Abstract
Rapid urbanization and industrialization have heightened concerns about air quality worldwide. Conventional air purification methods, reliant on chemicals or energy-intensive processes, fall short in open spaces and in combating emerging pollutants. Addressing these limitations, this study presents a novel water-film air purification prototype leveraging the adhesion between low-curvature liquid surfaces and air convection friction. Uniquely designed, this prototype effectively targets toxic gases (e.g., formaldehyde, SO2, NO2) and particulate matter (such as PM2.5) while allowing continuous airflow. This research explores the adhesion and sedimentation capabilities of a low-curvature water solution surface under convection friction, reducing the surface energy to remove airborne pollutants efficiently. The prototype was able to reduce the initial concentration in a 30 m³ chamber within 180 min by 91% for formaldehyde, 78% for nitrogen dioxide (NO2), 99% for sulfur dioxide (SO2), and 96% for PM2.5. Experimentally validated indicators—decay constants, CADR, and purification efficiency—enable a comprehensive evaluation of the purification device, demonstrating its efficacy in mitigating air pollution. This innovative design, which is cost-effective due to its use of easily accessible components and water as the primary medium, indicates strong potential for large-scale deployment. This study points to an environmentally friendly and economical approach to air purification, shedding light on a promising direction for enhancing indoor air quality. Further optimization and exploration of diverse pollutants and environmental conditions will propel the practical applications of this pioneering technology.
1. Introduction
Air pollution has become a significant global challenge, posing threats to both the environment and human health [1,2]. Harmful gases and particles, emitted from sources such as vehicles, industries, domestic heating, and daily activities, severely compromise air quality [3]. Consequently, there is an urgent need for the development of efficient, cost-effective, and environmentally friendly air purification technologies [4]. Addressing this necessity is crucial in safeguarding our environment and human well-being [5,6].
In recent decades, air purifiers have found extensive use in indoor and enclosed environments [7]. They employ physical and chemical methods to effectively remove pollutants from the air [8,9]. However, existing air purification technologies suffer from several drawbacks, including the introduction of additional pollutants, high energy consumption, and expensive maintenance costs [10]. Hence, the search for a novel and efficient air purification approach is of utmost significance [11,12].
In recent years, researchers have begun exploring the potential of the adhesive effect of liquid surfaces in air purification [13]. In particular, the adhesive effect resulting from convection friction between a low-curvature liquid surface and air has shown promising advantages for air purification. In this context, the low curvature of the liquid surface can capture pollutants from the air through convection friction within a certain velocity range, thus achieving air purification [14].
This paper aims to investigate the utilization of the adhesive effect derived from convection friction between a low-curvature liquid surface and air for air purification. We will focus on studying the adhesive mechanisms of the liquid surface and the purification efficiency of the liquid surface for major harmful substances in the air. By designing appropriate experimental setups and conducting systematic experimental investigations, we will assess the performance and effectiveness of this innovative air purification method.
The findings of this study are expected to provide new insights and approaches for the development of more efficient, cost-effective, and environmentally friendly air purification technologies [15,16]. We firmly believe that through a deeper understanding of the adhesive effect derived from convection friction between a low-curvature liquid surface and air, we can make significant contributions to improving air quality and protecting human health [17,18,19,20].
2. Materials and Methods
2.1. Intermolecular Forces
Figure 1 illustrates the concept of intermolecular forces, pivotal in air purification as they regulate interactions among airborne molecules. As illustrated in Figure 1, molecular interactions have a specific distance termed the equilibrium distance (often denoted as r0). At this distance, the forces between molecules reach a balance between repulsion and attraction, leading to a neutral net force between them. When the distance between two molecules is less than this equilibrium distance (r0), it usually leads to repulsion. This arises due to the overlap of electron clouds between atomic nuclei, causing an increase in repulsive forces between electron clouds. Conversely, when the distance between molecules exceeds the equilibrium distance (r0), they experience primarily attractive van der Waals forces. The pollutant molecules and water molecules exist within this distance range; hence, intermolecular force will enable the water film to possess the capability to adsorb pollutants.
