Analysis of Size Distribution, Chemical Composition, and Optical Properties of Mineral Dust Particles from Dry Deposition Measurement in Tenerife: Determined by Single-Particle Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Location and Time
2.2. Wind Measurements
2.3. Particle Sampling and Description of the Samplers
2.3.1. Flat-Plate Sampler
2.3.2. The Modified Wilson and Cook (MWAC) Sampler
2.3.3. Free-Wing Impactor (FWI)
2.4. SEM-Analysis
2.5. Particle Size Measurement
2.6. Mass Flux Calculation
2.7. Determining the Size Distributions for Mass Concentration from the Free-Wing Impactor Measurements
2.8. Determining the Atmospheric Concentration from Deposition Fluxes
2.9. Chemical Composition and Composition Classification
2.10. Calculation of the Refractive Index
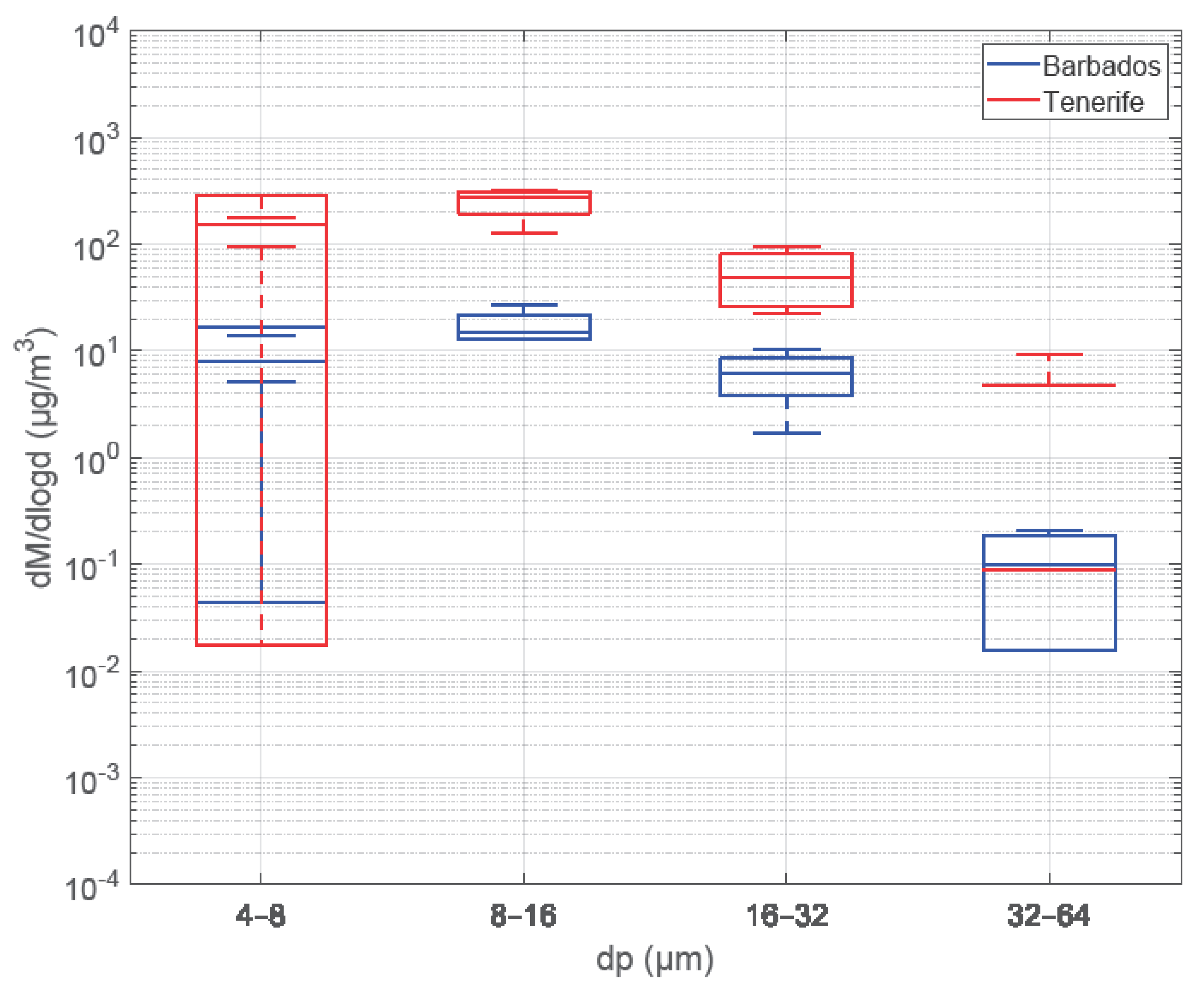
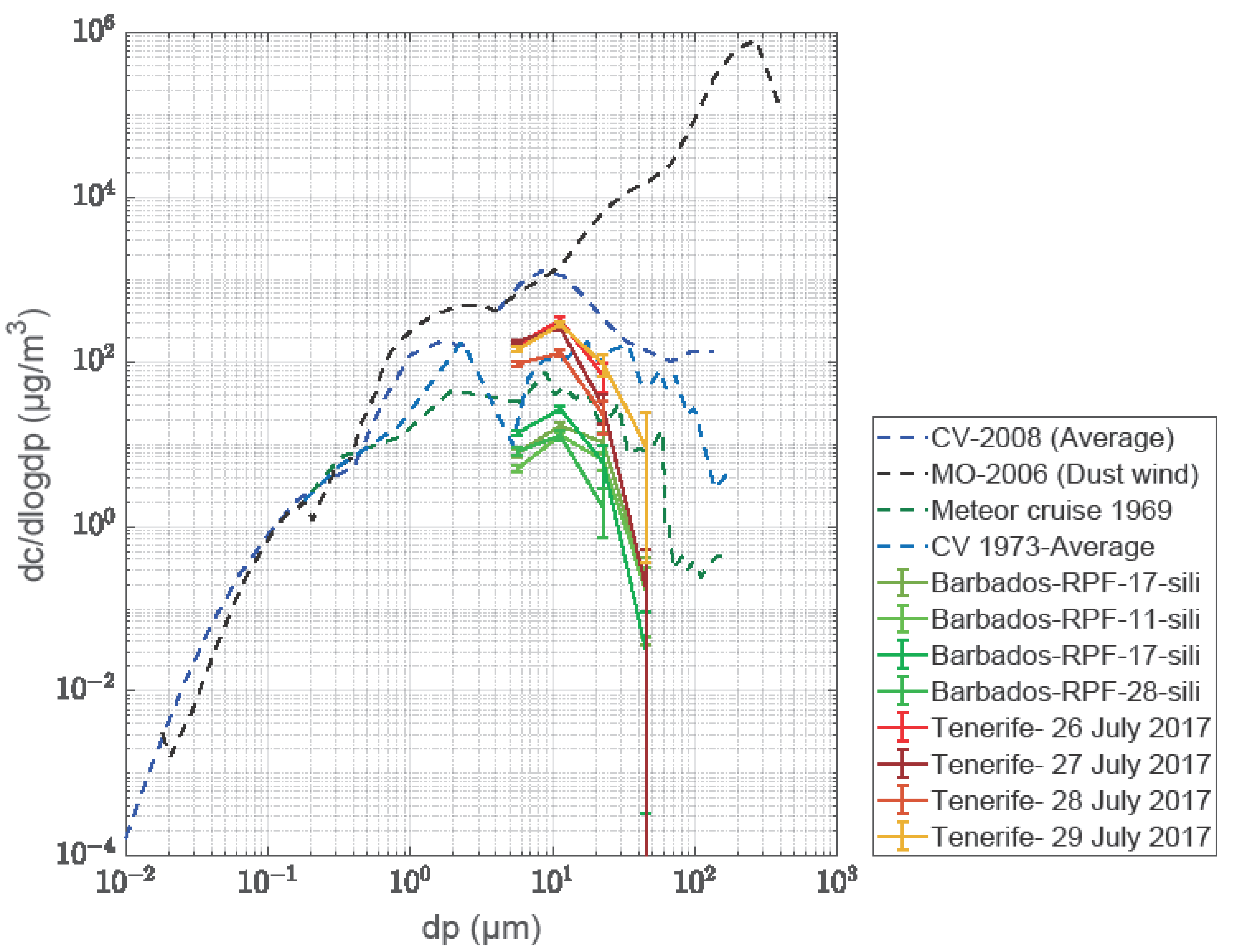
2.11. Comparison of Mineral Dust Concentrations—Tenerife vs. Barbados
2.12. Aerosol Buffering Potential
2.13. Mixing of Sulfate Particle with Dust
3. Results and Discussion
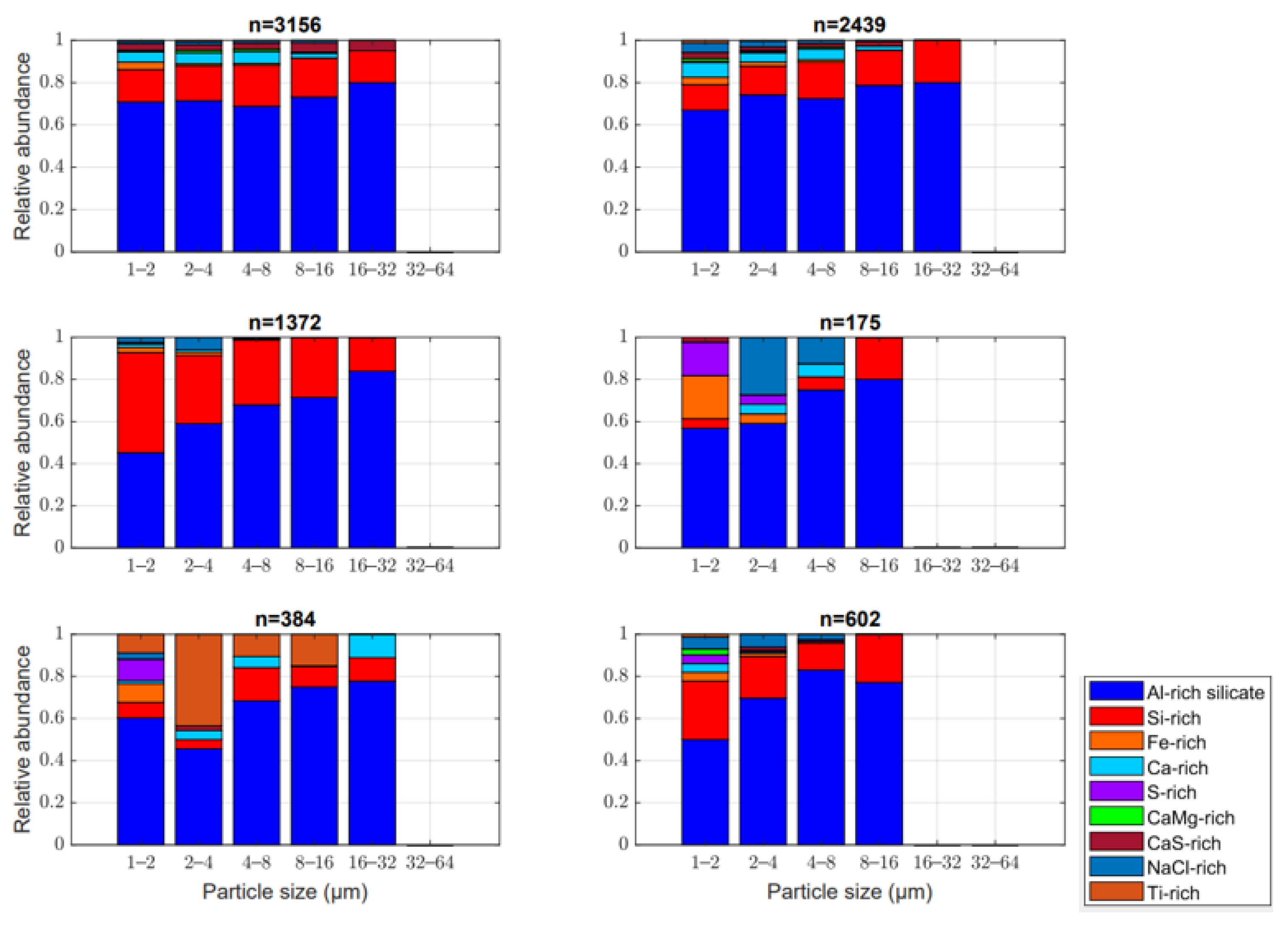
3.1. Chemical Composition
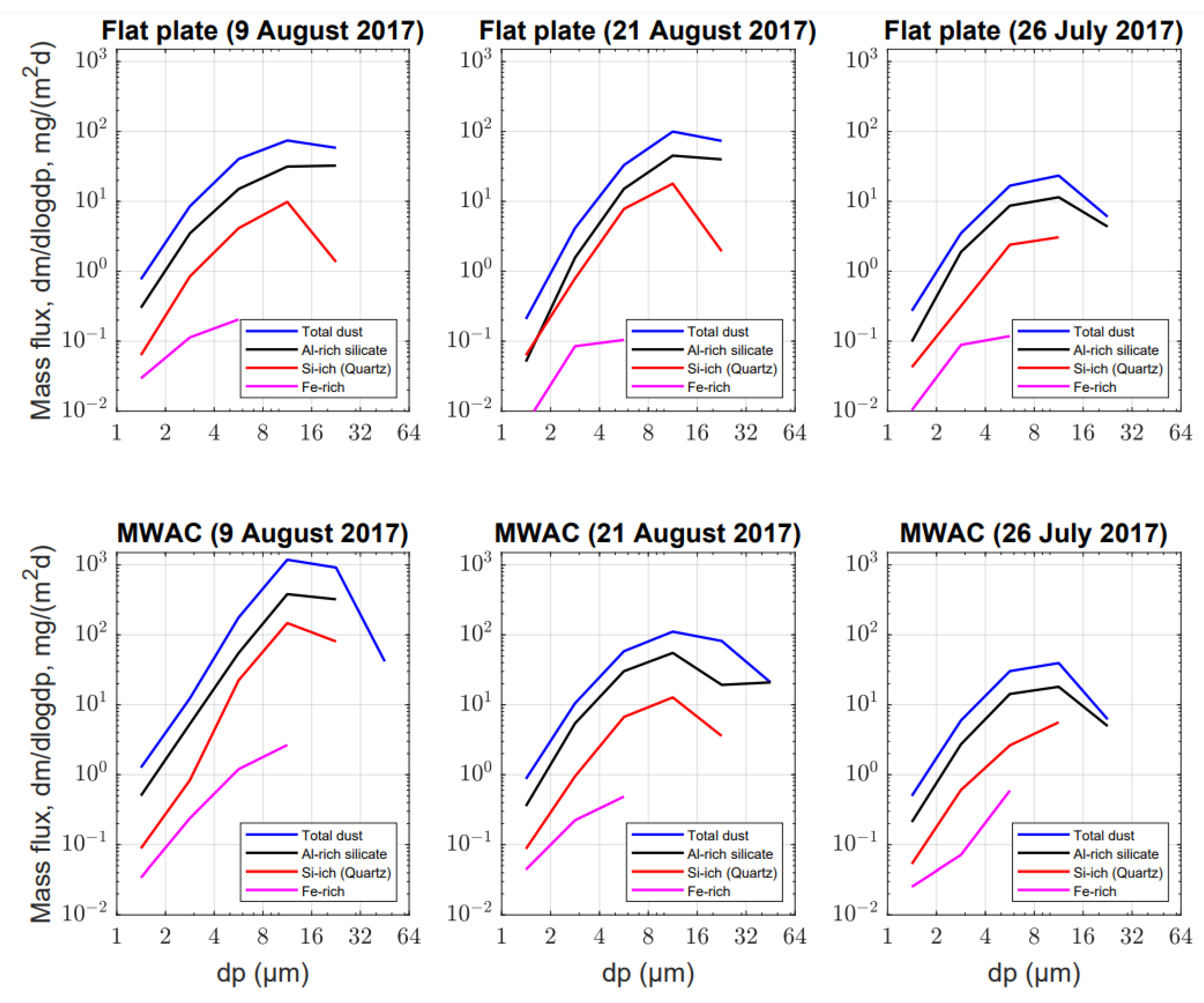
3.2. Composition and Flux Analysis (Horizontal vs. Vertical Dust Flux)
3.3. Size Distributions from the Free-Wing Impactor Measurements
3.4. Complex Index of Refraction
3.5. Aerosol Potential Buffering
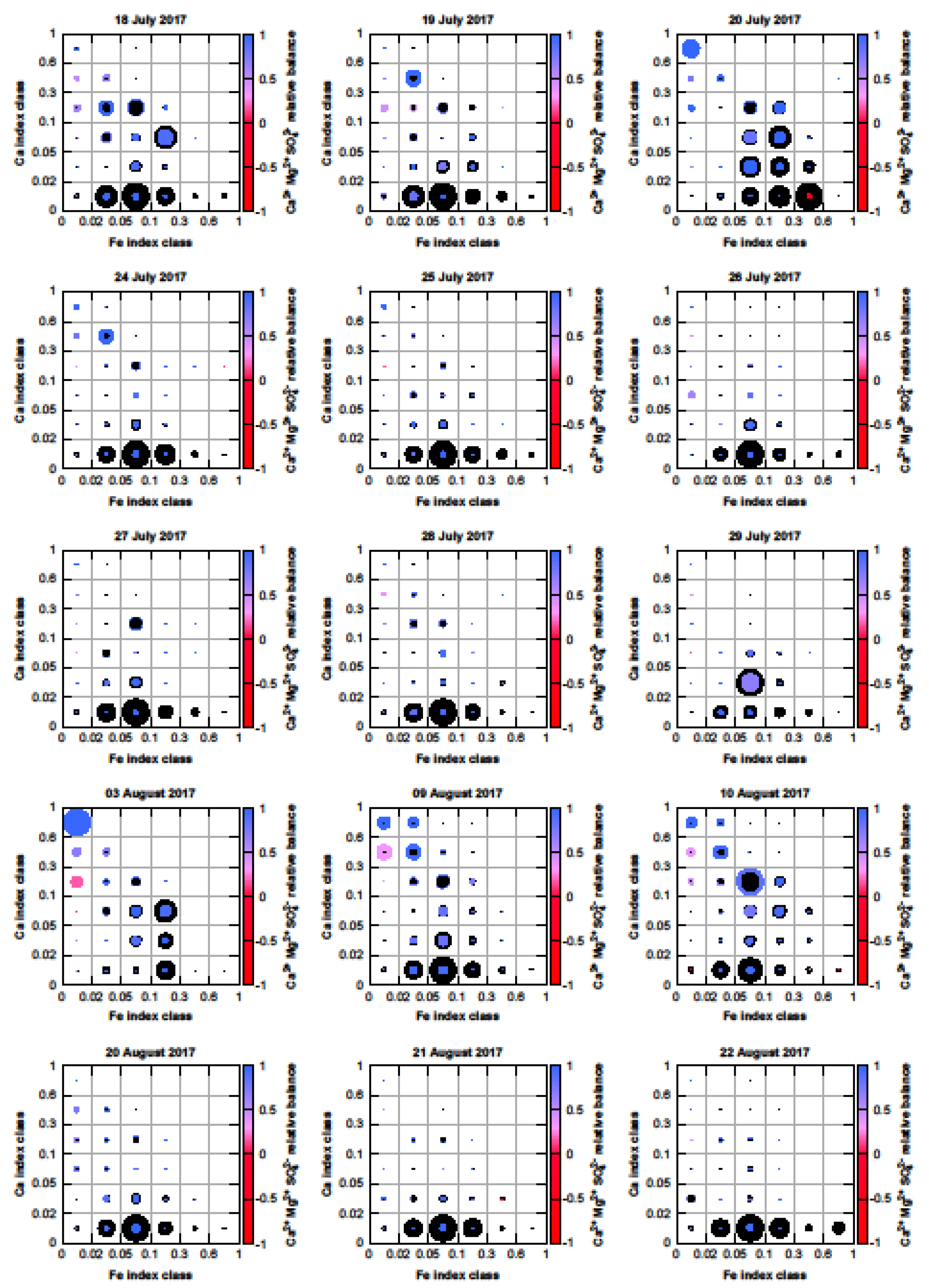
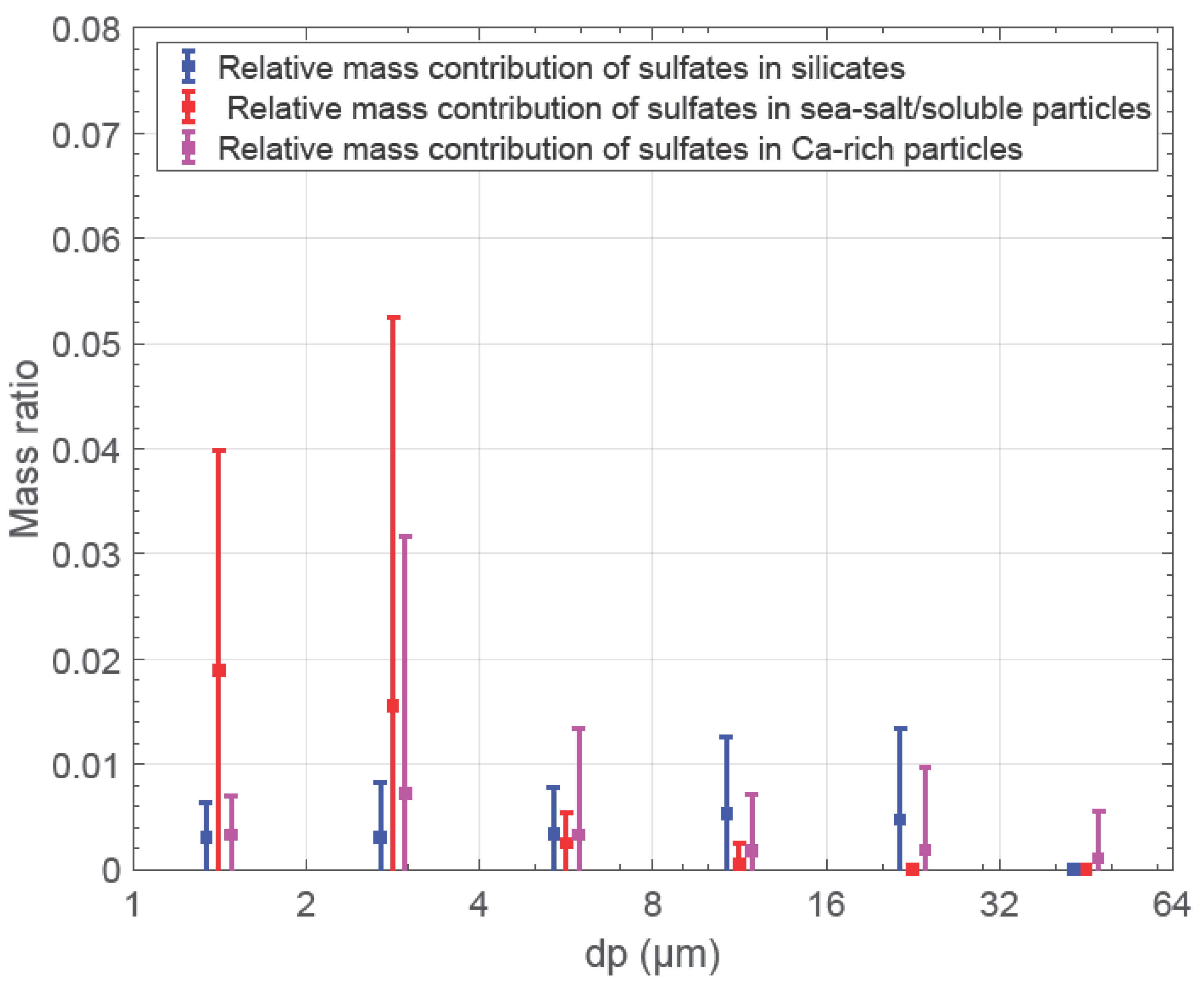
3.6. Mixing of Dust with Sulfate (SO42−)
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arimoto, R. Eolian Dust and Climate: Relationships to Sources, Tropospheric Chemistry, Transport and Deposition. Earth Sci. Rev. 2001, 54, 29–42. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A. Global Iron Connections between Desert Dust, Ocean Biogeochemistry, and Climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Haywood, J.; Boucher, O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev. Geophys. 2000, 38, 513–543. [Google Scholar] [CrossRef]
- Sokolik, I.; Winker, D.; Bergametti, G.; Gillette, D.; Carmichael, G.; Kaufman, Y.; Gomes, L.; Schuetz, L.; Penner, J. Introduction to special section: Outstanding problems in quantifying the radiative impacts of mineral dust. J. Geophys. Res. Atmos. 2001, 106, 18015–18027. [Google Scholar] [CrossRef]
- Penner, J.E.; Andreae, M.; Annegarn, H.; Barrie, L.; Feichter, J.; Hegg, D.; Jayaraman, A.; Leaitch, R.; Murphy, D.; Nganga, J. Aerosols, their direct and indirect effects. In Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; pp. 289–348. [Google Scholar]
