A Compact NO2 Gas Sensor with an Optimized Photoacoustic Cell and 450 nm-LD
Abstract
1. Introduction
2. Principle
2.1. Gas Absorption Line Selection
2.2. The Generation of Photoacoustic Signal
3. Experiment Configuration
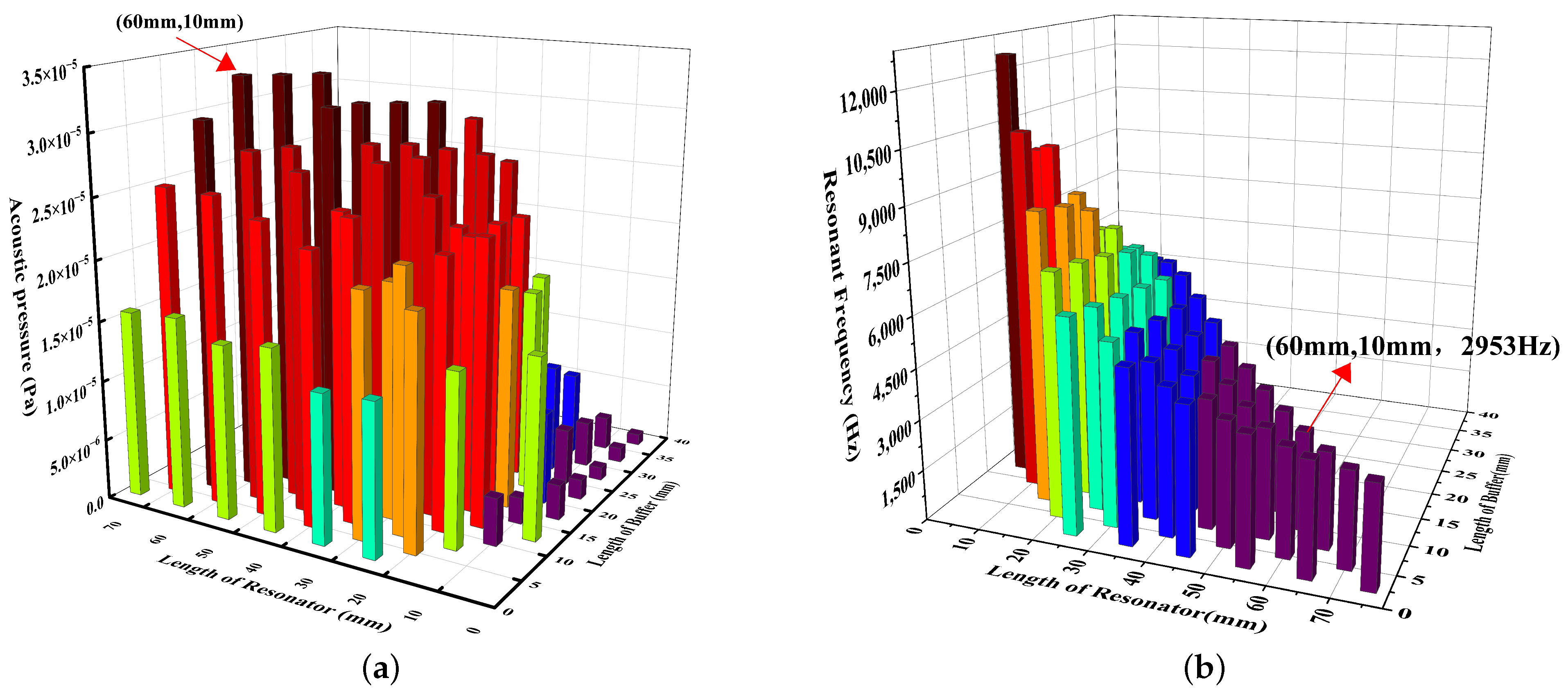
3.1. Photoacoustic Cell Design
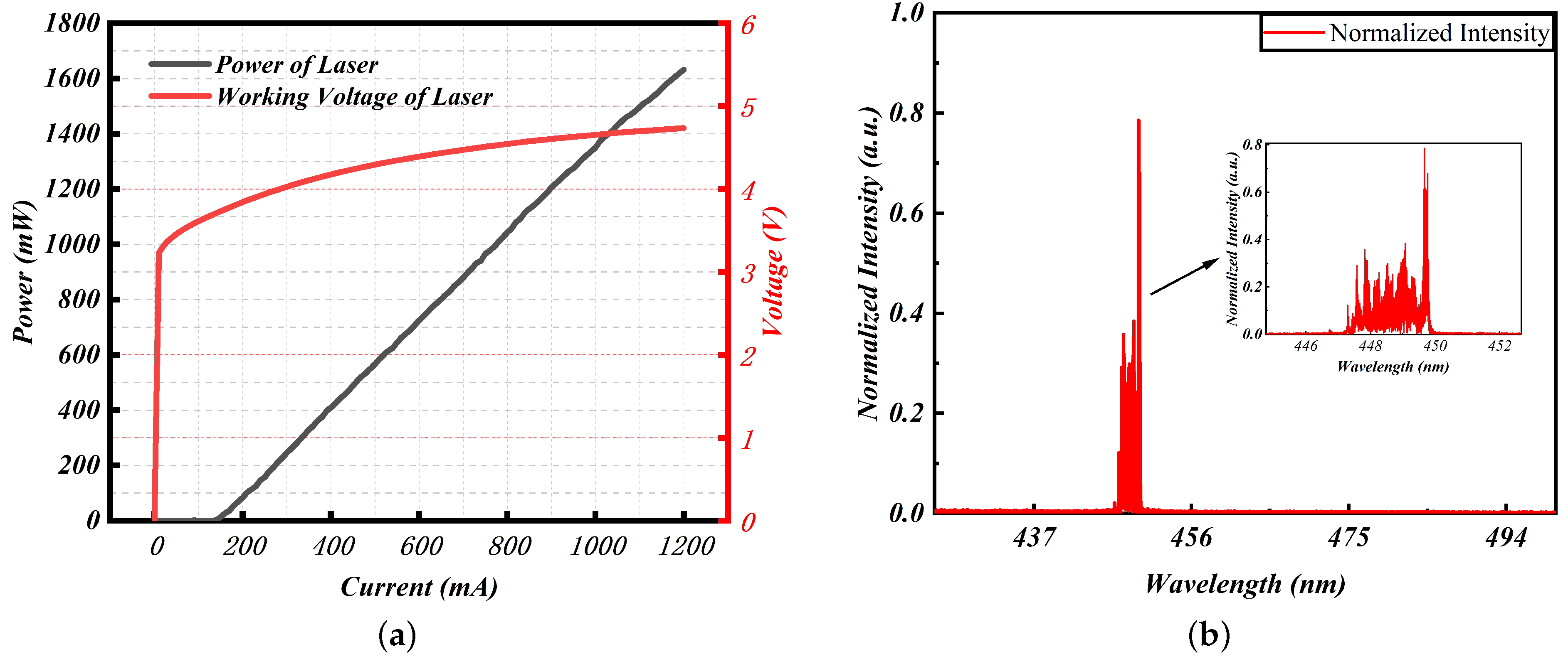
3.2. Excitation Light Source Selection
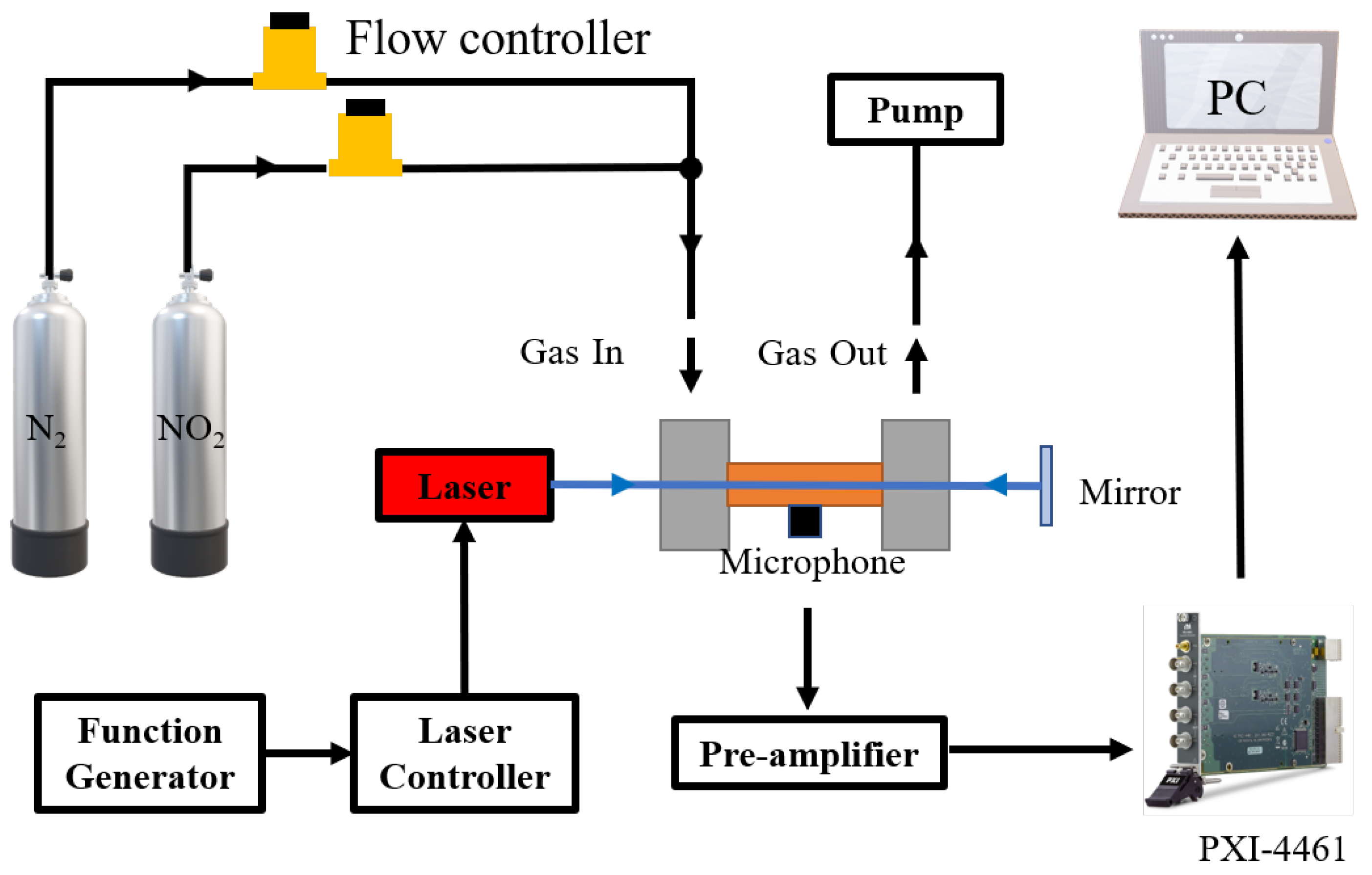
3.3. Experimental Set-Up
4. Results and Discussion
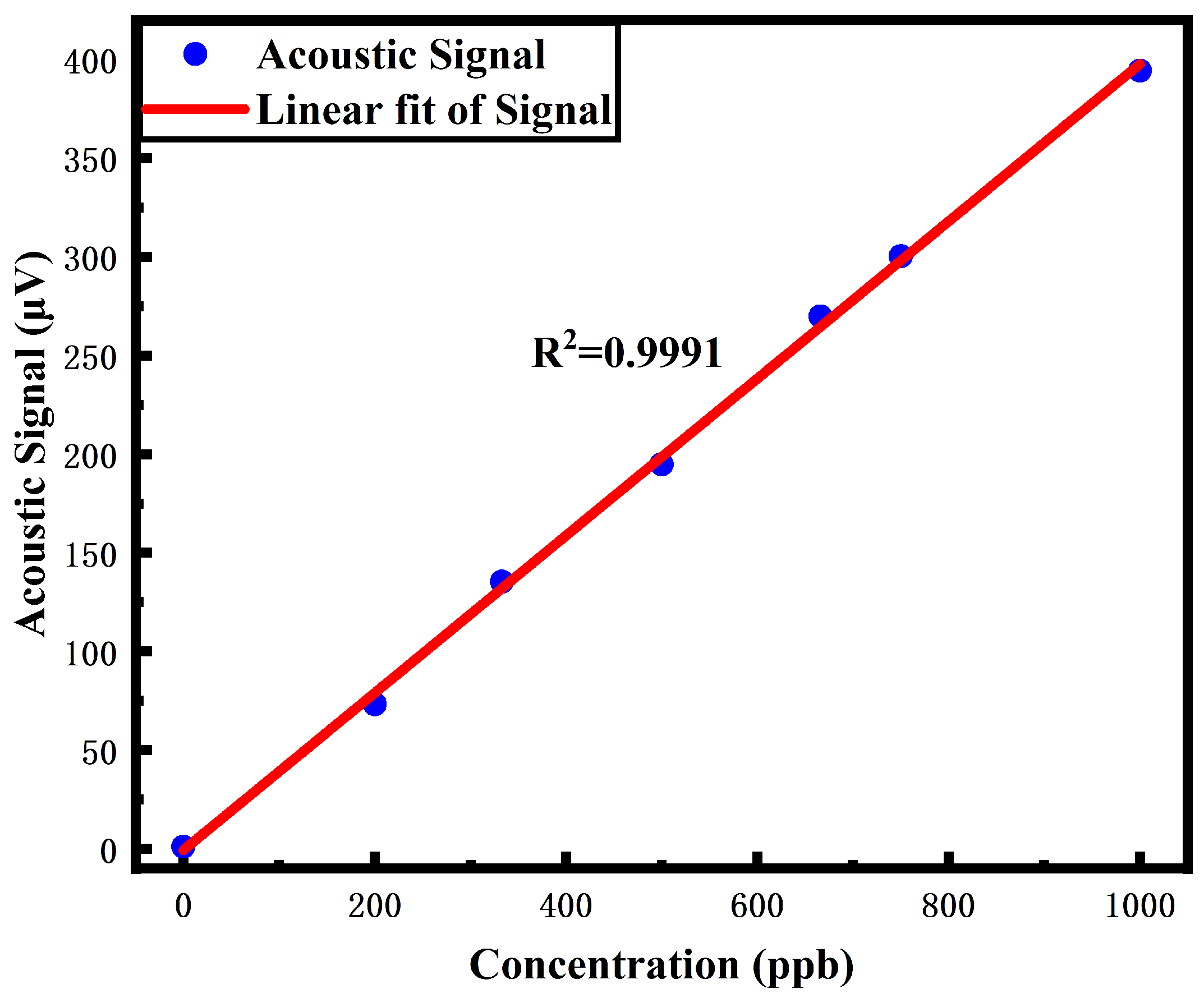
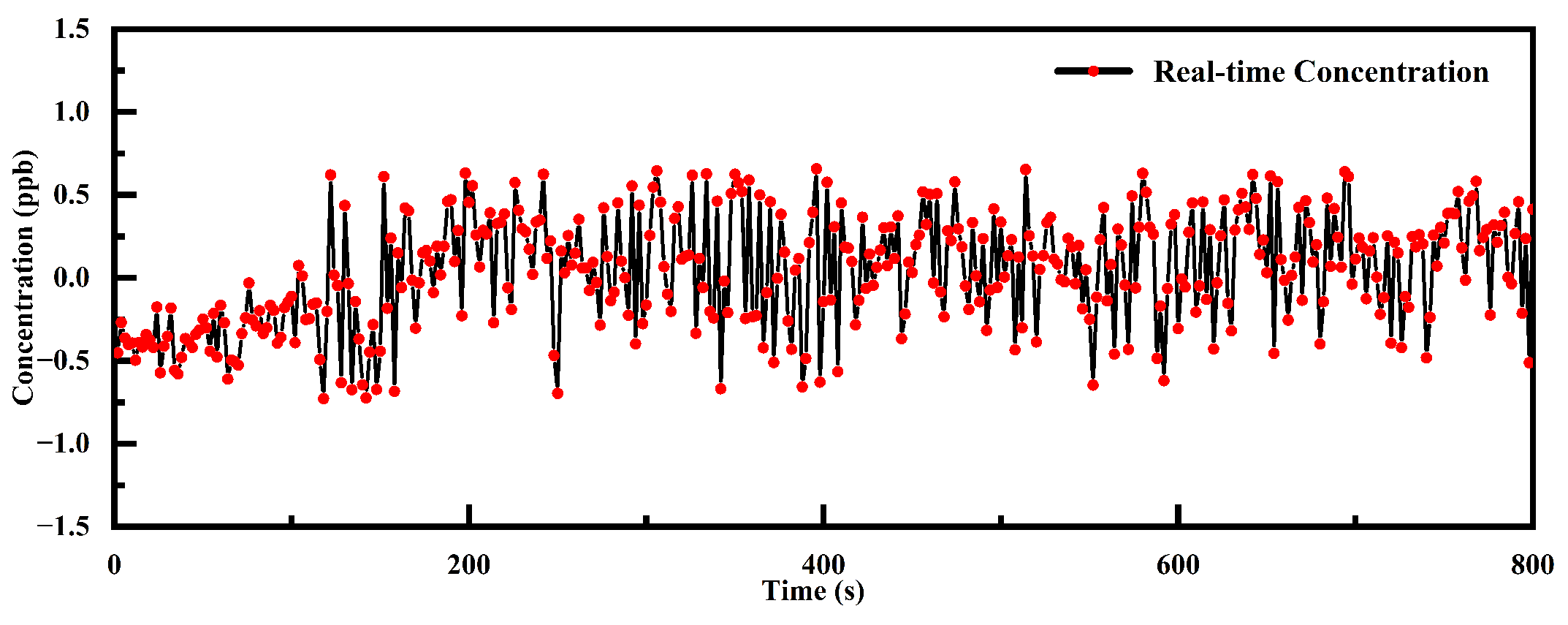
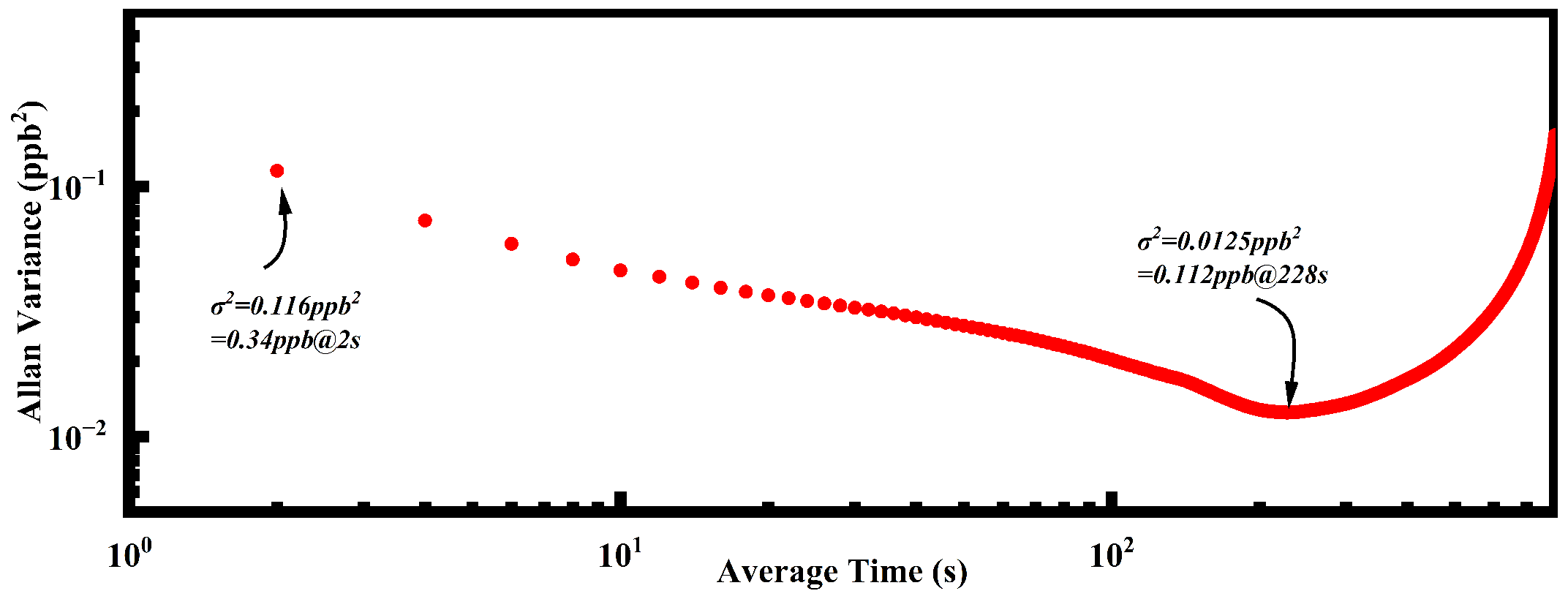
4.1. Performance Evaluation
4.2. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LD | laser diode |
| PAC | photoacoustic cell |
| NO | nitrogen dioxide |
| SNR | signal-to-noise |
| MDL | minimum detection limit |
| TDLAS | tunable diode laser absorption spectroscopy |
| FTIR | fourier transform infrared spectroscopy |
| NDIR | nondispersive infrared |
| CRDS | cavity ring-down spectroscopy |
| PAS | photoacoustic spectroscopy |
| QEPAS | quartz-enhanced photoacoustic |
| HITRAN | high resolution Transmission molecular absorption database |
| DAQ | data acquisition |
| L-I-V | light-current-voltage |
References