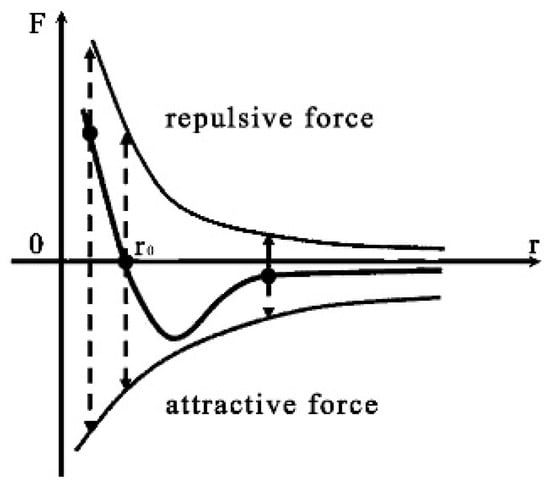
Figure 1.
Intermolecular forces.
In our research, we focus on the influence of intermolecular forces on air purification at the low-curvature liquid surface. A low-curvature liquid surface has a small curvature, placing the liquid surface in a nearly flat state. This characteristic allows the liquid surface to come into contact with pollutants in the air and adsorb them through intermolecular forces. In preliminary experiments, we observed that small water droplets tend to rapidly evaporate their surface substances into the air, while larger droplets with flatter surfaces exhibit lower rates of evaporation per unit area over time. Therefore, the relatively stable and low-curvature liquid surface enhances the intermolecular attraction and improves the adhesion capability of pollutants to the liquid surface. This provides a foundation for utilizing the adhesive effect derived from convection friction between a low-curvature liquid surface and air to achieve air purification.
The phenomenon of material accumulation on a surface is commonly referred to as adhesion or adsorption. In the case of a solution, the surface layer of the solution exhibits higher activity and possesses adhesion capability. Moreover, when a solute is added, it can reduce the surface tension, causing the solute to preferentially accumulate in the surface layer to minimize the system’s surface energy. Conversely, when the solute increases the surface tension, its concentration in the surface layer becomes lower than that in the bulk. In such cases, due to the concentration difference, the surface components of the solution tend to equilibrate with the internal concentration of the solution. To achieve dynamic equilibrium, the substances in the solution’s surface layer will diffuse into the solution’s interior, leading to adsorption at the solution’s surface. Furthermore, many harmful substances exhibit high solubility in water, further increasing the adsorption capacity of water surfaces for these harmful substances. Water is an excellent solvent, non-toxic, harmless, and environmentally friendly, making the flowing water surface highly suitable for adsorbing harmful substances.
2.2. Experimental Prototype Design for Air Purification
The structural and schematic diagrams of the indoor air purification device designed in this study are illustrated in Figure 2. In Figure 2a, the device uses an air-driven fan device (2) to draw indoor air into the device chamber through a pipe (1). A motor (3) in the device drives a large number of stainless steel blades (4) that are partially immersed in water. The rotation speed is set at 100 rpm. The blade surfaces exhibit strong water-absorbing properties, allowing the entire blade surface to form a layer of low-curvature water film during rotation.
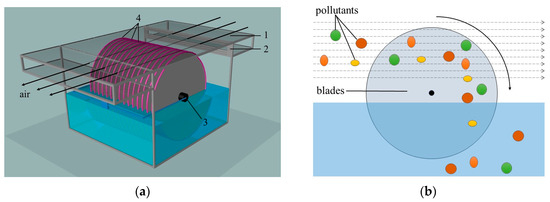
Figure 2.
(a) Structural diagram; (b) schematic diagram.
In the air purification device, it is essential to ensure thorough contact and friction between the low-curvature water surface and the air. Therefore, we utilized an air-driven fan device to draw air containing toxic gases or PM2.5 from the ventilation duct into the purification device’s chamber. As shown in Figure 2b, these pollutants were adsorbed and absorbed by the low-curvature water surface, carried into the water by the blades, and subsequently precipitated. Purified clean air was discharged from the opposite side of the purification device, facilitating the circulation of air and the continuous removal of harmful substances by the purification device.
Additionally, we established a stable experimental space and placed the experimental setup inside to assess its air purification characteristics. We ensured that the overall temperature during the experiments remained at (21 ± 2.5) °C, while the relative humidity was maintained at (40 ± 5)%. Throughout the testing process, specific concentrations of air pollutants were released into the ventilation duct of the test chamber. Using pollutant concentration measuring instruments, we recorded the air pollutant concentration data within the chamber over time to quantitatively analyze the results of air purification.
