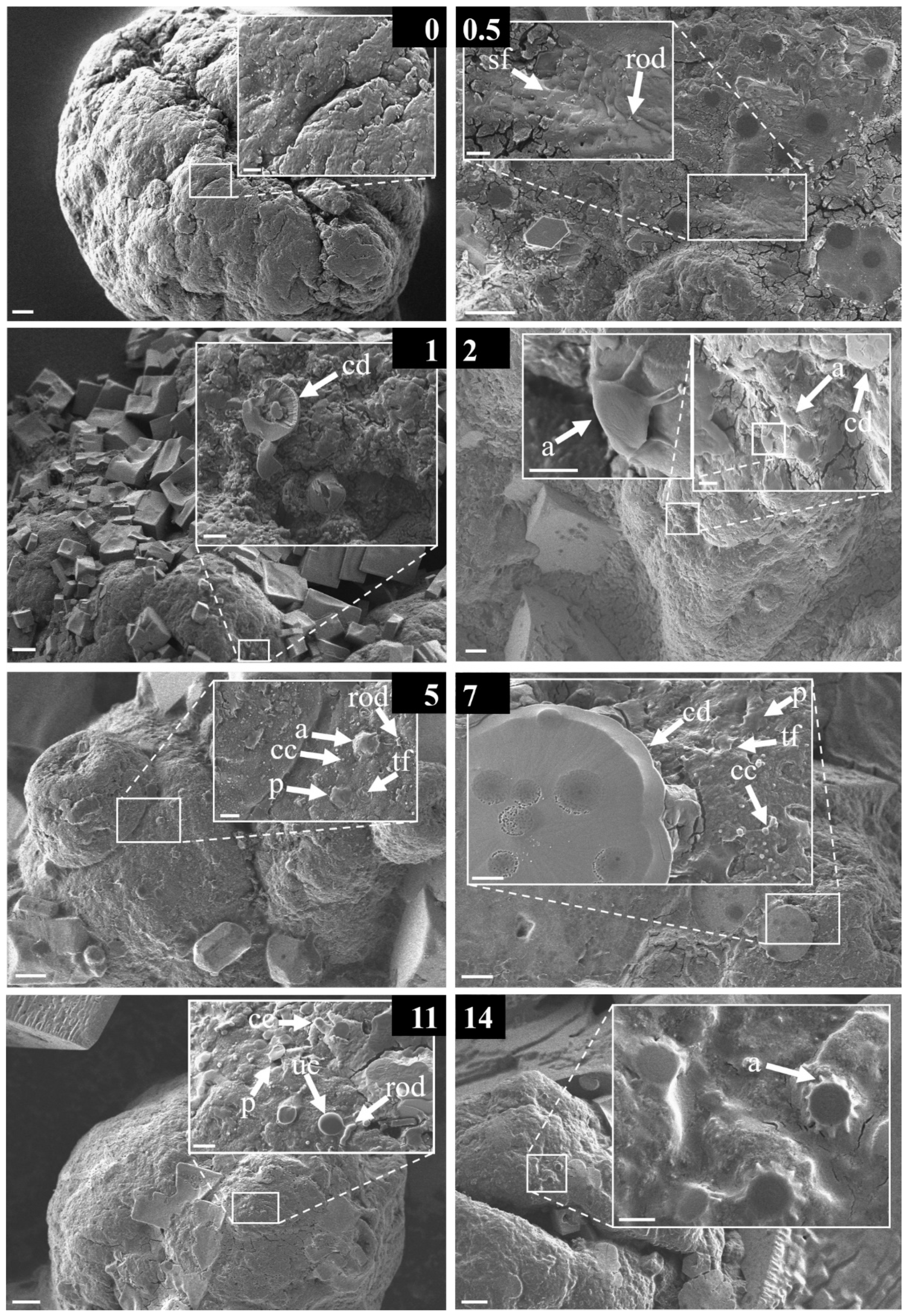
3.2. Structural Diversity of the Early-Stage Biofilm
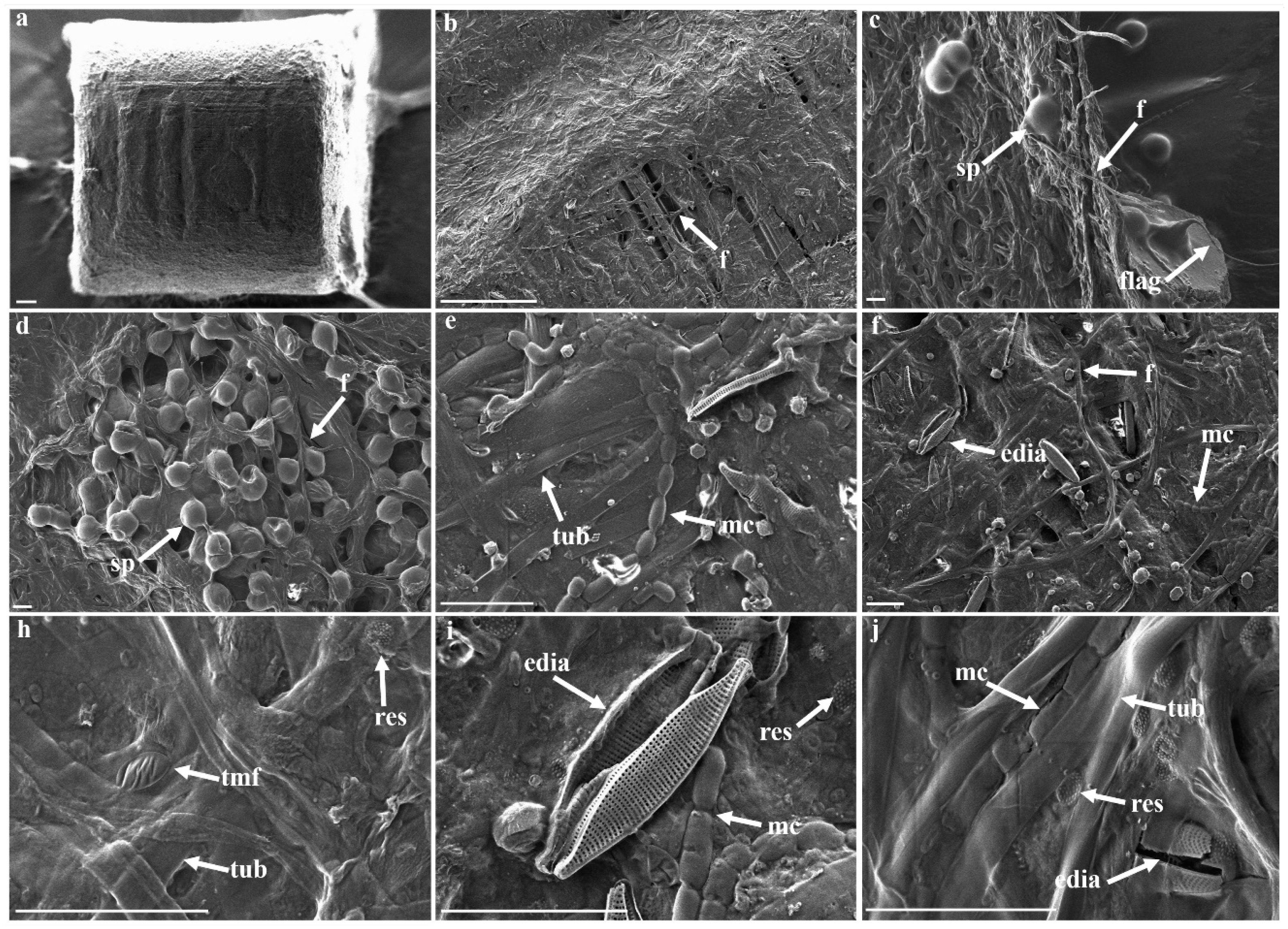
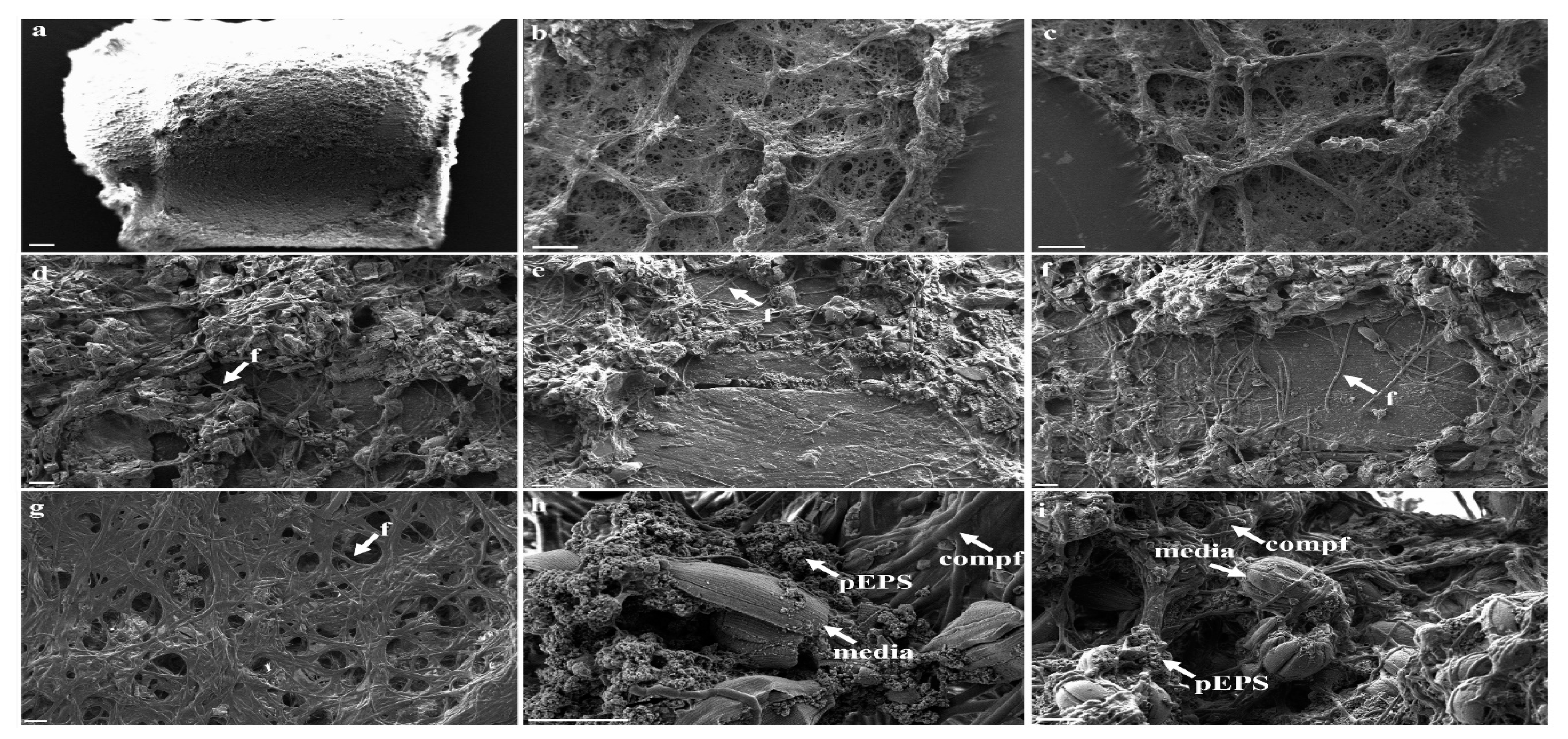
3.3. Morphological Strcutures Within Late-Stage Biofilms
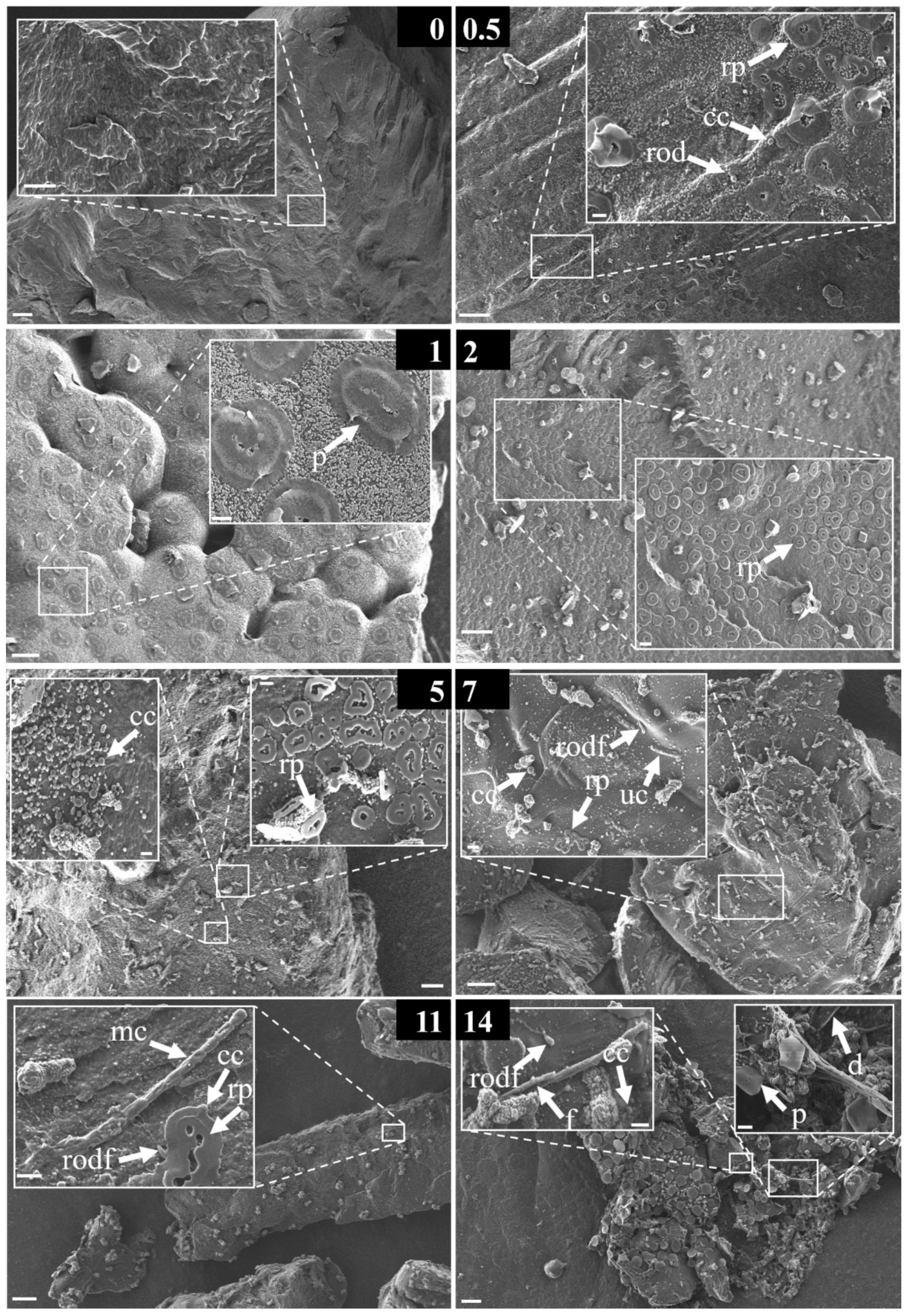
3.4. Morphological Strcutures Within Early-Stage Biofilms
4. Discussion
4.1. Correlation of Structural Diversity of Early-Stage Biofilms and Polymer Properties
4.2. Descriptive Analysis of the Observed Morphological Structures Forming a Late-Stage Biofilm
4.3. Descriptive Analysis of the Observed Morphological Structures Forming an Early-Stage Biofilm
4.4. Potential Ecological Implications of Our Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.J.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic. Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. In Conference Proceedings; Environmental Science: Silver Spring, MD, USA, 2008; p. 530. [Google Scholar]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environ. Chem. 2015. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef]
- Imhof, H.K.; Sigl, R.; Brauer, E.; Feyl, S.; Giesemann, P.; Klink, S.; Leupolz, K.; Löder, M.G.J.; Löschel, L.A.; Missun, J.; et al. Spatial and temporal variation of macro-, meso- and microplastic abundance on a remote coral island of the Maldives, Indian Ocean. Mar. Pollut. Bull. 2017, 116, 340–347. [Google Scholar] [CrossRef]
- Lacerda, A.L.d.F.; Rodrigues, L. dos S.; van Sebille, E.; Rodrigues, F.L.; Ribeiro, L.; Secchi, E.R.; Kessler, F.; Proietti, M.C. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 2019, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1. [Google Scholar] [CrossRef]
- Laist, D. Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine Debris—Sources, Impacts Solutions; Coe, J.M., Rogers, D.B., Eds.; Springer: New York, NY, USA, 1997; pp. 99–139. [Google Scholar] [CrossRef]
