Analysis of Volatile Organic Compounds from Compost
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Compost Setup
2.3. Sampling Design
2.4. Analytical Protocols
2.5. Calibration
2.6. Data Processing
2.7. Sampling Method Validation and Recovery
3. Results and Discussion
3.1. Sampling Method Validation
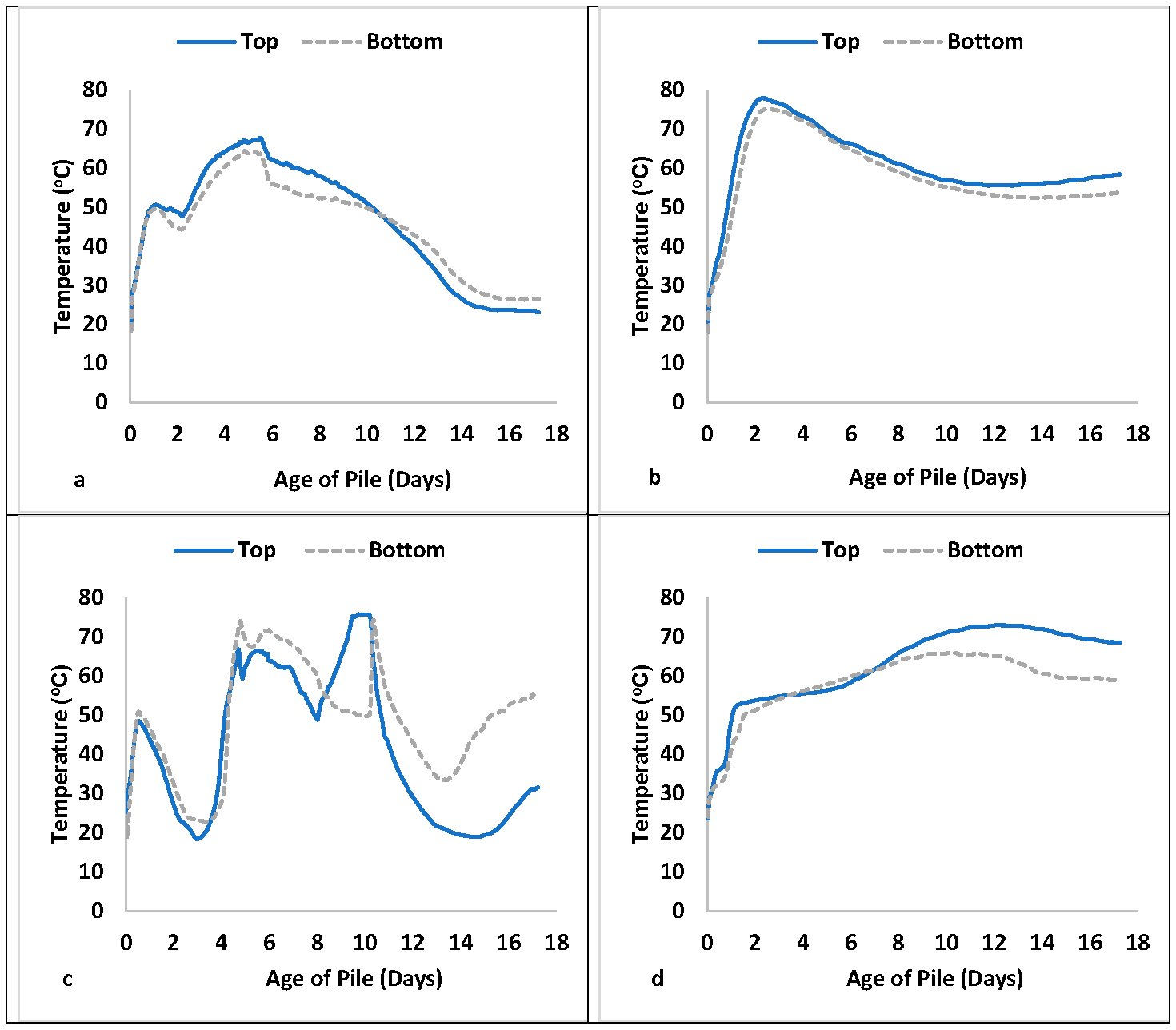
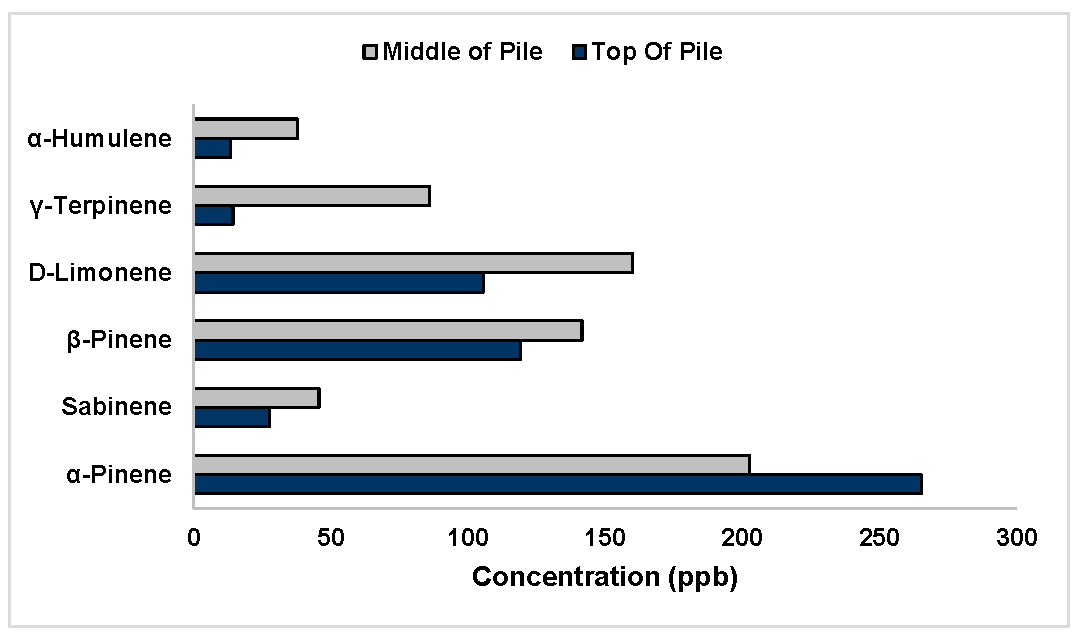
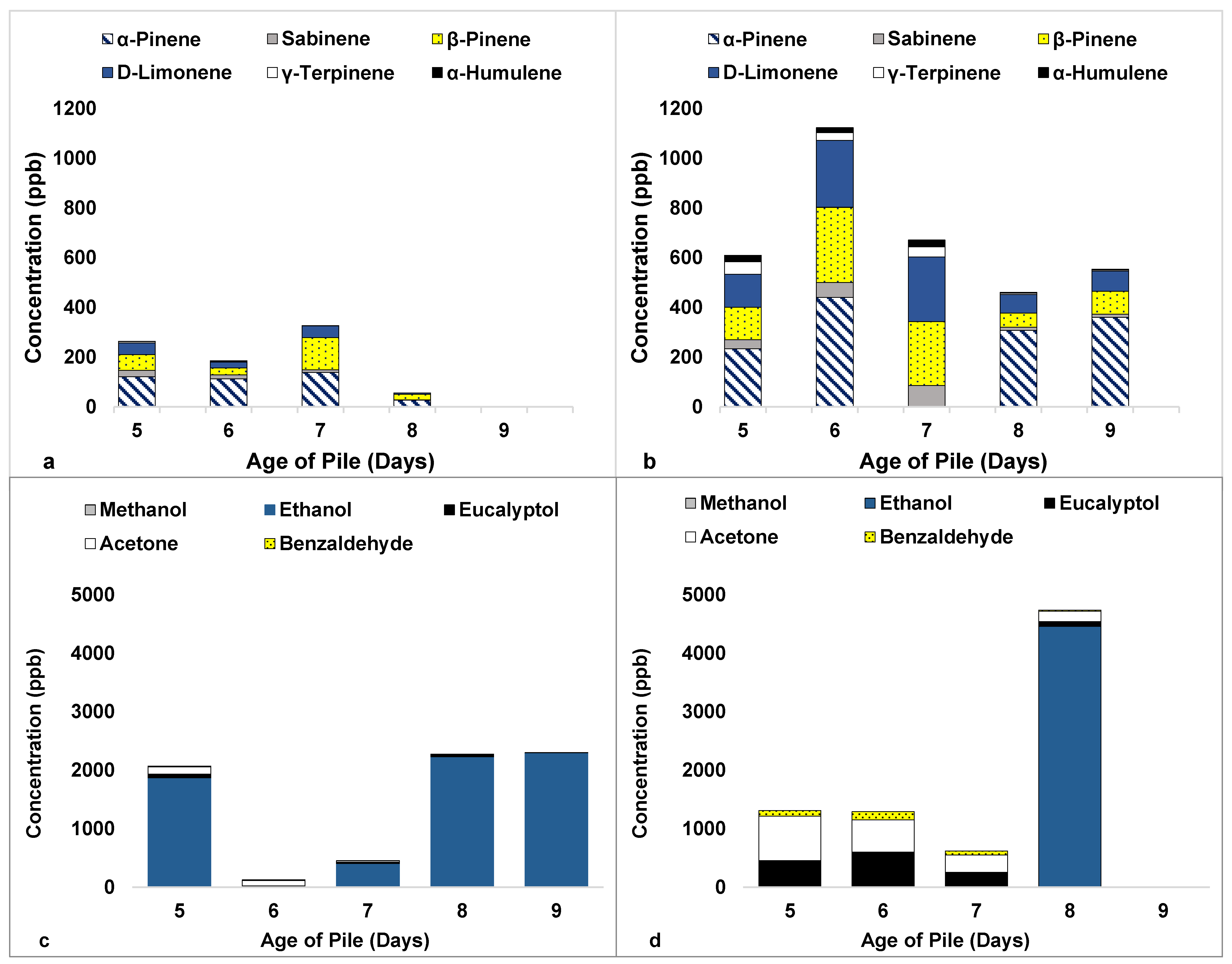
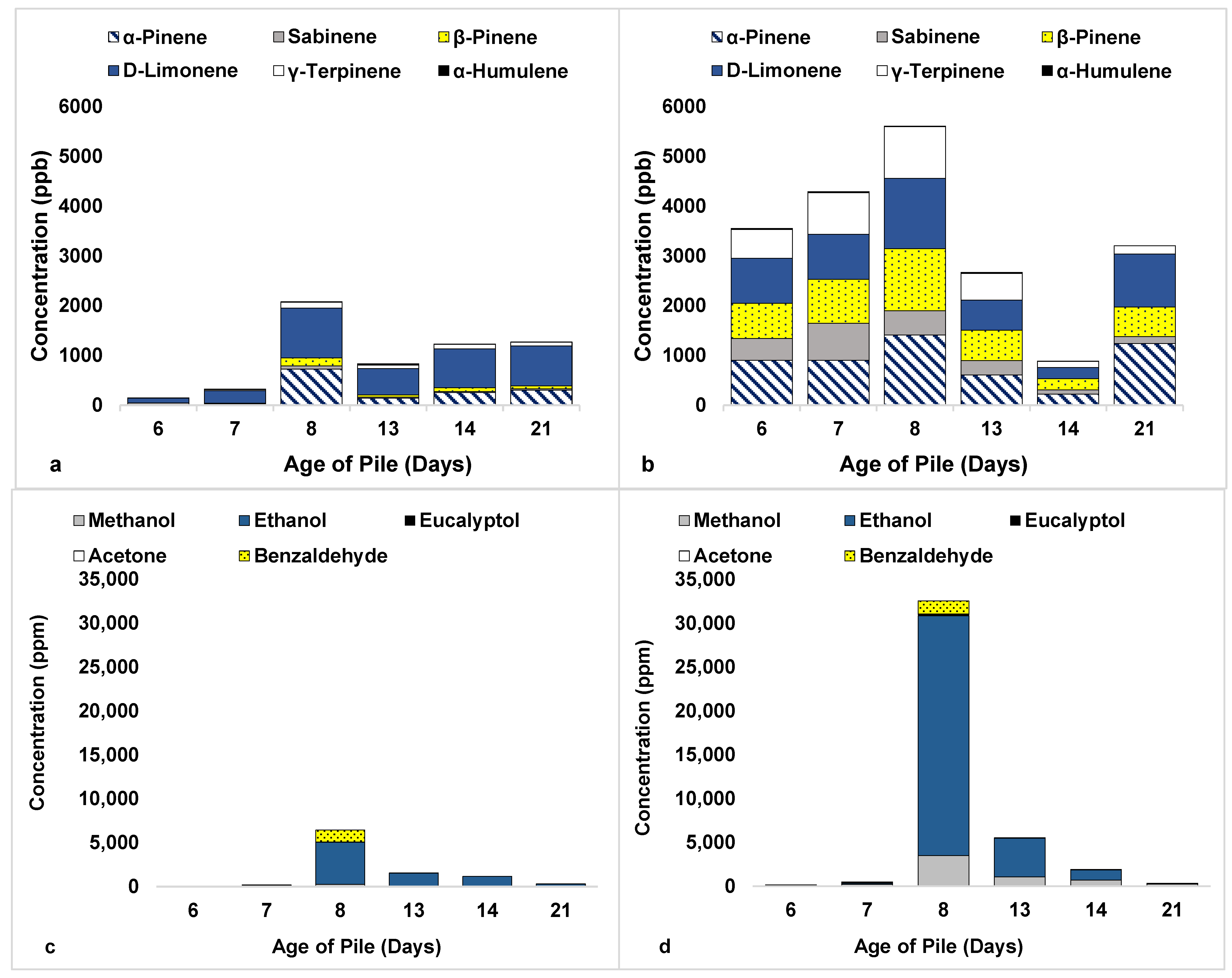
3.2. Compost Experiments
3.3. Comparison to Other Studies
3.4. Advantages and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOC | Volatile organic compound |
| TD-GC-MS | Thermal desorption–gas chromatography–mass spectrometry |
| HS-GCFID | Headspace gas chromatography–flame ionization detection |
| US EPA | United States Environmental Protection Agency |
| SCAQMD | South Coast Air Quality Management District |
| YW | Yard waste |
| FY | Combined food-yard waste |
| EPA | Environmental Protection Agency |
| OSHA | Occupational Safety and Health Administration |
| PEL | Permissible exposure limit |
| TWA | Time-weighted average |
| WSU CF | Washington State University Compost Facility |
| ppm | Parts per million |
| ppb | Parts per billion |
References