To enhance the water adsorption capacity and hydrophilicity of the blade surface, we performed a chemical oxidation treatment on the blades. This treatment was carried out at room temperature by using a strong oxidizing agent. Subsequently, we conducted contact angle measurements on the blades using a contact angle analyzer. The measured contact angle was 33.5° ± 0.5°, indicating good hydrophilicity of the blades. This enables the blades to strongly adsorb water during rotation, effectively forming a large water surface area. Stainless steel was chosen as the blade material due to its corrosion resistance, good wear resistance, and ease of processing, making it highly suitable for the device blades. By chemically oxidizing and optimizing the surface characteristics of the blades, we improved their hydrophilicity and water adsorption capacity, providing an important foundation for the performance of the air purification device.
The purification efficiency of the water film is influenced by several factors:
- Temperature: Higher temperatures generally lead to reduced adhesion forces.
- Surface Charge of Pollutants: Charged molecules or particles tend to adhere more readily to the water surface.
- pH Value of the Water Film: Acidic or alkaline conditions affect surface charge and subsequently impact adhesion.
- Hydrophilicity of Pollutants: Substances with strong hydrophilicity are less likely to adhere, while those with strong hydrophobicity are more prone to adhesion.
- Pollutants Adsorption: Special chemical adsorption can also increase adhesion forces.
Therefore, adjusting these factors allows for the control of the adhesive properties of the water surface. Ideal properties of the water surface should include the following:
- High surface tension, approximately 72 mN/m at 21 °C.
- An approaching contact angle of 0°, indicating high hydrophilicity of the blade surface.
- A water film with an ideal, smooth interface without geometric deformations.
- Uniform and consistent surface tension without irregularities or fluctuations.
- The ability to form highly ordered surface structures through hydrogen bonding.
- Low surface roughness, providing a smooth surface.
This design of the air purification device offers high purification efficiency and reliability, providing a simple, effective, and economical method for improving indoor air quality.
2.3. Air Purification Testing Standards
The test environment was set up according to the following standards: the experimental volume was 30 m3, the temperature was maintained at (21 ± 2.5) °C, and the relative humidity was kept within the range of (40 ± 5)%. Additionally, the air leakage in the test chamber should be less than 0.05 m3/h to ensure the stability and accuracy of the experimental environment.
For the evaluation of air purification devices, the following indicators will be considered for calculation:
- Decay Constant (k): The decay constant is used to assess the removal efficiency of pollutants by the purifier. Its calculated value represents the slope of the regression line depicting the decay of particulate matter or gaseous pollutants in the experimental environment. A larger decay constant indicates a faster pollutant removal efficiency. Therefore, the decay constant plays a crucial role in guiding the optimization and application of air purification technologies.
The calculation method for the pollutant’s decay constant (k) is as follows:
where Ct is the pollutant concentration (mg/m3 or ppm) at time t, C0 is the initial pollutant concentration (mg/m3 or ppm) at time t = 0, k is the decay constant (min−1), and t is the time (min).
The formula for calculating k is as follows:
- 2.
- Clean Air Delivery Rate (CADR): CADR refers to the volume of air that the air purification device can purify per unit time. The calculation method is as follows:
- 3.
- Purification Efficiency: The purification efficiency indicates the ability of the air purification device to remove specific pollutants. It can be calculated based on the pollutant concentration before and after passing through the purifier. The formula for calculating the removal efficiency is as follows:
Through a comprehensive assessment of these indicators, we can objectively evaluate the performance and purification effectiveness of the air purification device, providing a comprehensive display of its air purification capabilities. These data also play a crucial guiding role in optimizing and implementing air purification technology, offering robust support for further research and applications.
3. Results and Discussion
3.1. Purification Efficiency of Formaldehyde
Using the above air purification device, a gas containing an initial concentration of 0.6 mg/m3 formaldehyde was purified. The test data obtained are shown in Figure 3.
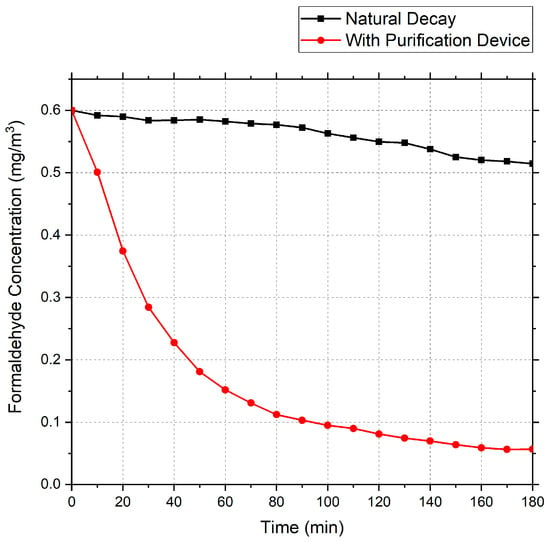
Figure 3.