- Stockdale, A.; Krom, M.D.; Mortimer, R.J.; Benning, L.G.; Carslaw, K.S.; Herbert, R.J.; Shi, Z.; Myriokefalitakis, S.; Kanakidou, M.; Nenes, A. Understanding the nature of atmospheric acid processing of mineral dusts in supplying bioavailable phosphorus to the oceans. Proc. Natl. Acad. Sci. USA 2016, 113, 14639–14644. [Google Scholar] [CrossRef]
- Boyd, P.; Jickells, T.; Law, C.; Blain, S.; Boyle, E.; Buesseler, K.; Coale, K.; Cullen, J.; De Baar, H.J.; Follows, M. A synthesis of mesoscale iron-enrichment experiments 1993–2005: Key findings and implications for ocean biogeochemistry. Science 2007, 315, 612–617. [Google Scholar] [CrossRef]
- Jeong, G.; Achterberg, E.P. Chemistry and mineralogy of clay minerals in Asian and Saharan dusts and the implications for iron supply to the oceans. Atmos. Chem. Phys. 2014, 14, 12415–12428. [Google Scholar] [CrossRef]
- Mahowald, N.M.; Baker, A.R.; Bergametti, G.; Brooks, N.; Duce, R.A.; Jickells, T.D.; Kubilay, N.; Prospero, J.M.; Tegen, I. Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Ito, A.; Feng, Y. Role of dust alkalinity in acid mobilization of iron. Atmos. Chem. Phys. 2010, 10, 9237–9250. [Google Scholar] [CrossRef]
- Nenes, A.; Krom, M.D.; Mihalopoulos, N.; Cappellen, P.V.; Shi, Z.; Bougiatioti, A.; Zarmpas, P.; Herut, B. Atmospheric acidification of mineral aerosols: A source of bioavailable phosphorus for the oceans. Atmos. Chem. Phys. 2011, 11, 6265–6272. [Google Scholar] [CrossRef]
- Pósfai, M.; Axisa, D.; Tompa, É.; Freney, E.; Bruintjes, R.; Buseck, P.R. Interactions of mineral dust with pollution and clouds: An individual-particle TEM study of atmospheric aerosol from Saudi Arabia. Atmos. Res. 2013, 122, 347–361. [Google Scholar] [CrossRef]
- Ingall, E.; Feng, Y.; Longo, A.; Lai, B.; Shelley, R.; Landing, W.; Morton, P.; Nenes, A.; Mihalopoulos, N.; Violaki, K. Enhanced Iron Solubility at Low pH in Global Aerosols. Atmosphere 2018, 9, 201. [Google Scholar] [CrossRef]
- Meskhidze, N.; Chameides, W.L.; Nenes, A.; Chen, G. Iron Mobilization in Mineral Dust: Can Anthropogenic SO2 Emissions Affect Ocean Productivity? Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Gao, Y.; Anderson, J.R. Characteristics of Chinese aerosols determined by individual-particle analysis. J. Geophys. Res. Atmos. 2001, 106, 18037–18045. [Google Scholar] [CrossRef]
