Seasonal Variations of Particulate Matter Capture and the Air Pollution Tolerance Index of Five Roadside Plant Species
Abstract
1. Introduction
2. Materials and Methods
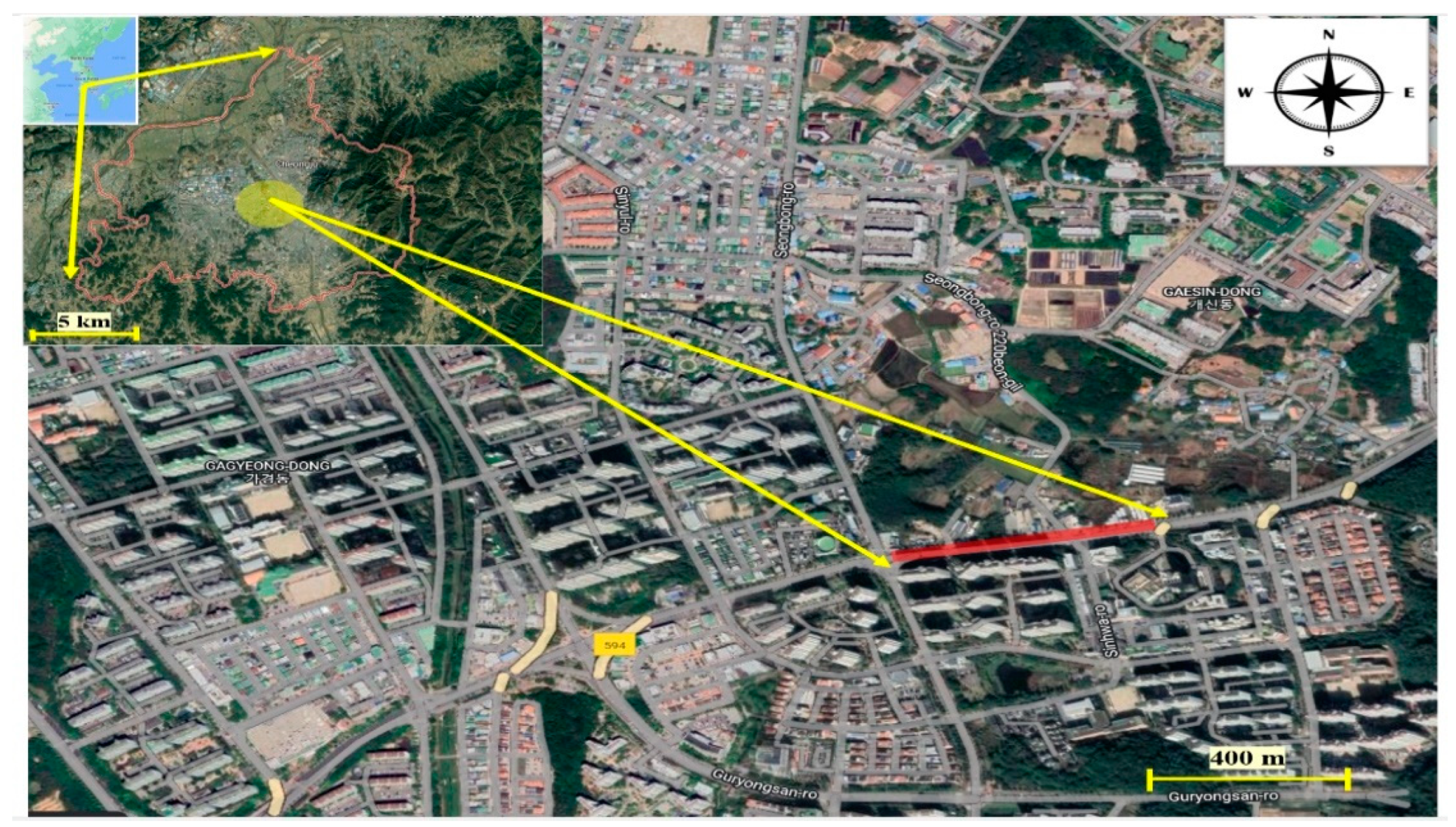
2.1. Study Area and Sampling Collection
2.2. Accumulation of Surface PM, In-Wax PM, and Epicuticular Waxes on Leaves
2.3. Leaves Traits
2.3.1. Leaf Extract pH (pH)
2.3.2. The Relative Leaf Water Content (RWC)