- Harper, P.C.; Fowler, J.A. Plastic pellets in New Zealand storm-killed prions (Pachyptila spp.) 1958–1977. Notornis 1987, 34, 65–70. [Google Scholar]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- von Moos, N.; Burkhardt-Holm, P.; Koehler, A. Uptake and E ff ects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 327–335. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2015. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Welden, N.A.; Lusher, A.L. Microplastics: From Origin to Impacts; Elsevier Inc.: New York, NY, USA, 2020; Volume 32, ISBN 9780128178805. [Google Scholar] [CrossRef]
- Ryan, P.G. Effects of ingested plastic on seabird feeding: Evidence from chickens. Mar. Pollut. Bull. 1988, 19, 125–128. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.J.; Bréchon, A.L.; Thompson, R.C. Ingestion and fragmentation of plastic carrier bags by the amphipod Orchestia gammarellus: Effects of plastic type and fouling load. Mar. Pollut. Bull. 2018, 127, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242. [Google Scholar] [CrossRef]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Loeder, M.G.J.; Gerdts, G.; Osborn, A.M. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol. Ecol. 2014, 2, 478–492. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-zettler, L.A. Life in the ‘ Plastisphere ’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Dang, H.; Li, T.; Chen, M.; Huang, G. Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters. Appl. Environ. Microbiol. 2008, 74, 52–60. [Google Scholar] [CrossRef]
- Webb, H.K.; Crawford, R.J.; Sawabe, T.; Ivanova, E.P. Poly(ethylene terephthalate) polymer surfaces as a substrate for bacterial attachment and biofilm formation. Microbes Environ. 2009, 24, 39–42. [Google Scholar] [CrossRef]