- Bauer, S.E.; Mishchenko, M.I.; Lacis, A.A.; Zhang, S.; Perlwitz, J.; Metzger, S.M. Do Sulfate and Nitrate Coatings on Mineral Dust Have Important Effects on Radiative Properties and Climate Modeling? J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Falkovich, A.H.; Ganor, E.; Levin, Z.; Formenti, P.; Rudich, Y. Chemical and mineralogical analysis of individual mineral dust particles. Geophys. Res. Atmos. 2001, 106, 18029–18036. [Google Scholar] [CrossRef]
- Dall’Osto, M.; Harrison, R.M.; Highwood, E.J.; O’Dowd, C.; Ceburnis, D.; Querol, X.; Achterberg, E.P. Variation of the mixing state of Saharan dust particles with atmospheric transport. Atmos. Environ. 2010, 44, 3135–3146. [Google Scholar] [CrossRef]
- Kandler, K.; Lieke, K.; Benker, N.; Emmel, C.; Küpper, M.; Müller-Ebert, D.; Ebert, M.; Scheuvens, D.; Schladitz, A.; Schütz, L. Electron microscopy of particles collected at Praia, Cape Verde, during the Saharan Mineral Dust Experiment: Particle chemistry, shape, mixing state and complex refractive index. Tellus B Chem. Phys. Meteorol. 2011, 63, 475–496. [Google Scholar] [CrossRef]
- Schütz, L.; Sebert, M. Mineral aerosols and source identification. J. Aerosol Sci. 1987, 18, 1–10. [Google Scholar] [CrossRef]
- Stuut, J.B.; Zabel, M.; Ratmeyer, V.; Helmke, P.; Schefuß, E.; Lavik, G.; Schneider, R. Provenance of present-day eolian dust collected off NW Africa. J. Geophys. Res. Atmos. 2005, 110. [Google Scholar] [CrossRef]
- Chou, C.; Formenti, P.; Maille, M.; Ausset, P.; Helas, G.; Harrison, M.; Osborne, S. Size distribution, shape, and composition of mineral dust aerosols collected during the African Monsoon Multidisciplinary Analysis Special Observation Period 0: Dust and Biomass-Burning Experiment field campaign in Niger, January 2006. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Kandler, K.; Benker, N.; Bundke, U.; Cuevas, E.; Ebert, M.; Knippertz, P.; Rodríguez, S.; Schütz, L.; Weinbruch, S. Chemical composition and complex refractive index of Saharan Mineral Dust at Izana, Tenerife (Spain) derived by electron microscopy. Atmos. Environ. 2007, 41, 8058–8074. [Google Scholar] [CrossRef]
- Eriksen Hammer, S.; Ebert, M.; Weinbruch, S. Comparison of operator-and computer-controlled scanning electron microscopy of particles from different atmospheric aerosol types. Anal. Bioanal. Chem. 2019, 411, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Goudie, A.; Middleton, N. Saharan dust storms: Nature and consequences. Earth Sci. Rev. 2001, 56, 179–204. [Google Scholar] [CrossRef]