2.3.3. Chlorophyll Contents
2.3.4. Ascorbic Acid (AA)
2.4. The Air Pollution Tolerance Index (APTI)
3. Statistical Analysis
4. Results and Discussion
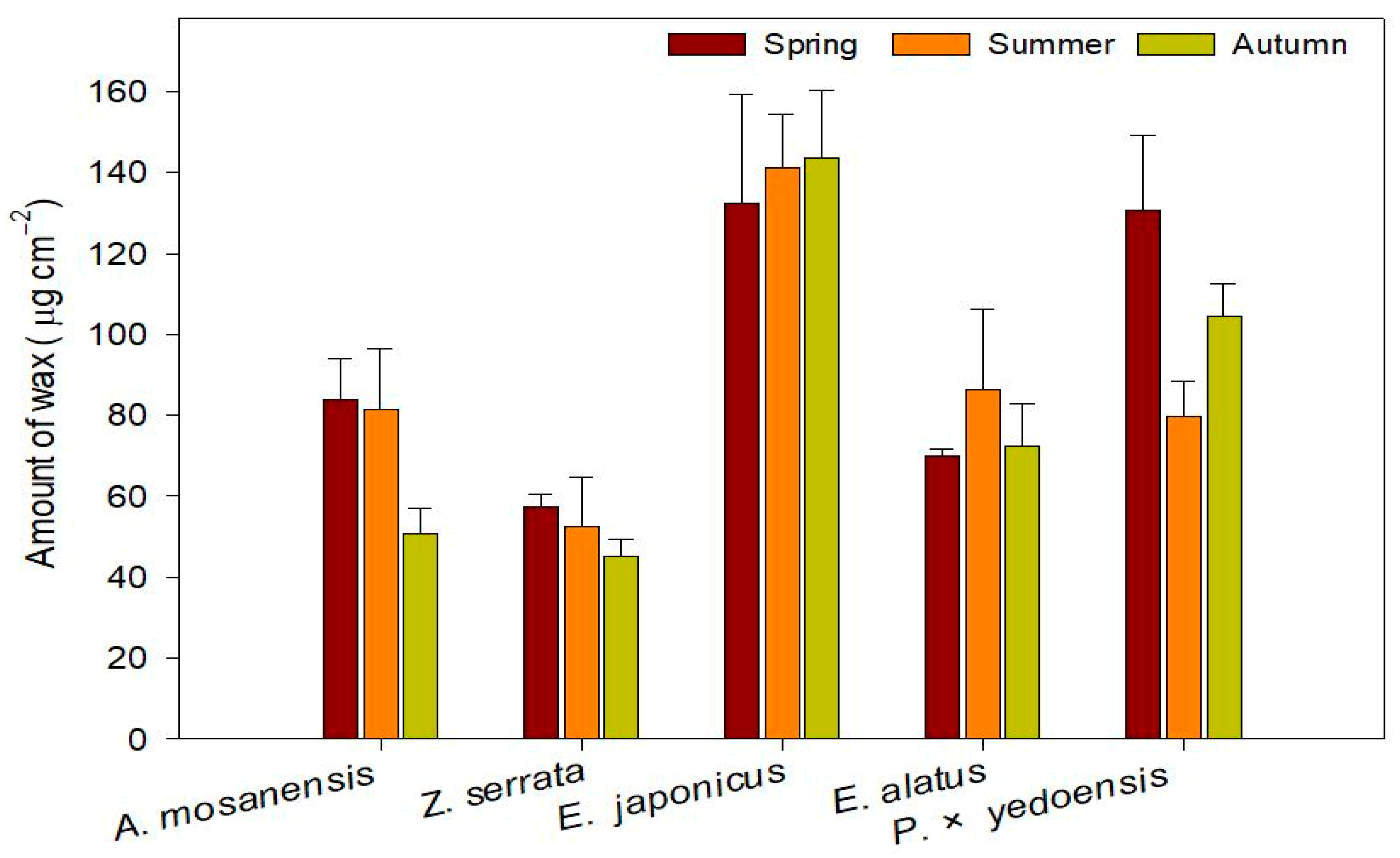
4.1. PM accumulation of Plants
4.2. Leaf Traits
4.3. The APTI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10). Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 1 October 2022.).