- Hossain, M.R.; Jiang, M.; Wei, Q.H.; Leff, L.G. Microplastic surface properties affect bacterial colonization in freshwater. J. Basic Microbiol. 2019, 59, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Artham, T.; Sudhakar, M.; Venkatesan, R.; Madhavan Nair, C.; Murty, K.V.G.K.; Doble, M. Biofouling and stability of synthetic polymers in sea water. Int. Biodeterior. Biodegrad. 2009, 63, 884–890. [Google Scholar] [CrossRef]
- Loeb, G.; Neihof, R. Marine conditioning films. Adv. Chem. 1975, 145, 319–335. [Google Scholar] [CrossRef][Green Version]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. The Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017. [Google Scholar] [CrossRef]
- PlasticsEurope (Association of Plastic Manufacturers). Conversio Market & Strategy GmbH Plastics—The Facts 2019; PlasticsEurope (Association of Plastic Manufacturers): Brussels, Belgium, 2019. [Google Scholar]
- González-Ramírez, A.I.; Ramírez-Granillo, A.; Medina-Canales, M.G.; Rodríguez-Tovar, A.V.; Martínez-Rivera, M.A. Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol. 2016, 16, 243. [Google Scholar] [CrossRef]
- Solmaz, K.; Ozcan, Y.; Mercan Dogan, N.; Bozkaya, O.; Ide, S. Characterization and Production of Extracellular Polysaccharides (EPS) by Bacillus Pseudomycoides U10. Environments 2018, 5, 63. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, C.; Santschi, P.H. Chemical composition and 234Th (IV) binding of extracellular polymeric substances (EPS) produced by the marine diatom Amphora sp. Mar. Chem. 2008, 112, 81–92. [Google Scholar] [CrossRef]
- Hoagland, K.D.; Rosowski, J.R.; Gretz, M.R.; Roemer, S.C. Diatom extracellular polymeric substances: Function, fine structure, chemistry, and physiology. J. Phycol. 1993, 29, 537–566. [Google Scholar] [CrossRef]
