Distributions of Microplastics in Surface Water, Fish, and Sediment in the Vicinity of a Sewage Treatment Plant
Abstract
1. Introduction
2. Materials and Methods
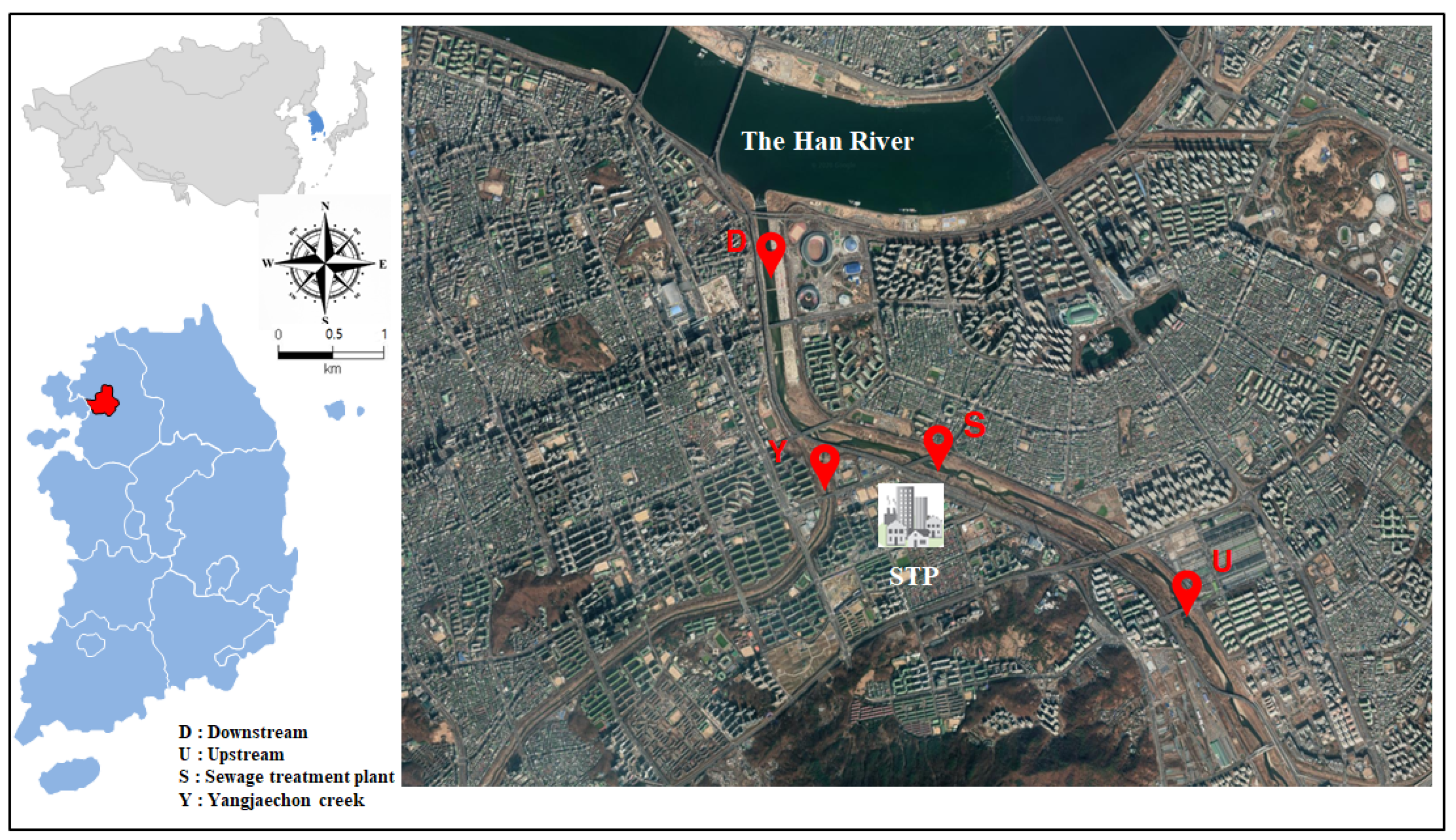
2.1. Study Area
2.2. Sample Collection
2.3. Sample Preparation
2.4. Characterization of Microplastics
3. Results and Discussion
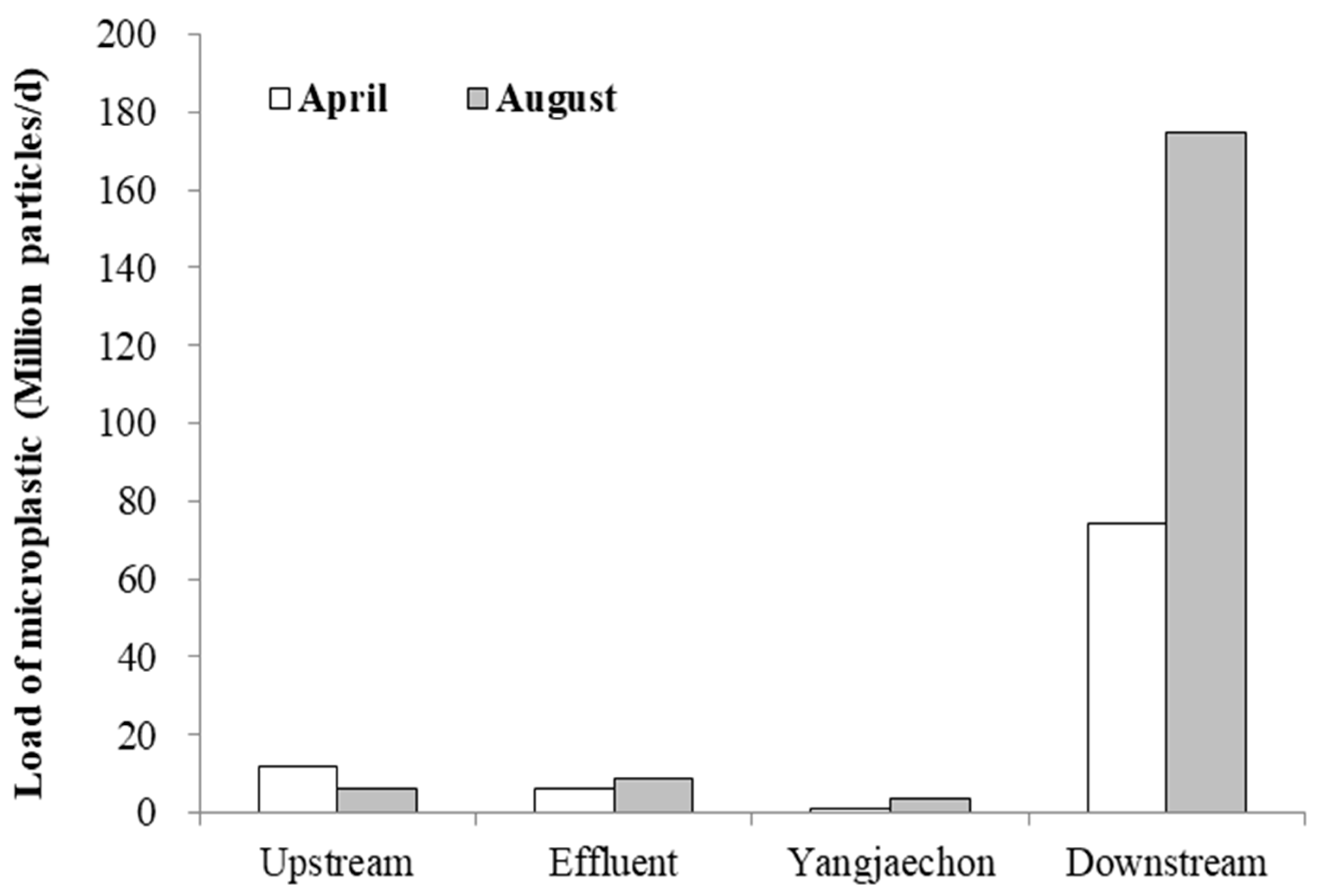
3.1. The Concentration of Microplastics in Water
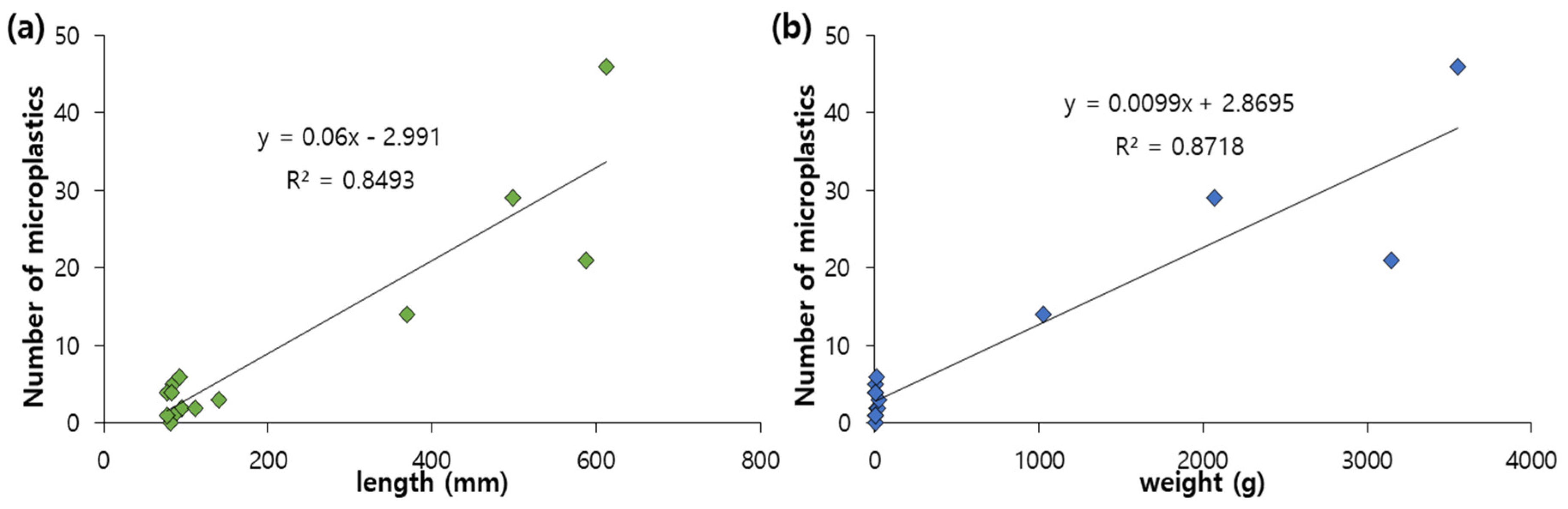
3.2. The Concentration of Microplastics in Fish
3.3. The Concentration of Microplastics in Sediment
3.4. Characteristics of Microplastics
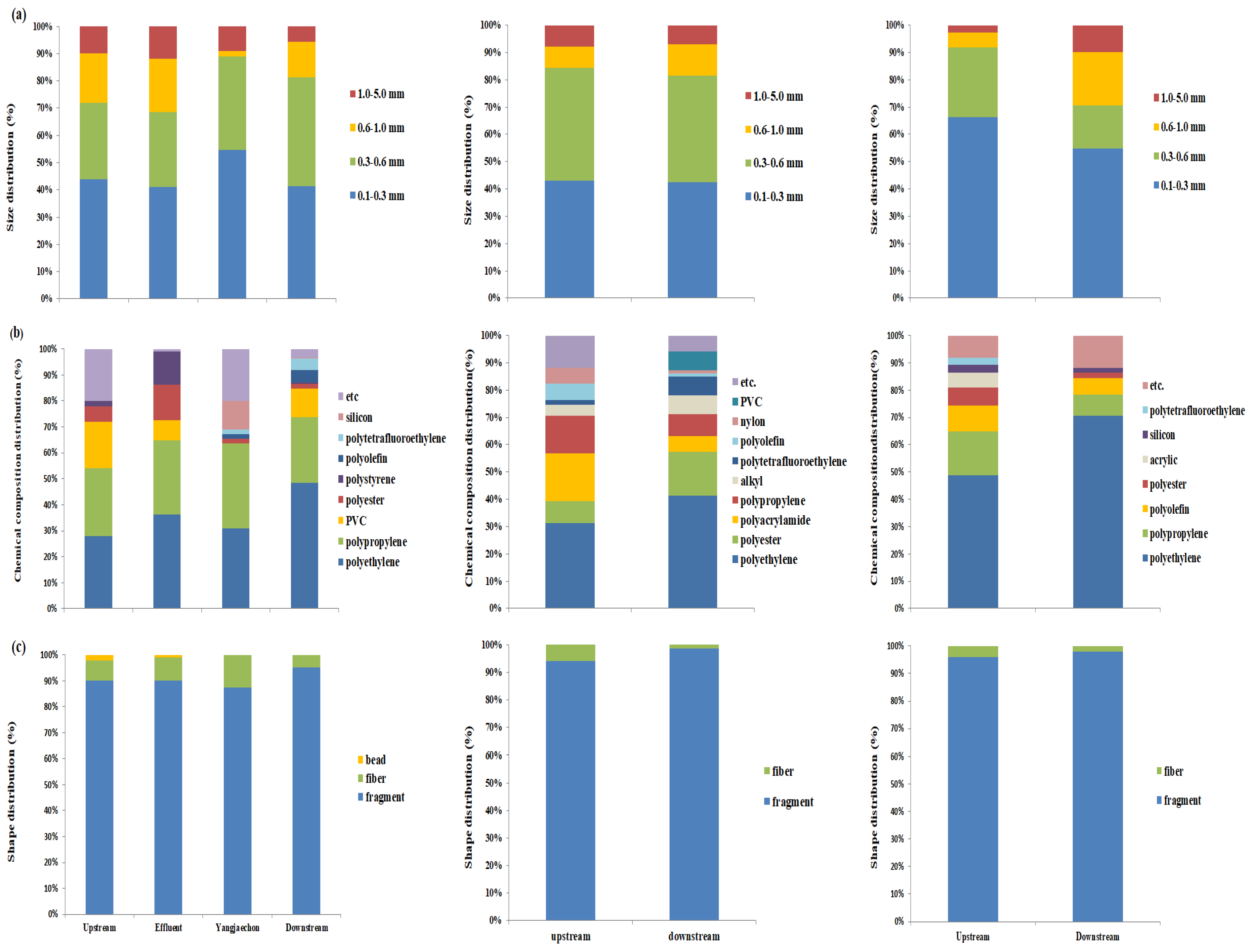
3.4.1. The Size Distribution of Microplastics
3.4.2. Shape of Microplastics
3.4.3. Polymer Type
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buist, J.M. Resume-the origins of polymers and their cellular derivates. Prog. Rubber Plast. Technol. 1986, 2, 17–30. [Google Scholar]
- Plastics—The Facts. An Analysis of European Plastics Production, Demand and Waste Data; PlasticEurope: Brussels, Belgium, 2018. [Google Scholar]
- Foekema, E.M.; Gruijter, C.D.; Mergia, M.T.; van Franeker, J.A.; Murk, A.T.J.; Kolemans, A.A. Plastic in North sea fish. Environ. Sci. Technol. 2013, 27, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- McNeish, R.E.; Kim, L.H.; Barrett, H.A.; Mason, S.A.; Kelly, J.J.; Hoellein, T.J. Microplastic in riverine fish is connected to species traits. Sci. Rep. 2018, 8, 11639. [Google Scholar] [CrossRef]
- Pinheiro, C.; Oliveira, U.; Vieira, M. Occurrence and impacts of microplastics in freshwater fish. J. Aquac. Mar. Biol. 2017, 5, 00138. [Google Scholar]
- Roch, S.; Water, T.; Ittner, L.D.; Friedrich, C.; Brinker, A. A systematic study of the microplastic burden in freshwater fishes of south-western Germany—Are we searching at the right scale? Sci. Total Environ. 2019, 689, 1001–1011. [Google Scholar] [CrossRef]