- Werle, P. A review of recent advances in semiconductor laser based gas monitors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 197–236. [Google Scholar] [CrossRef]
- Fiddler, M.N.; Begashaw, I.; Mickens, M.A.; Collingwood, M.S.; Assefa, Z.; Bililign, S. Laser spectroscopy for atmospheric and environmental sensing. Sensors 2009, 9, 10447–10512. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Mehta, B.; Tyagi, A.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Bielecki, Z.; Stacewicz, T.; Wojtas, J.; Mikołajczyk, J.; Szabra, D.; Prokopiuk, A. Selected optoelectronic sensors in medical applications. Opto-Electron. Rev. 2018, 26, 122–133. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2012, 24, 012004. [Google Scholar] [CrossRef]
- Frish, M.B.; Wainner, R.T.; Laderer, M.C.; Green, B.D.; Allen, M.G. Standoff and Miniature Chemical Vapor Detectors Based on Tunable Diode Laser Absorption Spectroscopy. IEEE Sens. J. 2010, 10, 639–646. [Google Scholar] [CrossRef]
- Faix, O. Fourier transform infrared spectroscopy. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 83–109. [Google Scholar]
- Jha, R.K. Non-dispersive infrared gas sensing technology: A review. IEEE Sens. J. 2021, 22, 6–15. [Google Scholar] [CrossRef]