Formaldehyde decay curve.
Based on the data, the total measured decay constant (Ke) for formaldehyde over three hours was calculated as 0.0128 min−1, the natural decay constant (Kn) was 0.00086 min−1, the clean air delivery rate (CADR) was 0.0953 m3/min, and the concentration was reduced by 91% after 180 min.
3.2. Purification Efficiency of NO2
Using the above air purification device, a gas containing an initial concentration of 40 ppm sulfur dioxide (NO2) was purified. The test data obtained are shown in Figure 4.
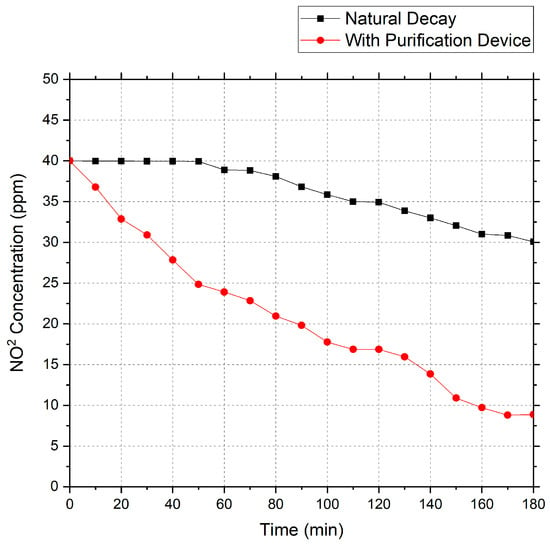
Figure 4.
NO2 decay curve.
Based on the data, the total measured decay constant (Ke) for NO2 over three hours was calculated as 0.0082 min−1, the natural decay constant (Kn) was 0.0018 min−1, the clean air delivery rate (CADR) was 0.0518 m3/min, and the concentration was reduced by 78% after 180 min.
3.3. Purification Efficiency of SO2
Using the above air purification device, a gas containing an initial concentration of 80 ppm SO2 was purified. The test data obtained are shown in Figure 5.
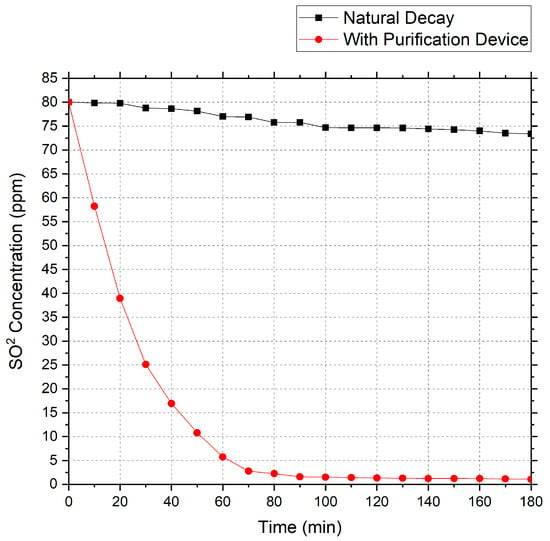
Figure 5.
SO2 decay curve.
Based on the data, the total measured decay constant (Ke) for SO2 over three hours was calculated as 0.0247 min−1, the natural decay constant (Kn) was 0.00052 min−1, the clean air delivery rate (CADR) was 0.1935 m3/min, and the concentration was reduced by 99% after 180 min.
3.4. Purification Efficiency of PM2.5
Using the above air purification device, a gas containing an initial concentration of 550 ppm PM2.5 was purified. The test data obtained are shown in Figure 6.
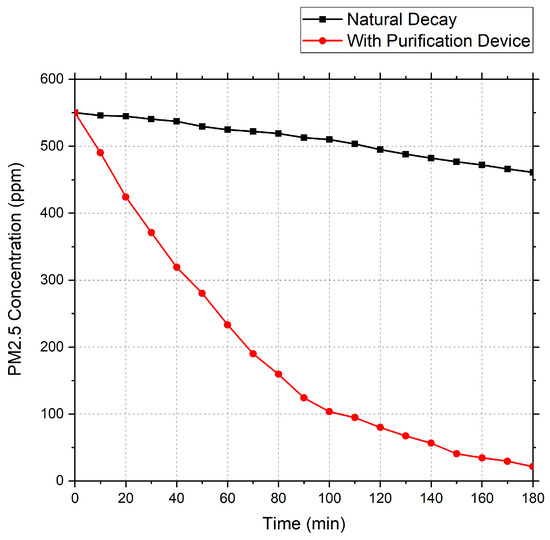
Figure 6.