- Sanchez, W.; Bender, C.; Porcher, J.M. Wild gudgeons (Gobio gobio) from French rivers are contaminated by microplastics: Preliminary study and first evidence. Environ. Res. 2014, 128, 98–100. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian subalpine lake. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devries, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Lu, Y.F.; Zhng, Y.; Deng, Y.F. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Jeong, C.B.; Kang, H.M.; Lee, Y.H. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer Brachionus koreanus via multixenobiotic resistance (MXR) disruption. Environ. Sci. Technol. 2018, 52, 11411–11418. [Google Scholar] [CrossRef]
- Ogonowski, M.; Schur, C.; Jarsen, A. The effects of natural and anthropogenic microplastics on individual fitness in Daphnia magna. PLoS ONE. 2016, 11, e0155063. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Shin, W.J.; Kwon, O.Y.; Kang, J.H. Size-dependent effects of micropolystyrene particles in the marine copepod Tigriopus japonicas. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Grbíc, J.; Hel, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Edwins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef]
- Alvim, C.B.; Mendoza-Roca, J.A.; Bes-Pia, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manag. 2020, 255, 109739. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Phoenix, V.; Guachotte-Lindsay, C. Micro- and nanoplastic pollution of freshwater and wastewater treatment systems. Springer Sci. Rev. 2017, 5, 19–30. [Google Scholar] [CrossRef]
- Watkins, L.; McGrattan, S.; Sullivan, P.; Walter, M.T. The effect of dams on river transport of microplastic pollution. Sci. Total Environ. 2019, 664, 834–840. [Google Scholar] [CrossRef]
- Mani, T.; Burkhardt-Holm, P. Seasonal microplastic variation in nival and pluvial stretches of the Rhine River—From the Swiss catchment towards the North Sea. Sci. Total Environ. 2020, 707, 135579. [Google Scholar] [CrossRef]
- Statistics of Sewerage. Korean Ministry of Environment. 2018. Available online: www.me.go.kr (accessed on 5 May 2020).