- Romanini, D.; Ventrillard, I.; Méjean, G.; Morville, J.; Kerstel, E. Introduction to cavity enhanced absorption spectroscopy. In Cavity-Enhanced Spectroscopy and Sensing; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–60. [Google Scholar]
- Li, J.; Chen, W.; Yu, B. Recent progress on infrared photoacoustic spectroscopy techniques. Appl. Spectrosc. Rev. 2011, 46, 440–471. [Google Scholar] [CrossRef]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ choice—Critical review—A critical review of solid state gas sensors. J. Electrochem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Copenhagen, Denmark, 2006. [Google Scholar]
- Li, B.; Kim, Y.L.; Lippert, A.R. Chemiluminescence measurement of reactive sulfur and nitrogen species. Antioxid. Redox Signal. 2022, 36, 337–353. [Google Scholar] [CrossRef]
- Ugale, A.D.; Umarji, G.G.; Jung, S.H.; Deshpande, N.G.; Lee, W.; Cho, H.K.; Yoo, J.B. ZnO decorated flexible and strong graphene fibers for sensing NO2 and H2S at room temperature. Sens. Actuators B Chem. 2020, 308, 127690. [Google Scholar] [CrossRef]
- Kalkman, J.; Van Kesteren, H. Relaxation effects and high sensitivity photoacoustic detection of NO2 with a blue laser diode. Appl. Phys. B 2008, 90, 197–200. [Google Scholar] [CrossRef]
- Peltola, J.; Hieta, T.; Vainio, M. Parts-per-trillion-level detection of nitrogen dioxide by cantilever-enhanced photo-acoustic spectroscopy. Opt. Lett. 2015, 40, 2933–2936. [Google Scholar] [CrossRef]