- Ho, T.T.K.; Tra, V.T.; Le, T.H.; Nguyen, N.K.Q.; Tran, C.S.; Nguyen, P.T.; Vo, T.D.H.; Thai, V.N.; Bui, X.T. Compost to improve sustainable soil cultivation and crop productivity. Case Stud. Chem. Environ. Eng. 2022, 6, 100211. [Google Scholar] [CrossRef]
- Stentiford, E.I. Composting Control: Principles and Practice. In The Science of Composting; Springer: Dordrecht, The Netherlands, 1996; pp. 49–59. [Google Scholar] [CrossRef]
- Abduli, M.A.; Naghib, A.; Yonesi, M.; Akbari, A. Life cycle assessment (LCA) of solid waste management strategies in Tehran: Landfill and composting plus landfill. Environ. Monit. Assess. 2011, 178, 487–498. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas emissions from organic waste composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef]
- Anglou, F.Z.; Sanders, R.E.; Stamatopoulos, I. Of the first five US states with food waste bans, Massachusetts alone has reduced landfill waste. Science 2024, 385, 1236–1240. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335. [Google Scholar] [CrossRef]
- Limbri, H.; Gunawan, C.; Thomas, T.; Smith, A.; Scott, J.; Rosche, B. Coal-packed methane biofilter for mitigation of green house gas emissions from coal mine ventilation air. PLoS ONE 2014, 9, e94641. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wu, Z.; Zhang, M.; Li, X.; Sun, Y.; Wu, R.; Zheng, Y.; Xia, Y. Microorganisms as bio-filters to mitigate greenhouse gas emissions from high-altitude permafrost revealed by nanopore-based metagenomics. IMeta 2022, 1, e24. [Google Scholar] [CrossRef]
- Kumar, A.; Alaimo, C.P.; Horowitz, R.; Mitloehner, F.M.; Kleeman, M.J.; Green, P.G. Volatile organic compound emissions from green waste composting: Characterization and ozone formation. Atmos. Environ. 2011, 45, 1841–1848. [Google Scholar] [CrossRef]
- Lou, X.F.; Nair, J. The impact of landfilling and composting on greenhouse gas emissions—A review. Bioresour. Technol. 2009, 100, 3792–3798. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, J.; Shao, Z.; Chen, T. Emission characteristics and health risk assessment of VOCs from a food waste anaerobic digestion plant: A case study of Suzhou, China. Environ. Pollut. 2020, 257, 113546. [Google Scholar] [CrossRef]
- Nair, A.T.; Senthilnathan, J.; Nagendra, S.M.S. Emerging perspectives on VOC emissions from landfill sites: Impact on tropospheric chemistry and local air quality. Process Saf. Environ. Prot. 2019, 121, 143–154. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Samuelsson, J.; Christensen, T.H.; Scheutz, C. Quantification of Greenhouse Gas Emissions from Windrow Composting of Garden Waste. J. Environ. Qual. 2010, 39, 713–724. [Google Scholar] [CrossRef]