- Dumack, K.; Siemensma, F.; Bonkowski, M. Rediscovery of the Testate Amoeba Genus Penardeugenia (Thaumatomonadida, Imbricatea). Protist 2018, 169, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, K.H. New and little-known marine and freshwater species of the silica-scaled genera Thaumatomastix and Reckertia (Cercozoa: Thaumatomonadida). J. Mar. Biol. Assoc. UK 2013, 93, 1231–1244. [Google Scholar] [CrossRef]
- Saraeva, I.; Kudryashov, S.I.; Danilov, P.; Busleev, N.; Tolordava, E.R.; Rudenko, A.A.; Zayarny, D.; Ionin, A.; Romanova, Y.M. Polarization-Sensitive Surface-Enhanced In Situ Photoluminescence Spectroscopy of S. aureus Bacteria on Gold Nanospikes. Sensors 2020, 20, 2466. [Google Scholar] [CrossRef]
- Bessudova, A.Y.; Domysheva, V.M.; Firsova, A.D.; Likhoshway, Y.V. Silica-scaled chrysophytes of Lake Baikal. Acta Biol. Sib. 2017, 3, 47. [Google Scholar] [CrossRef][Green Version]
- Reimers, C.E.; Li, C.; Graw, M.F.; Schrader, P.S.; Wolf, M. The identification of cable bacteria attached to the anode of a benthic microbial fuel cell: Evidence of long distance extracellular electron transport to electrodes. Front. Microbiol. 2017, 8, 2055. [Google Scholar] [CrossRef]
- Koon, M.A.; Almohammed Ali, K.; Speaker, R.M.; McGrath, J.P.; Linton, E.W.; Steinhilb, M.L. Preparation of prokaryotic and eukaryotic organisms using chemical drying for morphological analysis in scanning electron microscopy (SEM). J. Vis. Exp. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Margalef, R. Size of centric diatoms as an ecological indicator. SIL Commun. 1953–1996 1969, 17, 202–210. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Du, W.; Gao, K. Physiological response of marine centric diatoms to ultraviolet radiation, with special reference to cell size. J. Photochem. Photobiol. B Biol. 2015, 153, 1–6. [Google Scholar] [CrossRef]