- Thompson, J.E. Crowd-sourced air quality studies: A review of the literature & portable sensors. Trends Environ. Anal. Chem. 2016, 11, 23–34. [Google Scholar]
- Zheng, F.; Qiu, X.; Shao, L.; Feng, S.; Cheng, T.; He, X.; He, Q.; Li, C.; Kan, R.; Fittschen, C. Measurement of nitric oxide from cigarette burning using TDLAS based on quantum cascade laser. Opt. Laser Technol. 2020, 124, 105963. [Google Scholar] [CrossRef]
- Li, G.; Werwein, V.; Lüttschwager, A.; Eon Kim, M.; Nwaboh, J.; Werhahn, O.; Ebert, V. FTIR-based spectral line data of the v3 band of NO2 at 6.3 μm and multi-component impurity analysis of NO2 reference gases within the scope of the EMPIR MetNO2 project. In Proceedings of the EGU General Assembly Conference Abstracts, Online, 4–8 May 2020; p. 21718. [Google Scholar]
- Rück, T.; Bierl, R.; Matysik, F.M. Low-cost photoacoustic NO2 trace gas monitoring at the pptV-level. Sens. Actuators A Phys. 2017, 263, 501–509. [Google Scholar] [CrossRef]
- Karhu, J.; Hieta, T.; Manoocheri, F.; Vainio, M.; Ikonen, E. LED-based photoacoustic NO2 sensor with a sub-ppb detection limit. ACS Sens. 2021, 6, 3303–3307. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Z.; Tang, H.; Xue, H.; Chen, Y.; Hu, R. Compact, Fast Cavity Ring-Down Spectroscopy Monitor for Simultaneous Measurement of Ozone and Nitrogen Dioxide in the Atmosphere. Atmosphere 2022, 13, 2106. [Google Scholar] [CrossRef]