- Pagans, E.; Font, X.; Sánchez, A. Emission of volatile organic compounds from composting of different solid wastes: Abatement by biofiltration. J. Hazard. Mater. 2006, 131, 179–186. [Google Scholar] [CrossRef]
- Van Durme, G.P.; McNamara, B.F.; McGinley, C.M. Bench-Scale Removal of Odor and Volatile Organic Compounds at a Composting Facility. Water Environ. Res. 1992, 64, 19–27. Available online: https://www-jstor-org.uidaho.idm.oclc.org/stable/25044110?sid=primo (accessed on 2 February 2025). [CrossRef]
- Müller, T.; Thißen, R.; Braun, S.; Dott, W.; Fischer, G. (M)VOC and composting facilities: Part 1: (M)VOC emissions from municipal biowaste and plant refuse. Environ. Sci. Pollut. Res. 2004, 11, 91–97. [Google Scholar] [CrossRef]
- Schiavon, M.; Martini, L.M.; Corrà, C.; Scapinello, M.; Coller, G.; Tosi, P.; Ragazzi, M. Characterization of volatile organic compounds (VOCs) released by the composting of different waste matrices. Environ. Pollut. 2017, 231, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N.; Koziel, J.A.; Ahn, H.K.; Glanville, T.D.; Crawford, B.P.; Raman, D.R. Laboratory scale evaluation of volatile organic compound emissions as indication of swine carcass degradation inside biosecure composting units. Bioresour. Technol. 2010, 101, 71–78. [Google Scholar] [CrossRef] [PubMed]
- US EPA. (n.d.). Initial List of Hazardous Air Pollutants with Modifications|US EPA. Available online: https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications (accessed on 7 May 2025).
- Srinivasan, S.; Divahar, R. Nature Environment and Pollution Technology An International Quarterly Scientific Journal Odor Emissions from Municipal Solid Waste Open Dumps Constituting Health Problems Due to their Composition, Ecological Impacts and Potential Health Risks. Nat. Environ. Pollut. Technol. 2025, 24, 1–10. [Google Scholar] [CrossRef]
- US OSHA. (n.d.). TABLE Z-1—TABLE Z-1 Limits for Air Contaminants|Occupational Safety and Health Administration. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1000TABLEZ1 (accessed on 7 May 2025).
- Nordahl, S.L.; Preble, C.v.; Kirchstetter, T.W.; Scown, C.D. Greenhouse Gas and Air Pollutant Emissions from Composting. Environ. Sci. Technol. 2023, 57, 2235–2247. [Google Scholar] [CrossRef]
- Ham, R.K.; Komilis, D. A Laboratory Study to Investigate Gaseous Emissions and Solids Decomposition During Composting of Municipal Solid Wastes; US EPA Report; United States Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 2003.