- Oliver, P.G.; Rodrigues, C.F. Thyasiridae (mollusca: Bivalvia) from the Kemp caldera hydrothermal site, south sandwich islands, Antarctica. J. Conchol. 2017, 42, 267–282. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Drechsler, A.; Caspari, A.; Synytska, A. Influence of roughness and capillary size on the zeta potential values obtained by streaming potential measurements. Surf. Interface Anal. 2020, 1–5. [Google Scholar] [CrossRef]
- Heinrich, P.; Hanslik, L.; Kämmer, N.; Braunbeck, T. The tox is in the detail: Technical fundamentals for designing, performing, and interpreting experiments on toxicity of microplastics and associated substances. Environ. Sci. Pollut. Res. 2020, 27, 22292–22318. [Google Scholar] [CrossRef]
- Chiovitti, A.; Dugdale, T.M.; Wetherbee, R. Diatom Adhesives: Molecular and Mechanical Properties. Biol. Adhes. 2006, 1, 79–103. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Chepkwony, N.K.; Berne, C.; Brun, Y.V. Comparative analysis of ionic strength tolerance between freshwater and marine Caulobacterales adhesins. J. Bacteriol. 2019, 201, 18. [Google Scholar] [CrossRef]
- Sanni, O.; Chang, C.Y.; Anderson, D.G.; Langer, R.; Davies, M.C.; Williams, P.M.; Williams, P.; Alexander, M.R.; Hook, A.L. Bacterial attachment to polymeric materials correlates with molecular flexibility and hydrophilicity. Adv. Healthc. Mater. 2015, 4, 695–701. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, I.; Himanshu, P.; Ramteke, P.W.; Pandey, A.C.; Mishra, S.B.; Patil, S. Electrospun Nanofibrous Scaffold as a Potential Carrier of Antimicrobial Therapeutics for Diabetic Wound Healing and Tissue Regeneration; Elsevier Inc.: New York, NY, USA, 2017; ISBN 9780323527279. [Google Scholar] [CrossRef]
