Abstract
Pharmaceutically active compounds are only partially removed from wastewaters and hence may be major contaminants of freshwaters. Direct and indirect effects on aquatic organisms are reported at dilute concentrations. This study was focused on the possible effects of environmentally relevant concentrations (~1 µg L−1) of two psychoactive compounds on the behavior of freshwater crayfish. Experimental animals exposed to venlafaxine did not show any behavioral alteration. Crayfish exposed to the benzodiazepine oxazepam exhibited a significant alteration in the distance moved and activity, and the effects were different when individuals were ready for reproduction. Results suggested that even the low concentration of selected psychoactive pharmaceuticals could alter the behavioral patterns of crayfish, as reported for other pharmaceuticals. These results provide new information about the possible adverse effects of pharmaceuticals at dilute concentrations. From previous knowledge and our results, it is obvious that different compounds have different effects and the effects are even specific for different taxa. Detailed studies are therefore needed to assess the possible ecological consequences of particular substances, as well as for their mixtures.
1. Introduction
Pharmaceutically active compounds (PhAC) are considered emerging contaminants in aquatic environments [1,2]. PhACs originate mainly from human or animal excretion or runoff from hospitals [3,4] and penetrate freshwaters via effluents of sewage treatment plants (STPs) which are ineffective in their removal [5]. The residues have several non-lethal effects on aquatic organisms and, through them, on whole ecosystems [6,7]. Psychotropic substances are present often at much lower concentrations in surface waters [8,9,10] than, for example, antibiotics or hypertension drugs, [11,12] but they have important effects in very diluted concentrations as well [13,14].
The psychotropic substances venlafaxine and oxazepam alter the state of the brain by flooding it with the neurotransmitter serotonin (5-HT) or act on benzodiazepine receptors, having direct inhibitory effects on the central nervous system [15,16]. Invertebrates (including crayfish) have similar receptors for psychotropic compounds as mammals [17], even with the potential for bioaccumulation [14,18], which increases the probability of the apparent PhACs’ effects on these animals.
Some psychoactive PhACs are also bioactive and can persist in the sediments of surface waters [19], enabling their transfer via the food-web [20]. They are developed to modify behavioral patterns, so a behavioral alteration in aquatic organisms is likely [21,22]. However, the behavioral effects of these psychotropic compounds still remain less understood than eco-toxicity tests [23]. Behavioral effects, from an ecological point of view, can affect the survival of an individual, as well as the long-term sustainability of a population [24]. Crayfish seem to be good model organisms, having well known social and spatial behavior [25,26] and being similarly susceptible to the behavioral changes induced by PhACs [13,27,28].
In this study, the behavioral patterns of a clonal species, the marbled crayfish (Procambarus virginalis, Lyko 2017), were assessed using an ethological software where control animals and those exposed to environmentally relevant concentrations of venlafaxine and oxazepam were used. We hypothesized about the possible behavioral changes associated with the pollutants used at concentrations commonly detected in surface waters, as confirmed in our previous study with other PhACs.
2. Materials and Methods
2.1. Chemicals
Venlafaxine (VEN) and oxazepam (OXA) were obtained from AK Scientific (Union City, CA, USA) and Lipomed (Cambridge, MA, USA), respectively. Stock solutions of both compounds (concentration of 10 mg L−1) were prepared using ultra-pure water (AquaMax Basic 360 Series and Ultra 370 Series instrument, Young Lin, Anyang, Republic of Korea) and were stored at 4 °C. The exposure solutions of 1 µg L−1 were prepared by dilution of the stock solution in aged tap water. Concentration testing was utilized to evaluate reported [29] environmentally relevant concentrations.
Isotopically labeled venlafaxine (D6-VEN) and oxazepam (D5-OXA; both from Lipomed (USA)) were used as internal standards for the analyses of water samples. Ultra-pure water and acetonitrile (LC/MS grade purity, Merck, Kenilworth, NJ, USA), both acidified with formic acid (Sigma-Aldrich, Darmstadt, Germany), were used as the mobile phases in liquid chromatography (LC).