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. A global perspective of fine particulate matter pollution and its health effects. Rev. Environ. Contam. Toxicol. 2018, 244, 5–51. [Google Scholar] [CrossRef]
- Yang, J.; McBride, J.; Zhou, J.; Sun, Z. The urban forest in Beijing and its role in air pollution reduction. Urban For. Urban Green. 2005, 3, 65–78. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, J.; Han, Y.; Sun, N.; Sun, W.; Li, J.; Liu, C.; Yin, S. Effects of the leaf functional traits of coniferous and broadleaved trees in subtropical monsoon regions on PM2.5 dry deposition velocities. Environ. Pollut. 2020, 265, 114845. [Google Scholar] [CrossRef]
- Bui, H.T.; Odsuren, U.; Kim, S.Y.; Park, B.J. Particulate matter accumulation and leaf traits of ten woody species growing with different air pollution conditions in Cheongju City, South Korea. Atmosphere 2022, 13, 1351. [Google Scholar] [CrossRef]
- Popek, R.; Przybysz, A.; Gawrońska, H.; Klamkowski, K.; Gawroński, S.W. Impact of particulate matter accumulation on the photosynthetic apparatus of roadside woody plants growing in the urban conditions. Ecotoxicol. Environ. Saf. 2018, 163, 56–62. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Bao, L.; Mo, L.; Yu, X.; Fan, D.; Lun, X. Influence of rainfall duration and intensity on particulate matter removal from plant leaves. Sci. Total. Environ. 2017, 609, 11–16. [Google Scholar] [CrossRef]
- Kwon, K.J.; Odsuren, U.; Bui, H.T.; Kim, S.Y.; Park, B.J. Growth and physiological responses of four plant species to different sources of particulate matter. J. People Plants Environ. 2021, 24, 461–468. [Google Scholar] [CrossRef]
- Mulenga, C.; Clarke, C.; Meincken, M. Physiological and growth responses to pollutant-induced biochemical changes in plants: A review. Pollution 2020, 6, 827–848. [Google Scholar] [CrossRef]
- Panda, L.R.L.; Aggarwal, R.K.; Bhardwaj, D.R. A review on air pollution tolerance index (APTI) and anticipated performance index (API). Curr. World Environ. J. 2018, 13, 55–65. [Google Scholar] [CrossRef]
- Sahu, C.; Basti, S.; Sahu, S.K. Air pollution tolerance index (APTI) and expected performance index (EPI) of trees in Sambalpur town of India. SN Appl. Sci. 2020, 2, 1327. [Google Scholar] [CrossRef]
- Singh, S.; Rao, D.; Agrawal, M.; Pandey, J.; Naryan, D. Air pollution tolerance index of plants. J. Environ. Manag. 1991, 32, 45–55. [Google Scholar] [CrossRef]
- Han, D.; Shen, H.; Duan, W.; Chen, L.A. review on particulate matter removal capacity by urban forests at different scales. Urban For. Urban Green. 2019, 48, 126565. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Wang, Y. Effects of weather, time, and pollution level on the amount of particulate matter deposited on leaves of Ligustrum lucidum. Sci. World J. 2015, 2015, 935942. [Google Scholar] [CrossRef]
- Chungcheonbuk-Do Institute of Health and Environment. Available online: https://here.chungbuk.go.kr (accessed on 16 December 2022).