- Frank, E.; Merete, W.; Lotte, C.; Anne-lise, H.; Vibke, T.R.; Clemmensen, I. Attachment of staphylococci to different plastic tubes in vitro. Med. Microbiol. 1994, 40, 37–42. [Google Scholar] [CrossRef]
- Gorrell, H.A. Classification of Formation Waters Based on Sodium Chloride Content: GEOLOGICAL NOTES. Am. Assoc. Pet. Geol. Bull. 1958, 42, 2522. [Google Scholar] [CrossRef]
- Harris, L.G.; Foster, S.J.; Richards, R.G.; Lambert, P.; Stickler, D.; Eley, A. An introduction to Staphylococcus aureus, and techniques for identifyingand quantifying S. aureus adhesins in relation to adhesion to biomaterials:Review. Eur. Cells Mater. 2002, 4, 39–60. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]



| Polymer Type | Supplier | Trade Name | Size Distribution [µm] |
|---|---|---|---|
| Polyamide pellet * | Leibniz-Institut für Polymerforschung Dresden e.V. | - | 2000–2500 |
| Polyamid fragments | BASF, Ludwigshafen, Germany | Ultramid®, A3K, PA66 | <500 |
| Polyethylene terephtalate fragments | Neogroup, Klaipeda district, Lithuania | Neopet 80 | <500 |
| Polyvinyl chloride fragments | Vinnolit GmbH & Co.KG, Ismaning, Germany | ®Vinnolit S 3268 | < 500 |
| Biofilm Structures | ||
|---|---|---|
| Abbreviation | Associated Structure | |
| late-stage biofilm | compf | compartmented filaments |
| edia | elongated diatoms | |
| f | filamentous | |
| flag | flagella-like | |
| mc | multicellular | |
| media | marine elongated diatoms | |
| pEPS | particulate EPS | |
| res | rough elliptical structure | |
| sp | sporous | |
| tmf | thin mucus-like film | |
| tub | tubular | |
| early stage biofilm | a | non-assignable/exceptional microorganismal structure |
| cc | coccoid bacteria | |
| cd | centric diatom | |
| d | diatom | |
| f | filamentous structure | |