- Bordós, G.; Urbány, B.; Micsinai, A.; Kriszt, B.; Palotai, Z.; Szabó, I.; Hantosi, Z.; Szoboszlay, S. Identification of microplastics in fish ponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere 2019, 216, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wen, X.; Du, C.; Jiang, J.; Wu, L.; Zhang, Y.; Hu, Z.; Hu, S.; Feng, Z.; Zhou, Z.; et al. Comparison of the abundance of microplastics between rural and urban areas: A case study from East Dongting Lake. Chemosphere. 2019, 244, 125486. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Jassen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Lee, S.H.; Lee, M.S.; Lee, J.K.; Lee, S.H.; Zoh, K.D. Occurrence of microplastics in the Han River and riverine fish in Korea. Sci. Total Environ. 2020, 708, 134535. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.A.; Bratton, S.P. Urbanization is a major influence on microplastic ingestion by sunfish in the Brazos River Basin, Central Texas, USA. Environ. Pollut. 2016, 210, 380–387. [Google Scholar] [CrossRef]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef]
- Lima, A.R.; Costa, M.F.; Barletta, M. Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014, 132, 146–155. [Google Scholar] [CrossRef]
- Eo, S.; Hong, S.H.; Song, Y.K.; Han, G.M.; Shim, W.J. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, Korea. Water Res. 2019, 160, 228–237. [Google Scholar] [CrossRef]
- Piñon-Colin, T.J.; Rodriguez-Jimenez, R.; Rogel-Hernandez, E.R.; Alvarez-Andrade, A.; Wakida, F.T. Microplastics in stormwater runoff in a semiarid region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef]
- Akarsu, C.; Kumbur, H.; Gokdag, K.; Kideys, A.E.; Sanchez-Vidal, A. Microplastics composition and load from three wastewater treatment plants discharging into Mersin Bay, north eastern Mediterranean Sea. Mar. Pollut. Bull. 2020, 150, 110776. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Lasee, S.; Mauricio, J.; Thompson, W.A.; Karnjanapiboonwong, A.; Kasumba, J.; Subbiah, S.; Morse, A.N.; Anderson, T.A. Microplastic in a freshwater environment receiving treated wastewater effluent. Integ. Environ. Assess. Manag. 