- Bhattacharya, S.S.; Kim, K.H.; Ullah, M.A.; Goswami, L.; Sahariah, B.; Bhattacharyya, P.; Cho, S.B.; Hwang, O.H. The effects of composting approaches on the emissions of anthropogenic volatile organic compounds: A comparison between vermicomposting and general aerobic composting. Environ. Pollut. 2016, 208, 600–607. [Google Scholar] [CrossRef]
- Büyüksönmez, F. Full-Scale VOC Emissions from Green And Food Waste Windrow Composting [Article]. Compost Sci. Util. 2012, 20, 57–62. [Google Scholar] [CrossRef]
- Büyüksönmez, F.; Evans, J. Biogenic emissions from green waste and comparison to the emissions resulting from composting. Part II. Volatile organic compounds (VOCs) [Article]. Compost Sci. Util. 2007, 15, 191–199. [Google Scholar] [CrossRef]
- South Coast Air Quality Management District. Method 25.3 Determination of Low Concentration Non-Methane Non-Ethane Organic Compound Emissions from Clean Fueled Combustion Sources Monitoring and Engineering Branch Monitoring and Analysis. 2000. Available online: http://www.aqmd.gov/docs/default-source/laboratory-procedures/methods-procedures/stm-025-3.pdf (accessed on 30 November 2024).
- Johnston, N. LCSC VOC Compost Dataset 2022; V2; Mendeley Data: London, UK, 2025. [Google Scholar]
- US EPA. Compendium Method TO-17 Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling Onto Sorbent Tubes. 1999. Available online: https://www.epa.gov/sites/default/files/2019-11/documents/to-17r.pdf (accessed on 15 March 2022).
- Scott, P.S.; Andrew, J.P.; Bundy, B.A.; Grimm, B.K.; Hamann, M.A.; Ketcherside, D.T.; Li, J.; Manangquil, M.Y.; Nuñez, L.A.; Pittman, D.L.; et al. Observations of volatile organic and sulfur compounds in ambient air and health risk assessment near a paper mill in rural Idaho, U.S.A. Atmos. Pollut. Res. 2020, 11, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.N.; Miller, D.D.; Bajracharya, A.; Bruchard, W.; Durbin, T.A.; McGarry, J.K.P.; Moser, E.P.; Nuñez, L.A.; Pukkila, E.J.; Scott, P.S.; et al. Health Risk Implications of Volatile Organic Compounds in Wildfire Smoke During the 2019 FIREX-AQ Campaign and Beyond. GeoHealth 2022, 6, e2021GH000546. [Google Scholar] [CrossRef]
- González, D.; Guerra, N.; Colón, J.; Gabriel, D.; Ponsá, S.; Sánchez, A. Characterization of the Gaseous and Odour Emissions from the Composting of Conventional Sewage Sludge. Atmosphere 2020, 11, 211. [Google Scholar] [CrossRef]
- Biasioli, F.; Aprea, E.; Gasperi, F.; Mä, T.D.; Biasioli, A.F. Measuring odour emission and biofilter efficiency in composting plants by proton transfer reaction-mass spectrometry. Water Sci. Technol. 2009, 59, 1263–1269. [Google Scholar] [CrossRef]


| Experiment | Pile Build Date/Time (PDT) | Feed Stock/Composition | Pile 1 Temperature Profile | Pile 2 Temperature Profile | Pile 1 Aeration | Pile 2 Aeration | Pile Cover |
|---|---|---|---|---|---|---|---|
| A | 13 July 2022 12:00 | Yard Waste (1) | Low | High | Negative | Negative | No |
| B | 26 August 2022 15:00 | Mixed Food/Yard Waste (2,3) | Low | High | Negative | Negative | Yes, screened fines from A piles |
| Target Compounds | Water-Insoluble VOCs (Hydrocarbons, Terpenes) | Water-Soluble VOCs (Alcohols, Aldehydes, Ketones) |
|---|---|---|
| Sample Type | Dual Sorbent Tube (gas phase) | Impinger (aqueous) |
| Instrument | Markes International Unity 2 Thermal Desorption Unit (TD)/Agilent 7820 Gas Chromatograph/5977 Mass Spectrometer (GCMS) | Agilent 7697 HeadSpace/7890 Gas Chromatograph (GC-FID) |