PM2.5 decay curve.
Based on the data, the total measured decay constant (Ke) for PM2.5 over three hours was calculated as 0.0179 min−1, the natural decay constant (Kn) was 0.00101 min−1, the clean air delivery rate (CADR) was 0.1348 m3/min, and the concentration was reduced by 96% after 180 min.
3.5. Results and Discussion
The experimental data are presented in Table 1.

Table 1.
Purification data for different pollutants.
The test outcomes demonstrate a purification efficiency exceeding 90% for water-soluble pollutants like formaldehyde and SO2, indicating excellent removal capabilities. In the case of PM2.5, it is easy to be adsorbed by the water film, so the purification efficiency reaches 96%, effectively eliminating particulate matter pollution from the air. The removal efficiency for NO2 is comparatively lower, attributed to its lower initial concentration in the experiment and higher natural decay rate.
Comparing this study with similar research endeavors, a negative ion air purifier achieves a 54.67% purification efficiency for formaldehyde and a 95.35% purification efficiency for PM2.5 [19]. Another air purifier utilizing KOH-modified active carbon with active potassium permanganate ball as an adsorbent achieves an 80.2% purification efficiency for SO2 [20]. After a comparative experiment with the same test duration and conditions as consistent as possible in terms of the test environment, this experimental prototype still has demonstrated superior purification capabilities across these various pollutants. Notably, the components used in this prototype—fan, motor, piping, and stainless steel blades—are easily obtainable, reducing costs. Furthermore, the primary medium for air purification is water, further lowering the overall cost of air purification. This enhances the potential scalability of this air purification method for large-scale applications. Compared to other air purification devices, this prototype also has an advantage in terms of noise levels. Overall, this unit can simultaneously purify multiple pollutants, boasting high pollutant removal efficiency, low cost, minimal noise, and environmental friendliness.
4. Conclusions
In this study, we designed and developed an innovative air purification device based on the adhesion effect between low-curvature liquid surfaces and air convection friction. By harnessing the adhesion between liquid surfaces and air, we effectively removed common pollutants such as formaldehyde, NO2, SO2, and PM2.5 during the air purification process. The purification efficiency for formaldehyde, NO2, SO2, and PM2.5 reached 91%, 78%, 99%, and 96%, respectively. These test results were obtained within a 30 m3 space over a duration of 180 min. Furthermore, based on the test data, the total measured decay constants (Ke) for formaldehyde over three hours amounted to 0.0128 min−1 with a clean air delivery rate (CADR) of 0.0953 m3/min; for NO2, it was 0.0082 min−1 with a CADR of 0.0518 m3/min; for SO2, it was 0.0247 min−1 with a CADR of 0.1935 m3/min; and for PM2.5, it was 0.0179 min−1 with a CADR of 0.1348 m3/min. These validated indicators verify the reliability of the device and comprehensively assess its performance, confirming its significant impact in mitigating air pollution.
Compared to other air purification methods, this approach stands out due to its simplicity, efficiency, cost-effectiveness, and environmental friendliness. We believe this research offers a fresh perspective and method in the development of air purification technology, making a substantial contribution to improving air quality. The experimental prototype is straightforward to assemble and cost-effective. Components such as the fan, motor, piping, and stainless steel blades utilized in the prototype are easily accessible, contributing to its affordability. Moreover, water serves as the primary medium for purification, further lowering the overall purification cost. Consequently, this method exhibits high feasibility for large-scale application.
Future research directions could involve further optimizing the design of the purification device to enhance its purification efficiency and stability. Additionally, deeper investigations into different types of pollutants and various environmental conditions could expand the applicability and feasibility of this advanced technology in diverse practical applications.