2013, 13, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Park, H.J.; Kwon, O.K.; Lee, W.S.; Jeong, D.H.; Ju, B.K.; Kwon, J.H. Occurrence of microplastics in municipal sewage treatment plants: A review. Environ. Health Toxicol. 2018, 33, e2018013. [Google Scholar] [CrossRef] [PubMed]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with eluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Magnusson, K.; Noren, F. Screening of Microplastic Particles in and Downstream a Wastewater Treatment Plant; Report C55; Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef]
- Campbell, S.H.; Williamson, P.R.; Hall, B.D. Microplastics in the gastrointestinial tracts of fish and the water from an urban prairie creek. Facets 2017, 2, 359–409. [Google Scholar] [CrossRef]
- Horton, A.A.; Jurgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Silva-Cavalcanti, J.S.; Silva, J.D.B.; de Franca, E.J.; de Araujo, M.C.B.; Gusmao, F. Microplastics ingestion by a common tropical freshwater fishing resource. Environ. Pollut. 2017, 221, 218–226. [Google Scholar] [CrossRef]
- Slootmaekers, B.; Carteny, C.C.; Belpaire, C.; Saverwyns, S.; Fremout, W.; Blust, R.; Bervoets, L. Microplastic contamination in gudgeons (Gobio gobio) from Flemish rivers (Belgium). Environ. Pollut. 2019, 244, 675–684. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotox. Environ. Safety 2019, 170, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.B.; Bonner, T.H. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pazos, R.S.; Maiztegui, T.; Colautti, D.C.; Paracampo, A.H.; Gómez, N. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017, 122, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.S.; Bhatti, M.S.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation. Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Xue, B.; Wang, Y. Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: New implications for location, rhizosphere, and sediment compositions. Environ. Pollut. 2019, 244, 685–692. [Google Scholar] [CrossRef]