2.2. Experimental Animals
Marbled crayfish (with a carapace length of 16–22 mm, measured using a vernier caliper to the nearest 0.1 mm) were randomly selected from our laboratory culture. Crayfish weight (to the nearest 0.1 g) was obtained using an electronic balance (Kern & Sohn GmbH, Balingen, Germany). The mean lengths and weights of the animals used (Table 1) did not differ between the control and exposed groups.

Table 1.
The mean carapace length (CL) and weight (W) of marbled crayfish (Procambarus virginalis, Lyko 2017) animals used in the experimental groups in either the presence or absence of shelter. Data are presented as mean ± standard deviation. The t-test values and p-values are shown to demonstrate no differences between experimental groups.
2.3. Experimental Design
The exposition and experimental work was conducted in November (VEN) and December (OXA) 2017. In total, 55 crayfish were exposed to a concentration of ~1 μg L−1 of VEN for 21 days and 60 animals to an OXA compound for 7 days, respectively. The concentration was chosen based on previously reported environmental concentrations [9,14,18,30,31]. The exposure times were chosen in relation to the mode of action of the selected compounds. VEN acts when a steady-state plasma concentration is achieved (3–4 weeks) [32], while OXA acts immediately [16]. Crayfish maintained in aged tap water were used as controls, with the same handling as the exposed groups. The crayfish were held individually in transparent plastic boxes (190 × 140 × 75 mm) with 0.5 L of exposure solution or aged tap water. The numbers of animals that molted, spawned, or died during the exposure period were recorded.
During the exposure period, crayfish were fed ad libitum with fish pellets (Sera Granugreen, Sera, Heinsberg, Germany). Boxes were cleaned during exposure to the solution and the water exchange (every 48 h). The control group was always handled first to avoid its contamination. Crayfish which molted or spawned were discarded from the experiment. Water temperature was measured by an alcohol thermometer (to the nearest 0.1 °C) and did not differ (p > 0.05) between the control and the exposed group in both VEN and OXA studies. Water temperature ranged between 19.3 and 20.6 °C.
The real concentrations of VEN and OXA in the exposure solution, as well as in the control group’s water, was checked using liquid chromatography with a tandem mass spectrometer (LC-MS/MS, Research Institute of Fish Culture and Hydrobiology, Vodňany, Czech Republic) four (VEN) and three times (OXA) during the exposure period. The concentrations of the compounds were analyzed in freshly prepared solutions (at time 0) and after 48 h, when the used solution was exchanged (time 48). Collected samples were filtered (0.20 µm regenerated cellulose, Labicom, Olomouc, Czech Republic) and stored in a freezer at −20 °C until analysis. After thawing and the addition of the internal standards, the samples were measured using the 10 min method on a Hypersil Gold aQ column (50 × 2.1 mm; 5 mm particles) coupled with an Accela 1250 LC pump and a TSQ Quantum Ultra Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The concentrations of the tested compounds in the analyzed water samples from the exposed boxes at time 0 and time 48 did not differ. The concentrations of VEN and OXA in water samples from the control group were below the limit of detection (see Table 2).

Table 2.
The concentration of VEN and OXA in the water at time 0 (control, exposed) and after 48 h of exposure (control, exposed) (α = 0.05). Data are presented as mean ± standard deviation.
2.4. Behavioral Data Acquisition
The exposed crayfish were individually placed in circular plastic tanks (280 mm in diameter), with 2 L of aged tap water and 200 mL of fine sand (<1 mm). In total, 110 and 120 crayfish were used for video-tracking in the VEN and OXA experiments, respectively. Stocked crayfish were video-tracked using a digital video camera (Sony HDR-CX240, Sony, Tokyo, Japan) in trials of 20 parallel tracked tanks (10 control and 10 exposed animals), i.e., 6 trials were done for each compound. Half of the trials were made without shelter, while the other half were conducted with shelter (consisting of halved ceramic plant pots of a 60 mm entry width and a depth of 50 mm). Shelter is an essential resource of crayfish, being nocturnal animals which are usually only active for a period throughout the day, and affecting shelter use can make crayfish more prone to predation or cannibalism. After the video recording, the presence of glair glands (a mark of readiness for reproduction) was also recorded in the used crayfish, due to the possible consequences of upcoming reproduction on their behavior.