- Cordoba-Jabonero, C.; Andrey-Andres, J.; Gomez, L.; Adame, J.A.; Sorribas, M.; Navarro-Comas, M.; Puentedura, O.; Cuevas, E.; Gil-Ojeda, M. Vertical mass impact and features of Saharan dust intrusions derived from ground-based remote sensing in synergy with airborne in-situ measurements. Atmos. Environ. 2016, 142, 420–429. [Google Scholar] [CrossRef]
- Bergamaschi, P.; Bräunlich, M.; Marik, T.; Brenninkmeijer, C.A. Measurements of the carbon and hydrogen isotopes of atmospheric methane at Izaña, Tenerife: Seasonal cycles and synoptic-scale variations. J. Geophys. Res. Atmos. 2000, 105, 14531–14546. [Google Scholar] [CrossRef]
- Rodríguez, S.; Cuevas, E.; Prospero, J.; Alastuey, A.; Querol, X.; López-Solano, J.; García, M.; Alonso-Pérez, S.J.A.C. Modulation of Saharan dust export by the North African dipole. Atmos. Chem. Phys. 2015, 15, 7471–7486. [Google Scholar] [CrossRef]
- Waza, A.; Schneiders, K.; Kandler, K. Daily dust deposition fluxes at Izana, Tenerife collected by different techniques: Particle size and composition from single particle electron microscopy, PANGAEA. Pangaea 2019, 12, 6647–6665. [Google Scholar] [CrossRef]
- García, R.; García, O.; Cuevas, E.; Cachorro, V.; Barreto, A.; Guirado-Fuentes, C.; Kouremeti, N.; Bustos, J.; Romero-Campos, P.; de Frutos, A. Aerosol optical depth retrievals at the Izaña Atmospheric Observatory from 1941 to 2013 by using artificial neural networks. Atmos. Meas. Tech. 2016, 9, 53–62. [Google Scholar] [CrossRef]
- Wilson, S.J.; Cook, R.U. Wind erosion. In Soil Erosion; Morgan, P.R.C., Kirkby, M.J., Eds.; John Wiley & Sons: Chichester, UK, 1980; pp. 217–251. [Google Scholar]
- Ott, D.K.; Peters, T.M. A shelter to protect a passive sampler for coarse particulate matter, PM10−2.5. Aerosol Sci. Technol. 2008, 42, 299–309. [Google Scholar] [CrossRef]
- Kandler, K.; Schneiders, K.; Ebert, M.; Hartmann, M.; Weinbruch, S.; Prass, M.; Pöhlker, C. Composition and mixing state of atmospheric aerosols determined by electron microscopy: Method development and application to aged Saharan dust deposition in the Caribbean boundary layer. Atmos. Chem. Phys. 2018, 18, 13429–13455. [Google Scholar] [CrossRef]