- Pegado, T.S.S.; Shmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- De Vries, A.N.; Govoni, D.; Árnason, S.H.; Carlsson, P. Microplastic ingestion by fish: Body size, condition factor and gut fullness are not related to the amount of plastics consumed. Mar. Pollut. Bull. 2020, 151, 110827. [Google Scholar] [CrossRef]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Fok, L.; Yan, Y.; Li, H.X.; Xu, X.R. Occurrence and distribution of microplastics in an urgan river: A case study in the Pearl River along Guangzhou city, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef]
- Wang, J.D.; Peng, J.P.; Tan, Z.; Gao, Y.F.; Zhan, Z.W.; Chen, Q.Q.; Cai, L.Q. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 246–258. [Google Scholar] [CrossRef]
- He, B.; Goonetilleke, A.; Ayoko, G.A.; Rintoul, L. Abundance, distribution patterns and identification of microplastics in Brisbane River sediments, Australia. Sci. Total Environ. 2020, 700, 134467. [Google Scholar] [CrossRef]
- Nel, H.A.; Dalu, T.; Wasserman, R.J. Sinks and sources: Assessing microplastic abundance in river sediment and deposit feeders in an austral temperate urban river system. Sci. Total Environ. 2018, 612, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thmopson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelgaic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Russel, M.; Ewins, C.; Quinn, B. The uptake of macroplastic & microplastic by demersal and pelagic fish in the Northeast Atlantic around Scotland. Mar. Pollut. Bull. 2017, 122, 353–359. [Google Scholar] [PubMed]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Shi, Y.; Zhao, Y.; Zheng, S. Characteristics and retention of microplastics in the digestive extracts of fish from the Yellow Sea. Environ. Pollut. 2019, 249, 878–885. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Zhang, K.; Gong, W.; Lu, J.; Xiong, X.; Wu, C. Accumulation of floating microplastics behind the three gorges dam. Environ. Pollut. 