The video-recording lasted for 4 h. Light was provided, as permanent indirect illumination, by fluorescent tubes (daylight, 2310 lm). Video-recordings were analyzed later using EthoVision® XT 13.0 software (Noldus Information Technology by Wageningen, The Netherlands) with a multiple-arena module. The distance moved (cm), activity (percentage of time when crayfish locomotion was detected), and velocity (cm s−1) were evaluated. When conditions of shelter were present, the software also revealed results about the time spent inside/outside the shelter.
2.5. Statistical Analysis
A chi-square test was used for numbers of molted and spawned crayfish in comparison with control ones. The Kolmogorov-Smirnov normality test was done for the entire data set. The homogeneity of variances was tested using the Bartlett test for the parameters of behavioral patterns. The concentrations of the tested compound at time 0 and time 48 were compared through paired t-tests. A t-test for independent samples was used to compare the size and weight of the animals used in the exposed and control groups. The distance moved, velocity, activity, and time spent outside the shelter (replicate groups as a random factor, exposure as a fixed factor), were analyzed through factorial ANOVA. The null hypothesis was rejected at α = 0.05. The data were statistically analyzed by Statistica 12.0 software (StatSoft, Tulsa, OK, USA).
3. Results
3.1. Venlafaxine
No significant differences were detected in VEN-exposed crayfish in comparison with control animals in set-ups both with and without available shelter (Table 3). The only effect detected was that of developed glair glands in the set-up with shelter on activity (F1,44 = 6.95, p = 0.012) and time spent outside the shelter (F1,44 = 4.94, p = 0.031). No values are recorded for crayfish without shelter due to the absence of glair glands (Table 4). Data are shown in Table 3 and Table 4.

Table 3.
The values of the distance moved, velocity, activity, and time spent outside the shelter in crayfish exposed to venlafaxine and in control crayfish, in set-ups with and without available shelter. Data are shown as mean ± standard deviation.

Table 4.
The values of the distance moved, velocity, activity, and time spent outside the shelter in crayfish exposed to venlafaxine and in control crayfish in accordance with the presence of glair glands, in set-ups with and without available shelter. Data are shown as mean ± standard deviation.
3.2. Oxazepam
In the set-up without available shelter, crayfish moved longer distances (F1,56 = 4.17, p = 0.046) and showed higher activity (F1,56 = 4.75, p = 0.034) when exposed to OXA, compared to the control group. The effect of glair glands was observed only in the control animals in both the distance moved (F1,56 = 5.73, p = 0.024) and activity (F1,56 = 6.92, p = 0.013). In OXA-exposed animals, this effect was not so obvious and no differences in either the distance moved (F1,56 = 0.20, p = 0.656) or activity (F1,56 = 1.36, p = 0.248) were revealed. Similarly, no significant difference was revealed in the velocity, either between the control and exposed animals or between the animals with and without glair glands (Figure 1 and Figure 2).
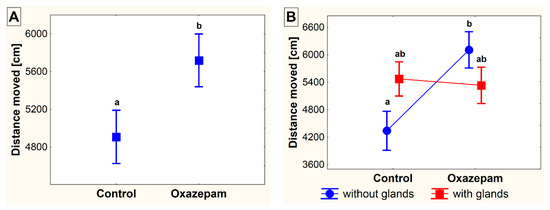
Figure 1.
The distance moved (in cm) of marbled crayfish (Procambarus virginalis, Lyko 2017) exposed to oxazepam (~1 µg L−1) and of the control animals in the conditions without available shelter (A). The differences detected between the groups of crayfish and in accordance with the presence of developed glair glands (B). The different superscripts show significant differences (α = 0.05) among groups. Data are presented as mean ± standard error of mean.
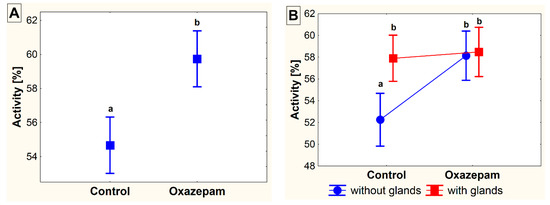
Figure 2.