- Waza, A.; Schneiders, K.; May, J.; Rodríguez, S.; Epple, B.; Kandler, K. Field comparison of dry deposition samplers for collection of atmospheric mineral dust: Results from single-particle characterization. Atmos. Meas. Tech. Discuss. 2019, 2019, 6647–6665. [Google Scholar] [CrossRef]
- Ott, D.K.; Kumar, N.; Peters, T.M. Passive sampling to capture spatial variability in PM10–2.5. Atmos. Environ. 2008, 42, 746–756. [Google Scholar] [CrossRef]
- Wagner, J.; Leith, D. Passive aerosol sampler. Part I: Principle of operation. Aerosol Sci. Technol. 2001, 34, 186–192. [Google Scholar] [CrossRef]
- Wagner, J.; Leith, D. Passive aerosol sampler. Part II: Wind tunnel experiments. Aerosol Sci. Technol. 2001, 34, 193–201. [Google Scholar] [CrossRef]
- Jaenicke, R.; Junge, C. Studien zur oberen Grenzgröße des natürlichen Aerosols. Contrib. Atmos. Phys. 1967, 40, 129–143. [Google Scholar]
- Kandler, K.; Schütz, L.; Deutscher, C.; Ebert, M.; Hofmann, H.; Jäckel, S.; Jaenicke, R.; Knippertz, P.; Lieke, K.; Massling, A. Size distribution, mass concentration, chemical and mineralogical composition and derived optical parameters of the boundary layer aerosol at Tinfou, Morocco, during SAMUM 2006. Tellus B Chem. Phys. Meteorol. 2009, 61, 32–50. [Google Scholar] [CrossRef]
- Ott, D.K.; Cyrs, W.; Peters, T.M. Passive measurement of coarse particulate matter, PM10–2.5. J. Aerosol Sci. 2008, 39, 156–167. [Google Scholar] [CrossRef]
- Davies, C. Particle-fluid interaction. J. Aerosol Sci. 1979, 10, 477–513. [Google Scholar] [CrossRef]
- Schulz, M.; Prospero, J.M.; Baker, A.R.; Dentener, F.; Ickes, L.; Liss, P.S.; Mahowald, N.M.; Nickovic, S.; Garcia-Pando, C.P.; Rodríguez, S. Atmospheric transport and deposition of mineral dust to the ocean: Implications for research needs. Environ. Sci. Technol. 2012, 46, 10390–10404. [Google Scholar] [CrossRef]
- Li, J.; Shao, L.; Chang, L.; Xing, J.; Wang, W.; Li, W.; Zhang, D. Physicochemical Characteristics and Possible Sources of Individual Mineral Particles in a Dust Storm Episode in Beijing, China. Atmosphere 2018, 9, 269. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Zhang, C.; Xu, T.; Zhou, Q. What is the real role of iron oxides in the optical properties of dust aerosols? Atmos. Chem. Phys. 2015, 15, 12159–12177. [Google Scholar] [CrossRef]
- Skiles, S.M.; Painter, T.; Okin, G.S. A method to retrieve the spectral complex refractive index and single scattering optical properties of dust deposited in mountain snow. J. Glaciol. 2017, 63, 133–147. [Google Scholar] [CrossRef]