2015, 204, 117–123. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Goncalves, F.J.M.; Nogueira, H.; Marques, J.C.; Goncalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Ganier, Y.; Jacob, H.; Guerra, A.S.; Bertucci, F.; Lecchini, D. Evaluation of microplastic ingestion by tropical fish from Moorea island, French Polynesia. Mar. Pollut. Bull. 2019, 140, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, J.; Krause, S.; Lynch, I.; Smith, G.H.S. Abundance, distribution, and drivers of microplastic contamination in urban river environment. Water 2018, 10, 1597. [Google Scholar] [CrossRef]
- Cannas, S.; Fastelli, P.; Guerranti, C.; Renzi, M. Plastic litter in sediments from the coasts of south Tuscany (Tyrhenian Sea). Mar. Pollut. Bull. 2017, 119, 372–375. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2017, 36, 947–951. [Google Scholar] [CrossRef]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A large-scale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Yang, B.; Lu, D.; Zong, Q. Distribution of microplastics in surface water and sediments of Qin river in Beibu Gulf, China. Sci. Total Environ. 2020, 708, 135176. [Google Scholar] [CrossRef]
- Plastics-The Facts. An Analysis of European Plastics Productions, Demand and Waste Data; Plastics-The Facts: Brussels, Belgium, 2019; pp. 1–42. [Google Scholar]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, J.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef]
- Zhang, K.; Xiong, X.; Hu, H.; Wu, C.; Bi, Y.; Wu, Y.; Zhou, B.; Lam, P.K.S.; Liu, J. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of three gorges reservoir, China. Environ. Sci. Technol. 2017, 51, 3794–3801. [Google Scholar] [CrossRef]
- Andray, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- SAPEA. A Scientific Perspective on Microplastics in Nature and Society; SAPEA (Science Advice for Policy by European Adcademies): Brussels, Belgium, 2019; pp. 1–176. [Google Scholar]