- Li, S.; Lu, J.; Shang, Z.; Zeng, X.; Yuan, Y.; Wu, H.; Pan, Y.; Sampaolo, A.; Patimisco, P.; Spagnolo, V.; et al. Compact quartz-enhanced photoacoustic sensor for ppb-level ambient NO2 detection by use of a high-power laser diode and a grooved tuning fork. Photoacoustics 2022, 25, 100325. [Google Scholar] [CrossRef]
- Bell, A.G. The production of sound by radiant energy. Science 1881, 48, 242–253. [Google Scholar] [CrossRef]
- Patimisco, P.; Scamarcio, G.; Tittel, F.K.; Spagnolo, V. Quartz-enhanced photoacoustic spectroscopy: A review. Sensors 2014, 14, 6165–6206. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Review of recent advances in QEPAS-based trace gas sensing. Appl. Sci. 2018, 8, 1822. [Google Scholar] [CrossRef]
- Ayache, D.; Rousseau, R.; Kniazeva, E.; Charensol, J.; Seoudi, T.; Bahriz, M.; Gouzi, F.; Spagnolo, V.; Vicet, A. Commercial and Custom Quartz Tuning Forks for Quartz Enhanced Photoacoustic Spectroscopy: Stability under Humidity Variation. Sensors 2023, 23, 3135. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Chen, K.; Chen, Y.; Mei, L.; Yu, Q. Integration of T-type half-open photoacoustic cell and fiber-optic acoustic sensor for trace gas detection. Opt. Express 2019, 27, 18222–18231. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Gao, T.; Chen, Y.; Zhang, B.; Peng, W.; Yu, Q.; Ma, F.; Mei, L.; Chen, K. Sub-ppb level detection of nitrogen dioxide based on an optimized H-type longitudinal acoustic resonator and a lock-in white-light interferometry demodulation algorithm. J. Quant. Spectrosc. Radiat. Transf. 2020, 253, 107136. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Q.; Wang, R.; Liu, K.; Chen, W.; Wang, G.; Gao, X. Development of a 443 nm diode laser-based differential photoacoustic spectrometer for simultaneous measurements of aerosol absorption and NO2. Photoacoustics 2021, 21, 100229. [Google Scholar] [CrossRef]
- Li, Z.; Si, G.; Ning, Z.; Liu, J.; Fang, Y.; Si, B.; Cheng, Z.; Yang, C. Highly sensitive sphere-tube coupled photoacoustic cell suitable for detection of a variety of trace gases: NO2 as an example. Sensors 2022, 22, 281. [Google Scholar] [CrossRef]
- Brewer, A.; McElroy, C.; Kerr, J. Nitrogen dioxide concentrations in the atmosphere. Nature 1973, 246, 129–133. [Google Scholar] [CrossRef]
- Pollack, I.B.; Lerner, B.M.; Ryerson, T.B. Evaluation of ultraviolet light-emitting diodes for detection of atmospheric NO2 by photolysis-chemiluminescence. J. Atmos. Chem. 2010, 65, 111–125. [Google Scholar] [CrossRef]