- Di Biagio, C.; Formenti, P.; Balkanski, Y.; Caponi, L.; Cazaunau, M.; Pangui, E.; Journet, E.; Nowak, S.; Andreae, M.O.; Kandler, K.; et al. Complex refractive indices and single scattering albedo of global dust aerosols in the shortwave spectrum and relationship to iron content and size. Atmos. Chem. Phys. Discuss. 2019, 19, 15503–15531. [Google Scholar] [CrossRef]
- Ebert, M.; Weinbruch, S.; Rausch, A.; Gorzawski, G.; Helas, G.; Hoffmann, P.; Wex, H. Complex refractive index of aerosols during LACE 98# x2010; as derived from the analysis of individual particles. J. Geophys. Res. 2002, 107. [Google Scholar] [CrossRef]
- Chýlek, P.; Srivastava, V.; Pinnick, R.G.; Wang, R. Scattering of electromagnetic waves by composite spherical particles: Experiment and effective medium approximations. Appl. Opt. 1988, 27, 2396–2404. [Google Scholar] [CrossRef]
- Nousiainen, T. Optical modeling of mineral dust particles: A review. J. Quant. Spectrosc. Ra. 2009, 110, 1261–1279. [Google Scholar] [CrossRef]
- Lindqvist, H.; Jokinen, O.; Kandler, K.; Scheuvens, D.; Nousiainen, T. Single scattering by realistic, inhomogeneous mineral dust particles with stereogrammetric shapes. Atmos. Chem. Phys. 2014, 14, 143–157. [Google Scholar] [CrossRef]
- Müller, T.; Schladitz, A.; Massling, A.; Kaaden, N.; Kandler, K.; Wiedensohler, A. Spectral absorption coefficients and imaginary parts of refractive indices of Saharan dust during SAMUM-1. Tellus B Chem. Phys. Meteorol. 2009, 61, 79–95. [Google Scholar] [CrossRef]
- Petzold, A.; Rasp, K.; Weinzierl, B.; Esselborn, M.; Hamburger, T.; Dörnbrack, A.; Kandler, K.; Schuütz, L.; Knippertz, P.; Fiebig, M. Saharan dust absorption and refractive index from aircraft-based observations during SAMUM 2006. Tellus B Chem. Phys. Meteorol. 2009, 61, 118–130. [Google Scholar] [CrossRef]
- Maring, H.; Savoie, D.; Izaguirre, M.; Custals, L.; Reid, J. Mineral dust aerosol size distribution change during atmospheric transport. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Kandler, K.; Schneiders, K.; Heuser, J.; Waza, A.; Aryasree, S.; Althausen, D.; Hofer, J.; Abdullaev, S.F.; Makhmudov, A.N. Differences and similarities of central Asian, African, and arctic dust composition from a single particle perspective. Atmosphere 2020, 11, 269. [Google Scholar] [CrossRef]
- Sokolik, I.N.; Toon, O.B. Incorporation of mineralogical composition into models of the radiative properties of mineral aerosol from UV to IR wavelengths. J. Geophys. Res. Atmos. 1999, 104, 9423–9444. [Google Scholar] [CrossRef]