- Tien, C.J.; Wnag, Z.X.; Chen, C.S. Microplastics in water, sediment and fish from the Fengshan River system: Relationship to aquatic factors and accumulation of polycyclic aromatic hydrocarbons by fish. Environ. Pollut. 2020, 265, 114962. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Leuten, E.L.; Tonkin, A.; Gallow, T.; Thompson, R.C. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]

| Name | Scientific Name | Feeding Habit | Length (mm) | Weight (g) | No | Concentration (Particles/Fish) |
|---|---|---|---|---|---|---|
| Carp | Cyrinus carpio | Omnivore | 566 ± 59 | 292.8 ± 767.7 | 3 | 32.0 ± 12.8 |
| Crucian carp carpcarp | Carassius auratus | Omnivore | 369 | 1024.3 | 1 | 14 |
| Goby minnow | Pseudogobio esocinus | Insectivore | 141 | 24.5 | 1 | 3 |
| Bass | Micropterus salmoides | Carnivore | 112 | 16.0 | 1 | 2 |
| Minnow | Zacco platypus | Omnivore | 84 ± 6 | 6.4 ± 2.0 | 8 | 2.9 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.-J.; Lee, S.-H.; Lee, M.-S.; Lee, J.-K.; Park, J.-H.; Zoh, K.-D. Distributions of Microplastics in Surface Water, Fish, and Sediment in the Vicinity of a Sewage Treatment Plant. Water 2020, 12, 3333. https://doi.org/10.3390/w12123333
Park T-J, Lee S-H, Lee M-S, Lee J-K, Park J-H, Zoh K-D. Distributions of Microplastics in Surface Water, Fish, and Sediment in the Vicinity of a Sewage Treatment Plant. Water. 2020; 12(12):3333. https://doi.org/10.3390/w12123333
Chicago/Turabian StylePark, Tae-Jin, Seung-Hyun Lee, Myung-Sung Lee, Jae-Kwan Lee, Ji-Hyoung Park, and Kyung-Duk Zoh. 2020. "Distributions of Microplastics in Surface Water, Fish, and Sediment in the Vicinity of a Sewage Treatment Plant" Water 12, no. 12: 3333. https://doi.org/10.3390/w12123333
APA StylePark, T.-J., Lee, S.-H., Lee, M.-S., Lee, J.-K., Park, J.-H., & Zoh, K.-D. (2020). Distributions of Microplastics in Surface Water, Fish, and Sediment in the Vicinity of a Sewage Treatment Plant. Water, 12(12), 3333. https://doi.org/10.3390/w12123333