- Rosencwaig, A.; Griffiths, P.R. Photoacoustics and photoacoustic spectroscopy. Phys. Today 1981, 34, 64. [Google Scholar] [CrossRef]
- Cai, Y.; Arsad, N.; Li, M.; Wang, Y. Buffer structure optimization of the photoacoustic cell for trace gas detection. Optoelectron. Lett. 2013, 9, 233–237. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, L.; Yin, X.; Wu, H. Compact and highly sensitive NO2 photoacoustic sensor for environmental monitoring. Molecules 2020, 25, 1201. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.; Lamard, L.; Balslev-Harder, D.; Peremans, A.; Petersen, J.C. Mid-infrared photoacoustic spectroscopy for atmospheric NO2 measurements. In Proceedings of the Photonic Instrumentation Engineering V, San Francisco, CA, USA, 27 January–1 February 2018; Volume 10539, pp. 108–114. [Google Scholar]
- Keeratirawee, K.; Hauser, P.C. Piezoelectric tube as resonant transducer for gas-phase photoacoustics. Anal. Chim. Acta 2021, 1147, 165–169. [Google Scholar] [CrossRef]






| Reference | PAC Type | Frequency (Hz) | Volume (mm × mm × mm) | Gas Capacity (mL) | MDL (ppb) |
|---|---|---|---|---|---|
| [37] | differential | 1780 | 170 × 50 × 50 | 55.35 | 0.25 |