The activity (in %) of marbled crayfish (Procambarus virginalis, Lyko 2017) exposed to oxazepam (~1 µg L−1) and control crayfish in the conditions without available shelter (A). The differences detected between the groups of crayfish in accordance with the presence of developed glair glands (B). The different superscripts show significant differences (α = 0.05) among groups. Data are presented as mean ± standard error of mean.
In the set-up with available shelter, no differences were detected between the control and OXA-exposed animals. Only the effect of glair glands was detected in all of the parameters observed; the distance moved (F1,56 = 8.74, p = 0.005), velocity (F1,56 = 4.24, p = 0.044), activity (F1,56 = 9.47, p = 0.003), and the time spent outside the shelter (F1,56 = 6.51, p = 0.014).

Table 5.
The values of the distance moved, velocity, activity, and time spent outside the shelter in crayfish exposed to oxazepam and in control crayfish, in set-ups with and without available shelter. Data are shown as mean ± standard deviation.

Table 6.
The values of the distance moved, velocity, activity, and time spent outside the shelter in crayfish exposed to oxazepam and in control crayfish in accordance with the presence of glair glands, in set-ups with and without available shelter. Data are shown as mean ± standard deviation.
3.3. Life History Traits
During the exposure period, the number of molted crayfish did not vary significantly in both VEN (χ2 = 0.08, p = 0.783) and OXA (χ2 = 0.72, p = 0.398) exposed crayfish compared to control ones. However, the number of crayfish which spawned eggs during the exposure period was slightly lower but not statistically significant (χ2 = 2.81, p = 0.094) in the VEN-exposed group compared to the control group. The number of spawned animals in the OXA-exposed group did not differ from the controls (χ2 = 0.15, p = 0.703). There was no reported mortality (Table 7).

Table 7.
The total number of molted, spawned, and dead crayfish in the control and exposed groups during the exposure period to venlafaxine and oxazepam.
4. Discussion
Pharmaceuticals accessing natural ecosystems via sewage water treatment plant effluents [5,33] are reported as drivers of ecological changes [18,34], and psychotropic drugs are often confirmed as inducing behavioral changes in fish and other aquatic invertebrates [13,35]. Knowledge about the behavioral endpoint of these drugs is still too scarce to summarize their ecological consequences [13].
The present study assessed the behavioral effects of two psychoactive compounds, VEN and OXA, on clonal marbled crayfish exposed to dilute concentrations which can be found in natural conditions. The study also follows up on previous studies [13,36] exploring the compound-specific effects on the behavior of a model invertebrate in comparable, defined conditions. The results observed again confirmed that the low concentrations observed in natural conditions can have important consequences. Exploratory behavior, expressed as the activity and distance moved, should affect the wasting of energy, leading to a shorter life span in more active individuals [37]. In addition, the visibility of an animal to predators, especially in conditions of invaded ecosystems, can have effects on food resources, which are under greater pressure due to the higher activity [38,39]. Such alterations can then change the ecosystem’s functioning [40,41]. The elevated activity, followed by altered foraging behavior can, therefore, lead to the breakdown of food chains, loss of biodiversity, and ecosystem instability [41,42].
Our experimental data helped to identify the environmental risks of OXA, but surprisingly no behavioral changes have been observed for the antidepressant VEN. Venlafaxine effects are reported at higher concentrations [43] compared to other antidepressants, citalopram [13] and sertraline [36], which were tested at the same (or even lower concentrations in the case of fluoxetine and sertraline) [44].
Earlier research demonstrated different, or even opposite results among similar compounds or compounds with similar modes of action, as well as among different taxa observed [21,35,45]. This can be due, in part, to different experimental conditions, ways of application (injection, oral application, passively from a water solution), doses (lower than in environmentally detected, environmentally relevant, elevated), and approaches used to determine the behavioral effects. We, therefore, tried to minimize the variables and use similar, relatively easy approaches for the observation, an application imitating the environmental intoxication from the dilute solutions, and the use of genetically uniform marbled crayfish, to erase the effect of different genotypes. However, the results presented, compared with previous ones produced with the same methodology, reveal again the differences among individual compounds. In fact, there is a need to investigate the mechanisms of action of these substances in detail and to elaborate on studies dealing with different mixtures of pharmaceuticals as they act simultaneously on aquatic biota [46].