| mc | multicellular | |
| p | platelet | |
| rp | round platelet | |
| rod | rod-/vibrio-shaped bacteria | |
| rodf | rod-/vibrio-shaped bacteria with flagella | |
| sf | solid film | |
| tf | thin film | |
| uc | unicellular | |
| Polymer Type | ζ-Potential (pH 8) | Contact Angle [56] | Environmental Media | Biofilm Structural Diversity [14 Days] |
|---|---|---|---|---|
| PA | −55 mV | 70° | FW | 9 |
| SW | 7 | |||
| PET | −43 mV | 81° | FW | 6 |
| SW | 6 | |||
| PVC | −15 mV | 87° | FW | 3 |
| SW | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsperger, A.F.R.M.; Stellwag, A.C.; Caspari, A.; Fery, A.; Lueders, T.; Kress, H.; Löder, M.G.J.; Laforsch, C. Structural Diversity in Early-Stage Biofilm Formation on Microplastics Depends on Environmental Medium and Polymer Properties. Water 2020, 12, 3216. https://doi.org/10.3390/w12113216
Ramsperger AFRM, Stellwag AC, Caspari A, Fery A, Lueders T, Kress H, Löder MGJ, Laforsch C. Structural Diversity in Early-Stage Biofilm Formation on Microplastics Depends on Environmental Medium and Polymer Properties. Water. 2020; 12(11):3216. https://doi.org/10.3390/w12113216
Chicago/Turabian StyleRamsperger, Anja F. R. M., Anja C. Stellwag, Anja Caspari, Andreas Fery, Tillmann Lueders, Holger Kress, Martin G. J. Löder, and Christian Laforsch. 2020. "Structural Diversity in Early-Stage Biofilm Formation on Microplastics Depends on Environmental Medium and Polymer Properties" Water 12, no. 11: 3216. https://doi.org/10.3390/w12113216
APA StyleRamsperger, A. F. R. M., Stellwag, A. C., Caspari, A., Fery, A., Lueders, T., Kress, H., Löder, M. G. J., & Laforsch, C. (2020). Structural Diversity in Early-Stage Biofilm Formation on Microplastics Depends on Environmental Medium and Polymer Properties. Water, 12(11), 3216. https://doi.org/10.3390/w12113216