| [30] | H-type | 1415 | 240 × 24 × 24 | 55.79 | 1.26 |
| [31] | H-type | 1430 | 220 × 70 × 70 | 431.58 | 300 |
| [32] | (sphere + tube) | 1199 | 100 × 50 × 50 | 71.73 | 0.7 |
| [38] | H-type | 14,400 | 45 × 8 × 8 | 1.877 | 1600 |
| [39] | H-type | 5238 | 80 × 26 × 26 | 31.85 | 83 |
| This paper | H-type | 3150 | 80 × 30 × 30 | 18.85 | 4.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Yao, J.; Gong, P.; Jiao, M.; Lei, C.; Jiang, Y.; Xie, L. A Compact NO2 Gas Sensor with an Optimized Photoacoustic Cell and 450 nm-LD. Atmosphere 2023, 14, 704. https://doi.org/10.3390/atmos14040704
Yuan W, Yao J, Gong P, Jiao M, Lei C, Jiang Y, Xie L. A Compact NO2 Gas Sensor with an Optimized Photoacoustic Cell and 450 nm-LD. Atmosphere. 2023; 14(4):704. https://doi.org/10.3390/atmos14040704
Chicago/Turabian StyleYuan, Wenyang, Jiaqi Yao, Ping Gong, Mingqi Jiao, Congbiao Lei, Yuxuan Jiang, and Liang Xie. 2023. "A Compact NO2 Gas Sensor with an Optimized Photoacoustic Cell and 450 nm-LD" Atmosphere 14, no. 4: 704. https://doi.org/10.3390/atmos14040704
APA StyleYuan, W., Yao, J., Gong, P., Jiao, M., Lei, C., Jiang, Y., & Xie, L. (2023). A Compact NO2 Gas Sensor with an Optimized Photoacoustic Cell and 450 nm-LD. Atmosphere, 14(4), 704. https://doi.org/10.3390/atmos14040704