The present study can be helpful for the set-up of new studies aimed not only at behavioral patterns but at life history traits, including reproduction. Behavioral alterations provide the potential to assess the risk of ecological effects of pharmaceutical products, thus it might be useful for the generalization of the impacts on the aquatic environment. In our present study, we also observed changed behavior in crayfish with developed glair glands, indicative of the preparation for reproduction [37,47]. To safeguard their future offspring, crayfish limit their activity, which was expressed as a lower distance moved and less activity. In the exposed animals, this pattern was missing, which can affect the reproductive success in a population due to a higher risk of predation. Life history traits and affected reproduction have also been confirmed from other studies with several psychoactive drugs on different aquatic taxa [36,43,48].
To summarize, our results together with those previously published show high variability in the type, strength, and direction of the effects of pharmaceuticals on aquatic biota. The use of passive application due to exposure through diluted, environmentally relevant concentrations of tested compounds seems to be appropriate for the assessment of their environmental effects. The main pollutants should also be tested individually, as well as their mixtures as found in field sampling [46]. The large number of compounds in surface waters and their different/specific modes of action is motivation for the better understanding of their real ecological impact on ecosystems.
Author Contributions
Conceptualization, T.R., R.G. and M.B.; formal analysis, J.K.; funding acquisition, T.R.; investigation, J.K., M.S.H., A.K., S.R. and M.B.; methodology, K.G., T.R., A.K. and M.B.; project administration, T.R.; resources, K.G., T.R. and R.G.; supervision, T.R., R.G. and M.B.; validation, K.G., R.G. and M.B.; visualization, J.K. and M.S.H.; writing—original draft, J.K., M.S.H., A.K. and M.B.; writing—review and editing, K.G., A.K. and M.B.
Funding
The study was financially supported by the Czech Science Foundation (project No. 16-06498S), the Ministry of Education, Youth and Sports of the Czech Republic—projects CENAKVA (No. CZ.1.05/2.1.00/01.0024), CENAKVA II (No. LO1205 under the NPU I program), CENAKVA Center Development (No. CZ.1.05/2.1.00/19.0380) and by the Grant Agency of University of South Bohemia No. 012/2016/Z.
Acknowledgments
We would like to thank our colleagues Wei Guo and Filip Ložek who helped us during experimental work. We also deeply appreciate the help of Julian D. Reynolds, not only in language corrections of the manuscript but for friendly mentoring too.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Ethical code
No specific permissions were required for the locations and activities involved in this study. All experiments were conducted according to the principles of the Ethics Committee for the Protection of Animals in Research of the University of South Bohemia in České Budějovice, FFPW, Vodňany, based on the EU-harmonized Animal Welfare Act of the Czech Republic, No. 53100/2013-MZE-17214.
References
- Burkina, V.; Zlabek, V.; Zamaratskaia, G. Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish. Environ. Toxicol. Pharmacol. 2015, 40, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Collado, N.; Rodriguez-Mozaz, S.; Gros, M.; Rubirola, A.; Barcelo, D.; Comas, J.; Rodriguez-Roda, I.; Buttiglieri, G. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014, 185, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Frederic, O.; Yves, P. Pharmaceuticals in hospital wastewater: Their ecotoxicity and contribution to the environmental hazard of the effluent. Chemosphere 2014, 115, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Removal and seasonal variability of selected analgesics/anti-inflammatory, anti-hypertensive/cardiovascular pharmaceuticals and UV filters in wastewater treatment plant. Environ. Sci. Pollut. Res. 2014, 21, 7578–7585. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Rodriguez-Mozaz, S.; Barcelo, D. Pharmaceuticals in biota in the aquatic environment: Analytical methods and environmental implications. Anal. Bioanal. Chem. 2012, 404, 2611–2624. [Google Scholar] [CrossRef]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care 2012, 16, R136. [Google Scholar] [CrossRef]