- Textor, C.; Schulz, M.; Guibert, S.; Kinne, S.; Balkanski, Y.; Bauer, S.; Berntsen, T.; Berglen, T.; Boucher, O.; Chin, M. Analysis and quantification of the diversities of aerosol life cycles within AeroCom. Atmos. Chem. Phys. 2006, 6, 1777–1813. [Google Scholar] [CrossRef]
- Weinzierl, B.; Petzold, A.; Esselborn, M.; Wirth, M.; Rasp, K.; Kandler, K.; Schuetz, L.; Koepke, P.; Fiebig, M. Airborne measurements of dust layer properties, particle size distribution and mixing state of Saharan dust during SAMUM 2006. Tellus B Chem. Phys. Meteorol. 2009, 61, 96–117. [Google Scholar] [CrossRef]
- Ryder, C.L.; Highwood, E.J.; Rosenberg, P.D.; Trembath, J.; Brooke, J.K.; Bart, M.; Dean, A.; Crosier, J.; Dorsey, J.; Brindley, H. Optical properties of Saharan dust aerosol and contribution from the coarse mode as measured during the Fennec 2011 aircraft campaign. Atmos. Chem. Phys. 2013, 13, 303–325. [Google Scholar] [CrossRef]
- Weinzierl, B.; Sauer, D.; Esselborn, M.; Petzold, A.; Veira, A.; Rose, M.; Mund, S.; Wirth, M.; Ansmann, A.; Tesche, M. Microphysical and optical properties of dust and tropical biomass burning aerosol layers in the Cape Verde region—An overview of the airborne in situ and lidar measurements during SAMUM-2. Tellus B Chem. Phys. Meteorol. 2011, 63, 589–618. [Google Scholar] [CrossRef]
- Kandler, K.; Schütz, L.; Jäckel, S.; Lieke, K.; Emmel, C.; Müller-Ebert, D.; Ebert, M.; Scheuvens, D.; Schladitz, A.; Šegvić, B. Ground-based off-line aerosol measurements at Praia, Cape Verde, during the Saharan Mineral Dust Experiment: Microphysical properties and mineralogy. Tellus B Chem. Phys. Meteorol. 2011, 63, 459–474. [Google Scholar] [CrossRef]
- Weinzierl, B.; Ansmann, A.; Prospero, J.M.; Althausen, D.; Benker, N.; Chouza, F.; Dollner, M.; Farrell, D.; Fomba, W.; Freudenthaler, V. The Saharan aerosol long-range transport and aerosol–cloud-interaction experiment: Overview and selected highlights. Bull. Am. Meteorol. Soc. 2017, 98, 1427–1451. [Google Scholar] [CrossRef]
- Gasteiger, J.; Groß, S.; Sauer, D.; Haarig, M.; Ansmann, A.; Weinzierl, B. Particle settling and vertical mixing in the Saharan Air Layer as seen from an integrated model, lidar, and in situ perspective. Atmos. Chem. Phys. 2017, 17, 297–311. [Google Scholar] [CrossRef]