- Kwon, K.J.; Urrintuya, O.; Kim, S.Y.; Yang, J.C.; Sung, J.W.; Park, B.J. Removal potential of particulate matter of 12 woody plant species for landscape planting. J. People Plants Environ. 2020, 23, 647–654. [Google Scholar] [CrossRef]
- Bui, H.T.; Odsuren, U.; Kim, S.Y.; Park, B.J. Seasonal variations in the particulate matter accumulation and leaf traits of 24 plant species in urban green space. Land 2022, 11, 1981. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediation 2011, 13, 1037–1046. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Dinesh, B.; Yadav, B.; Reddy, R.D.; Padma, A.S.; Sukumaran, M.K. Determination of ascorbic acid content in some Indian spices. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 864–868. [Google Scholar]
- Ghafari, S.; Kaviani, B.; Sedaghathoor, S.; Allahyari, M.S. Assessment of air pollution tolerance index (APTI) for some ornamental woody species in green space of humid temperate region (Rasht, Iran). Environ. Dev. Sustain. 2021, 23, 1579–1600. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, F.; Zhang, X.; Gao, T. Difference of airborne particulate matter concentration in urban space with different green coverage rates in Baoji, China. Int. J. Environ. Res. Public Health 2019, 16, 1465. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.J.; Yoon, J.H.; Bae, E.J.; Jeong, B.R.; Yong, S.H.; Choi, M.S. Particulate matter removal ability of ten evergreen trees planted in Korea urban greening. Forests 2021, 12, 438. [Google Scholar] [CrossRef]
- Popek, R.; Gawrońska, H.; Wrochna, M.; Gawroński, S.W.; Sæbø, A. Particulate matter on foliage of 13 woody species: Deposition on surfaces and phytostabilisation in waxes–a 3-year study. Int. J. Phytoremediation 2013, 15, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Particulate pollution capture by urban trees: Effect of species and windspeed. Glob. Chang. Biol. 2000, 6, 995–1003. [Google Scholar] [CrossRef]
- Gourdji, S. Review of plants to mitigate particulate matter, ozone as well as nitrogen dioxide air pollutants and applicable recommendations for green roofs in Montreal, Quebec. Environ. Pollut. 2018, 241, 378–387. [Google Scholar] [CrossRef]
- Rahul, J.; Jain, M.K. An investigation in to the impact of particulate matter on vegetation along the national highway: A review. Environ. Sci. 2014, 8, e372. [Google Scholar] [CrossRef]
- Kwak, M.J.; Lee, J.K.; Park, S.; Kim, H.; Lim, Y.J.; Lee, K.A.; Son, J.-a.; Oh, C.Y.; Kim, I.; Woo, S.Y. Surface-based analysis of leaf microstructures for adsorbing and retaining capability of airborne particulate matter in ten woody species. Forests 2020, 11, 946. [Google Scholar] [CrossRef]
- Łukowski, A.; Popek, R.; Karolewski, P. Particulate matter on foliage of Betula pendula, Quercus robur, and Tilia cordata: Deposition and ecophysiology. Environ. Sci. Pollut. Res. 2020, 27, 10296–10307. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in tree species ability to capture and retain airborne fine particulate matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, G.; An, H.; Yin, W.; Xia, X. Quantifying the particulate matter accumulation on leaf surfaces of urban plants in Beijing, China. Atmos. Pollut. Res. 2017, 8, 836–842. [Google Scholar] [CrossRef]