- Fedorova, G.; Golovko, O.; Randak, T.; Grabic, R. Storage effect on the analysis of pharmaceuticals and personal care products in wastewater. Chemosphere 2014, 111, 55–60. [Google Scholar] [CrossRef]
- Yadav, M.K.; Short, M.D.; Aryal, R.; Gerber, C.; van den Akker, B.; Saint, C.P. Occurrence of illicit drugs in water and wastewater and their removal during wastewater treatment. Water Res. 2017, 124, 713–727. [Google Scholar] [CrossRef]
- Marti, E.; Huerta, B.; Rodriguez-Mozaz, S.; Barcelo, D.; Marce, R.; Balcazar, J.L. Abundance of antibiotic resistance genes and bacterial community composition in wild freshwater fish species. Chemosphere 2018, 196, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, A.; Giebultowicz, J.; Stankiewicz, U.; Wroczynski, P.; Nalecz-Jawecki, G. Determination of selected cardiovascular active compounds in environmental aquatic samples—Methods and results, a review of global publications from the last 10 years. Chemosphere 2015, 138, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Buric, M.; Grabicova, K.; Kubec, J.; Kouba, A.; Kuklina, I.; Kozak, P.; Grabic, R.; Randak, T. Environmentally relevant concentrations of tramadol and citalopram alter behaviour of an aquatic invertebrate. Aquat. Toxicol. 2018, 200, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.P.; Ford, A.T. The biological effects of antidepressants on the molluscs and crustaceans: A review. Aquat. Toxicol. 2014, 151, 4–13. [Google Scholar] [CrossRef]
- Hyttel, J. Pharmacological Characterization of Selective Serotonin Reuptake Inhibitors (SSRIs). Int. Clin. Psychopharm. 1994, 9, 19–26. [Google Scholar] [CrossRef]
- Skerritt, J.H.; Johnston, G.A. Enhancement of GABA binding by benzodiazepines and related anxiolytics. Eur. J. Pharmacol. 1983, 89, 193–198. [Google Scholar] [CrossRef]
- Rosi-Marshall, E.J.; Snow, D.; Bartelt-Hunt, S.L.; Paspalof, A.; Tank, J.L. A review of ecological effects and environmental fate of illicit drugs in aquatic ecosystems. J. Hazard. Mater. 2015, 282, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Blaha, M.; Kumar, V.; Cerveny, D.; Fedorova, G.; Randak, T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015, 72, 145–153. [Google Scholar] [CrossRef]
- Klaminder, J.; Brodin, T.; Sundelin, A.; Anderson, N.J.; Fahlman, J.; Jonsson, M.; Fick, J. Long-Term Persistence of an Anxiolytic Drug (Oxazepam) in a Large Freshwater Lake. Environ. Sci. Technol. 2015, 49, 10406–10412. [Google Scholar] [CrossRef]
- Lagesson, A.; Fahlman, J.; Brodin, T.; Fick, J.; Jonsson, M.; Bystrom, P.; Klaminder, J. Bioaccumulation of five pharmaceuticals at multiple trophic levels in an aquatic food web—Insights from a field experiment. Sci. Total. Environ. 2016, 568, 208–215. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems-impacts through behavioural alterations. Philos. Trans. R. Soc. B 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Nordling, J.; Lagesson, A.; Klaminder, J.; Hellstrom, G.; Christensen, B.; Fick, J. Environmental relevant levels of a benzodiazepine (oxazepam) alters important behavioral traits in a common planktivorous fish, (Rutilus rutilus). J. Toxicol. Environ. Health A 2017, 80, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Megharaj, M.; Kirkbride, K.P.; Naidu, R. Illicit drugs and the environment—A review. Sci. Total Environ. 2013, 463, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.V.; Kellner, M.; Henriksen, P.G.; Olsen, H.; Hansen, S.H.; Baatrup, E. The psychoactive drug Escitalopram affects swimming behaviour and increases boldness in zebrafish (Danio rerio). Ecotoxicology 2018, 27, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Patoka, J.; Kouba, A.; Buric, M. Clonal crayfish as biological model: A review on marbled crayfish. Biologia 2018, 73, 841–855. [Google Scholar] [CrossRef]
- Kubec, J.; Kouba, A.; Buřič, M. Communication, behaviour, and decision making in crayfish: A review. Zool. Anz. 2018, 278, 28–37. [Google Scholar] [CrossRef]