- Sokolik, I.N. The spectral radiative signature of wind-blown mineral dust: Implications for remote sensing in the thermal IR region. Geophys. Res. Lett. 2002, 29, 7-1–7-4. [Google Scholar] [CrossRef]
- Li, L.; Mahowald, N.M.; Miller, R.L.; Pérez García-Pando, C.; Klose, M.; Hamilton, D.S.; Gonçalves Ageitos, M.; Ginoux, P.; Balkanski, Y.; Green, R.O. Quantifying the range of the dust direct radiative effect due to source mineralogy uncertainty. Atmos. Chem. Phys. 2021, 21, 3973–4005. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; di Lorenzo, F.; Elert, K. Mineralogy and physicochemical features of Saharan dust wet deposited in the Iberian Peninsula during an extreme<? xmltex\break?> red rain event. Atmos. Chem. Phys. 2018, 18, 10089–10122. [Google Scholar]

| Particle Group | Wavelength | |||
|---|---|---|---|---|
| 350 nm | 630 nm | 870 nm | 1640 nm | |
| Silicates | 1.60 | 1.56 | 1.54 | 1.54 |
| Si-rich (Quartz) | 1.57 | 1.55 | 1.53 | 1.53 |
| Ca-rich (Calcite) | 1.69 | 1.66 | 1.65 | 1.63 |
| S-rich (Sulfate) | 1.54 | 1.53 | 1.52 | 1.49 |
| Fe-rich (Hematite) | 2.30 | 2.8 | 2.6 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waza, A.; Schneiders, K.; Heuser, J.; Kandler, K. Analysis of Size Distribution, Chemical Composition, and Optical Properties of Mineral Dust Particles from Dry Deposition Measurement in Tenerife: Determined by Single-Particle Characterization. Atmosphere 2023, 14, 700. https://doi.org/10.3390/atmos14040700
Waza A, Schneiders K, Heuser J, Kandler K. Analysis of Size Distribution, Chemical Composition, and Optical Properties of Mineral Dust Particles from Dry Deposition Measurement in Tenerife: Determined by Single-Particle Characterization. Atmosphere. 2023; 14(4):700. https://doi.org/10.3390/atmos14040700
Chicago/Turabian StyleWaza, Andebo, Kilian Schneiders, Johannes Heuser, and Konrad Kandler. 2023. "Analysis of Size Distribution, Chemical Composition, and Optical Properties of Mineral Dust Particles from Dry Deposition Measurement in Tenerife: Determined by Single-Particle Characterization" Atmosphere 14, no. 4: 700. https://doi.org/10.3390/atmos14040700
APA StyleWaza, A., Schneiders, K., Heuser, J., & Kandler, K. (2023). Analysis of Size Distribution, Chemical Composition, and Optical Properties of Mineral Dust Particles from Dry Deposition Measurement in Tenerife: Determined by Single-Particle Characterization. Atmosphere, 14(4), 700. https://doi.org/10.3390/atmos14040700