| Carrier Gas | Ultra-high purity helium, 1.5 mL/min | Ultra-high purity helium, 1 mL/min |
| Split Ratio | Varied (split-less to 1:89) | Varied 1:2–1:100 |
| Column | Agilent DB-624, 60 m, 0.32 mm ID, 1.8 µm thickness | J&W DB-624, 30 m, 0.32 mm ID, 1.8 µm thickness |
| Temperature (°C) Programming | TD Pre-purge 2 min, Cold trap −5 °C Tube desorption: 50 °C for 5 min then 200 °C for 10 min Cold trap desorption 250 °C for 10 min GC 40 °C hold 2 min Ramp1—40–195 °C Ramp2—195–250 °C, hold 2 min MS 230 °C source 150 °C quadruple | HS 70 °C 80 °C 200 °C transfer line GC 40 °C to 195 °C Ramp—10 °C/min FID 250 °C |
| Detector | Quadruple Electron Ionization Mass Spectrometer 45–300 amu/z Voltage = 1801 volts | Flame Ionization Detector with ultra-high purity hydrogen/zero air fuel |
| Compound | HS-GC-FID Concentration (ppb) | TD-GC-MS Concentration (ppb) | Total Concentration (ppb) | Percent Recovery (%) Impinger | Mean Percent Recovery (%) Impinger |
|---|---|---|---|---|---|
| Ethanol | 548,080 | 2155 | 550,235 | 99 | 99.0 |
| Guaiacol | 66,950 | 0 | 66,950 | 100 | 100.0 |
| Eucalyptol | 50,482 | 19 | 50,501 | 99 | 99.9 |
| 1-Butanol | 549 | 0 | 549 | 100 | 100.0 |
| Isopropanol | 35,052 | 130 | 35,182 | 99 | 99.5 |
| D-limonene | 1413 | 89,863 | 91,276 | 1.5 | 48.3 |
| alpha-Pinene | 208 | 1413 | 1621 | 12.8 | 7.7 |
| beta-Pinene | 46 | 413 | 459 | 10.0 | 31.6 |
| Study | Compost Type | Sampling/Analytical Technique | Gases Measured | Observations (Maximum or Ranges) |
|---|---|---|---|---|
| Current study | Yard and food/yard waste | Impinger water trap/HS-GC-FID, Tenax sorbent/TD-GC-MS | Methanol Ethanol Alpha-pinene Beta-pinene D-limonene | 3800 ppm 27,400 ppm 1638 ppb 1637 ppb 1750 ppb |
| Büyüksönmez and Evans [26] | Green waste | Coconut shell sorbent/GC-MS | Alpha-pinene Beta-pinene D-limonene | 10–153 mg/kg 1–49 mg/kg 0.1–58 mg/kg |
| Büyüksönmez [25] | Green and food waste | SCAQMD method 25.3/Tenax sorbent/GC-FID | Total non-methane/ethane VOC | 2–40 ppm |
| Kumar et al. [9] | Green waste | Water trap, canisters, GC-MS | Speciated VOCs, alcohols, terpenes, acids | 2.6–13 mg/m2/min |
| Gonzalez et al. [32] | Benchtop sewage-sludge | Air bags/sorbent tubes/TD-GC-MS | Alpha-pinene Beta-pinene D-limonene Eucalyptol | 13,299 ppb 6390 ppb 5490 ppb 13,605 ppb |
| Biasioli et al. [33] | Compost piles with and without biofilter | Air bags, proton transfer reaction–mass spectrometry | Dimethyl sulfide methanethiol acetic acid acetaldehyde | 20 ppb 10 ppb 100 ppb 200 ppb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, S.K.; Morales, R.C.; McCown, K.A.; Wilson, K.C.; Jobson, B.T.; Johnston, N.A.C. Analysis of Volatile Organic Compounds from Compost. Atmosphere 2025, 16, 591. https://doi.org/10.3390/atmos16050591
Berger SK, Morales RC, McCown KA, Wilson KC, Jobson BT, Johnston NAC. Analysis of Volatile Organic Compounds from Compost. Atmosphere. 2025; 16(5):591. https://doi.org/10.3390/atmos16050591
Chicago/Turabian StyleBerger, Shastine K., Rosario C. Morales, Katherine A. McCown, Kylie C. Wilson, Bertram T. Jobson, and Nancy A. C. Johnston. 2025. "Analysis of Volatile Organic Compounds from Compost" Atmosphere 16, no. 5: 591. https://doi.org/10.3390/atmos16050591
APA StyleBerger, S. K., Morales, R. C., McCown, K. A., Wilson, K. C., Jobson, B. T., & Johnston, N. A. C. (2025). Analysis of Volatile Organic Compounds from Compost. Atmosphere, 16(5), 591. https://doi.org/10.3390/atmos16050591