- Barry, M.J. Effects of fluoxetine on the swimming and behavioural responses of the Arabian killifish. Ecotoxicology 2013, 22, 425–432. [Google Scholar] [CrossRef]
- Jonsson, M.; Fick, J.; Klaminder, J.; Brodin, T. Antihistamines and aquatic insects: Bioconcentration and impacts on behavior in damselfly larvae (Zygoptera). Sci. Total Environ. 2014, 472, 108–111. [Google Scholar] [CrossRef]
- Cunha, D.L.; Mendes, M.P.; Marques, M. Environmental risk assessment of psychoactive drugs in the aquatic environment. Environ. Sci. Pollut. Res. 2019, 26, 78–90. [Google Scholar] [CrossRef]
- Grabic, R.; Fick, J.; Lindberg, R.H.; Fedorova, G.; Tysklind, M. Multi-residue method for trace level determination of pharmaceuticals in environmental samples using liquid chromatography coupled to triple quadrupole mass spectrometry. Talanta 2012, 100, 183–195. [Google Scholar] [CrossRef]
- Sehonova, P.; Svobodova, Z.; Dolezelova, P.; Vosmerova, P.; Faggio, C. Effects of waterborne antidepressants on non-target animals living in the aquatic environment: A review. Sci. Total Environ. 2018, 631–632, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Courtney, D.B. Selective serotonin reuptake inhibitor and venlafaxine use in children and adolescents with major depressive disorder: A Systematic Review of Published Randomized Controlled Trials. Can. J. Psychiatry 2004, 49, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; Antonio, D.C.; Cornero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of psychoactive pharmaceuticals in fish in an effluent dominated stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Kubec, J.; Grabicova, K.; Grabic, R.; Randak, T.; Guo, W.; Kouba, A.; Buřič, M. Environmentally relevant concentrations of methamphetamine and sertraline modify the behavior and life history traits of an aquatic invertebrate. Sci. Total Environ. 2019. under review. [Google Scholar]
- Gherardi, F. Behaviour. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 258–290. [Google Scholar]
- Holdich, D.M. Background and functional morphology. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 3–29. [Google Scholar]
- Craddock, N.; Jones, I. Genetics of bipolar disorder. J. Med. Genet. 1999, 36, 585–594. [Google Scholar] [CrossRef]
- Manning, A.; Dawkins, M.S. An Introduction to Animal Behaviour, 6th ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; p. ix. 458p. [Google Scholar]
- Schmitz, O.J. Predator diversity and trophic interactions. Ecology 2007, 88, 2415–2426. [Google Scholar] [CrossRef]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thebault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef]
- Fong, P.P.; Bury, T.B.; Dworkin-Brodsky, A.D.; Jasion, C.M.; Kell, R.C. The antidepressants venlafaxine (“Effexor”) and fluoxetine (“Prozac”) produce different effects on locomotion in two species of marine snail, the oyster drill (Urosalpinx cinerea) and the starsnail (Lithopoma americanum). Mar. Environ. Res. 2015, 103, 89–94. [Google Scholar] [CrossRef]
- Bossus, M.C.; Guler, Y.Z.; Short, S.J.; Morrison, E.R.; Ford, A.T. Behavioural and transcriptional changes in the amphipod Echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquat. Toxicol. 2014, 151, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Imeh-Nathaniel, A.; Rincon, N.; Orfanakos, V.B.; Brechtel, L.; Wormack, L.; Richardson, E.; Huber, R.; Nathaniel, T.I. Effects of chronic cocaine, morphine and methamphetamine on the mobility, immobility and stereotyped behaviors in crayfish. Behav. Brain Res. 2017, 332, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, T.; Castaño-Sánchez, A.; Di Marzio, W.D.; García-Doncel, P.; Martínez, L.N.; Galassi, D.M.P.; Iepure, S. The role of freshwater copepods in the environmental risk assessment of caffeine and propranolol mixtures in the surface water bodies of Spain. Chemosphere 2019, 220, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.; Holdich, D. Growth and reproduction. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 152–191. [Google Scholar]
- Carfagno, G.L.F.; Fong, P.P. Growth Inhibition of Tadpoles Exposed to Sertraline in the Presence of Conspecifics. J. Herpetol. 2014, 48, 571–576. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).