Author Contributions
Conceptualization, Y.Z. (Yaozhong Zhang); methodology, H.Y.; validation, H.W.; formal analysis, H.W.; investigation, X.H.; resources, Y.Z. (Yafei Zhang); data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, Y.Z. (Yaozhong Zhang); supervision, Y.Z. (Yafei Zhang); project administration, Y.Z. (Yafei Zhang). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Z.; Tang, Z.; Song, Y.; Yang, G.; Qian, W.; Yang, M.; Zhu, Y.; Ran, R.; Wang, W.; Zhou, W.; et al. High-Entropy Perovskite Oxide: A New Opportunity for Developing Highly Active and Durable Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nano-Micro Lett. 2022, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.P.; Perumpully, S.J.; Samuel, C.; Gautam, S. Exposure and health: A progress update by evaluation and scientometric analysis. Stoch. Environ. Res. Risk Assess. 2023, 37, 453–465. [Google Scholar] [CrossRef]
- Friedler, E.; Gilboa, Y.; Muklada, H. Quality of Roof-Harvested Rainwater as a Function of Environmental and Air Pollution Factors in a Coastal Mediterranean City (Haifa, Israel). Water 2017, 9, 896. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ning, C.; Shen, Y.; Yao, C.; Chen, D.; Cheng, S. Projection of the Co-Reduced Emissions of CO2 and Air Pollutants from Civil Aviation in China. Sustainability 2023, 15, 7082. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Sun, R.; Li, M.; Liu, H.; Shi, Y.; Zhu, D.; Li, J.; Ma, L.; Fu, S. Influence of nitrogen addition on the functional diversity and biomass of fine roots in warm-temperate and subtropical forests. For. Ecol. Manag. 2023, 545, 121309. [Google Scholar] [CrossRef]
- Lee, J.J.; Hwang, H.; Hong, S.C.; Lee, J.Y. Effect of Air Purification Systems on Particulate Matter and Airborne Bacteria in Public Buses. Atmosphere 2022, 13, 55. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Cao, J.-J.; Huang, Y.; Zhang, Q. Ambient Air Purification by Nanotechnologies: From Theory to Application. Catalysts 2021, 11, 1276. [Google Scholar] [CrossRef]
- Niu, J.; Tung, T.; Burnett, J. Quantification of dust removal and ozone emission of ionizer air-cleaners by chamber testing. J. Electrost. 2001, 51–52, 20–24. [Google Scholar] [CrossRef]
- Jin, H.; Yu, J.; Cui, D.; Gao, S.; Yang, H.; Zhang, X.; Hua, C.; Cui, S.; Xue, C.; Zhang, Y.; et al. Remote Tracking Gas Molecular via the Standalone-Like Nanosensor-Based Tele-Monitoring System. Nano-Micro Lett. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zou, J.; Yang, W.; Li, C.-Q. A Review of Recent Advances in Research on PM2.5 in China. Int. J. Environ. Res. Public Health 2018, 15, 438. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-Y.; Li, C.-H.; Chang, L.-M.; Jau, H.-C.; Lo, W.-C.; Lin, W.-C.; Wang, C.-T.; Lin, T.-H. Functional Superhydrophobic Surfaces with Spatially Programmable Adhesion. Polymers 2020, 12, 2968. [Google Scholar] [CrossRef] [PubMed]
- Kaim, S.D. The Molecular Theory of Liquid Nanodroplets Energetics in Aerosols. Entropy 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Oliveira, G.M.; Monteiro, H.; Silva, G.V.; Caetano, N.S.; Martins, A.A. Indoor Air Quality Improvement Using Nature-Based Solutions: Design Proposals to Greener Cities. Int. J. Environ. Res. Public Health 2021, 18, 8472. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.F.; Huang, N.; Niu, Z.; Qin, Y.C.; Pei, J.; Wang, J. Mapping Winter Crops in China with Multi-Source Satellite Imagery and Phenology-Based Algorithm. Remote Sens. 2019, 11, 820. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, T.; Yi, S.; Huang, Y.; Guo, Y.; Huang, S.; Zhou, C.; Zhou, R.; Cao, H. Reducing the Effectiveness of Ward Particulate Matter, Bacteria and Influenza Virus by Combining Two Complementary Air Purifiers. Int. J. Environ. Res. Public Health 2022, 19, 10446. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, A.; Chen, H.; Zhao, Z.; Cai, J.; Wang, C.; Yang, C.; Li, H.; Xu, X.; Ha, S.; et al. Cardiopulmonary Benefits of Reducing Indoor Particles of Outdoor Origin: A randomized, double-blind crossover trial of air purifiers. J. Am. Coll. Cardiol. 2015, 65, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. Design and Optimization of Desktop Negative Ion Air Purifier. Master’s Thesis, Beijing Institute of Petrochemical Technology, Beijing, China, 2018. [Google Scholar]
- Chen, J.; Xue, X.; Li, M. Removal of SO2 from fresh air system by a novel integrated air cleaner. J. Environ. Health 2017, 34, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).