- Lu, T.; Lin, X.; Chen, J.; Huang, D.; Li, M. Atmospheric particle retention capacity and photosynthetic responses of three common greening plant species under different pollution levels in Hangzhou. Glob. Ecol. Conserv. 2019, 20, e00783. [Google Scholar] [CrossRef]
- Sgrigna, G.; Baldacchini, C.; Dreveck, S.; Cheng, Z.; Calfapietra, C. Relationships between air particulate matter capture efficiency and leaf traits in twelve tree species from an Italian urban-industrial environment. Sci. Total Environ. 2020, 718, 137310. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Ki Király, L.; Künstler, A. The role of plant grwoth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Das, M.; Das, M.; Mukherjee, A. Air pollution tolerance index (APTI) used for assessing air quality to alleviate climate change: A review. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 45–54. [Google Scholar]
- Mei, P.; Malik, V.; Harper, R.W.; Jiménez, J.M. Air pollution, human health and the benefits of trees: A biomolecular and physiologic perspective. Arboric. J. 2021, 43, 19–40. [Google Scholar] [CrossRef]
- Shannigrahi, A.S.; Fukushima, T.; Sharma, R.C. Anticipated air pollution tolerance of some plant species considered for green belt development in and around an industrial/urban area in India: An overview. Int. J. Environ. Stud. 2004, 61, 125–137. [Google Scholar] [CrossRef]
- Tripathi, A.; Gautam, M. Biochemical parameters of plants as indicators of air pollution. J. Environ. Biol. 2007, 28, 127–132. [Google Scholar]
- Pandit, J.; Sharma, A.K. A review of effects of air pollution on physical and biochemical characteristics of plants. Int. J. Chem. Stud. 2020, 8, 1684–1688. [Google Scholar] [CrossRef]
- Bui, H.T.; Odsuren, U.; Jeong, M.; Seo, J.W.; Kim, S.Y.; Park, B.J. Evaluation of the air pollution tolerance index of 12 plant species grwoing in environments with different airr pollution levels. J. People Plants Environ. 2022, 25, 23–31. [Google Scholar] [CrossRef]
- Karmakar, D.; Padhy, P.K. Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 2019, 237, 124522. [Google Scholar] [CrossRef] [PubMed]

| Plant species | Family | Foliage | Habit | Height |
|---|---|---|---|---|
| Abelia mosanensis T.H.Chung | Caprifoliaceae | Deciduous shrub | Shrub | 1 m |
| Zelkova serrata (Thunb.) Makino | Ulmaceae | Deciduous broad leaved | Tree | 10 m |
| Euonymus japonicus Thunb. | Celastraceae | Evergreen broad leaved | Shrub | 1 m |
| Euonymus alatus (Thunb.) Siebold | Celastraceae | Deciduous shrub | Shrub | 1 m |
| Prunus × yedoensis Matsum. | Rosaceae | Deciduous broad leaved | Tree | 10 m |
| Tolerance Level | Calculated Method |
|---|---|
| Tolerant (T) | APTI > mean APTI + SD |
| Moderately tolerant (MT) | Mean APTI < APTI < mean APTI + SD |
| Intermediate (T) | Mean APTI − SD < APTI < mean APTI |
| Sensitive (S) | APTI < mean APTI − SD |
| Leaf Traits | Seasons | A. mosanensis | Z. serrata | E. japonicus | E. alatus | P. × yedoensis |
|---|---|---|---|---|---|---|
| AA (mg·g−1) | Spring | 0.15 ± 0.01 b † | 0.22 ± 0.06 b | 0.47 ± 0.09 b | 0.60 ± 0.07 a | 0.23 ± 0.02 b |
| Summer | 0.35 ± 0.05 a | 0.49 ± 0.09 a | 0.67 ± 0.09 a | 0.48 ± 0.09 b | 0.45 ± 0.08 a | |
| Autumn | 0.36 ± 0.04 a | 0.43 ± 004 a | 0.55 ± 0.20 ab | 0.42 ± 0.05 b | 0.44 ± 0.05 a | |
| significant | *** | ** | ns | *** | *** | |
| pH | Spring | 5.82 ± 0.05 b | 5.69 ± 0.19 a | 5.92 ± 0.14 a | 5.87 ± 0.17 b | 5.62 ± 0.14 a |
| Summer | 6.01 ± 0.08 a | 5.87 ± 0.13 a | 5.82 ± 0.10 a | 6.12 ± 0.18 a | 5.82 ± 0.16 a | |
| Autumn | 5.71 ± 0.04 c | 5.69 ± 0.13 a | 5.83 ± 0.12 a | 5.92 ± 0.08 ab | 5.60 ± 0.28 a | |
| significant | *** | ns | ns | ns | ns | |
| RWC (%) | Spring | 51.66 ± 3.56 c | 74.13 ± 7.41 a | 66.83 ± 4.82 b | 55.29 ± 2.70 b | 61.15 ± 2.55 b |
| Summer | 75.13 ± 3.17 a | 69.44 ± 2.68 a | 74.56 ± 2.82 a | 77.65 ± 2.72 a | 78.04 ± 3.17 a | |
| Autumn | 65.96 ± 2.22 b | 65.16 ± 8.03 a | 77.45 ± 4.20 a | 76.30 ± 3.09 a | 78.12 ± 1.86 a | |
| significant | *** | ns | ** | *** | *** | |
| TChl (mg·g−1) | Spring | 0.17 ± 0.02 a | 0.34 ± 0.07 a | 0.16 ± 0.02 a | 0.13 ± 0.03 a | 0.21 ± 0.03 a |
| Summer | 0.16 ± 0.02 a | 0.16 ± 0.07 b | 0.13 ± 0.04 ab | 0.12 ± 0.01 a | 0.17 ± 0.04 ab | |
| Autumn | 0.16 ± 0.02 a | 0.23 ± 0.04 b | 0.11 ± 0.04 b | 0.11 ± 0.02 a | 0.14 ± 0.00 b | |
| significant | ns | ** | ns | ns | * |
| AA | pH | RWC | TChl | APTI | ||
|---|---|---|---|---|---|---|
| Spring | sPM (10–100 µm) | 0.390 * | 0.448 | 0.107 | −0.344 * | 0.154 |
| sPM (2.5–10 µm) | 0.546 * | 0.268 | −0.475 | −0.445 | −0.408 | |
| wPM (10–100 µm) | 0.539 * | 0.452 | 0.087 * | −0.411 ** | 0.151 * | |
| wPM (2.5–10 µm) | 0.322 | 0.122 | −0.074 | −0.435 ** | −0.036 | |
| Summer | sPM (10–100 µm) | 0.450 * | 0.330 | 0.256 * | −0.475 * | 0.338 |
| sPM (2.5–10 µm) | 0.444 * | 0.109 | 0.377 | −0.379 | 0.454 * | |
| wPM (10–100 µm) | 0.427 * | 0.255 * | 0.286 * | −0.445 * | 0.363 | |
| wPM (2.5–10 µm) | 0.029 | 0.108 | −0.167 * | −0.518 ** | −0.162 | |
| Autumn | sPM (10–100 µm) | 0.329 | 0.553 ** | 0.385 | −0.563 ** | 0.408 * |
| sPM (2.5–10 µm) | 0.244 | 0.054 | 0.266 | −0.153 | 0.282 | |
| wPM (10–100 µm) | 0.298 | 0.410 * | 0.497 * | −0.532 ** | 0.514 ** | |
| wPM (2.5–10 µm) | 0.510 ** | −0.226 | 0.522 | −0.414 * | 0.552 ** |
| Species | Spring | Summer | Autumn | |||
|---|---|---|---|---|---|---|
| APTI | Tolerant Level | APTI | Tolerant Level | APTI | Tolerant Level | |
| A. mosanensis | 5.26 | Sensitive | 7.73 | Intermediate | 6.81 | Sensitive |
| Z. serrata | 7.54 | Tolerant | 7.24 | Sensitive | 6.77 | Sensitive |
| E. japonicus | 6.97 | Moderately tolerant | 7.86 | Moderately tolerant | 8.07 | Moderately tolerant |
| E. alatus | 5.89 | Intermediate | 8.06 | Moderately tolerant | 7.88 | Moderately tolerant |
| P. × yedoensis | 6.25 | Intermediate | 8.08 | Moderately tolerant | 8.06 | Moderately tolerant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, H.-T.; Jeong, N.-R.; Park, B.-J. Seasonal Variations of Particulate Matter Capture and the Air Pollution Tolerance Index of Five Roadside Plant Species. Atmosphere 2023, 14, 138. https://doi.org/10.3390/atmos14010138
Bui H-T, Jeong N-R, Park B-J. Seasonal Variations of Particulate Matter Capture and the Air Pollution Tolerance Index of Five Roadside Plant Species. Atmosphere. 2023; 14(1):138. https://doi.org/10.3390/atmos14010138
Chicago/Turabian StyleBui, Huong-Thi, Na-Ra Jeong, and Bong-Ju Park. 2023. "Seasonal Variations of Particulate Matter Capture and the Air Pollution Tolerance Index of Five Roadside Plant Species" Atmosphere 14, no. 1: 138. https://doi.org/10.3390/atmos14010138
APA StyleBui, H.-T., Jeong, N.-R., & Park, B.-J. (2023). Seasonal Variations of Particulate Matter Capture and the Air Pollution Tolerance Index of Five Roadside Plant Species. Atmosphere, 14(1), 138. https://doi.org/10.3390/atmos14010138





